Densification of Magnesium Aluminate Spinel Using Manganese and Cobalt Fluoride as Sintering Aids
Abstract
1. Introduction
2. Materials and Methods
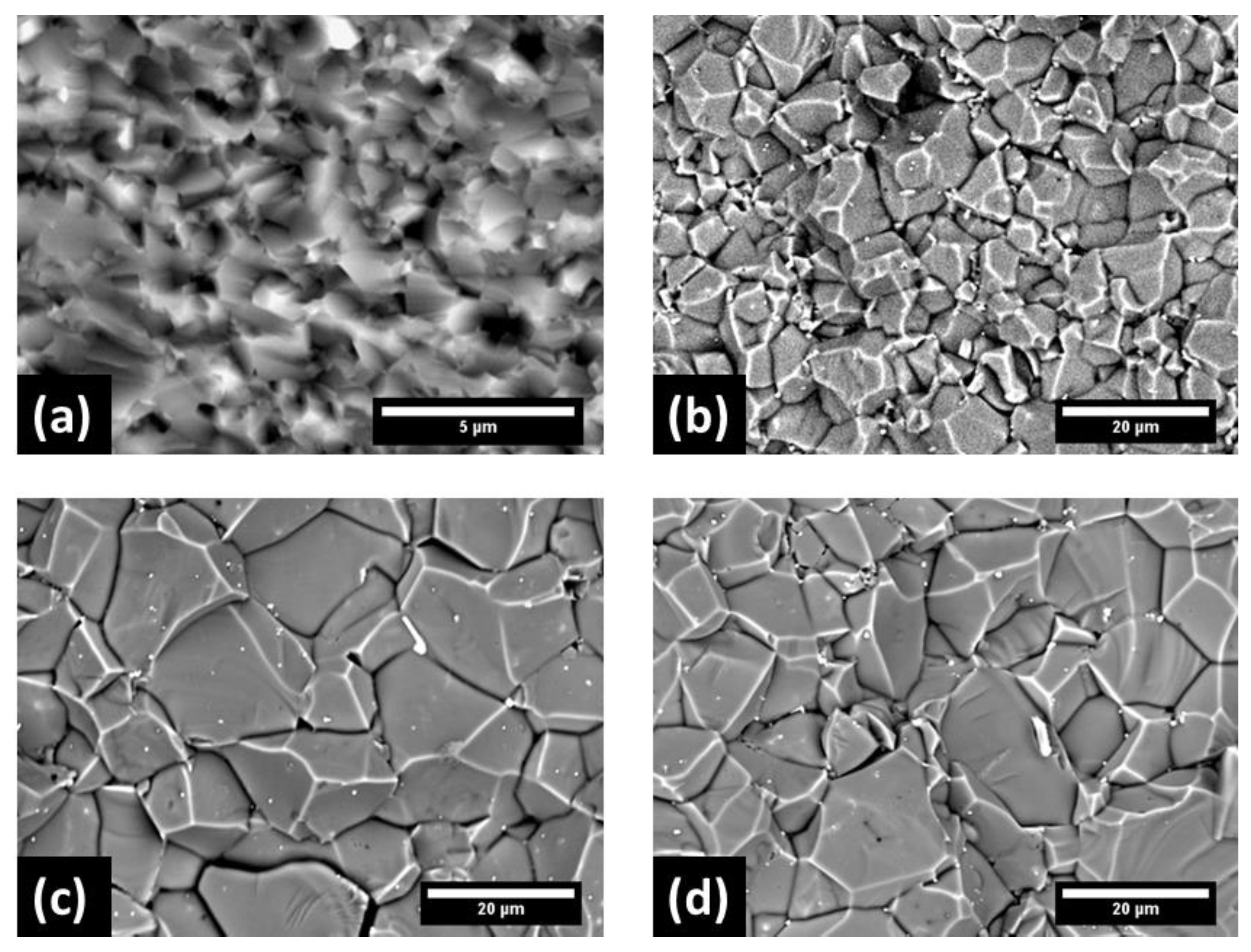
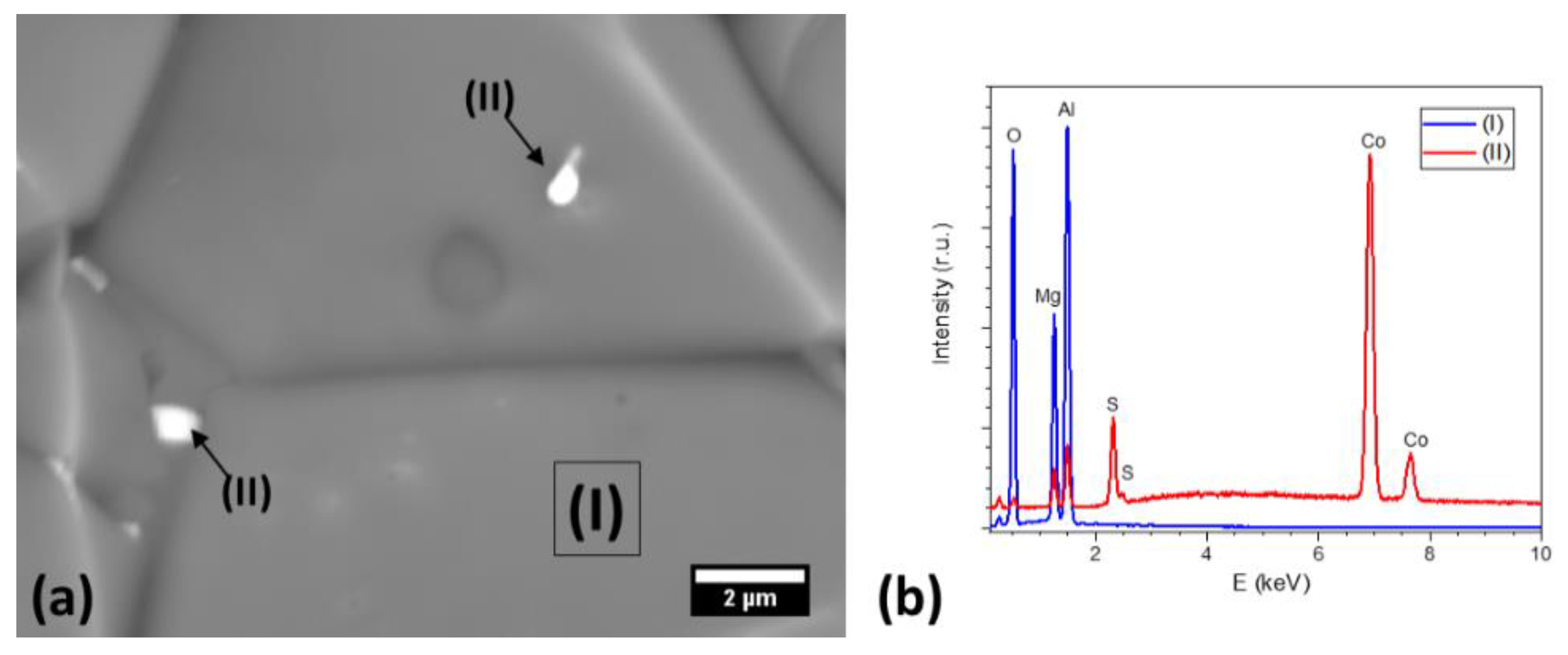
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Goldstein, A. Correlation between MgAl2O4-spinel structure, processing factors and functional properties of transparent parts (progress review). J. Eur. Ceram. Soc. 2012, 32, 2869–2886. [Google Scholar] [CrossRef]
- Reimanis, I.; Kleebe, H.-J. A Review on the Sintering and Microstructure Development of Transparent Spinel (MgAl2O4). J. Eur. Ceram. Soc. 2009, 92, 1472–1480. [Google Scholar] [CrossRef]
- Gilde, G.; Patel, P.; Patterson, P.; Blodgett, D.; Duncan, D.; Hahn, D. Evaluation of Hot Pressing and Hot Isostastic Pressing Parameters on the Optical Properties of Spinel. J. Eur. Ceram. Soc. 2005, 88, 2747–2751. [Google Scholar] [CrossRef]
- Krell, A.; Waetzig, K.; Klimke, J. Influence of the structure of MgO·nAl2O3 spinel lattices on transparent ceramics processing and properties. J. Eur. Ceram. Soc. 2012, 32, 2887–2898. [Google Scholar] [CrossRef]
- Hosseini, S.M. Structural, electronic and optical properties of spinel MgAl2O4 oxide. Phys. Status Solidi 2008, 245, 2800–2807. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Wills, J.M.; Grimes, N.W. Structure of Spinel. J. Eur. Ceram. Soc. 1999, 82, 3279–3292. [Google Scholar] [CrossRef]
- Tomita, A.; Sato, T.; Tanaka, K.; Kawabe, Y.; Shirai, M.; Tanaka, K.; Hanamura, E. Luminescence channels of manganese-doped spinel. J. Lumin. 2004, 109, 19–24. [Google Scholar] [CrossRef]
- Jouini, A.; Yoshikawa, A.; Brenier, A.; Fukuda, T.; Boulon, G. Optical properties of transition metal ion-doped MgAl2O4 spinel for laser application. Phys. Status Solidi C 2007, 4, 1380–1383. [Google Scholar] [CrossRef]
- Hanamura, E.; Kawabe, Y.; Takashima, H.; Sato, T.; Tomita, A. Optical properties of transition-metal doped spinels. J. Nonlinear Opt. Phys. Mater. 2003, 12, 467–473. [Google Scholar] [CrossRef]
- Sokol, M.; Ratzker, B.; Kalabukhov, S.; Dariel, M.P.; Galun, E.; Frage, N. Transparent Polycrystalline Magnesium Aluminate Spinel Fabricated by Spark Plasma Sintering. Adv. Mater. 2018, 30, 1706283. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.F.; Zhang, J.; Luo, D.W.; Gu, F.; Tang, D.Y.; Dong, Z.L.; Tan, G.E.B.; Que, W.X.; Zhang, T.S.; Li, S.; et al. Transparent ceramics: Processing, materials and applications. Prog. Solid State Chem. 2013, 41, 20–54. [Google Scholar] [CrossRef]
- Krell, A.; Klimke, J.; Hutzler, T. Transparent compact ceramics: Inherent physical issues. Opt. Mater. 2009, 31, 1144–1150. [Google Scholar] [CrossRef]
- Morita, K.; Kim, B.-N.; Yoshida, H.; Hiraga, K. Densification behavior of a fine-grained MgAl2O4 spinel during spark plasma sintering (SPS). Scr. Mater. 2010, 63, 565–568. [Google Scholar] [CrossRef]
- Kim, J.-M.; Kim, H.-N.; Park, Y.-J.; Ko, J.-W.; Lee, J.-W.; Kim, H.-D. Fabrication of transparent MgAl2O4 spinel through homogenous green compaction by microfluidization and slip casting. Ceram. Int. 2015, 41, 13354–13360. [Google Scholar] [CrossRef]
- Gajdowski, C.; Böhmler, J.; Lorgouilloux, Y.; Lemonnier, S.; d’Astorg, S.; Barraud, E.; Leriche, A. Influence of post-HIP temperature on microstructural and optical properties of pure MgAl2O4 spinel: From opaque to transparent ceramics. J. Eur. Ceram. Soc. 2017, 37, 5347–5351. [Google Scholar] [CrossRef]
- Sokol, M.; Halabi, M.; Kalabukhov, S.; Frage, N. Nano-structured MgAl2O4 spinel consolidated by high pressure spark plasma sintering (HPSPS). J. Eur. Ceram. Soc. 2017, 37, 755–762. [Google Scholar] [CrossRef]
- Rubat du Merac, M.; Reimanis, I.E.; Smith, C.; Kleebe, H.-J.; Müller, M.M. Effect of Impurities and LiF Additive in Hot-Pressed Transparent Magnesium Aluminate Spinel. Int. J. Appl. Ceram. Technol. 2013, 10, E33–E48. [Google Scholar] [CrossRef]
- Bonnefont, G.; Fantozzi, G.; Trombert, S.; Bonneau, L. Fine-grained transparent MgAl2O4 spinel obtained by spark plasma sintering of commercially available nanopowders. Ceram. Int. 2012, 38, 131–140. [Google Scholar] [CrossRef]
- Waetzig, K.; Hutzler, T. Highest UV-vis transparency of MgAl2O4 spinel ceramics prepared by hot pressing with LiF. J. Eur. Ceram. Soc. 2017, 37, 2259–2263. [Google Scholar] [CrossRef]
- Rozenburg, K.; Reimanis, I.E.; Kleebe, H.-J.; Cook, R.L. Sintering Kinetics of a MgAl2O4 Spinel Doped with LiF. J. Eur. Ceram. Soc. 2008, 91, 444–450. [Google Scholar] [CrossRef]
- Reimanis, I.E.; Kleebe, H.-J. Reactions in the sintering of MgAl2O4 spinel doped with LiF. IJMR 2007, 98, 1273–1278. [Google Scholar] [CrossRef]
- Villalobos, G.R.; Sanghera, J.S.; Aggarwal, I.D. Degradation of Magnesium Aluminum Spinel by Lithium Fluoride Sintering Aid. J. Eur. Ceram. Soc. 2005, 88, 1321–1322. [Google Scholar] [CrossRef]
- C21 Committee. Test Method for Specific Gravity of Fired Ceramic Whiteware Materials; ASTM International: West Conshohocken, PA, USA, 1988. [Google Scholar]
- Maca, K.; Trunec, M.; Chmelik, R. Processing and Properties of Fine-Grained Transparent MgAl2O4 Ceramics. Available online: http://www.ceramics-silikaty.cz/index.php?page=cs_detail_doi&id=502 (accessed on 3 December 2019).
- Chaim, R.; Marder, R.; Estournés, C.; Shen, Z. Densification and preservation of ceramic nanocrystalline character by spark plasma sintering. Adv. Appl. Ceram. 2012, 111, 280–285. [Google Scholar] [CrossRef]
- Scardi, P.; Lutterotti, L.; Maggio, R.D. Size-Strain and Quantitative Phase Analysis by the Rietveld Method. Adv. X-ray Anal. 1991, 35, 69–76. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A Friendly Java Program for Material Analysis Using Diffraction; (IUCr) Newsletter: Buffalo, NY, USA, 1999; pp. 14–15. [Google Scholar]
- Benameur, N.; Bernard-Granger, G.; Addad, A.; Raffy, S.; Guizard, C. Sintering Analysis of a Fine-Grained Alumina–Magnesia Spinel Powder. J. Eur. Ceram. Soc. 2011, 94, 1388–1396. [Google Scholar] [CrossRef]
- Talimian, A.; Pouchly, V.; El-Maghraby, H.F.; Maca, K.; Galusek, D. Impact of high energy ball milling on densification behaviour of magnesium aluminate spinel evaluated by master sintering curve and constant rate of heating approach. Ceram. Int. 2019, 45, 23467–23474. [Google Scholar] [CrossRef]
- Meir, S.; Kalabukhov, S.; Froumin, N.; Dariel, M.P.; Frage, N. Synthesis and Densification of Transparent Magnesium Aluminate Spinel by SPS Processing. J. Eur. Ceram. Soc. 2009, 92, 358–364. [Google Scholar] [CrossRef]
- Rozenburg, K.; Reimanis, I.E.; Kleebe, H.-J.; Cook, R.L. Chemical Interaction Between LiF and MgAl2O4 Spinel During Sintering. J. Eur. Ceram. Soc. 2007, 90, 2038–2042. [Google Scholar] [CrossRef]
- Ting, C.-J.; Lu, H.-Y. Defect Reactions and the Controlling Mechanism in the Sintering of Magnesium Aluminate Spinel. J. Eur. Ceram. Soc. 1999, 82, 841–848. [Google Scholar] [CrossRef]
- Mordekovitz, Y.; Shelly, L.; Halabi, M.; Kalabukhov, S.; Hayun, S. The Effect of Lithium Doping on the Sintering and Grain Growth of SPS-Processed, Non-Stoichiometric Magnesium Aluminate Spinel. Materials 2016, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.; Loiko, P.; Burshtein, Z.; Skoptsov, N.; Glazunov, I.; Galun, E.; Kuleshov, N.; Yumashev, K. Development of Saturable Absorbers for Laser Passive Q-Switching near 1.5 μm Based on Transparent Ceramic Co2+:MgAl2O4. J. Eur. Ceram. Soc. 2016, 99, 1324–1331. [Google Scholar] [CrossRef]
- Sai, Q.; Xia, C.; Rao, H.; Xu, X.; Zhou, G.; Xu, P. Mn, Cr-co-doped MgAl2O4 phosphors for white LEDs. J. Lumin. 2011, 131, 2359–2364. [Google Scholar] [CrossRef]
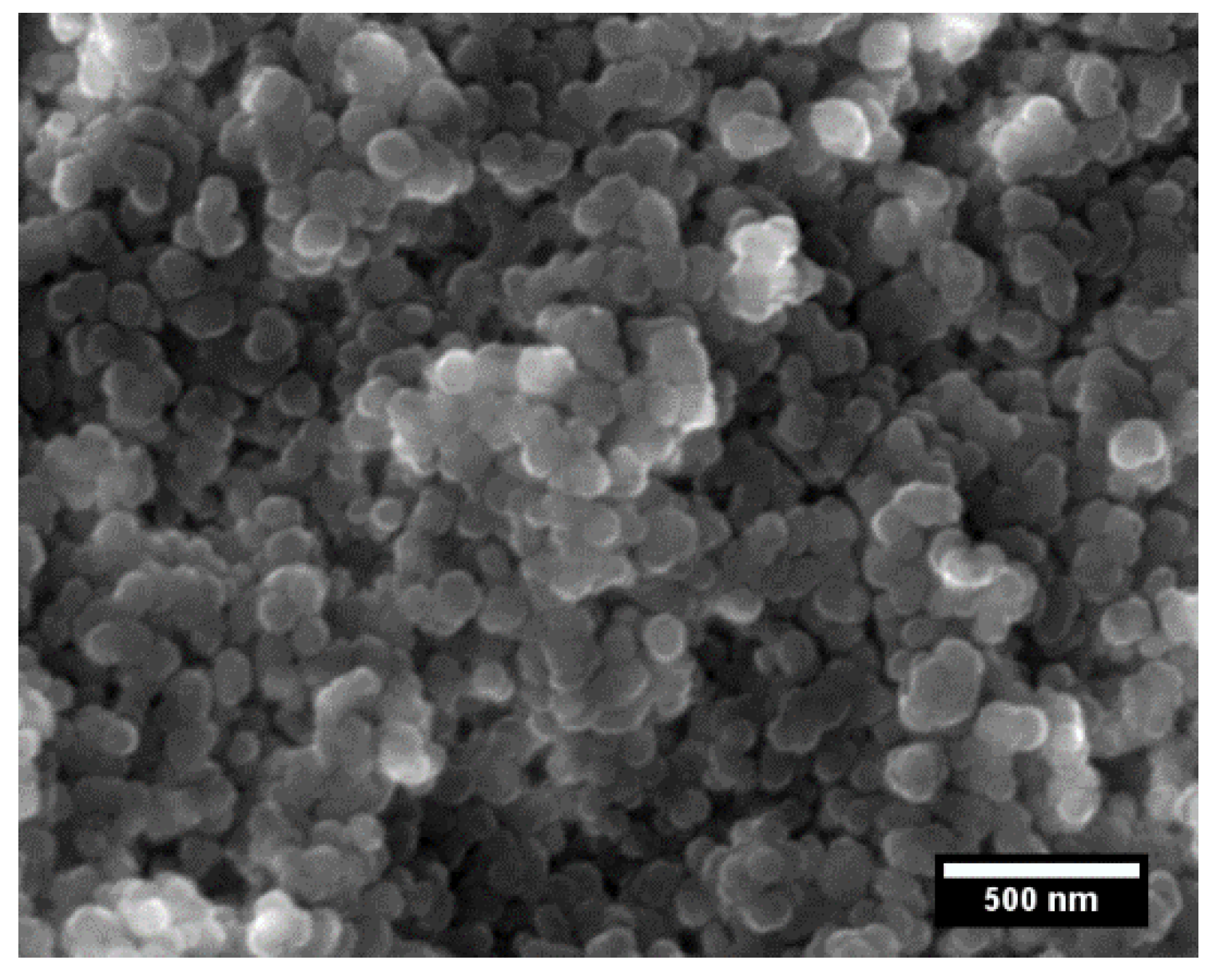
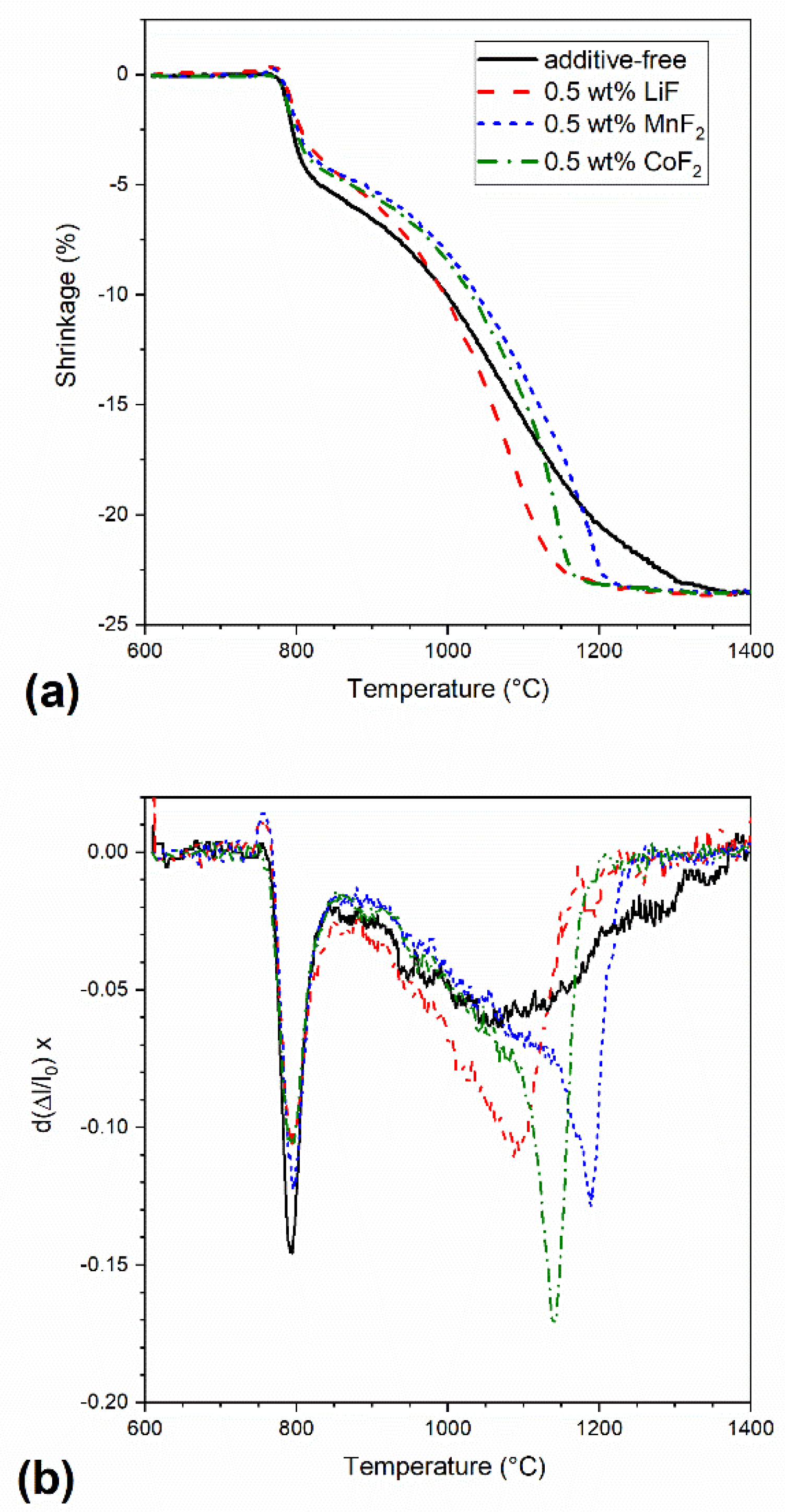
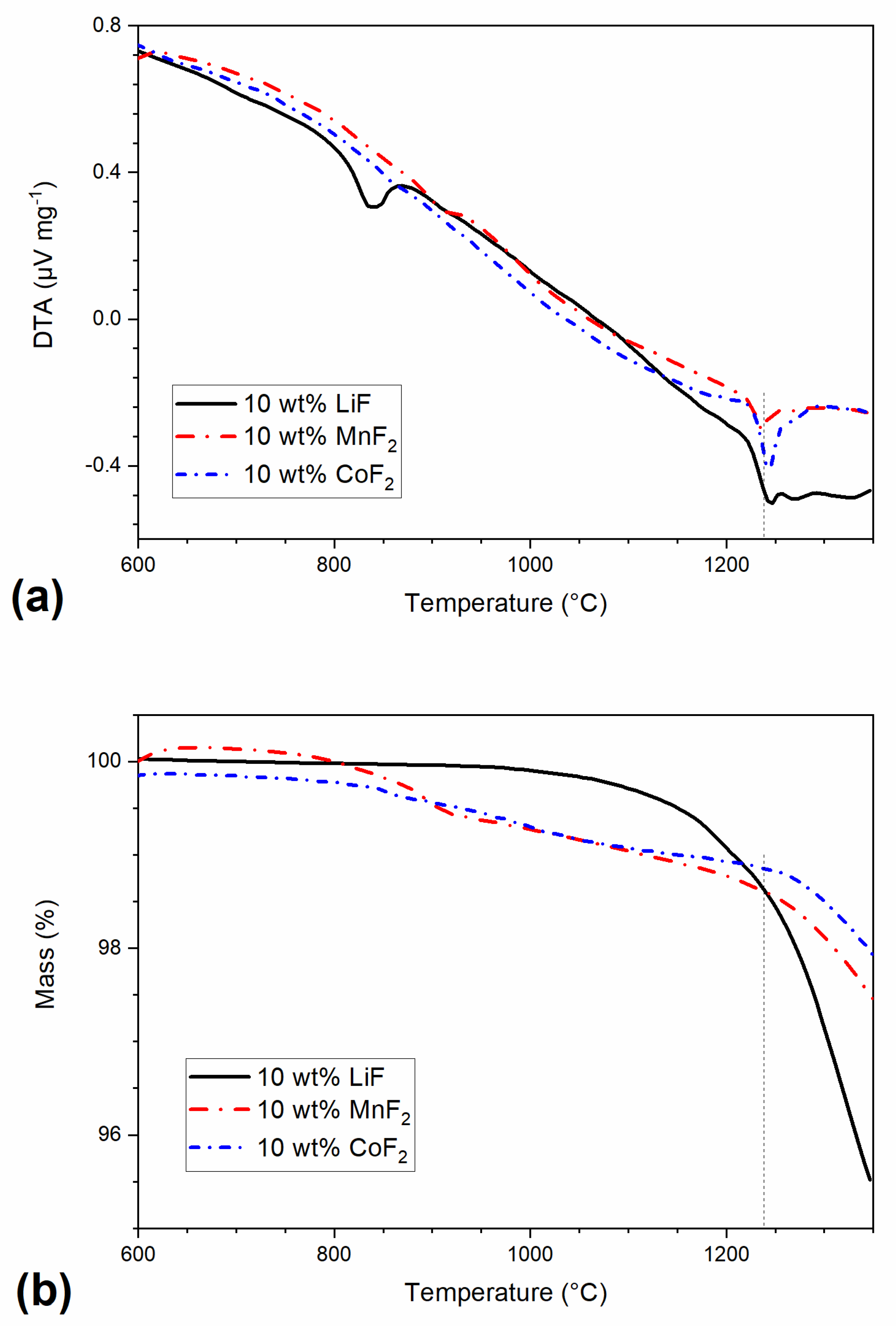
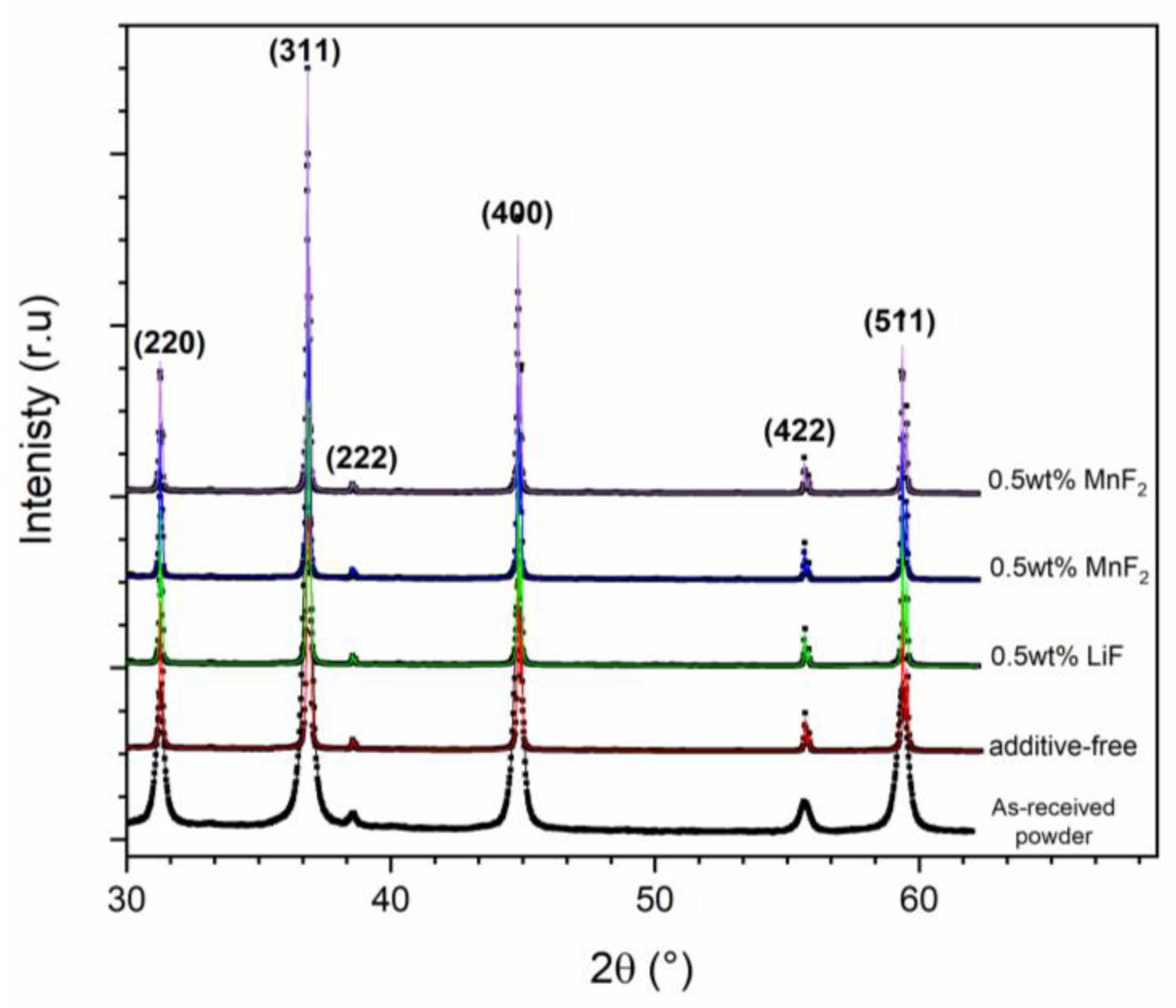
| Sample | Relative Density (%) | Apparent Porosity (%) |
|---|---|---|
| Additive-free | 99.90 (0.02) | 0.07 (0.03) |
| 0.5 wt% LiF | 99.6 (0.1) | 0.43 (0.00) |
| 0.5 wt% MnF2 | 99.4 (0.7) | 0.57 (0.06) |
| 0.5 wt% CoF2 | 99.7 (0.2) | 0.33(0.07) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talimian, A.; Pouchly, V.; Maca, K.; Galusek, D. Densification of Magnesium Aluminate Spinel Using Manganese and Cobalt Fluoride as Sintering Aids. Materials 2020, 13, 102. https://doi.org/10.3390/ma13010102
Talimian A, Pouchly V, Maca K, Galusek D. Densification of Magnesium Aluminate Spinel Using Manganese and Cobalt Fluoride as Sintering Aids. Materials. 2020; 13(1):102. https://doi.org/10.3390/ma13010102
Chicago/Turabian StyleTalimian, Ali, Vaclav Pouchly, Karel Maca, and Dusan Galusek. 2020. "Densification of Magnesium Aluminate Spinel Using Manganese and Cobalt Fluoride as Sintering Aids" Materials 13, no. 1: 102. https://doi.org/10.3390/ma13010102
APA StyleTalimian, A., Pouchly, V., Maca, K., & Galusek, D. (2020). Densification of Magnesium Aluminate Spinel Using Manganese and Cobalt Fluoride as Sintering Aids. Materials, 13(1), 102. https://doi.org/10.3390/ma13010102








