Study on Bainitic Transformation by Dilatometer and In Situ LSCM
Abstract
1. Introduction
2. Materials and Experimental Procedures
3. Results and Analysis
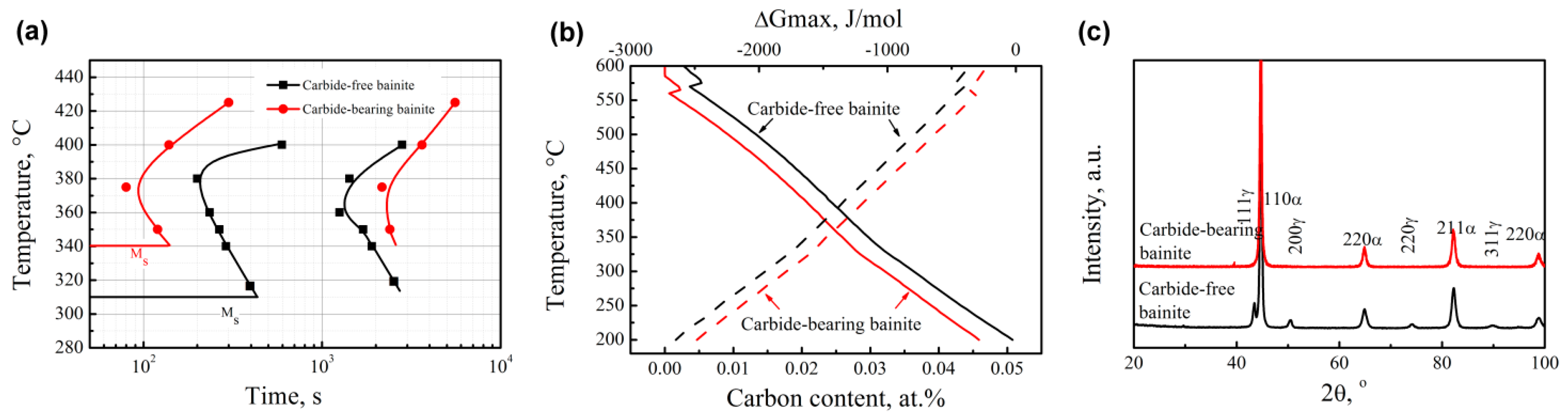
3.1. Transformation Kinetics
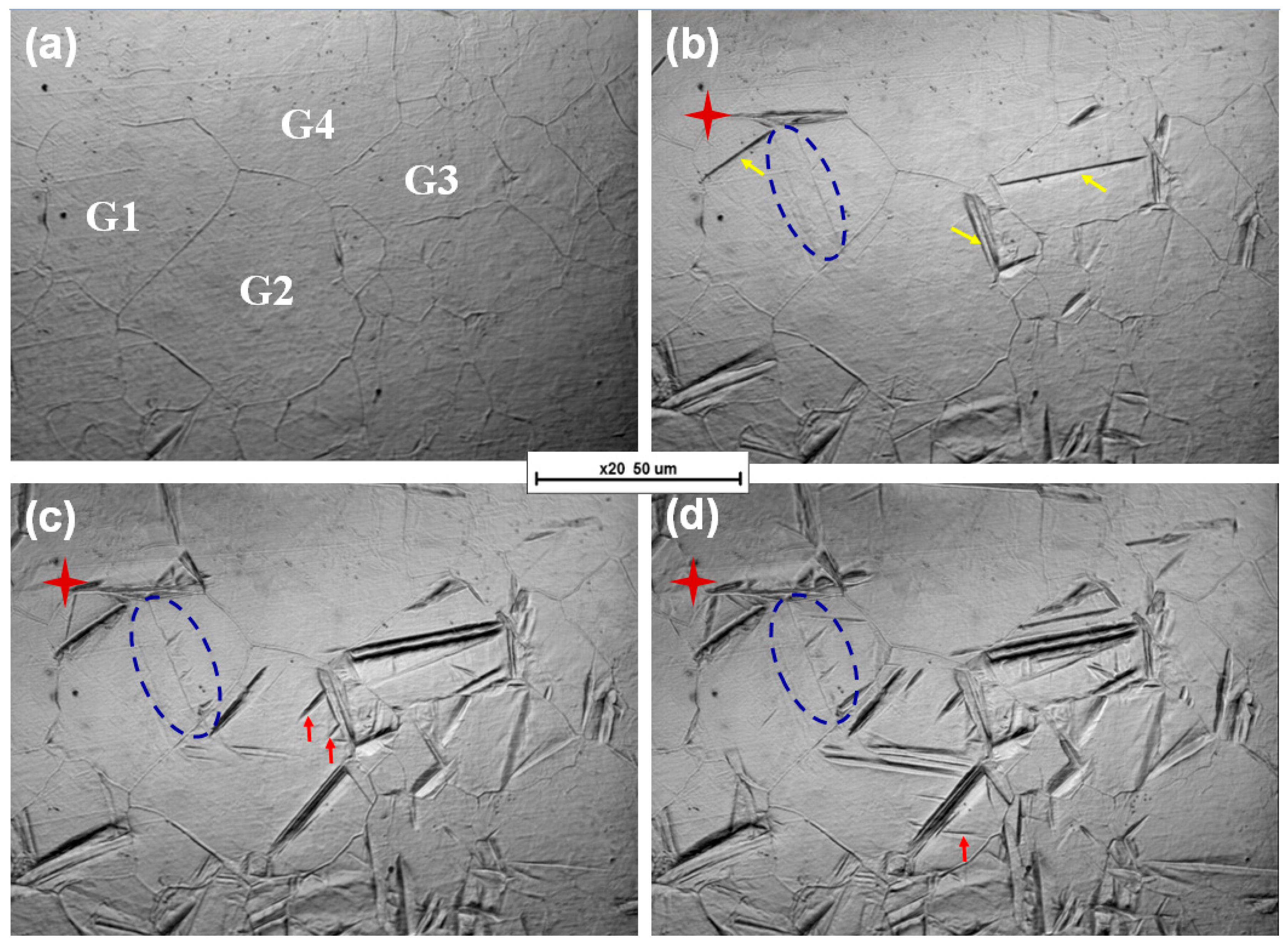
3.2. In Situ Observation
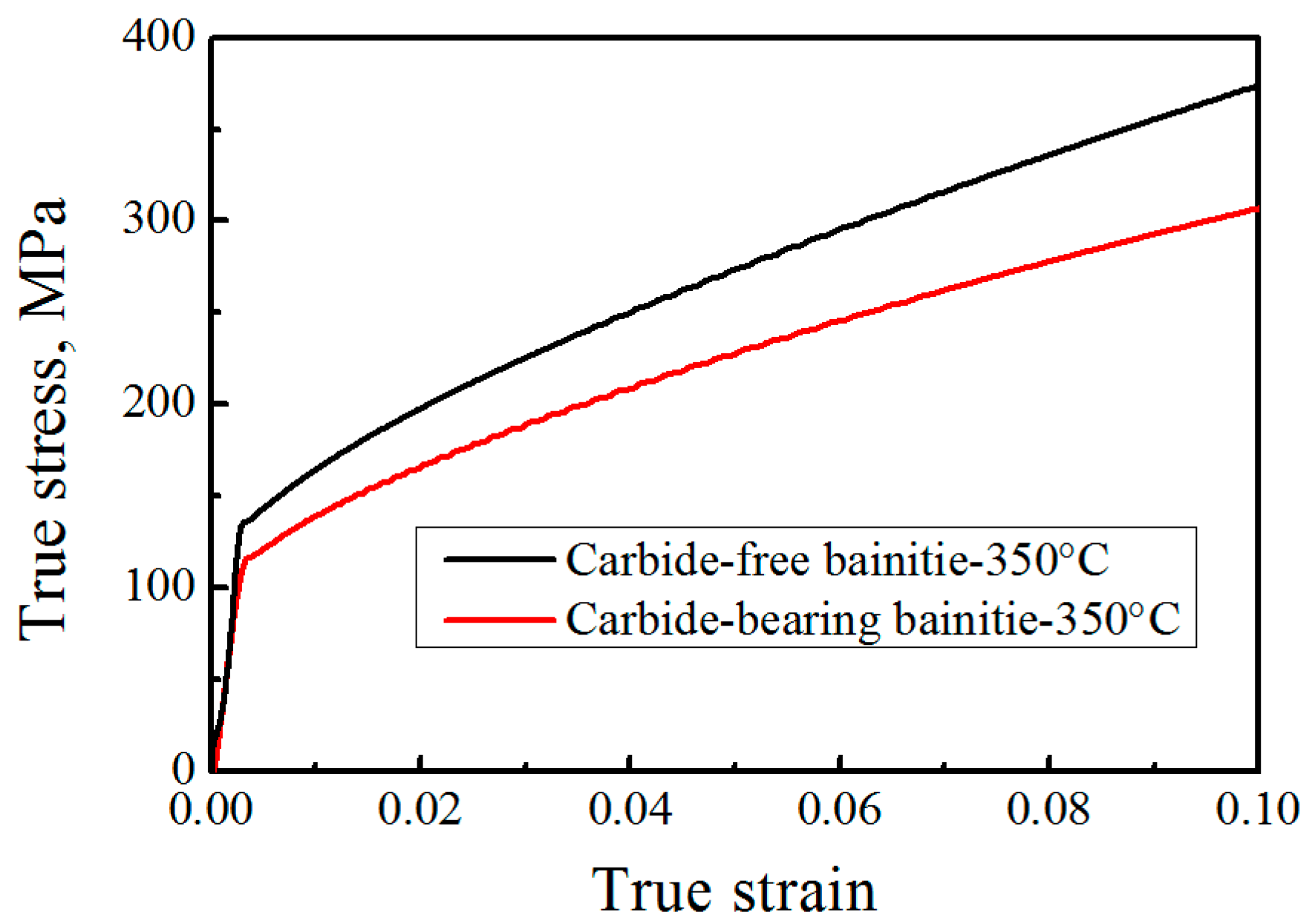
3.3. Relationship between Transformation, Kinetics and Microstructure
4. Conclusions
- Carbide-free bainitic steel requires a long incubation period, because Si and Al hinder C diffusion, making the distribution of carbon atoms more homogeneous. Carbide-bearing bainitic steel exhibits a long transformation time.
- Bainitic ferrite plates nucleate preferentially on the grain boundary and new plates nucleate on previously formed ones. Compared to nucleation at grain boundaries, nucleation on previously formed bainitic ferrite is faster in some grains.
- The location and number of nucleation points have an important influence on the thickness of bainitic ferrite. The greater the number of positions of preferential nucleation on the original austenite grain boundary in the early transformation stage, the greater the thickness of the bainitic ferrite.
Author Contributions
Funding
Conflicts of Interest
References
- Garciamateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Acceleration of low-temperature bainite. ISIJ. Int. 2003, 43, 285–288. [Google Scholar]
- Long, X.; Zhang, F.; Yang, Z.; Lv, B. Study on microstructures and properties of carbide-free and carbide-bearing bainitic steels. Mater. Sci. Eng. A 2018, 715, 10–16. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, X.; Zhao, X.; Dong, C.; Kang, S. Effects of nucleation site and morphology of carbide-free bainite on microstructures and properties of bainite/martensite multi-phase steels. Mater. Sci. Eng. A 2019, 744, 86–93. [Google Scholar] [CrossRef]
- Yang, Z.N.; Zhao, X.J.; Zhang, F.C.; Li, Y.G.; Long, X.Y.; Zhang, M. Review on method of accelerating bainite transformation. J. Yanshan Uni. 2018, 42, 471–478. [Google Scholar] [CrossRef]
- Peet, M.; Babu, S.; Miller, M.; Bhadeshia, H. Three-dimensional atom probe analysis of carbon distribution in low-temperature bainite. Scr. Mater. 2004, 50, 1277–1281. [Google Scholar] [CrossRef]
- Caballero, F.; Miller, M.; Garcia-Mateo, C.; Cornide, J.; Santofimia, M.; Caballero, F. Temperature dependence of carbon supersaturation of ferrite in bainitic steels. Scr. Mater. 2012, 67, 846–849. [Google Scholar] [CrossRef]
- Hulme-Smith, C.; Lonardelli, I.; Dippel, A.; Bhadeshia, H. Experimental evidence for non-cubic bainitic ferrite. Scr. Mater. 2013, 69, 409–412. [Google Scholar] [CrossRef]
- Sampath, S.; Rementeria, R.; Huang, X.; Poplawsky, J.; Garcia-Mateo, C.; Caballero, F.; Janisch, R.; Poplawsky, J. The role of silicon, vacancies, and strain in carbon distribution in low temperature bainite. J. Alloy. Compd. 2016, 673, 289–294. [Google Scholar] [CrossRef]
- Jang, J.H.; Bhadeshia, H.; Suh, D.-W. Solubility of carbon in tetragonal ferrite in equilibrium with austenite. Scr. Mater. 2013, 68, 195–198. [Google Scholar] [CrossRef]
- Pereloma, E.; Timokhina, I.; Miller, M.; Hodgson, P. Three-dimensional atom probe analysis of solute distribution in thermomechanically processed TRIP steels. Acta Mater. 2007, 55, 2587–2598. [Google Scholar] [CrossRef]
- Timokhina, I.B.; Liss, K.-D.; Raabe, D.; Rakha, K.; Beladi, H.; Xiong, X.Y.; Hodgson, P.D. Growth of bainitic ferrite and carbon partitioning during the early stages of bainite transformation in a 2 mass% silicon steel studied byin situneutron diffraction, TEM and APT. J. Appl. Crystallogr. 2016, 49, 399–414. [Google Scholar] [CrossRef]
- Guo, L.; Bhadeshia, H.K.D.H.; Roelofs, H.; Lembke, M.I. In situ synchrotron X-ray study of bainite transformation kinetics in a low-carbon Si-containing steel. Mater. Sci. Technol. 2017, 33, 2147–2156. [Google Scholar] [CrossRef]
- Xu, G.; Liu, F.; Wang, L.; Hu, H. A new approach to quantitative analysis of bainitic transformation in a superbainite steel. Scr. Mater. 2013, 68, 833–836. [Google Scholar] [CrossRef]
- Sainis, S.; Farahani, H.; Gamsjager, E.; Van Der Zwaag, S. An In-Situ LSCM Study on Bainite Formation in a Fe-0.2C-1.5Mn-2.0Cr Alloy. Metals 2018, 8, 498. [Google Scholar] [CrossRef]
- Zhou, W.; Hou, T.; Tao, L.; Wu, K. In Situ Observation on the Effects of Prior Martensite Formation on Nanostructured Low–Temperature Bainite Transformation. Metals 2018, 8, 818. [Google Scholar] [CrossRef]
- Long, X.; Kang, J.; Lv, B.; Zhang, F. Carbide-free bainite in medium carbon steel. Mater. Des. 2014, 64, 237–245. [Google Scholar] [CrossRef]
- De, A.K.; Murdock, D.C.; Mataya, M.C.; Speer, J.G.; Matlock, D.K. Quantitative measurement of deformation-induced martensite in 304 stainless steel by X-ray diffraction. Scr. Mater. 2004, 50, 1445–1449. [Google Scholar] [CrossRef]
- Zhang, M.X.; Wang, J.; Kang, M.K. The study on effect of silicon in steels (Part 1)-Influence of silicon on the continuous cooling transformation dynamics of undercooled austenite. Heat Treat. Metals 1992, 8, 3–7. [Google Scholar]
- Liu, P.; Xing, W.; Cheng, X.; Li, D.; Li, Y.; Chen, X.-Q. Effects of dilute substitutional solutes on interstitial carbon in α -Fe: Interactions and associated carbon diffusion from first-principles calculations. Phys. Rev. B 2014, 90, 024103. [Google Scholar] [CrossRef]
- Peet, M.; Bhadeshia, H.K.D.H. Available online: https://www.msm.cam.ac.uk/map/steel/tar/mucg83.exe (accessed on 10 November 2018).
- Rementeria, R.; Poplawsky, J.D.; Aranda, M.M.; Guo, W.; Jimenez, J.A.; Garcia-Mateo, C.; Caballero, F.G. Carbon concentration measurements by atom probe tomography in the ferritic phase of high-silicon steels. Acta Mater. 2017, 125, 359–368. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels, 3rd ed.; Maney Publishing: Leeds, UK, 2015. [Google Scholar]
- Chu, C.; Qin, Y.; Li, X.; Yang, Z.; Zhang, F.; Guo, C.; Long, X.; You, L. Effect of Two-Step Austempering Process on Transformation Kinetics of Nanostructured Bainitic Steel. Materials 2019, 12, 166. [Google Scholar] [CrossRef] [PubMed]
- Suehiro, M.; Liu, Z.-K.; Ågren, J. A mathematical model for the solute drag effect on recrystallization. Met. Mater. Trans. A 1998, 29, 1029–1034. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Diffusion-controlled growth of ferrite plates in plain-carbon steels. Mater. Sci. Technol. 1985, 1, 497–504. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, W.; Yang, Z.; Zhang, C.; Van Der Zwaag, S. A 2D analysis of the competition between the equiaxed ferritic and the bainitic morphology based on a Gibbs Energy Balance approach. Acta Mater. 2016, 105, 317–327. [Google Scholar] [CrossRef]
- Xia, Y.; Miyamoto, G.; Yang, Z.; Zhang, C.; Furuhara, T. Direct measurement of carbon enrichment in the incomplete bainite transformation in Mo added low carbon steels. Acta Mater. 2015, 91, 10–18. [Google Scholar] [CrossRef]




| Materials | C | Si | Mn | Cr | Ni | Mo | Al |
|---|---|---|---|---|---|---|---|
| Carbide-free bainite | 0.34 | 1.48 | 1.52 | 1.15 | 0.93 | 0.40 | 0.71 |
| Carbide-bearing bainite | 0.34 | 0.01 | 1.61 | 1.24 | 0.96 | 0.45 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, X.; Zhang, F.; Yang, Z.; Zhang, M. Study on Bainitic Transformation by Dilatometer and In Situ LSCM. Materials 2019, 12, 1534. https://doi.org/10.3390/ma12091534
Long X, Zhang F, Yang Z, Zhang M. Study on Bainitic Transformation by Dilatometer and In Situ LSCM. Materials. 2019; 12(9):1534. https://doi.org/10.3390/ma12091534
Chicago/Turabian StyleLong, Xiaoyan, Fucheng Zhang, Zhinan Yang, and Ming Zhang. 2019. "Study on Bainitic Transformation by Dilatometer and In Situ LSCM" Materials 12, no. 9: 1534. https://doi.org/10.3390/ma12091534
APA StyleLong, X., Zhang, F., Yang, Z., & Zhang, M. (2019). Study on Bainitic Transformation by Dilatometer and In Situ LSCM. Materials, 12(9), 1534. https://doi.org/10.3390/ma12091534





