Correlative Confocal Raman and Scanning Probe Microscopy in the Ionically Active Particles of LiMn2O4 Cathodes
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
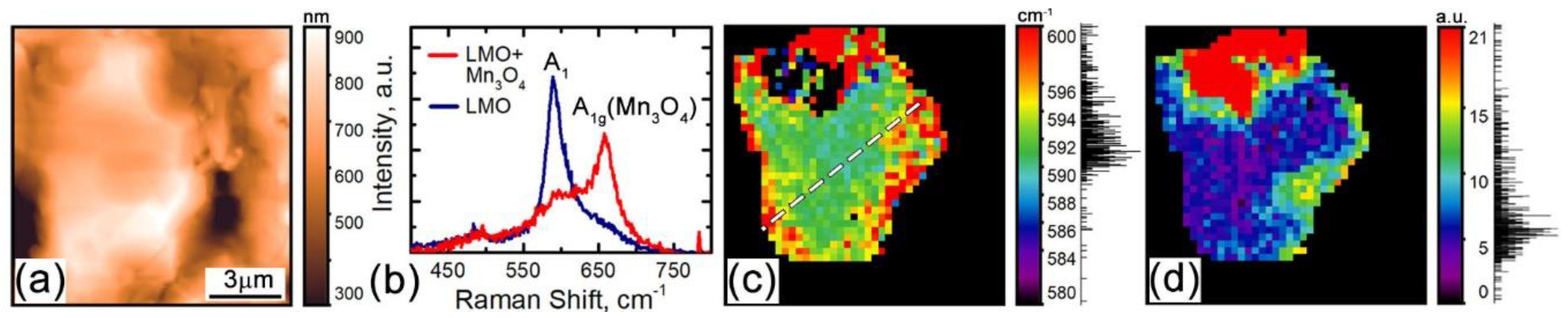
3.1. Confocal Raman Microscopy
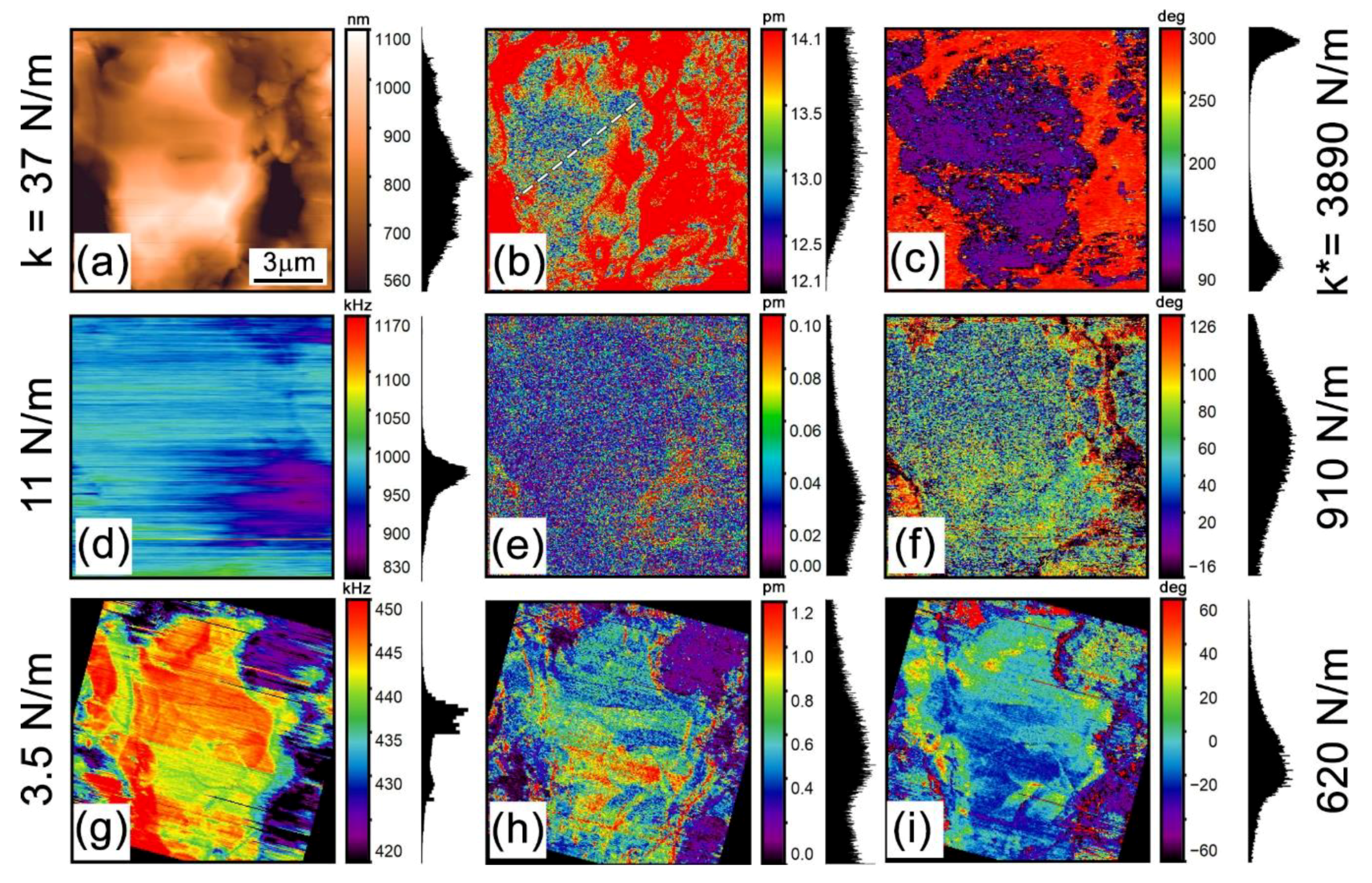
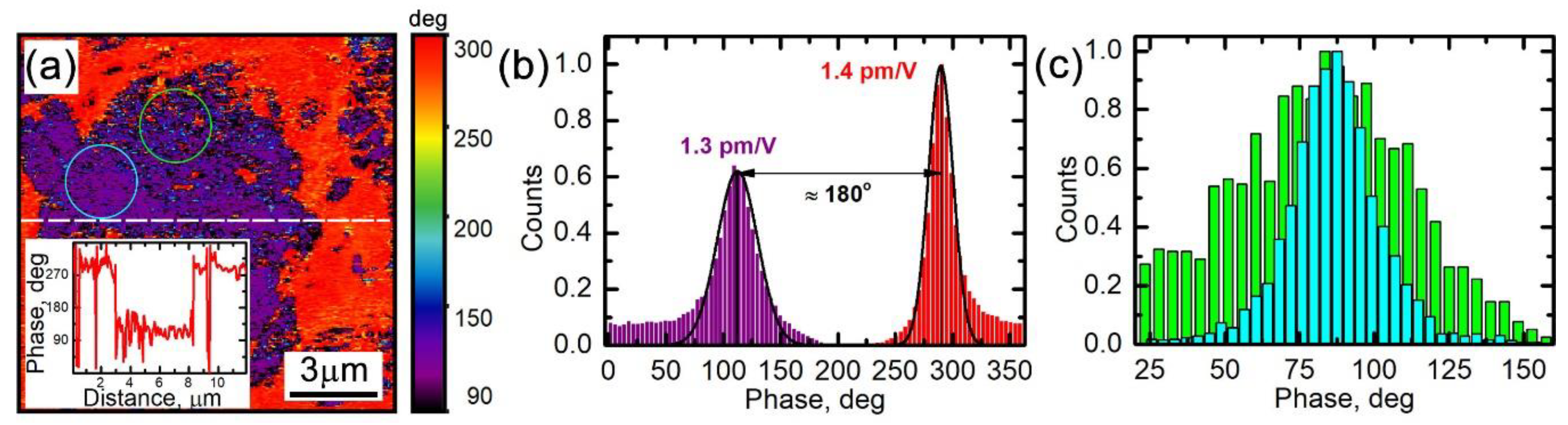
3.2. Low-Frequency Electrochemical Strain Microscopy
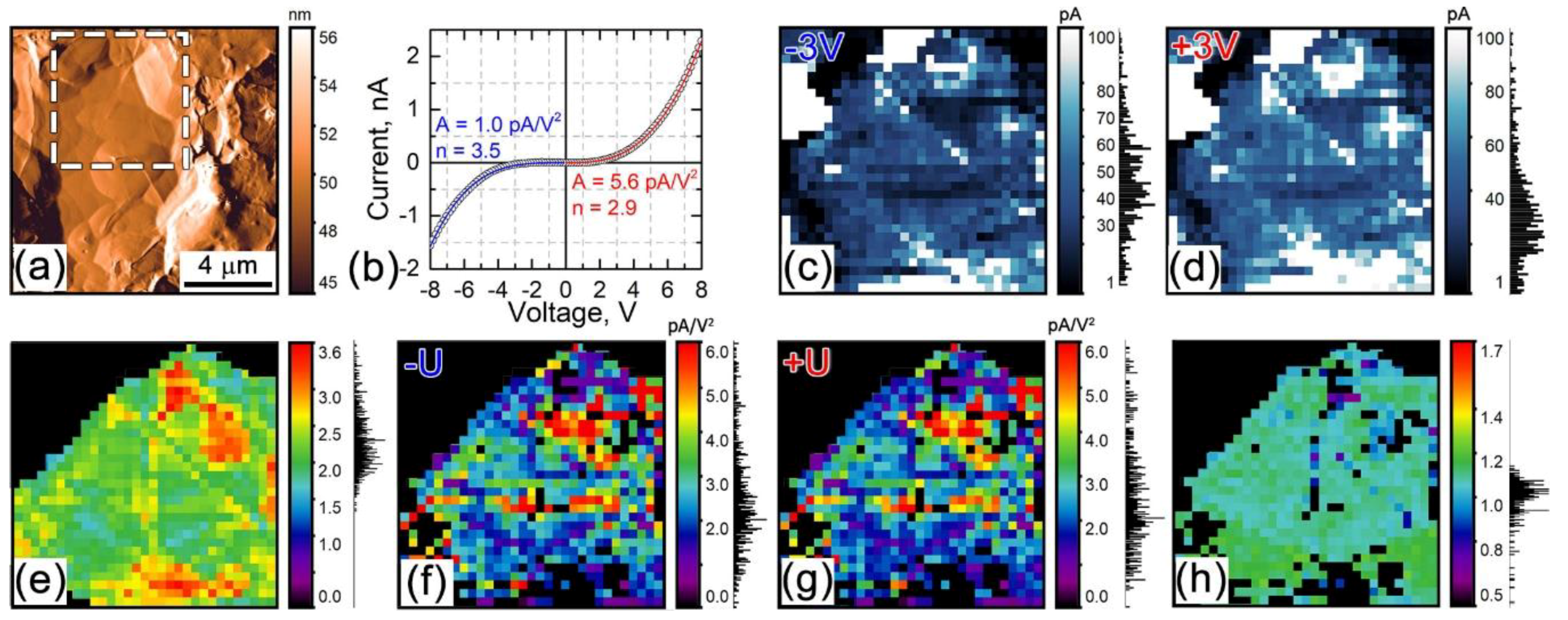
3.3. Parasitic Contributions to the ESM Response
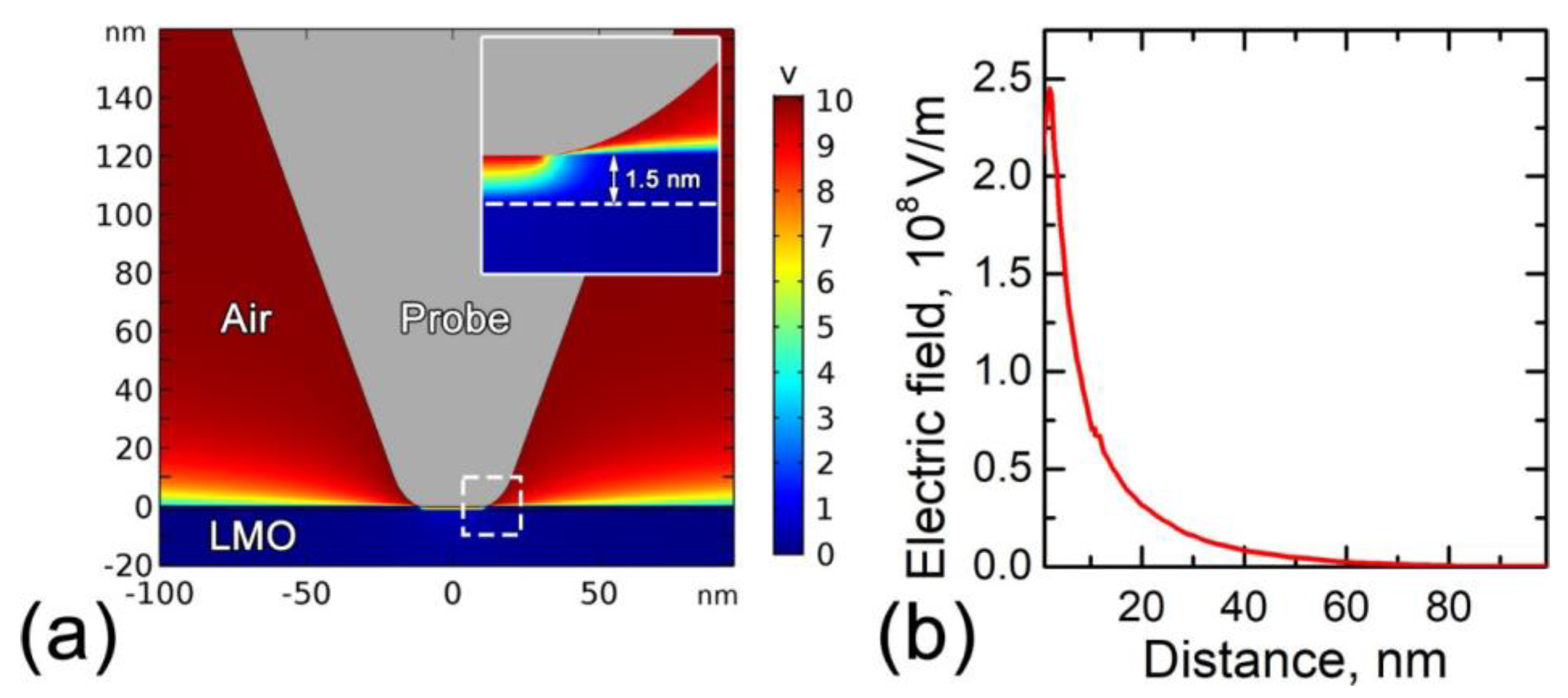
3.3.1. Electrostatic Tip-Surface Interaction
3.3.2. Local Joule Heating
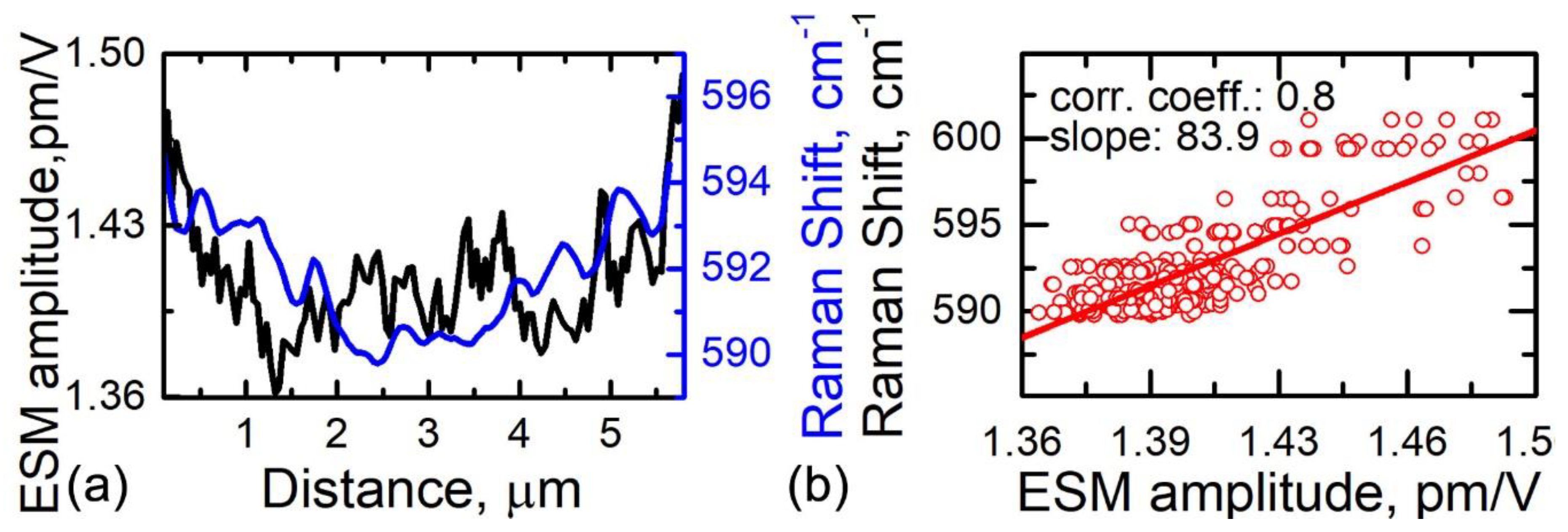
3.4. Relationship between Electrochemical Strain, Composition, and Lithiation State
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Probe Model | Scansens HA_NC, W2C Coated | Scansens HA_FM, W2C Coated | Scansens HA_HR, W2C Coated |
|---|---|---|---|
| Spring Constant (N/m) | 3.5 | 11 | 37 |
| Free Resonance Frequency (kHz) | 95 | 235 | 372 |
| Free Resonance Quality Factor | 356 | 392 | 694 |
| First Contact Resonance Frequency (kHz) | 442 | 1154 | 1912 |
| First Contact Resonance Quality Factor | 65 | 32 | 10 |
| Cantilever Length (μm) | 223 | 94 | 93 |
| Length to Tip (μm) | 219 | 90 | 89 |
| Tip Height (μm) | 10 | 10 | 10 |
| Contact Stiffness (N/m) | 620 | 910 | 3890 |
| Shape Factor for Contact Resonance | 1.37 | 1.06 | - |
| Shape Factor for 3.5 kHz | - | - | 0.14 |
Appendix B
Appendix C

Appendix D

References
- Sakti, A.; Azevedo, I.M.L.; Fuchs, E.R.H.; Michalek, J.J.; Gallagher, K.G.; Whitacre, J.F. Consistency and Robustness of Forecasting for Emerging Technologies: The Case of Li-ion batteries for Electric Vehicles. Energy Policy 2017, 106, 415–426. [Google Scholar] [CrossRef]
- Pelegov, D.; Pontes, J. Main Drivers of Battery Industry Changes: Electric Vehicles—A Market Overview. Batteries 2018, 4, 65. [Google Scholar] [CrossRef]
- Danis, L.; Gateman, S.M.; Kuss, C.; Schougaard, S.B.; Mauzeroll, J. Nanoscale Measurements of Lithium-Ion-Battery Materials using Scanning Probe Techniques. ChemElectroChem 2017, 4, 6–19. [Google Scholar] [CrossRef]
- Kuriyama, K.; Onoue, A.; Yuasa, Y.; Kushida, K. Atomic Force Microscopy Study of Surface Morphology Change in Spinel LiMn2O4: Possibility of Direct Observation of Jahn-Teller Instability. Surf. Sci. 2007, 601, 2256–2259. [Google Scholar] [CrossRef]
- Ventosa, E.; Schuhmann, W. Scanning Electrochemical Microscopy of Li-ion Batteries. Phys. Chem. Chem. Phys. 2015, 17, 28441–28450. [Google Scholar] [CrossRef]
- Balke, N.; Jesse, S.; Kim, Y.; Adamczyk, L.; Tselev, A.; Ivanov, I.N.; Dudney, N.J.; Kalinin, S.V. Real Space Mapping of Li-ion Transport in Amorphous Si Anodes with Nanometer Resolution. Nano Lett. 2010, 10, 3420–3425. [Google Scholar] [CrossRef] [PubMed]
- Balke, N.; Jesse, S.; Morozovska, A.N.; Eliseev, E.; Chung, D.W.; Kim, Y.; Adamczyk, L.; García, R.E.; Dudney, N.; Kalinin, S.V. Nanoscale Mapping of Ion Diffusion in a Lithium-Ion Battery Cathode. Nat. Nanotechnol. 2010, 5, 749–754. [Google Scholar] [CrossRef]
- Black, J.; Strelcov, E.; Balke, N.; Kalinin, S.V. Electrochemistry at the Nanoscale: The Force Dimension. Interface Mag. 2014, 23, 53–59. [Google Scholar] [CrossRef]
- Morozovska, A.N.; Eliseev, E.A.; Balke, N.; Kalinin, S.V. Local Probing of Ionic Diffusion by Electrochemical Strain Microscopy: Spatial Resolution and Signal Formation Mechanisms. J. Appl. Phys. 2010, 108, 053712. [Google Scholar] [CrossRef]
- Tselev, A.; Morozovska, A.N.; Udod, A.; Eliseev, E.A.; Kalinin, S.V. Self-consistent Modeling of Electrochemical Strain Microscopy of Solid Electrolytes. Nanotechnology 2014, 25, 445701. [Google Scholar] [CrossRef]
- Yang, S.; Yan, B.; Wu, J.; Lu, L.; Zeng, K. Temperature-Dependent Lithium-Ion Diffusion and Activation Energy of Li1.2Co0.13Ni0.13Mn0.54O2 Thin-Film Cathode at Nanoscale by Using Electrochemical Strain Microscopy. ACS Appl. Mater. Interfaces 2017, 9, 13999–14005. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yan, B.; Li, T.; Zhu, J.; Lu, L.; Zeng, K. In Situ Studies of Lithium-Ion Diffusion in a Lithium-Rich Thin Film Cathode by Scanning Probe Microscopy Techniques. Phys. Chem. Chem. Phys. 2015, 17, 22235–22242. [Google Scholar] [CrossRef]
- Chen, Q.N.; Liu, Y.; Liu, Y.; Xie, S.; Cao, G.; Li, J. Delineating Local Electromigration for Nanoscale Probing of Lithium Ion Intercalation and Extraction by Electrochemical Strain Microscopy. Appl. Phys. Lett. 2012, 101, 063901. [Google Scholar] [CrossRef][Green Version]
- Doria, S.; Yang, N.; Kumar, A.; Jesse, S.; Tebano, A.; Aruta, C.; Di Bartolomeo, E.; Arruda, T.M.; Kalinin, S.V.; Licoccia, S.; et al. Nanoscale Mapping of Oxygen Vacancy Kinetics in Nanocrystalline Samarium Doped Ceria Thin Films. Appl. Phys. Lett. 2013, 103. [Google Scholar] [CrossRef]
- Kumar, A.; Ciucci, F.; Morozovska, A.N.; Kalinin, S.V.; Jesse, S. Measuring Oxygen Reduction/Evolution Reactions on the Nanoscale. Nat. Chem. 2011, 3, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.N.; Adler, S.B.; Li, J. Imaging Space Charge Regions in Sm-doped Ceria Using Electrochemical Strain Microscopy. Appl. Phys. Lett. 2014, 105, 1–5. [Google Scholar] [CrossRef]
- Luchkin, S.Y.; Romanyuk, K.; Ivanov, M.; Kholkin, A.L. Li Transport in Fresh and Aged LiMn2O4 Cathodes via Electrochemical Strain Microscopy. J. Appl. Phys. 2015, 118, 072016. [Google Scholar] [CrossRef]
- Alikin, D.O.; Ievlev, A.V.; Luchkin, S.Y.; Turygin, A.P.; Shur, V.Y.; Kalinin, S.V.; Kholkin, A.L. Characterization of LiMn2O4 Cathodes by Electrochemical Strain Microscopy. Appl. Phys. Lett. 2016, 108, 113106. [Google Scholar] [CrossRef]
- Schön, N.; Gunduz, D.C.; Yu, S.; Tempel, H.; Schierholz, R.; Hausen, F. Correlative Electrochemical Strain and Scanning Electron Microscopy for Local Characterization of the Solid State Electrolyte Li1.3Al0.3Ti1.7(PO4)3. Beilstein J. Nanotechnol. 2018, 9, 1564–1572. [Google Scholar] [CrossRef]
- Giridharagopal, R.; Flagg, L.Q.; Harrison, J.S.; Ziffer, M.E.; Onorato, J.; Luscombe, C.K.; Ginger, D.S. Electrochemical Strain Microscopy Probes Morphology-Induced Variations in Ion Uptake and Performance in Organic Electrochemical Transistors. Nat. Mater. 2017, 16, 737–742. [Google Scholar] [CrossRef]
- Kumar, A.; Arruda, T.M.; Tselev, A.; Ivanov, I.N.; Lawton, J.S.; Zawodzinski, T.A.; Butyaev, O.; Zayats, S.; Jesse, S.; Kalinin, S.V. Nanometer-Scale Mapping of Irreversible Electrochemical Nucleation Processes on Solid Li-Ion Electrolytes. Sci. Rep. 2013, 3, 1621. [Google Scholar] [CrossRef]
- Yang, S.M.; Paranthaman, M.P.; Noh, T.W.; Kalinin, S.V.; Strelcov, E. Nanoparticle Shape Evolution and Proximity Effects During Tip-Induced Electrochemical Processes. ACS Nano 2016, 10, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Li, T.; Tian, T. In situ Study of Li-Ions Diffusion and Deformation in Li-rich Cathode Materials by using Scanning Probe Microscopy Techniques. J. Phys. D Appl. Phys. 2017, 50, 313001. [Google Scholar] [CrossRef]
- Seol, D.; Park, S.; Varenyk, O.V.; Lee, S.; Lee, H.N.; Morozovska, A.N.; Kim, Y. Determination of Ferroelectric Contributions to Electromechanical Response by Frequency Dependent Piezoresponse Force Microscopy. Sci. Rep. 2016, 6, 30579. [Google Scholar] [CrossRef]
- Alikin, D.O.; Romanyuk, K.N.; Slautin, B.N.; Rosato, D.; Shur, V.Y.; Kholkin, A.L. Quantitative Characterization of the Ionic Mobility and concentration in Li-battery Cathodes via Low Frequency Electrochemical Strain Microscopy. Nanoscale 2018, 10, 2503–2511. [Google Scholar] [CrossRef]
- Lushta, V.; Bradler, S.; Roling, B.; Schirmeisen, A. Correlation between drive amplitude and Resonance Frequency in Electrochemical Strain Microscopy: Influence of Electrostatic Forces. J. Appl. Phys. 2017, 121, 224302. [Google Scholar] [CrossRef]
- Balke, N.; Jesse, S.; Li, Q.; Maksymovych, P.; Baris Okatan, M.; Strelcov, E.; Tselev, A.; Kalinin, S.V. Current and Surface Charge Modified Hysteresis Loops in Ferroelectric Thin Films. J. Appl. Phys. 2015, 118, 072013. [Google Scholar] [CrossRef]
- Kim, Y.; Kumar, A.; Tselev, A.; Kravchenko, I.I.; Han, H.; Vrejoiu, I.; Lee, W.; Hesse, D.; Alexe, M.; Kalinin, S.V.; et al. Nonlinear Phenomena in Multiferroic Nanocapacitors: Joule Heating and Electromechanical Effects. ACS Nano 2011, 5, 9104–9112. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, A.; Wang, Z.; Hovington, P.; Miyahara, Y.; Paolella, A.; Gariepy, V.; Feng, Z.; Enright, T.; Aiken, C.; Zaghib, K.; et al. Measuring Spatially Resolved Collective Ionic Transport on Lithium Battery Cathodes Using Atomic Force Microscopy. Nano Lett. 2017, 17, 4489–4496. [Google Scholar] [CrossRef]
- Amanieu, H.Y.; Aramfard, M.; Rosato, D.; Batista, L.; Rabe, U.; Lupascu, D.C. Mechanical Properties of Commercial LixMn2O4 Cathode Under Different States of Charge. Acta Mater. 2015, 89, 153–162. [Google Scholar] [CrossRef]
- Mughal, M.Z.; Moscatelli, R.; Amanieu, H.Y.; Sebastiani, M. Effect of Lithiation on Micro-Scale Fracture Toughness of LixMn2O4 Cathode. Scr. Mater. 2016, 116, 62–66. [Google Scholar] [CrossRef]
- Slautin, B.; Alikin, D.; Rosato, D.; Pelegov, D.; Shur, V.; Kholkin, A. Local Study of Lithiation and Degradation Paths in LiMn2O4 Battery Cathodes: Confocal Raman Microscopy Approach. Batteries 2018, 4, 21. [Google Scholar] [CrossRef]
- Gannepalli, A.; Yablon, D.G.; Tsou, A.H.; Proksch, R. Mapping Nanoscale Elasticity and Dissipation Using Dual Frequency Contact Resonance AFM. Nanotechnology 2011, 22, 355705. [Google Scholar] [CrossRef]
- Balke, N.; Jesse, S.; Yu, P.; Carmichael, B.; Kalinin, S.V.; Tselev, A. Quantification of Surface Displacements and Electromechanical Phenomena via Dynamic Atomic Force Microscopy. Nanotechnology 2016, 27, 425707. [Google Scholar] [CrossRef] [PubMed]
- Proksch, R. In-situ Piezoresponse Force Microscopy Cantilever Mode Shape Profiling. J. Appl. Phys. 2015, 118, 072011. [Google Scholar] [CrossRef]
- Zhan, C.; Wu, T.; Lu, J.; Amine, K. Dissolution, Migration, and Deposition of Transition Metal Ions in Li-Ion Batteries Exemplified by Mn-Based Cathodes—A critical review. Energy Environ. Sci. 2018, 11, 243–257. [Google Scholar] [CrossRef]
- Balke, N.; Kalnaus, S.; Dudney, N.J.; Daniel, C.; Jesse, S.; Kalinin, S.V. Local Detection of Activation Energy for Ionic Transport in Lithium Cobalt Oxide. Nano Lett. 2012, 12, 3399–3403. [Google Scholar] [CrossRef] [PubMed]
- Romanyuk, K.; Luchkin, S.Y.; Ivanov, M.; Kalinin, A.; Kholkin, A.L. Single- and Multi-Frequency Detection of Surface Displacements via Scanning Probe Microscopy. Microsc. Microanal. 2015, 21, 154–163. [Google Scholar] [CrossRef]
- Soergel, E. Piezoresponse Force Microscopy (PFM). J. Phys. D Appl. Phys. 2011, 44, 464003. [Google Scholar] [CrossRef]
- Vasudevan, R.K.; Balke, N.; Maksymovych, P.; Jesse, S.; Kalinin, S.V. Ferroelectric or Non-Ferroelectric: Why So Many Materials Exhibit “Ferroelectricity” on the Nanoscale. Appl. Phys. Rev. 2017, 4, 021302. [Google Scholar] [CrossRef]
- Abdollahi, A.; Domingo, N.; Arias, I.; Catalan, G. Converse Flexoelectricity Yields Large Piezoresponse Force Microscopy Signals in Non-Piezoelectric Materials. Nat. Commun. 2019, 10, 1266. [Google Scholar] [CrossRef] [PubMed]
- Balke, N.; Jesse, S.; Carmichael, B.; Okatan, M.B.; Kravchenko, I.I.; Kalinin, S.V.; Tselev, A. Quantification of In-Contact Probe-Sample Electrostatic Forces with Dynamic Atomic Force Microscopy. Nanotechnology 2017, 28, 065704. [Google Scholar] [CrossRef] [PubMed]
- Op de Beeck, J.; Labyedh, N.; Sepúlveda, A.; Spampinato, V.; Franquet, A.; Conard, T.; Vereecken, P.M.; Celano, U. Direct Imaging and Manipulation of Ionic Diffusion in Mixed Electronic–Ionic Conductors. Nanoscale 2018, 10, 12564–12572. [Google Scholar] [CrossRef]
- Lampert, M.A.; Schilling, R.B. Chapter 1 Current Injection in Solids: The Regional Approximation Method. Semicond. Semimetals 1970, 6, 1–96. [Google Scholar] [CrossRef]
- Rose, A. Space-Charge-Limited Currents in Solids. Phys. Rev. 1955, 97, 1538–1544. [Google Scholar] [CrossRef]
- Reid, O.G.; Munechika, K.; Ginger, D.S. Space Charge Limited Current Measurements on Conjugated Polymer Films using Conductive Atomic Force Microscopy. Nano Lett. 2008, 8, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Amanieu, H.-Y.; Thai, H.N.M.; Luchkin, S.Y.; Rosato, D.; Lupascu, D.C.; Keip, M.-A.; Schröder, J.; Kholkin, A.L. Electrochemical Strain Microscopy Time Spectroscopy: Model and experiment on LiMn2O4. J. Appl. Phys. 2015, 118, 055101. [Google Scholar] [CrossRef]
- García, R.E.; Chiang, Y.-M.; Craig Carter, W.; Limthongkul, P.; Bishop, C.M. Microstructural Modeling and Design of Rechargeable Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A255–A263. [Google Scholar] [CrossRef]
- Fukuma, T.; Kimura, M.; Kobayashi, K.; Matsushige, K.; Yamada, H. Development of Low Noise Cantilever Deflection Sensor for Multienvironment Frequency-Modulation Atomic Force Microscopy. Rev. Sci. Instrum. 2005, 76, 053704. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alikin, D.; Slautin, B.; Abramov, A.; Rosato, D.; Shur, V.; Tselev, A.; Kholkin, A. Correlative Confocal Raman and Scanning Probe Microscopy in the Ionically Active Particles of LiMn2O4 Cathodes. Materials 2019, 12, 1416. https://doi.org/10.3390/ma12091416
Alikin D, Slautin B, Abramov A, Rosato D, Shur V, Tselev A, Kholkin A. Correlative Confocal Raman and Scanning Probe Microscopy in the Ionically Active Particles of LiMn2O4 Cathodes. Materials. 2019; 12(9):1416. https://doi.org/10.3390/ma12091416
Chicago/Turabian StyleAlikin, Denis, Boris Slautin, Alexander Abramov, Daniele Rosato, Vladimir Shur, Alexander Tselev, and Andrei Kholkin. 2019. "Correlative Confocal Raman and Scanning Probe Microscopy in the Ionically Active Particles of LiMn2O4 Cathodes" Materials 12, no. 9: 1416. https://doi.org/10.3390/ma12091416
APA StyleAlikin, D., Slautin, B., Abramov, A., Rosato, D., Shur, V., Tselev, A., & Kholkin, A. (2019). Correlative Confocal Raman and Scanning Probe Microscopy in the Ionically Active Particles of LiMn2O4 Cathodes. Materials, 12(9), 1416. https://doi.org/10.3390/ma12091416









