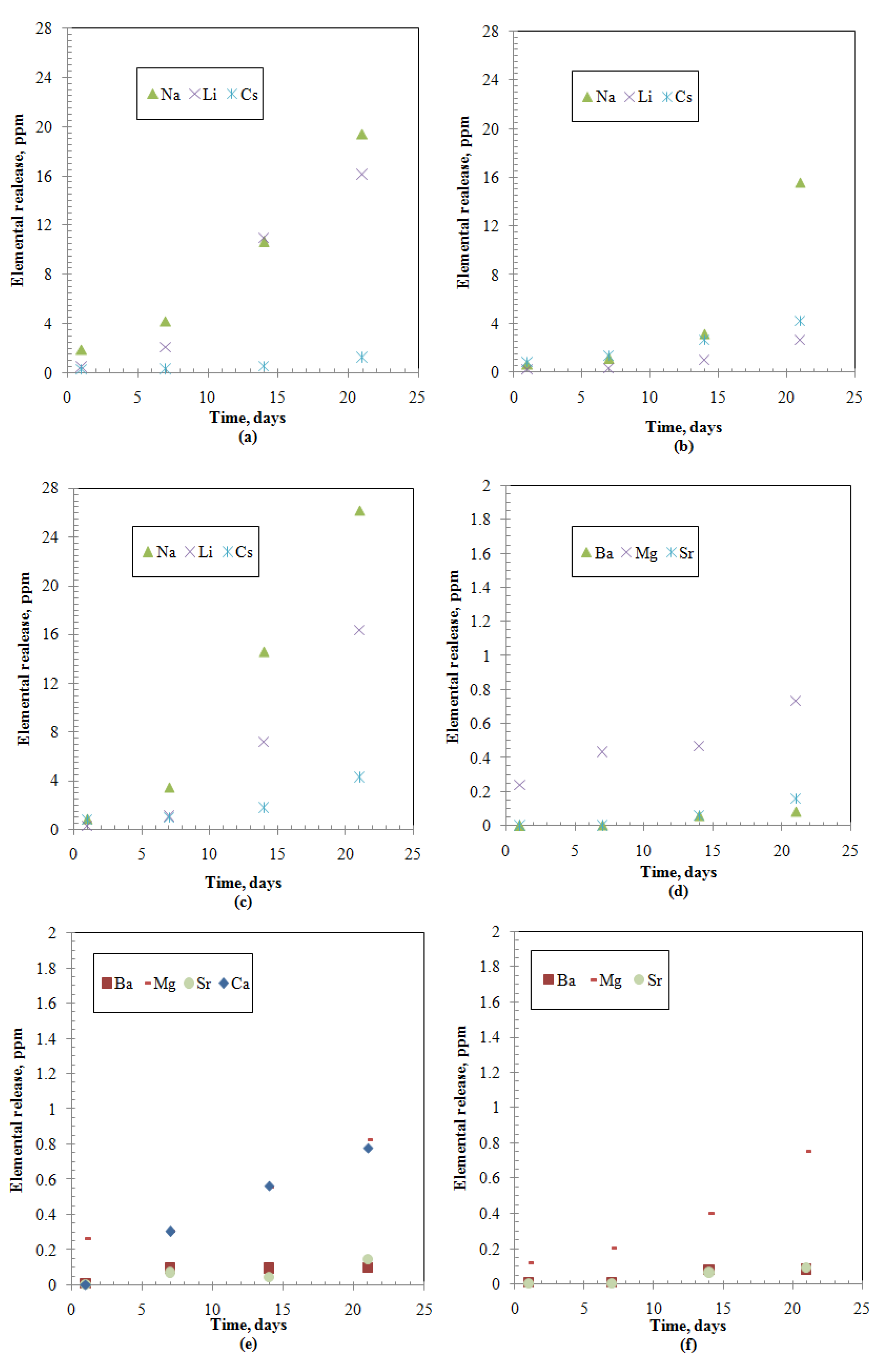
Figure 1.
Elements releases from the studied samples: (a) Group I-Alkali-Borosilicate (ABS)-Waste (17); (b) Group I- Modified Alkali-Borosilicate (MABS)-Waste (20); (c) Group I-ABS-Waste (25); (d) Group II-ABS-Waste (17); (e) Group II-MABS- Waste (20); (f) Group II-ABS-Waste (25).
Figure 1.
Elements releases from the studied samples: (a) Group I-Alkali-Borosilicate (ABS)-Waste (17); (b) Group I- Modified Alkali-Borosilicate (MABS)-Waste (20); (c) Group I-ABS-Waste (25); (d) Group II-ABS-Waste (17); (e) Group II-MABS- Waste (20); (f) Group II-ABS-Waste (25).
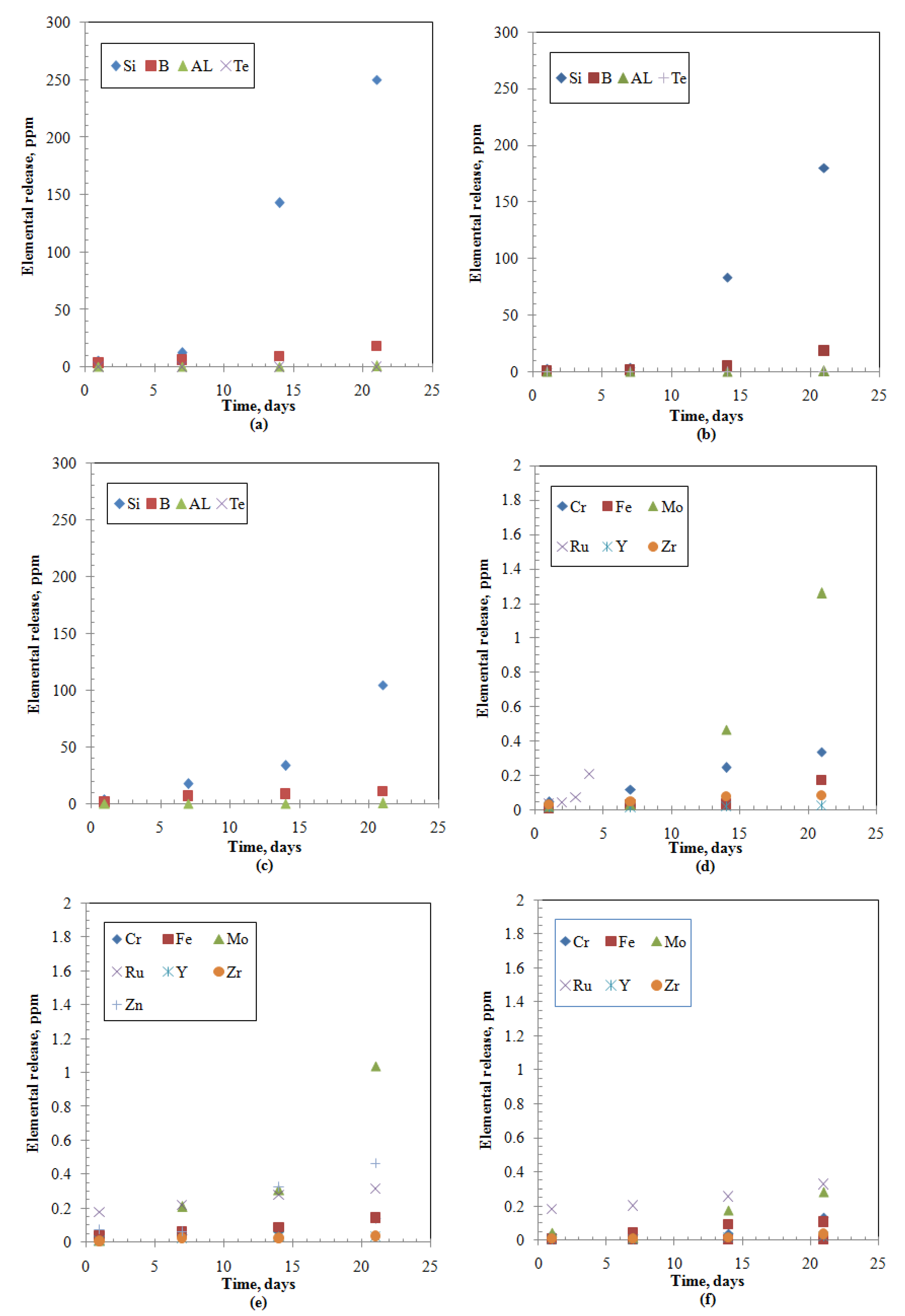
Figure 2.
Elemental releases from the studied samples: (a) Metalloid and post-transition-ABS-Waste (17); (b) Metalloid and post-transition-MABS-Waste (20); (c) Metalloid and post-transition-ABS-Waste (25); (d) Transition-ABS-Waste (17); (e) Transition-MABS-Waste (20); (f) Transition-ABS-Waste (25).
Figure 2.
Elemental releases from the studied samples: (a) Metalloid and post-transition-ABS-Waste (17); (b) Metalloid and post-transition-MABS-Waste (20); (c) Metalloid and post-transition-ABS-Waste (25); (d) Transition-ABS-Waste (17); (e) Transition-MABS-Waste (20); (f) Transition-ABS-Waste (25).
Figure 3.
Rare earth elements releases from the studied samples: (a) ABS-Waste (17); (b) MABS-Waste (20); (c) ABS-Waste (25).
Figure 3.
Rare earth elements releases from the studied samples: (a) ABS-Waste (17); (b) MABS-Waste (20); (c) ABS-Waste (25).
Figure 4.
The evolution of glass matrix composition during the leaching process: (a) temporal changes in non-bridging oxygen (NBO); (b) The NBO fraction as a function of former fraction.
Figure 4.
The evolution of glass matrix composition during the leaching process: (a) temporal changes in non-bridging oxygen (NBO); (b) The NBO fraction as a function of former fraction.
Figure 5.
Contribution of the waste matrix constituents to the hydration free energy.
Figure 5.
Contribution of the waste matrix constituents to the hydration free energy.
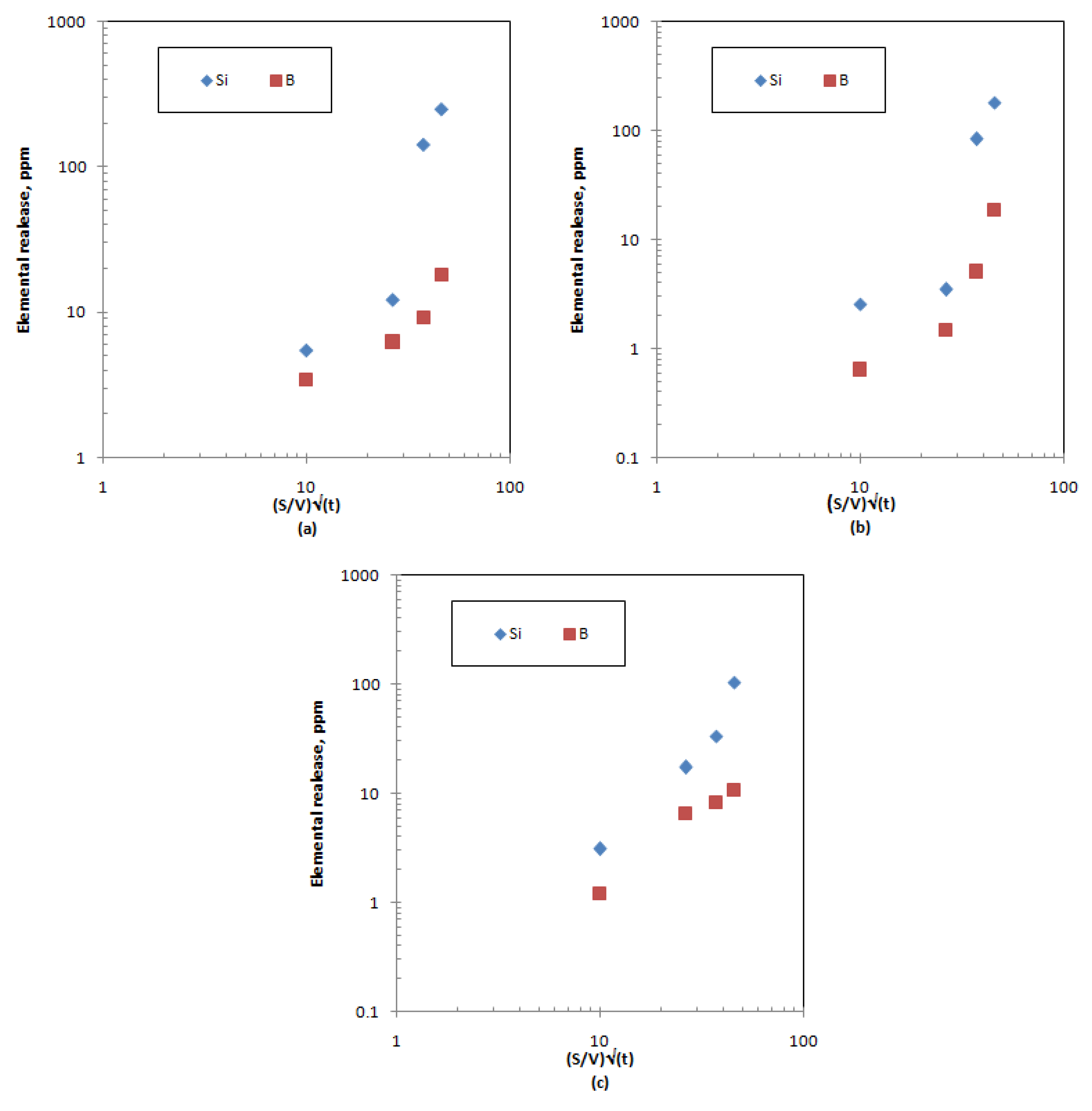
Figure 6.
Preliminary investigation of structural element leaching mechanisms for samples: (a) ABS-waste (17); (b) MABS-waste (20); (c) ABS-waste (25).
Figure 6.
Preliminary investigation of structural element leaching mechanisms for samples: (a) ABS-waste (17); (b) MABS-waste (20); (c) ABS-waste (25).
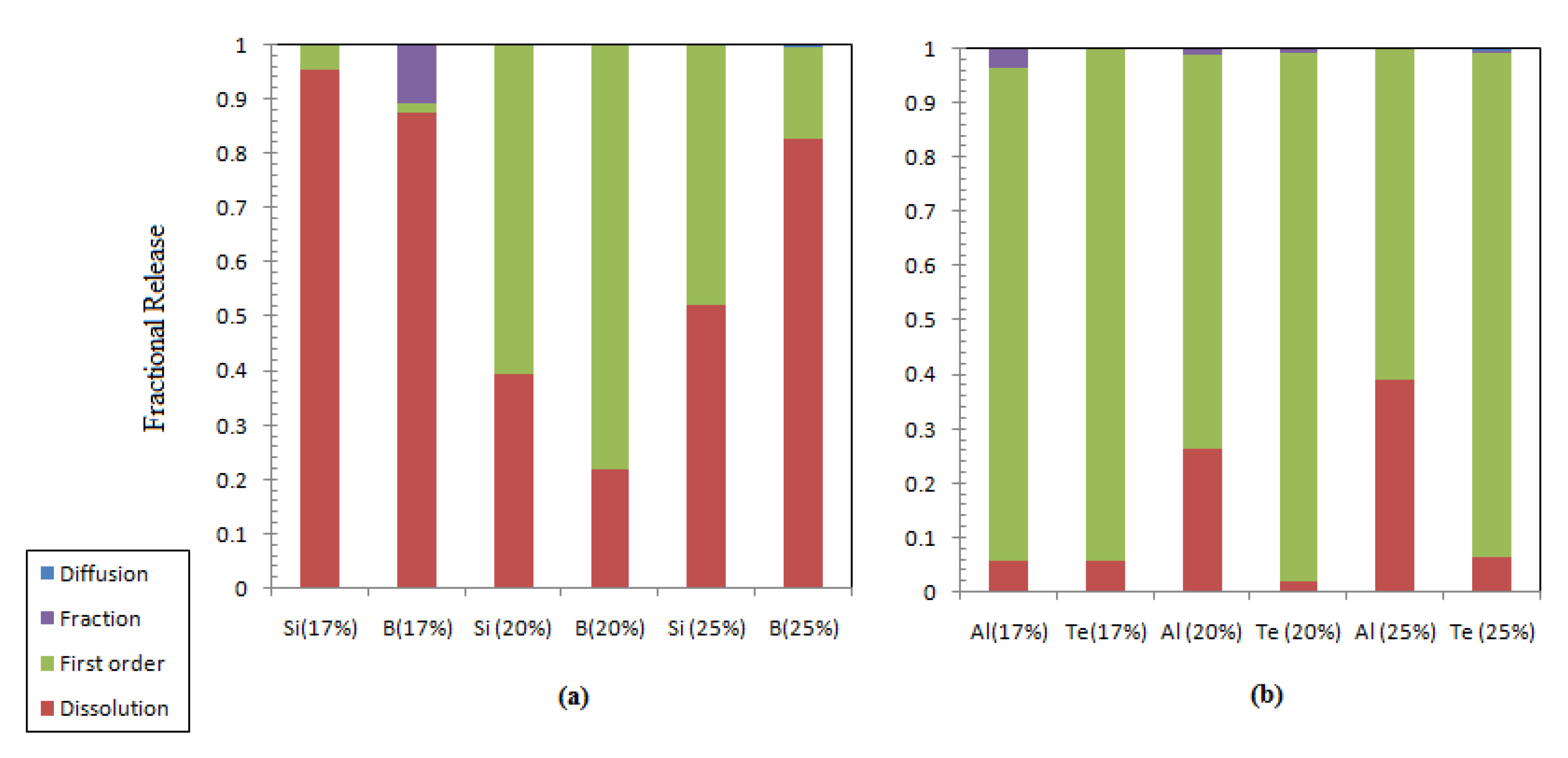
Figure 7.
Contribution of different leaching processes to the fractional release: (a) structural element; (b) post-transition and other metalloid elements.
Figure 7.
Contribution of different leaching processes to the fractional release: (a) structural element; (b) post-transition and other metalloid elements.
Figure 8.
Contribution of different leaching processes to the fractional release: (a) alkali elements; (b) alkaline earth metals.
Figure 8.
Contribution of different leaching processes to the fractional release: (a) alkali elements; (b) alkaline earth metals.
Figure 9.
Contribution of different leaching processes to the fractional release of transition metals.
Figure 9.
Contribution of different leaching processes to the fractional release of transition metals.
Figure 10.
Contribution of different leaching processes to the fractional release of rare earth elements.
Figure 10.
Contribution of different leaching processes to the fractional release of rare earth elements.
Table 1.
The normalized release rate (mg·m−2·d−1) of different elements from different alkali-borosilicate glasses matrices (ABS), including calcined Prototype Fast Reactor-Raffinate (PFR), Reactor Bolshoy Moshchnosty Kanalny-concentrate (RBMK), Water-Water Energetic Reactor-concentrate (WWER), RBMK-evaporator concentrate (K-26), High Level Waste Simulant (BS-5), and PyroGreen salt waste (PG).
Table 1.
The normalized release rate (mg·m−2·d−1) of different elements from different alkali-borosilicate glasses matrices (ABS), including calcined Prototype Fast Reactor-Raffinate (PFR), Reactor Bolshoy Moshchnosty Kanalny-concentrate (RBMK), Water-Water Energetic Reactor-concentrate (WWER), RBMK-evaporator concentrate (K-26), High Level Waste Simulant (BS-5), and PyroGreen salt waste (PG).
| ABS Glass Waste | PFR | RBMK | WWER | K-26 | BS-5 | PG |
|---|
| Test Type | PCT | ISO-6961 | PCT | PCT | PCT | Field Data |
|---|
| Alkali | Na | 16.9–21.7 | 101–102 | 102 | 59.3–90.9 | 378 | 1.42–8.57 |
| Li | - | - | - | - | - | 5.7–37.14 |
| Cs | - | 101–102 | 102 | - | - | - |
| Alkaline earth metal | Ca | 3.62–5.89 | - | - | - | - | - |
| Sr | - | 100–101 | 101 | - | - | - |
| Post-transition | Al | 0.29 | - | - | - | - | - |
| Transition | Mo | 4.44–6.38 | 100–10−1 | 100 | - | - | - |
| Ba | 1.47–4.43 | - | - | - |
| Cr | 0.16–0.35 | - | - | - |
| Metalloid | Si | 7.18–8.4 | - | - | 28.1–29.3 | 174 | 4.28–17.1 |
| B | 32.4–33.3 | <10−1 | <10−1 | 31.3–40.5 | 435 | 1.42–18.57 |
| Rare earth elements | - | 10−1 | 10−1 | - | 7.11 | - |
| Reference | [29] | [30] | [30] | [31] | [32] | [33] |
Table 2.
Chemical composition of the studied glasses (structural elements and modifiers).
Table 2.
Chemical composition of the studied glasses (structural elements and modifiers).
| Compound | SiO2 | B2O3 | Na2O | Li2O | CaO | ZnO | Total |
|---|
| ABS-Waste (17) | 50.200 | 15.400 | 8.800 | 8.700 | -- | -- | 83.100 |
| MABS-Waste (20) | 44.260 | 17.950 | 9.010 | 2.110 | 1.390 | 4.430 | 79.150 |
| ABS-Waste (25) | 46.280 | 16.430 | 8.330 | 3.980 | -- | -- | 75.020 |
Table 3.
Chemical composition of the studied glasses (waste components: alkali, alkaline, post-transitions, and metalloids).
Table 3.
Chemical composition of the studied glasses (waste components: alkali, alkaline, post-transitions, and metalloids).
| Compound | Alkali | Alkaline Earth Metals | Post-Transitions and Metalloids |
|---|
| Cs2O | BaO | MgO | SrO | Total | Al2O3 | TeO2 | Total |
|---|
| ABS-Waste (17) | 0.300 | 0.200 | 8.200 | 0.200 | 8.60 | 3.100 | 0.100 | 3.200 |
| MABS-Waste (20) | 0.890 | 0.40 | 4.100 | 0.240 | 4.740 | 4.110 | 0.150 | 4.260 |
| ABS-Waste (25) | 1.590 | 0.470 | 1.610 | 0.410 | 2.490 | 1.910 | 0.280 | 2.190 |
Table 4.
Chemical composition of the studied glasses (waste components: transitions and rare earth elements).
Table 4.
Chemical composition of the studied glasses (waste components: transitions and rare earth elements).
| Compound | Transition Metals* | Rare Earth * |
|---|
| Cr2O3 | Fe2O3 | MoO3 | RuO2 | ZrO2 | Y2O3 | Total | CeO2 | La2O3 | Nd2O3 | Total |
|---|
| ABS-Waste (17) | 0.300 | 1.300 | 0.700 | 0.200 | 0.800 | 0.100 | 3.400 | 0.500 | 0.100 | 0.400 | 1.000 |
| MABS-Waste (20) | 0.630 | 2.790 | 1.320 | 0.520 | 1.240 | 0.160 | 6.660 | 0.960 | 0.520 | 1.530 | 3.010 |
| ABS-Waste (25) | 0.510 | 2.060 | 2.490 | 0.550 | 2.820 | 0.310 | 8.740 | 1.450 | 0.730 | 2.170 | 4.350 |
Table 5.
The normalized release rate (mg·m−2·d−1) for structural elements and modifiers.
Table 5.
The normalized release rate (mg·m−2·d−1) for structural elements and modifiers.
| Compound | SiO2 | B2O3 | Na2O | Li2O | CaO | ZnO |
|---|
| ABS-Waste (17) | 50.814 | 36.024 | 28.301 | 37.940 | - | - |
| MABS-Waste (20) | 41.361 | 31.541 | 22.256 | 25.977 | 1.041 | 0.397 |
| ABS-Waste (25) | 22.918 | 20.121 | 40.363 | 32.122 | - | - |
Table 6.
Normalized release rate (mg·m−2·d−1) for waste components: Alkali, alkaline, post-transitions, and metalloids.
Table 6.
Normalized release rate (mg·m−2·d−1) for waste components: Alkali, alkaline, post-transitions, and metalloids.
| Compound | Alkali | Alkaline Earth Metals | Post-Transitions and Metalloids |
|---|
| Cs2O | BaO | MgO | SrO | Al2O3 | TeO2 |
|---|
| ABS-Waste (17) | 40.927 | 2.588 | 0.706 | 4.479 | 3.443 | 4.568 |
| MABS-Waste (20) | 47.539 | 1.288 | 1.587 | 3.447 | 2.038 | 1.493 |
| ABS-Waste (25) | 27.198 | 0.928 | 3.686 | 1.266 | 3.464 | 0.857 |
Table 7.
Normalized release rate (mg·m−2·d−1) for waste components: transitions and rare earth elements.
Table 7.
Normalized release rate (mg·m−2·d−1) for waste components: transitions and rare earth elements.
| Compound | Transition Metals | Rare Earth |
|---|
| Cr2O3 | Fe2O3 | MoO3 | RuO2 | ZrO2 | Y2O3 | CeO2 | La2O3 | Nd2O3 |
|---|
| ABS-Waste (17) | 15.648 | 1.766 | 12.841 | 6.458 | 0.670 | 2.818 | 3.781 | 6.639 | 8.578 |
| MABS-Waste (20) | 3.146 | 0.708 | 5.624 | 3.790 | 0.187 | 2.668 | 8.044 | 1.924 | 3.457 |
| ABS-Waste (25) | 3.590 | 0.696 | 0.811 | 3.726 | 0.071 | 0.702 | 3.852 | 2.017 | 1.670 |
Table 8.
The evolution and dependency of glass degradation measures.
Table 8.
The evolution and dependency of glass degradation measures.
| Glass Sample | AGF% × 10−4 | ET (μm) |
|---|
| 1 d | 7 d | 14 d | 21 d | Time Dependency | R2 | Boron Release Dependency | R2 |
|---|
| ABS-Waste (17) | 5.419 | 10.437 | 16.423 | 50.337 | | 0.969 | ET = 9.032CLFB − 0.842 | 0.976 |
| MABS-Waste (20) | 0.780 | 1.832 | 6.889 | 42.672 | | 0.983 | ET = 8.023CLFB − 0.235 | 0.989 |
| ABS-Waste (25) | 1.629 | 10.448 | 13.540 | 18.685 | | 0.943 | ET = 5.395CLFB − 0.072 | 0.997 |
Table 9.
Nonlinear curve fitting parameters of the cumulative leach fraction of metalloid and post-transition elements: ABS-Waste (17).
Table 9.
Nonlinear curve fitting parameters of the cumulative leach fraction of metalloid and post-transition elements: ABS-Waste (17).
| Element | D (m2·h−1), × 10−13 | U (m·h−1) × 10−7 | Qo, | K (h−1) × 10−8 | C × 10−4 | R2 |
|---|
| Si | 0 | 1.583 | 0.004 | 938.143 | 0 | 0.912 |
| B | 0 | 1.013 | 0.001 | 619.759 | 62.200 | 0.940 |
| Te | 0 | 0.127 | 0.105 | 49.556 | 0 | 0.830 |
| Al | 0 | 0.074 | 0.058 | 0.845 | 22.400 | 0.879 |
Table 10.
Nonlinear curve fitting parameters of the cumulative leach fraction of metalloid and post-transition elements: MABS-Waste (20).
Table 10.
Nonlinear curve fitting parameters of the cumulative leach fraction of metalloid and post-transition elements: MABS-Waste (20).
| Element | D (m2·h−1), × 10−13 | U (m·h−1) × 10−7 | Qo, | K (h−1) × 10−8 | C × 10−4 | R2 |
|---|
| Si | 0 | 1.211 | 0.094 | 10.397 | 0 | 0.934 |
| B | 0 | 0.851 | 0.152 | 4.011 | 0 | 0.899 |
| Te | 0 | 0.033 | 0.084 | 0.122 | 7.264 | 0.806 |
| Al | 0 | 0.065 | 0.009 | 14.107 | 1.576 | 0.917 |
Table 11.
Nonlinear curve fitting parameters of the cumulative leach fraction of metalloid and post-transition elements: ABS-Waste (25).
Table 11.
Nonlinear curve fitting parameters of the cumulative leach fraction of metalloid and post-transition elements: ABS-Waste (25).
| Element | D (m2·h−1), × 10−13 | U (m·h−1) × 10−7 | Qo, | K (h−1) × 10−8 | C × 10−4 | R2 |
|---|
| Si | 0 | 0.648 | 0.003 | 1.630 | 0 | 0.868 |
| B | 0.678 | 0.303 | 3.20 × 10−4 | 0.149 | 0 | 0.967 |
| Te | N* | 0.028 | 0.002 | 1.592 | 1.879 | 0.902 |
| Al | 0 | 0.102 | 8.05 × 10−4 | 0.747 | 0 | 0.886 |
Table 12.
Nonlinear curve fitting parameters of the cumulative leach fraction of alkali and alkaline earth metals: ABS-Waste (17).
Table 12.
Nonlinear curve fitting parameters of the cumulative leach fraction of alkali and alkaline earth metals: ABS-Waste (17).
| Group | Element | D (m2·h−1), × 10−13 | U (m·h−1), × 10−7 | Qo, | K (h−1), × 10−8 | C × 10−4 | R2 |
|---|
| Alkali metals | Na | 0 | 0.904 | 0.055 | 3.444 | 3.192 | 0.966 |
| Li | 0 | 1.266 | 0.038 | 0.0001 | 0 | 0.952 |
| Cs | 0 | 1.119 | 0.028 | 1.935 | 49.301 | 0.870 |
| Alkaline earth metals | Ba | 0 | 0.075 | 0.034 | 0.112 | 0 | 0.907 |
| Mg | 1.570 | 0.009 | 0.223 | 2.407 | 0.975 | 0.914 |
| Sr | 0 | 0.126 | 0.054 | 0.124 | 0 | 0.838 |
Table 13.
Nonlinear curve fitting parameters of the cumulative leach fraction of alkali and alkaline earth metals: MABS-Waste (20).
Table 13.
Nonlinear curve fitting parameters of the cumulative leach fraction of alkali and alkaline earth metals: MABS-Waste (20).
| Group | Element | D (m2·h−1), × 10−13 | U (m·h−1), × 10−7 | Qo, | K (h−1), × 10−8 | C × 10−4 | R2 |
|---|
| Alkali metals | Na | 0 | 0.576 | 0.009 | 0.287 | 0 | 0.867 |
| Li | 0 | 0.752 | 0.006 | 0.558 | 0 | 0.876 |
| Cs | 0 | 1.399 | 0.002 | 0.193 | 0.009 | 0.974 |
| Alkaline earth metals | Ca | 0 | 0.133 | 0.088 | 39.593 | 0.039 | 0.989 |
| Ba | 9.159 | 0.002 | 8.2*10-4 | 0.196 | 0 | 0.805 |
| Mg | 0 | 0.041 | 0.084 | 0.289 | 5.844 | 0.968 |
| Sr | 0 | 0.093 | 0.041 | 5.769 | 0 | 0.902 |
Table 14.
Nonlinear curve fitting parameters of the cumulative leach fraction of alkali and alkaline earth metals: ABS-Waste (25).
Table 14.
Nonlinear curve fitting parameters of the cumulative leach fraction of alkali and alkaline earth metals: ABS-Waste (25).
| Group | Element | D (m2·h−1), × 10−13 | U (m·h−1), × 10−7 | Qo, | K (h−1), × 10−8 | C × 10−4 | R2 |
|---|
| Alkali metals | Na | 0 | 1.273 | 0.084 | 2.157 | 0 | 0.949 |
| Li | 0 | 1.595 | 0.075 | 37.659 | 0 | 0.890 |
| Cs | 0 | 0.743 | 0.008 | 3.074 | 0.003 | 0.867 |
| Alkaline earth metals | Ba | 0 | 0.029 | 0.758 | 0.629 | 0.333 | 0.869 |
| Mg | 0 | 0.109 | 0.006 | 0.139 | 4.169 | 0.952 |
| Sr | 0 | 0.042 | 0.001 | 359.254 | 0 | 0.907 |
Table 15.
Nonlinear curve fitting parameters of the cumulative leach fraction of transition and rare earth elements: ABS-Waste (17).
Table 15.
Nonlinear curve fitting parameters of the cumulative leach fraction of transition and rare earth elements: ABS-Waste (17).
| Group | Element | D (m2·h−1), × 10−13 | U (m·h−1), × 10−7 | Qo, | K (h−1), × 10−8 | C × 10−4 | R2 |
|---|
| Transition elements | Cr | 0 | 0.516 | 0.415 | 2.883 | 16.600 | 0.994 |
| Fe | 0 | 0.005 | 0.006 | 0.239 | 0 | 0.876 |
| Mo | 0 | 0.359 | 0.001 | 1.302 | 0 | 0.917 |
| Ru | 0 | 0.172 | 0.001 | 0.747 | 5.654 | 0.898 |
| Zr | 2.047 | N* | 0.022 | 0.609 | 0.600 | 0.961 |
| Y | 0 | 0.063 | 0.0247 | 0.913 | 15.000 | 0.892 |
| Rare earth Elements | Ce | 0 | 0.100 | 0.070 | 0.583 | 0 | 0.885 |
| La | 0 | 0.027 | 0.241 | 0.003 | 2.326 | 0.926 |
| Nd | 0 | 0.026 | 0.110 | 0.352 | 1.033 | 0.971 |
Table 16.
Nonlinear curve fitting parameters of the cumulative leach fraction of transition and rare earth elements: MABS-Waste (20).
Table 16.
Nonlinear curve fitting parameters of the cumulative leach fraction of transition and rare earth elements: MABS-Waste (20).
| Group | Element | D (m2·h−1), × 10−13 | U (m·h−1), × 10−7 | Qo, | K (h−1), × 10−8 | C × 10−4 | R2 |
|---|
| Transition elements | Zn | 0 | 0.003 | 0.033 | 0.341 | 0.103 | 0.968 |
| Cr | 0 | 0.078 | 0.087 | 2.343 | 8.234 | 0.857 |
| Fe | 0 | 0.018 | 0.017 | 0.105 | 2.321 | 0.914 |
| Mo | 1.294 | 0.060 | 0.007 | 0.157 | 0 | 0.962 |
| Ru | 27.347 | 0.005 | 0.014 | 42.141 | 22.100 | 0.837 |
| Zr | 0.118 | N* | 0.034 | 0.137 | 0 | 0.946 |
| Y | 0 | 0 | 0.005 | 358 × 103 | 8.762 | 0.953 |
| Rare earth Elements | Ce | 0 | 0.212 | 0.048 | 0.005 | 24.600 | 0.929 |
| La | 0 | 0.005 | 0.020 | 350.372 | 0 | 0.83 |
| Nd | 0 | 0.089 | 0.144 | 7.292 | 11.900 | 0.936 |
Table 17.
Nonlinear curve fitting parameters of the cumulative leach fraction of transition and rare earth elements: ABS-Waste (25).
Table 17.
Nonlinear curve fitting parameters of the cumulative leach fraction of transition and rare earth elements: ABS-Waste (25).
| Group | Element | D (m2·h−1), × 10−13 | U (m·h−1), × 10−7 | Qo, | K (h−1), × 10−8 | C × 10−4 | R2 |
|---|
| Transition elements | Cr | 0 | 0.085 | 0.008 | 742.683 | 6.376 | 0.721 |
| Fe | N* | 0.019 | 0.021 | 21.050 | 0 | 0.974 |
| Mo | 0 | 0.025 | 0.072 | 9.901 | 0.313 | 0.871 |
| Ru | 0 | 0.071 | 0.583 | 0.135 | 28.400 | 0.823 |
| Zr | 0 | 0.023 | 0.018 | 2.098 | 0 | 0.919 |
| Y | 0 | 0.002 | 0.015 | 4.655 | 0.128 | 0.815 |
| Rare earth Elements | Ce | 64.407 | 0.003 | 0.062 | 393.147 | 0.207 | 0.983 |
| La | 2.602 | 0.045 | 0.010 | 0.135 | 0 | 0.993 |
| Nd | 3.063 | 0.019 | 0.038 | 0.279 | 5.912 | 0.944 |