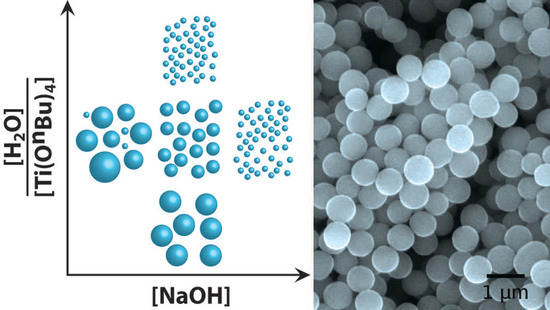
Titanium Oxide Microspheres with Tunable Size and Phase Composition
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
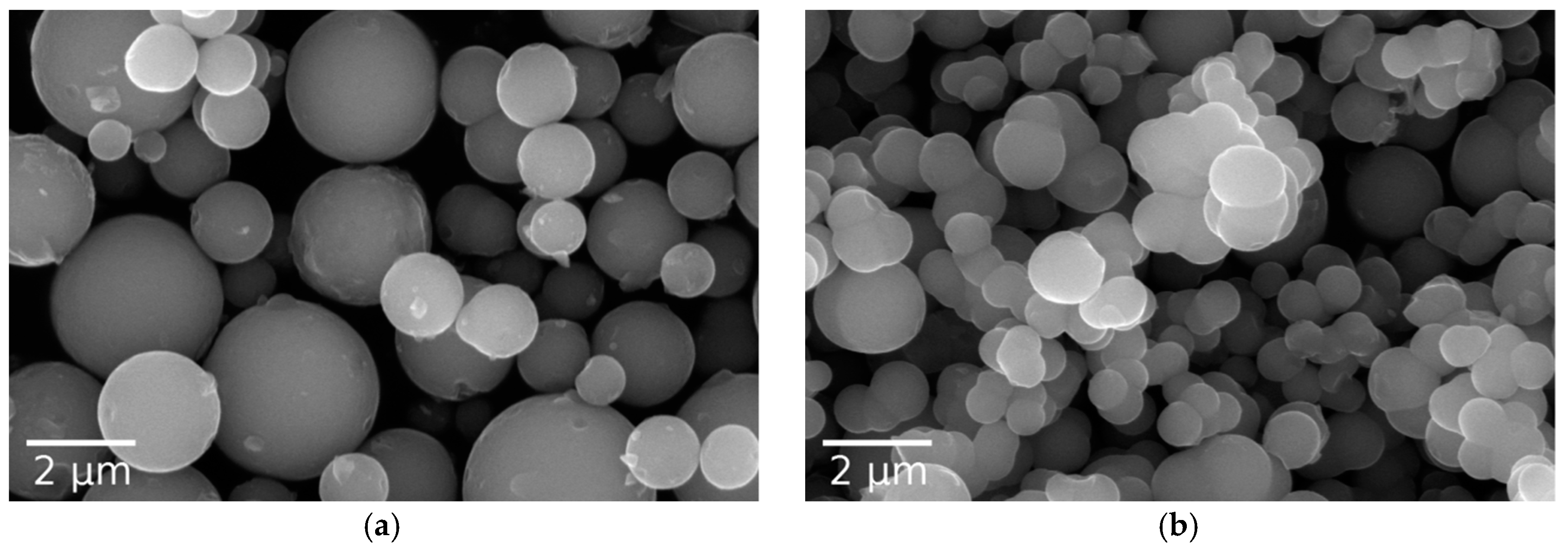
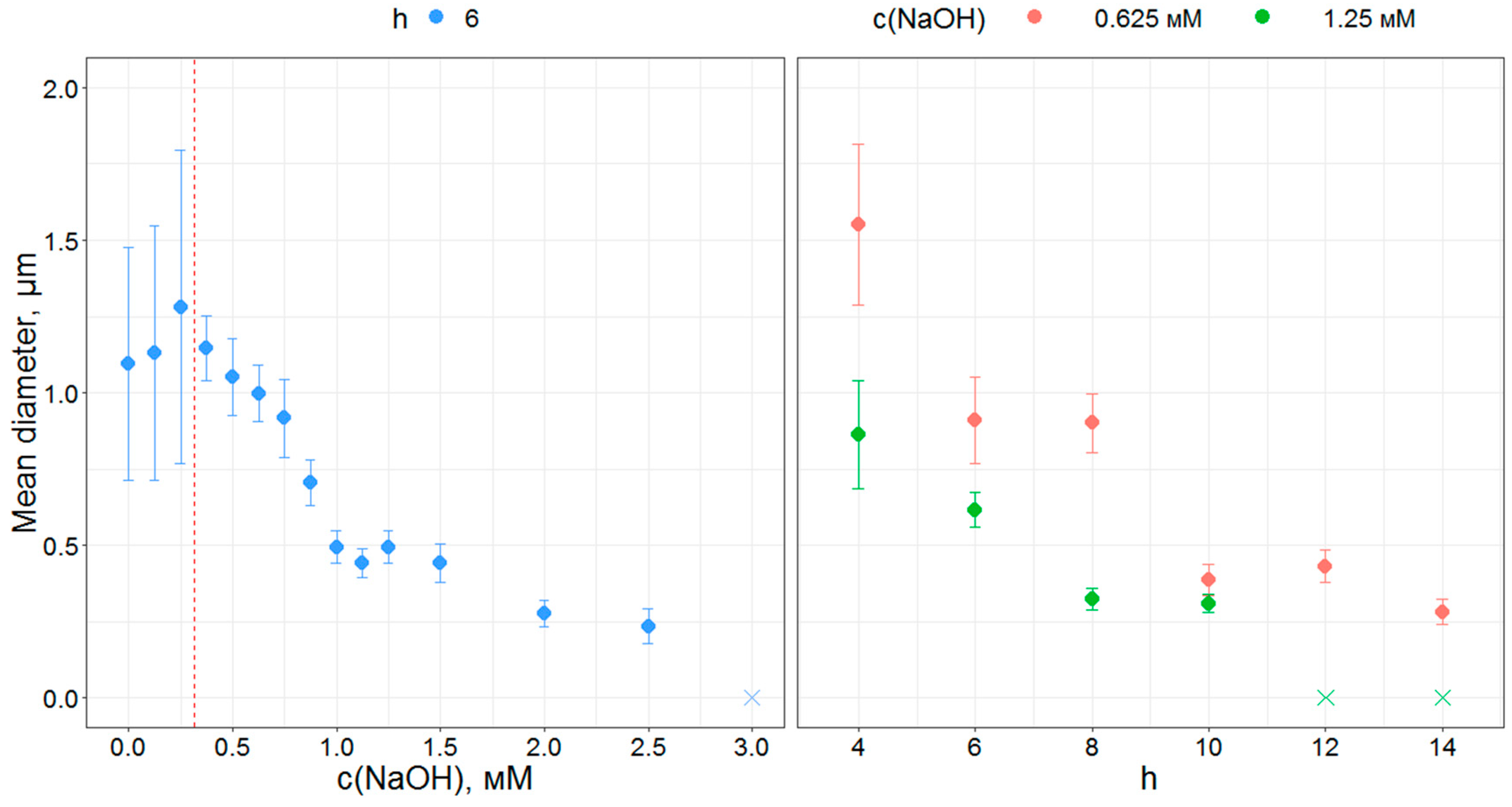
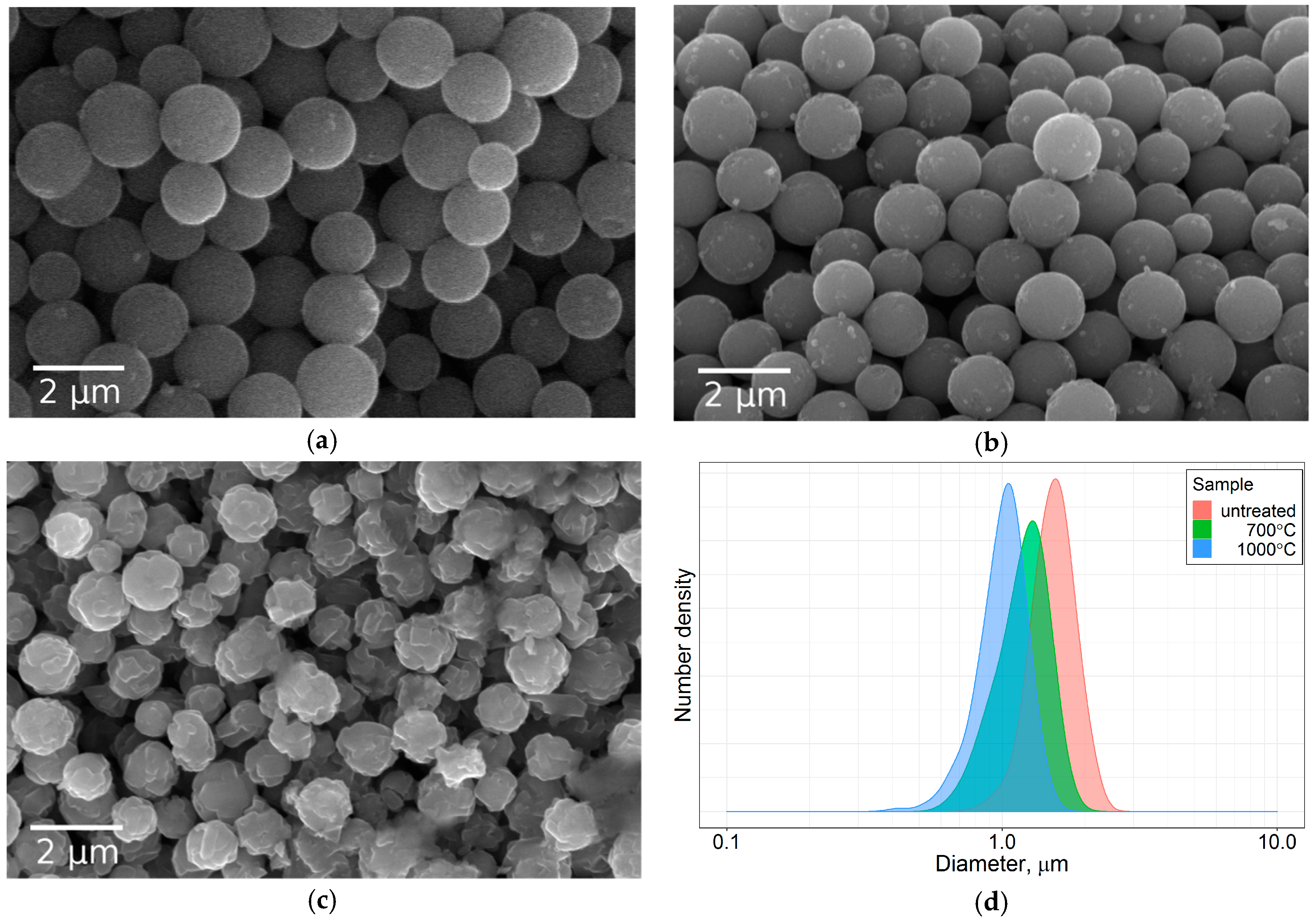
3.1. Particle Morphology and Size
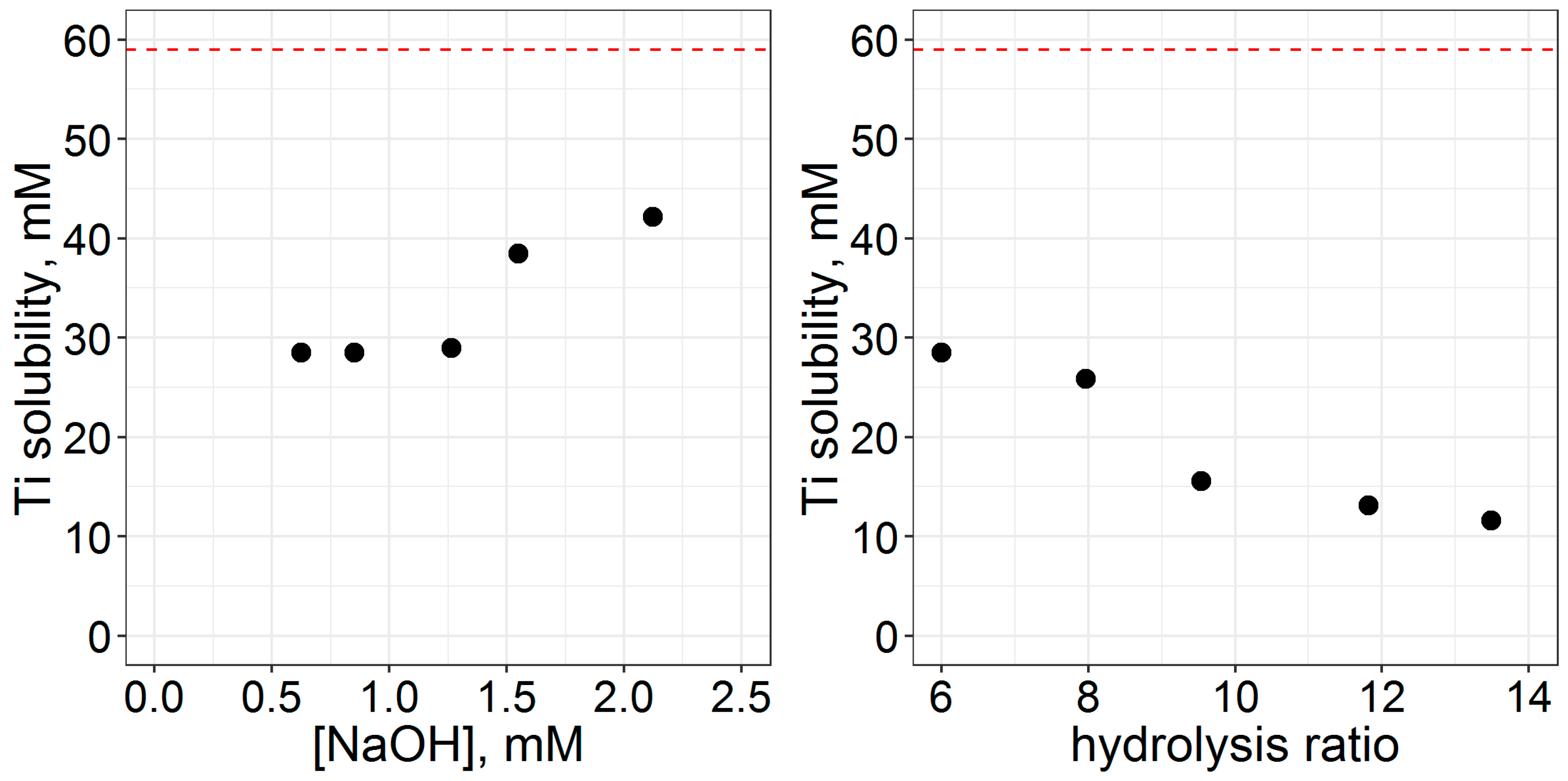
3.2. Processing
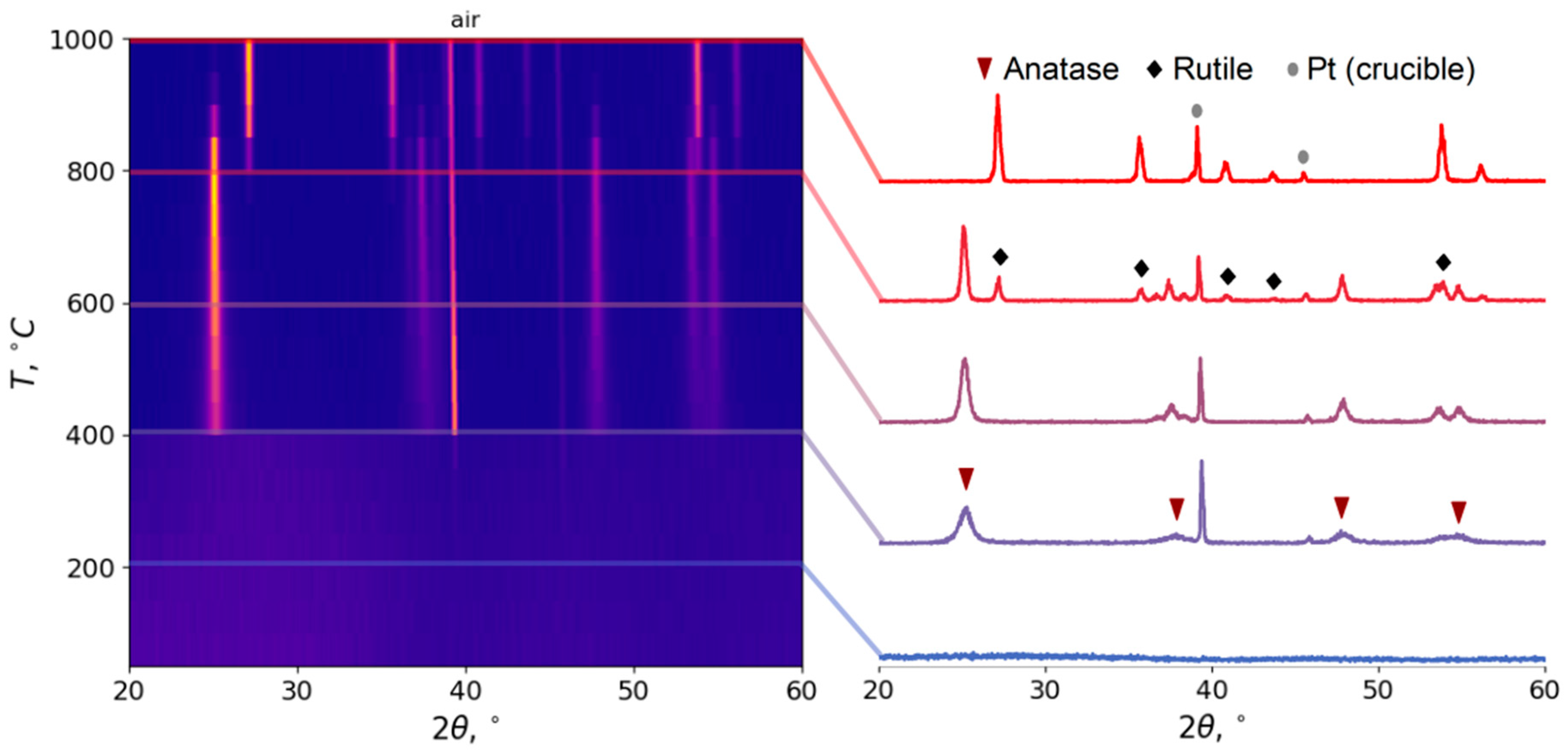
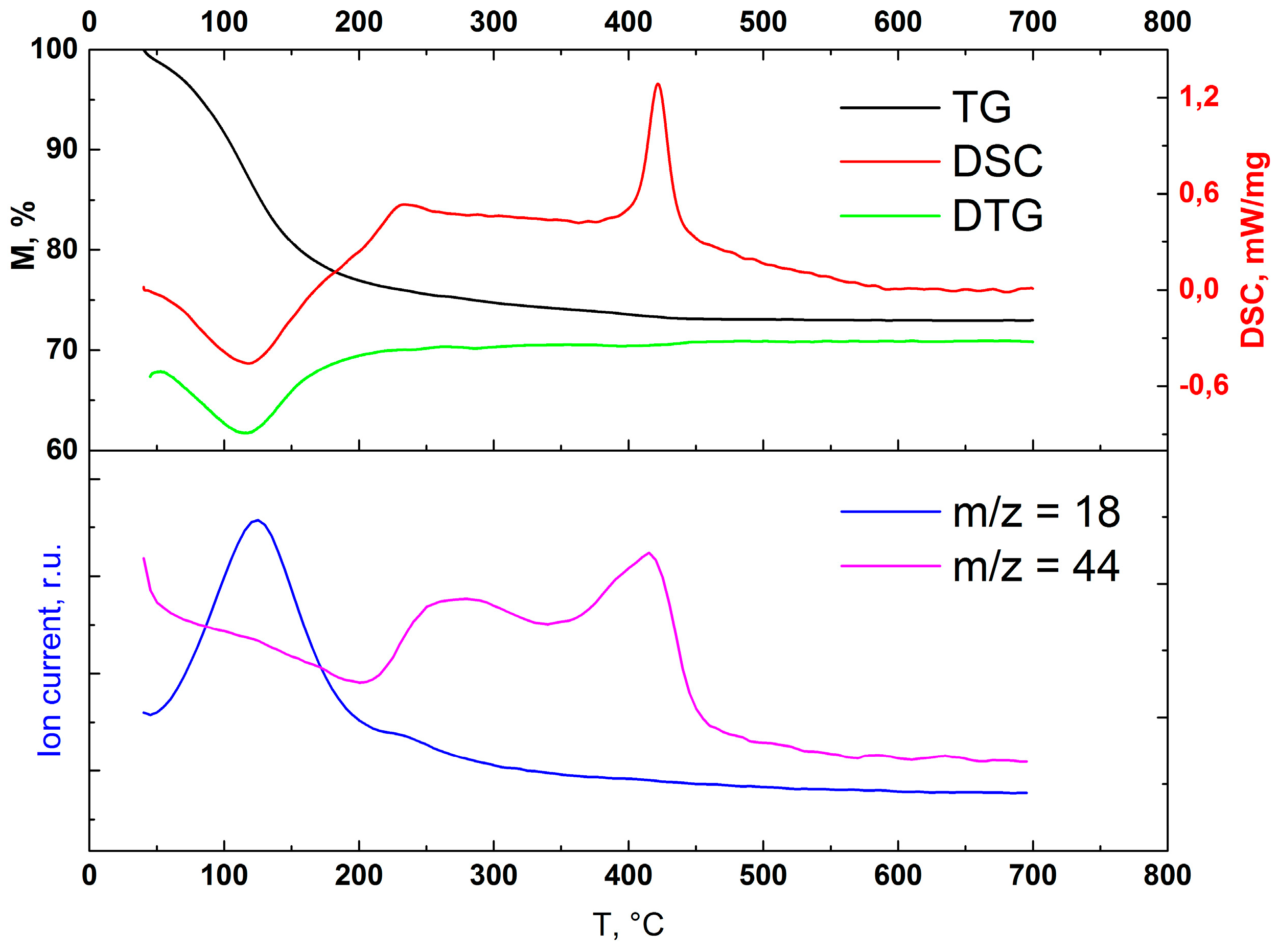
3.2.1. Choosing Optimal Conditions: High Temperature XRD
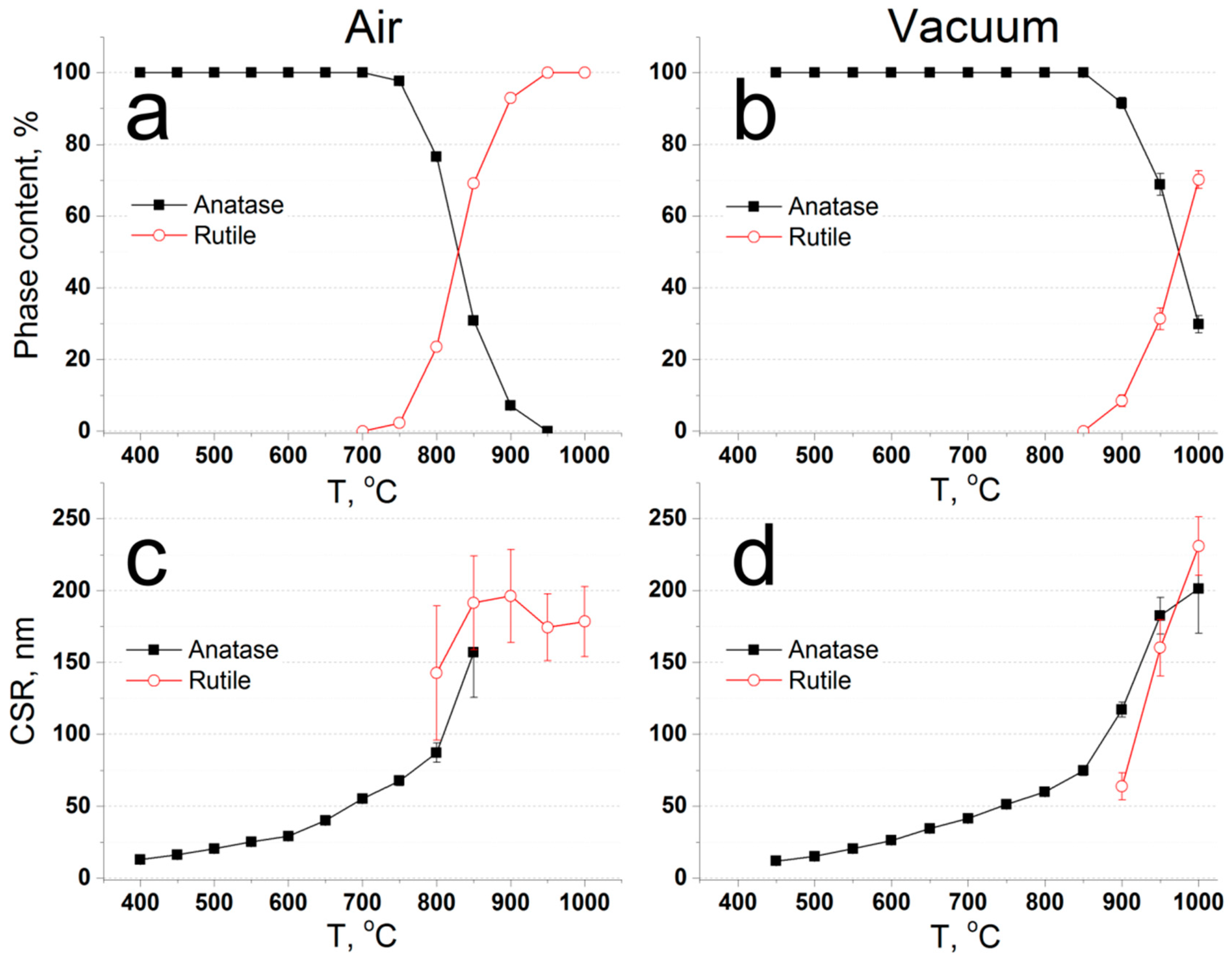
3.2.2. Annealing: Phase Composition
3.2.3. Annealing: Shrinkage
3.2.4. Hydrothermal Treatment
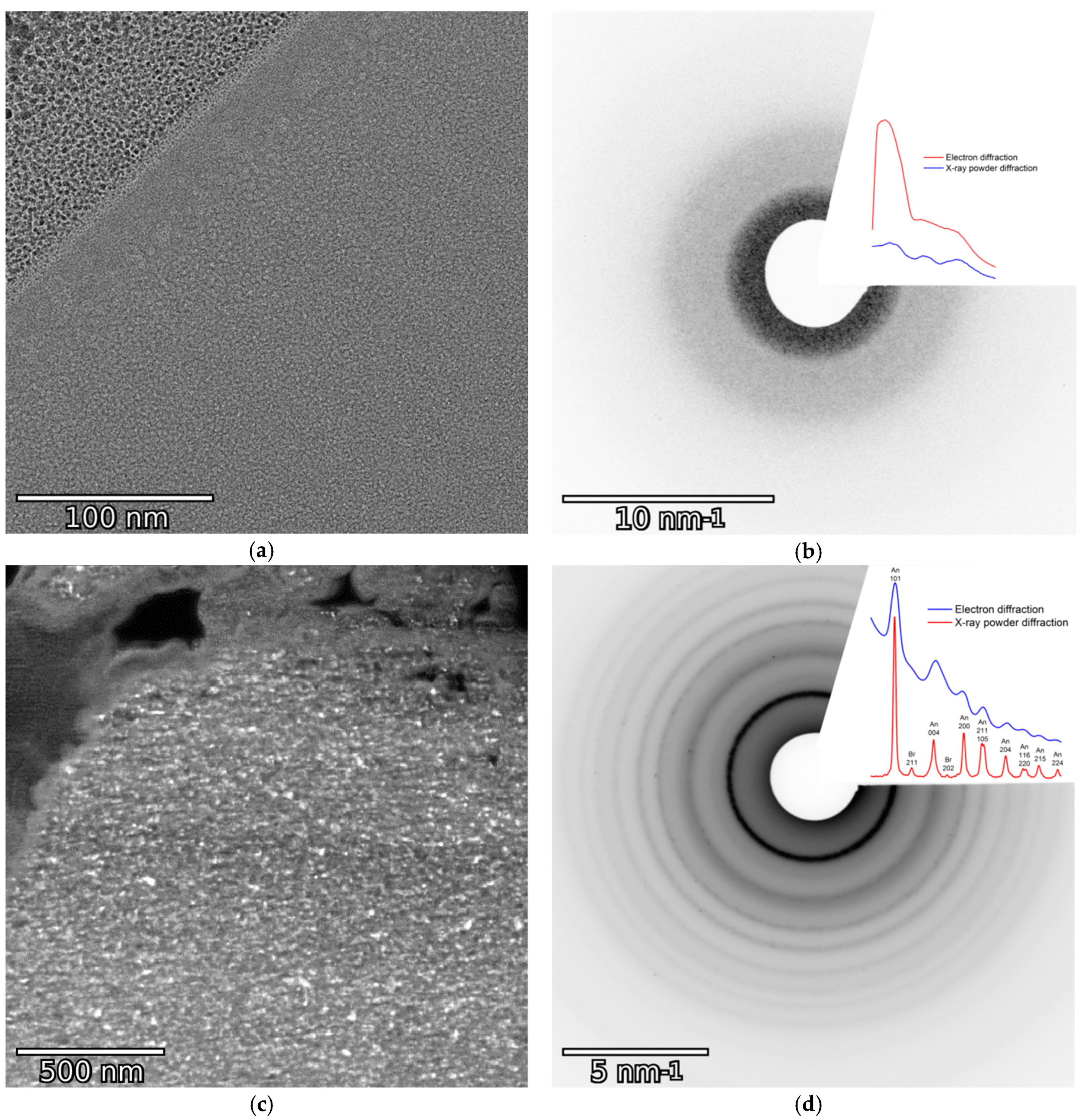
3.3. Internal Structure of the Microspheres
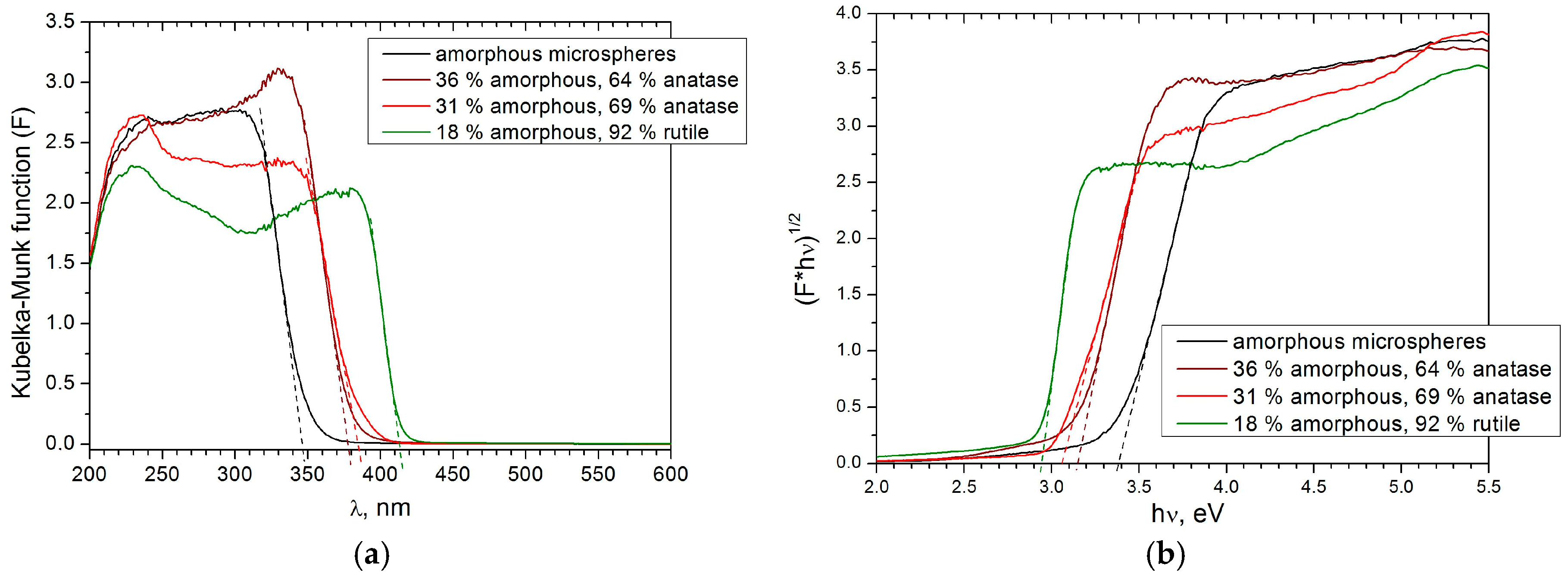
3.4. Optical and Photoprotector Properties of the Microspheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Huang, S.; Chen, Y.; Chiueh, L.; Shih, D.Y. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [PubMed]
- Morsella, M.; Alessandro, N.; Lanterna, A.E.; Scaiano, J.C. Improving the Sunscreen Properties of TiO2 through an Understanding of Its Catalytic Properties. ACS Omega 2016, 1, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-M.; Suryanarayanan, V.; Ho, K.-C. Influences of different TiO2 morphologies and solvents on the photovoltaic performance of dye-sensitized solar cells. J. Power Sources 2009, 188, 635–641. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. An analytical study of the porosity effect on dye-sensitized solar cell performance. Sol. Energy Mater. Sol. Cells 2006, 90, 1331–1344. [Google Scholar] [CrossRef]
- Mascolo, M.; Ring, T.; Mascolo, M.C.; Ring, T.A. Recyclable Aggregates of Mesoporous Titania Synthesized by Thermal Treatment of Amorphous or Peptized Precursors. Materials 2018, 11, 381. [Google Scholar] [CrossRef]
- Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R.; Niu, B.; Wang, X.; Wu, K.; He, X.; Zhang, R. Mesoporous Titanium Dioxide: Synthesis and Applications in Photocatalysis, Energy and Biology. Materials 2018, 11, 1910. [Google Scholar] [CrossRef] [PubMed]
- Du, L.X.; Jiang, Z.T.; Li, R. Preparation of porous titania microspheres for HPLC packing by sol-gel method. Mater. Lett. 2013, 95, 17–20. [Google Scholar] [CrossRef]
- Jiang, Z.T.; Zuo, Y.M. Synthesis of porous titania microspheres for HPLC packings by polymerization-induced colloid aggregation (PICA). Anal. Chem. 2001, 73, 686–688. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, Z.T.; Li, R.; Tan, J. Simultaneous Determination of Cyclamate, Acesulfame, and Aspartame in Beverages by Titania-Based RP-HPLC. Food Anal. Methods 2014, 7, 1400–1406. [Google Scholar] [CrossRef]
- Winkler, J.; Marmé, S. Titania as a sorbent in normal-phase liquid chromatography. J. Chromatogr. A 2000, 888, 51–62. [Google Scholar] [CrossRef]
- Lebedev, V.A.; Kozlov, D.A.; Kolesnik, I.V.; Poluboyarinov, A.S.; Becerikli, A.E.; Grünert, W.; Garshev, A.V. The amorphous phase in titania and its influence on photocatalytic properties. Appl. Catal. B Environ. 2016, 195, 39–47. [Google Scholar] [CrossRef]
- Nakamura, N. Chapter 34—Makeup Cosmetics; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128020050. [Google Scholar]
- Lebedev, V.A.; Sudin, V.V.; Kozlov, D.A.; Garshev, A.V. Photocatalytic properties of nanocrystalline TiO2 modified with CuO and WO3. Nanotechnol. Russ. 2016, 11, 20–28. [Google Scholar] [CrossRef]
- Gracheva, N.N.; Romanchuk, A.Y.; Smirnov, E.A.; Meledina, M.A.; Garshev, A.V.; Shirshin, E.A.; Fadeev, V.V.; Kalmykov, S.N. Am(III) sorption onto TiO2 samples with different crystallinity and varying pore size distributions. Appl. Geochem. 2014, 42, 69–76. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Green, M.; Just, M.; Li, Y.Y.; Chen, X. Titanium dioxide nanomaterials for photocatalysis. J. Phys. D. Appl. Phys. 2017, 50, 19. [Google Scholar] [CrossRef]
- Gomes, J.; Lincho, J.; Domingues, E.; Quinta-Ferreira, M.R.; Martins, C.R. N–TiO2 Photocatalysts: A Review of Their Characteristics and Capacity for Emerging Contaminants Removal. Water 2019, 11, 373. [Google Scholar] [CrossRef]
- Nasr, M.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Recent Progress on Titanium Dioxide Nanomaterials for Photocatalytic Applications. ChemSusChem 2018, 11, 3023–3047. [Google Scholar] [CrossRef]
- Nawrocki, J.; Dunlap, C.; McCormick, A.; Carr, P.W. Part I. Chromatography using ultra-stable metal oxide-based stationary phases for HPLC. J. Chromatogr. A 2004, 1028, 1–30. [Google Scholar] [CrossRef]
- Romanchuk, A.Y.; Kalmykov, S.N.; Egorov, A.V.; Zubavichus, Y.V.; Shiryaev, A.A.; Smirnov, E.A.; Garshev, A.V. Photoreduction of Pu(V,VI) by TiO2. Radiochim. Acta 2016, 104, 843. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Que, Y.-P.; Mo, L.-E.; Chen, W.-C.; Ding, Y.; Ma, Y.-M.; Jiang, L.; Hu, L.-H.; Dai, S.-Y. One-Pot Synthesis of Mesoporous TiO2 Micropheres and Its Application for High-Efficiency Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 10928–10934. [Google Scholar] [CrossRef]
- Liu, S.; Han, G.; Shu, M.; Han, L.; Che, S. Monodispersed inorganic/organic hybrid spherical colloids: Versatile synthesis and their gas-triggered reversibly switchable wettability. J. Mater. Chem. 2010, 20, 10001. [Google Scholar] [CrossRef]
- Barringer, E.A.; Bowen, H.K. Formation, Packing, and Sintering of Monodisperse TiO2 Powders. J. Am. Ceram. Soc. 1982, 65, C-199–C-201. [Google Scholar] [CrossRef]
- Tanaka, S.; Nogami, D.; Tsuda, N.; Miyake, Y. Synthesis of highly-monodisperse spherical titania particles with diameters in the submicron range. J. Colloid Interface Sci. 2009, 334, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Look, J.L.; Zukoski, C.F. Shear induced aggregation during the precipitation of titanium alkoxides. J. Colloid Interface Sci. 1992, 153, 461–482. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, J.; Liu, X.; Sun, A.; Xu, G.; Cui, P. Preparation of uniform titania microspheres with good electrorheological performance and their size effect. J. Mater. Chem. 2011, 21, 5051. [Google Scholar] [CrossRef]
- Jiang, X.; Herricks, T.; Xia, Y. Monodispersed spherical colloids of titania: Synthesis, characterization, and crystallization. Adv. Mater. 2003, 15, 1205–1209. [Google Scholar] [CrossRef]
- Pal, M.; Garcı, J. Size-Controlled Synthesis of Spherical TiO2 Nanoparticles: Morphology, Crystallization, and Phase Transition. J. Phys. Chem. C 2007, 111, 96–102. [Google Scholar] [CrossRef]
- EDELSON, L.H.; GLAESER, A.M. Role of Particle Substructure in the Sintering of Monosized Titania. J. Am. Ceram. Soc. 1988, 71, 225–235. [Google Scholar] [CrossRef]
- Eiden-Assmann, S.; Widoniak, J.; Maret, G. Synthesis and Characterization of Hollow and Non-Hollow Monodisperse Colloidal TiO2 Particles. J. Dispers. Sci. Technol. 2005, 25, 535–545. [Google Scholar] [CrossRef]
- Kim, H.T.; Park, H.; Bae, S.Y.; Yoo, K.O. Growth mechanism of monodispersed TiO2 fine particles by the hydrolysis of Ti(OC2H5)4. Korean J. Chem. Eng. 1995, 12, 516–522. [Google Scholar] [CrossRef]
- Look, J.L.; Zukoski, C.F. Alkoxide-Derived Titania Particles: Use of Electrolytes to Control Size and Agglomeration Levels. J. Am. Ceram. Soc. 1992, 75, 1587–1595. [Google Scholar] [CrossRef]
- Lakshminarasimhan, N.; Bae, E.; Choi, W. Enhanced photocatalytic production of H2 on mesoporous TiO2 prepared by template-free method: Role of interparticle charge transfer. J. Phys. Chem. C 2007, 111, 15244–15250. [Google Scholar] [CrossRef]
- Junqi, L.; Zuoli, H.; Liying, G.; Zhenfeng, Z. Synthesis and Characterization of Size-Controlled TiO2 Microspheres with Wormhole Mesoporous Structure. Mater. Manuf. Process. 2010, 25, 990–993. [Google Scholar] [CrossRef]
- Kumazawa, H.; Otsuki, H.; Sada, E. Preparation of monosized spherical titania fine particles by controlled hydrolysis of titanium tetraethoxide in ethanol. J. Mater. Sci. Lett. 1993, 12, 839–840. [Google Scholar] [CrossRef]
- Kojima, T.; Sugimoto, T. Formation Mechanism of Amorphous TiO2 Spheres in Organic Solvents. 1. Roles of Ammonia. J. Phys. Chem. C 2008, 112, 18760–18771. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, L.; Mo, L.; Jiang, L.; Hu, L.; Li, Z.; Chen, S.; Dai, S. TiO2 microspheres with controllable surface area and porosity for enhanced light harvesting and electrolyte diffusion in dye-sensitized solar cells. Adv. Funct. Mater. 2015, 25, 5946–5953. [Google Scholar] [CrossRef]
- Sanchez, C.; Livage, J.; Henry, M.; Babonneau, F. Chemical modification of alkoxide precursors. J. Non. Cryst. Solids 1988, 100, 65–76. [Google Scholar] [CrossRef]
- Smirnov, E.A.; Meledina, M.A.; Garshev, A.V.; Chelpanov, V.I.; Frost, S.; Wieneke, J.U.; Ulbricht, M. Grafting of titanium dioxide microspheres with a temperature-responsive polymer via surface-initiated atom transfer radical polymerization without the use of silane coupling agents. Polym. Int. 2013, 62, 836–841. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Cent. Eur. J. Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Sultanova, N.; Kasarova, S.; Nikolov, I. Dispersion Properties of Optical Polymers. Acta Phys. Pol. A 2009, 116, 585–587. [Google Scholar] [CrossRef]
- Demirörs, A.F.; Jannasch, A.; Van Oostrum, P.D.J.; Schäffer, E.; Imhof, A.; Van Blaaderen, A. Seeded growth of titania colloids with refractive index tunability and fluorophore-free luminescence. Langmuir 2011, 27, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman spectra of titanium dioxide (anatase, rutile) with identified oxygen isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Kojima, T.; Sugimoto, T. Formation Mechanism of Amorphous TiO2 Spheres in Organic Solvents 3. Effects of Water, Temperature, and Solvent Composition. J. Phys. Chem. C 2008, 112, 18445–18454. [Google Scholar] [CrossRef]
- Golubko, N.V.; Yanovskaya, M.I.; Golubko, L.A.; Kovsman, E.P.; Listoshina, M.B.; Rotenberg, B.A. Preparation of barium titanate and related materials by the alkoxide-hydroxide route. J. Sol-Gel Sci. Technol. 2001, 20, 135–143. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Phase transformation of nanocrystalline anatase-to-rutile via combined interface and surface nucleation. J. Mater. Res. 2000, 15, 437–448. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Akl, A.A. Effect of Se addition on optical and electrical properties of chalcogenide CdSSe thin films. Superlattices Microstruct. 2016, 89, 153–169. [Google Scholar] [CrossRef]
- Khatun, N.; Rini, E.G.; Shirage, P.; Rajput, P.; Jha, S.N.; Sen, S. Effect of lattice distortion on bandgap decrement due to vanadium substitution in TiO2 nanoparticles. Mater. Sci. Semicond. Process. 2016, 50, 7–13. [Google Scholar] [CrossRef]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Ferraro, S.; Seghetti, C.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Enhancement of visible-light photoactivity by polypropylene coated plasmonic Au/TiO2 for dye degradation in water solution. Appl. Surf. Sci. 2018, 441, 575–587. [Google Scholar] [CrossRef]
- Hecht, H.G. Comparison of continuum models in quantitative diffuse reflectance spectrometry. Anal. Chem. 1976, 48, 1775–1779. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Palmisano, L.; Parrino, F. Junction Effect on the Photocatalytic Activity of Mixed-Phase TiO2 Nanoparticles. ECS Trans. 2010, 25, 29–35. [Google Scholar]
- Serpone, N.; Lawless, D.; Khairutdinov, R. Size Effects on the Photophysical Properties of Colloidal Anatase TiO2 Particles: Size Quantization versus Direct Transitions in This Indirect Semiconductor? J. Phys. Chem. 1995, 99, 16646–16654. [Google Scholar] [CrossRef]
- Monticone, S.; Tufeu, R.; Kanaev, A.V.; Scolan, E.; Sanchez, C. Quantum size effect in TiO2 nanoparticles: Does it exist? Appl. Surf. Sci. 2000, 162–163, 565–570. [Google Scholar] [CrossRef]
- Serpone, N. Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lévy, F.; Berger, H.; Schmid, P.E. Urbach tail of anatase TiO2. Phys. Rev. B 1995, 52, 7771–7774. [Google Scholar] [CrossRef]

| Sample Name | Phase Composition | CSR (anatase), nm | BET Surface Area, m2/g | Vpore, cm3/g | Dpore, nm |
|---|---|---|---|---|---|
| untreated | amorphous | - | 220 | 0.10 | 2.6 |
| an-400 | anatase | 24 ± 5 | 85 | 0.08 | 2.3 |
| an-500 | anatase | 29 ± 4 | 65 | 0.13 | 4.9 |
| an-625 | anatase | 45 ± 8 | 40 | 0.11 | 6.6 |
| an-700 | anatase | 50 ± 11 | 20 | 0.08 | 7.9 |
| ht-200-24 | 80 ± 2% anatase 20 ± 2% brookite | 18 ± 1 | 115 | 0.34 | 9.8 |
| ht-200-96 | 83 ± 2% anatase 17 ± 2% brookite | 21 ± 1 | 80 | 0.33 | 9.8 |
| Phase Composition | Band Gap, eV | Urbach Energy, meV |
|---|---|---|
| amorphous | 3.39 | - |
| 64% anatase, 36% amorphous | 3.15 | 225 |
| 69% anatase, 31% amorphous | 3.09 | 179 |
| 82% rutile, 18% amorphous | 2.94 | 228 |
| Phase Composition | Particle Mean Diameter, μm | SPF |
|---|---|---|
| amorphous | 0.42 | 21 |
| 78% anatase, 22% amorphous | 0.35 | 4 |
| 80% rutile, 20% amorphous | 0.35 | 3 |
| commercial cosmetic pigment (77% of rutile, 23% amorphous) | <0.2 | 8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poluboyarinov, A.S.; Chelpanov, V.I.; Lebedev, V.A.; Kozlov, D.A.; Khazova, K.M.; Volkov, D.S.; Kolesnik, I.V.; Garshev, A.V. Titanium Oxide Microspheres with Tunable Size and Phase Composition. Materials 2019, 12, 1472. https://doi.org/10.3390/ma12091472
Poluboyarinov AS, Chelpanov VI, Lebedev VA, Kozlov DA, Khazova KM, Volkov DS, Kolesnik IV, Garshev AV. Titanium Oxide Microspheres with Tunable Size and Phase Composition. Materials. 2019; 12(9):1472. https://doi.org/10.3390/ma12091472
Chicago/Turabian StylePoluboyarinov, Anton S., Vitaly I. Chelpanov, Vasily A. Lebedev, Daniil A. Kozlov, Kristina M. Khazova, Dmitry S. Volkov, Irina V. Kolesnik, and Alexey V. Garshev. 2019. "Titanium Oxide Microspheres with Tunable Size and Phase Composition" Materials 12, no. 9: 1472. https://doi.org/10.3390/ma12091472
APA StylePoluboyarinov, A. S., Chelpanov, V. I., Lebedev, V. A., Kozlov, D. A., Khazova, K. M., Volkov, D. S., Kolesnik, I. V., & Garshev, A. V. (2019). Titanium Oxide Microspheres with Tunable Size and Phase Composition. Materials, 12(9), 1472. https://doi.org/10.3390/ma12091472