Hydrogen Accumulation and Distribution in Pipeline Steel in Intensified Corrosion Conditions
Abstract
:1. Introduction
Methane Dissociation
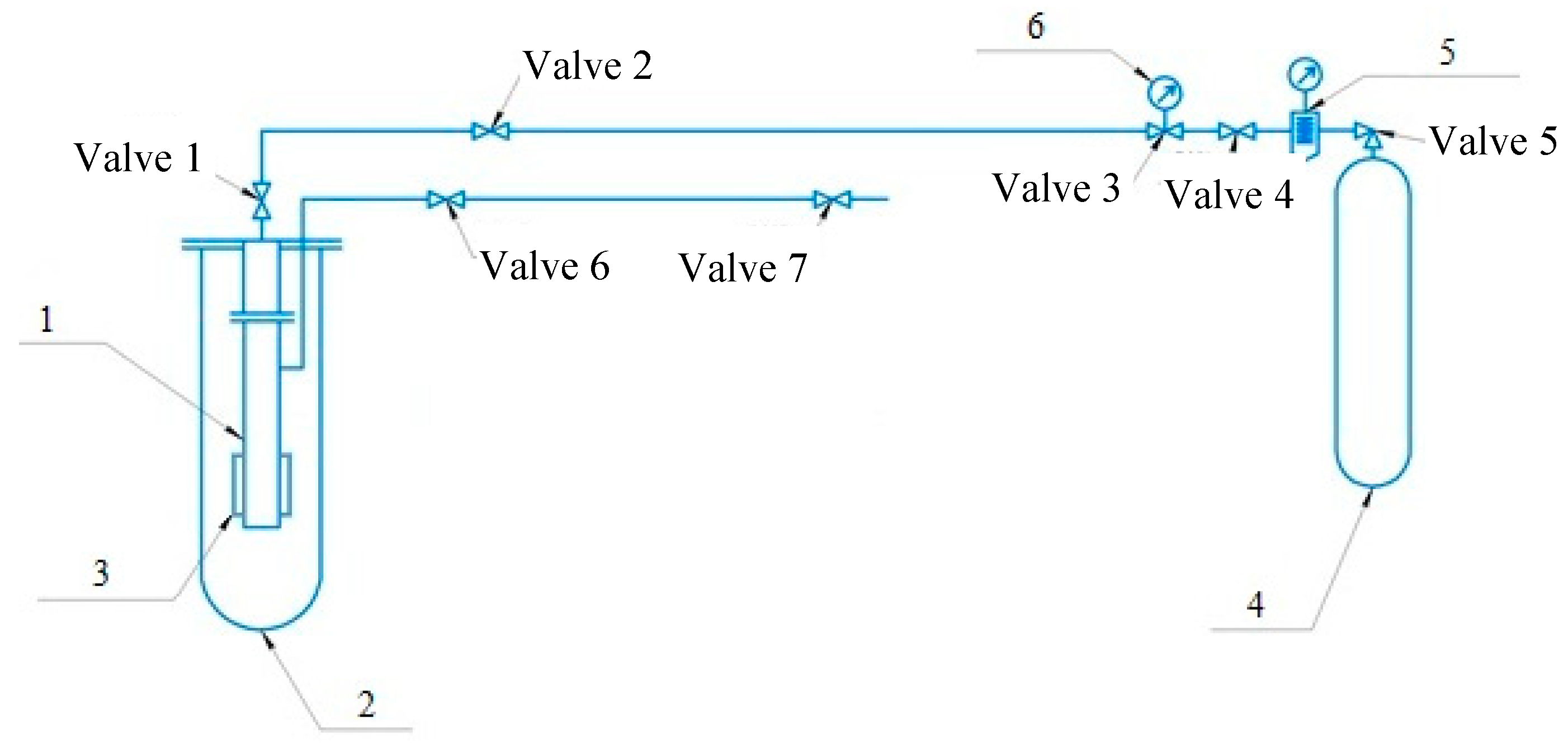
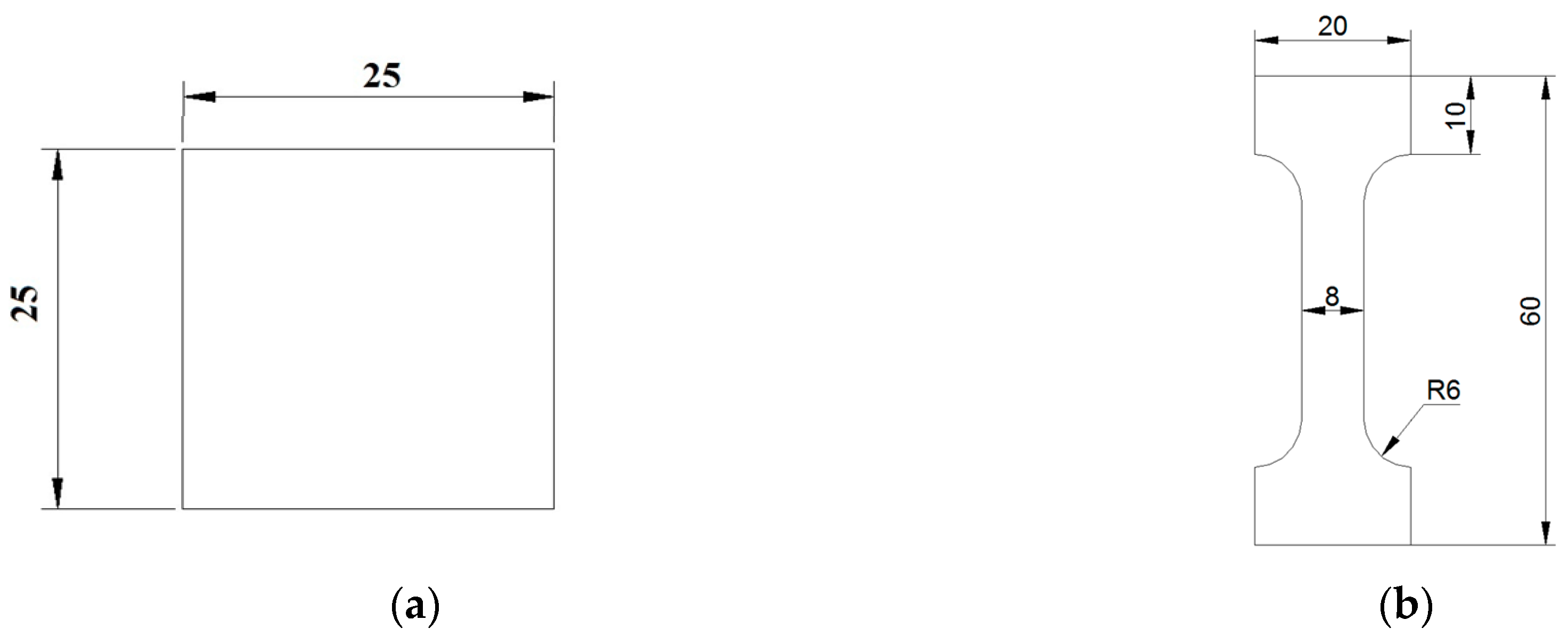
2. Materials and Methods
3. Results and Discussion
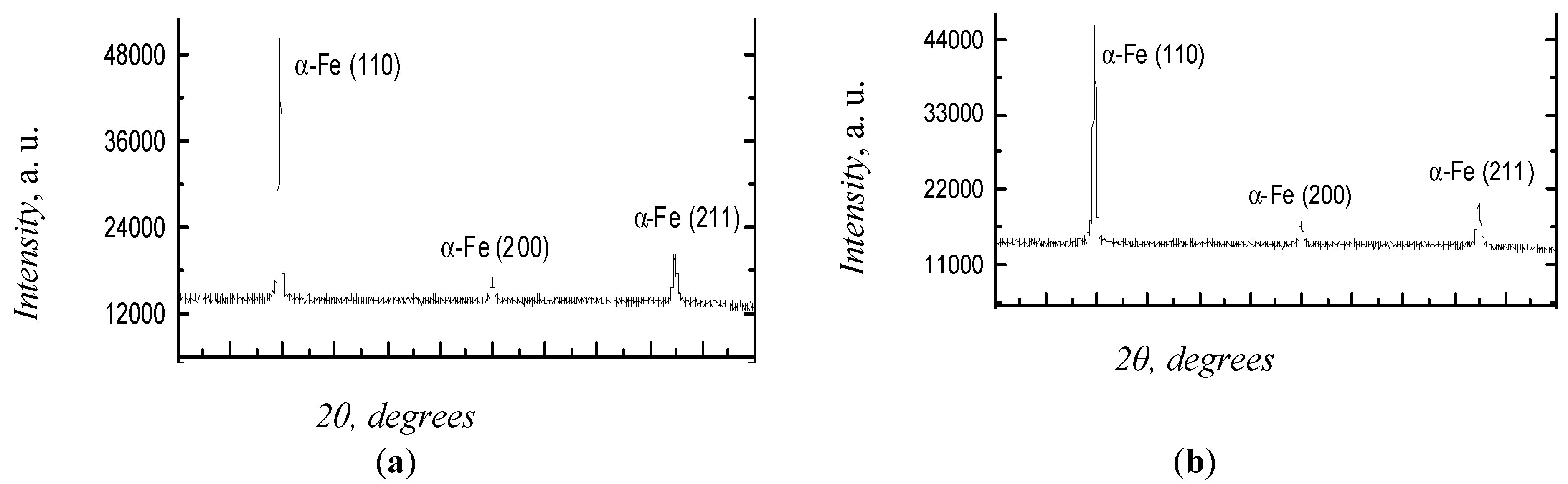
3.1. XRD Analysis
3.2. Elemental Composition
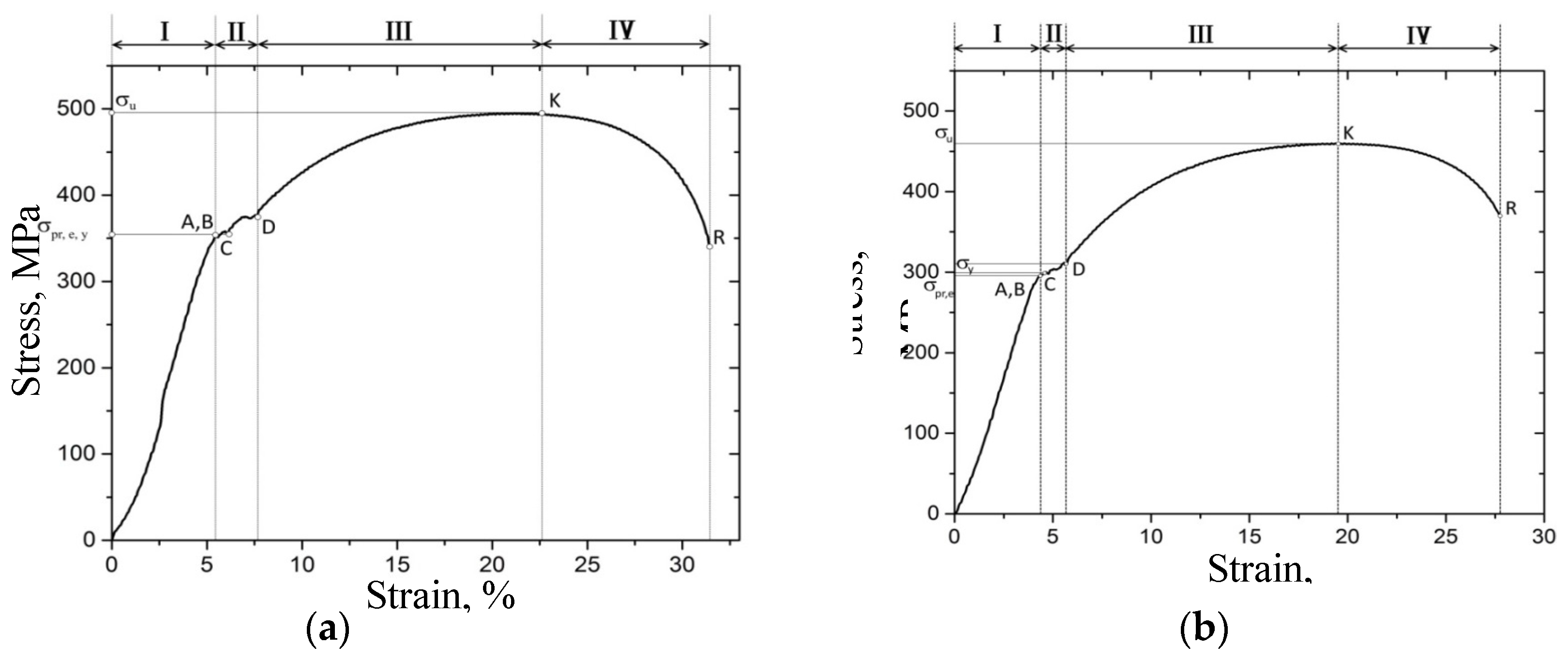
3.3. Uniaxial Tension Tests
3.4. Pendulum Impact Testing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Corrosion Problems During Oil and Gas Production and its Mitigation. Int. J. Ind. Chem. 2013, 4–35. [Google Scholar] [CrossRef]
- Walsh, M.R.; Hancock, S.H.; Wilson, S.J.; Patil, S.L.; Moridis, G.J.; Boswell, R.; Sloan, E.D. Preliminary report on the commercial viability of gas production from natural gas hydrates. Energy Econ. 2009, 31, 815–823. [Google Scholar] [CrossRef]
- Popoola, L.T.; Grema, A.S.; Latinwo, G.K.; Gutti, B.; Balogun, A.S. Opened Report: Corrosion Mitigation for Complex Environments; Champion Technologies: Houston, TX, USA, 2012. [Google Scholar]
- US Department of Transportation Pipeline and Hazardous Materials Safety Administration. Opened Report: Development of Guidelines for Identification of SCC Sites and Estimation of Re-Inspection Intervals for SCC Direct Assessment; US Department of Transportation Pipeline and Hazardous Materials Safety Administration: New Jersey Ave, Washington, USA, 2010. [Google Scholar]
- CEPA Pipeline Integrity Working Group. Recommended Practices for Managing Near-neutral pH Stress Corrosion Cracking, 3rd ed.; CEPA Pipeline Integrity Working Group: Calgary, AB, Canada, 2015; p. 162. [Google Scholar]
- Sandana, D. Stress Corrosion Cracking of Pipeline Steels in Contaminated Aqueous CO2 Environments. Ph.D. Thesis, School of Chemical Engineering and Advanced Materials, Newcastle University, Newcastle, UK, July 2016. [Google Scholar]
- Cheng, Y.F. (Ed.) Stress Corrosion Cracking of Pipelines; John Wiley & Sons, Inc.: Hoboken, TX, Canada, 2013; p. 275. [Google Scholar]
- Raja, V.S.; Shoji, T. (Eds.) Stress Corrosion Cracking, Theory and Practice; Woodhead Publishing Ltd.: Cambridge, UK, 2011; p. 816. [Google Scholar]
- Jones, R.H. (Ed.) Stress-Corrosion Cracking: Materials Performance and Evaluation; ASM International: Materials Park, OH, USA, 2017. [Google Scholar]
- Liu, Y.; Li, Q.; Cui, Z.Y.; Wua, W.; Li, Z.; Du, C.W.; Li, X.G. Field Experiment of Stress Corrosion Cracking Behavior of High Strength Pipeline Steels in Typical Soil Environments. Constr. Build. Mater. 2017, 148, 131–139. [Google Scholar] [CrossRef]
- Gao, S.-J.; Dong, C.-F.; Fu, A.-Q.; Xiao, K.; Li, X.-G. Corrosion Behavior of the Expandable Tubular in Formation Water. Int. J. Min. Met. Mater. 2015, 22, 149–156. [Google Scholar] [CrossRef]
- Zoski, C.G. Handbook of Electrochemistry, 1st ed.; Elsevier: Amsterdam, The Netherlands; Kidlington, UK, 2007; p. 935. [Google Scholar]
- Mazur, I.I.; Ivantsev, O.M. Pipeline System Safety [in Russian]; ELIMA: Moscow, Russia, 2004; p. 1104. [Google Scholar]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.D.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; Liverani, C.; Stopher, M.; Sweeney, F. Understanding and Mitigating Hydrogen Embrittlement of Steels: A Review of Experimental, Modelling and Design Progress From Atomistic to Continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Nechaev, Y.S. NATO Advanced Research Workshop on Hydrogen Materials Science and Chemistry of Metal Hydrides; Ser. II, Kluwer Acad. Publ.: Dordrecht, The Netherlands, 2002; p. 161. [Google Scholar]
- Malkin, A.I. Regularities and Mechanisms of the Rehbinder’s Effect. Colloid J. 2012, 74, 223–238. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement; Springer: Berlin, Germany, 2016; p. 239. [Google Scholar]
- Gangloff, R.P.; Somerday, B.P. Gaseous Hydrogen Embrittlement of Materials in Energy Technologies: The Problem, its Characterisation and Effects on Particular Alloy Classes; Elsevier, Woodhead Publishing: Sawston, Cambridge, UK, 2012; p. 964. [Google Scholar]
- Briottet, L.; Moro, I.; Lemoine, P. Quantifying the Hydrogen Embrittlement of Pipeline Steels for Safety Considerations. Int. J. Hydrogen Energy 2012, 37, 616–623. [Google Scholar] [CrossRef]
- Ashwani, S.N.; Tiwari, K.; Jackson, B. Dissociative Chemisorption of Methane on Ni and Pt Surfaces: Mode-Specific Chemistry and the Effects of Lattice Motion. J. Phys. Chem. A 2014, 118, 9615–9631. [Google Scholar]
- Guo, H. The Dissociative Chemisorption of Methane and its Isotopologues on Metal Surfaces. Doctoral Dissertation, University of Massachusetts Amherst, Amherst, MA, USA, 2018. [Google Scholar]
- Germain, J.E. Catalytic Conversion of Hydrocarbons; Academic Press Inc.: London, UK, 1969; p. 322. [Google Scholar]
- Kauzmann, W. Kinetic Theory of Gases; Dover Publications: Mineola, NY, USA, 2013; p. 272. [Google Scholar]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw Hill, Inc.: New York, NY, USA, 1999; p. 1291. [Google Scholar]
- Bao, S.; Fan, C.; Li, H.; Xu, Y. The Adsorption and Decomposition of Methane on Fe/Cu(110) Bimetallic Surface. Sci. China Math. Phys. Astron. Technol. Sci. 1995, 38, 813. [Google Scholar] [CrossRef]
- Galaktionova, L.V.; Arcatova, L.A.; Kurina, L.N.; Gorbunova, E.I.; Belousova, V.N.; Nyborodenko, Yu.S.; Kasatsky, N.G.; Golobokov, N.N. Fe-Containing Intermetallic Compounds as Catalysts for Carbon Dioxide Reforming of Methane. J. Phys. Chem. A 2008, 82, 271–275. [Google Scholar]
- Anderson, J.R.; Boudart, M. (Eds.) Catalysis Science and Technology; Springer-Verlag: Berlin/Heidelberg, Germany, 1984; p. 281. [Google Scholar]
- Xu, J.; Froment, G.F. Methane Steam Reforming, Methanation and Water-Gas Shift: 1. Intrinsic Kinetics. AICHE J. 1989, 35, 88–96. [Google Scholar] [CrossRef]
- Arutyunov, V.C.; Krylov, O.V. Oxidative Transformations of Methane; Nauka: Moscow, Russia, 1998; p. 362. [Google Scholar]
- Tikhomirov, D.V.; Krashennikov, S.V.; Don, B.D.; Makinsky, A.A. Combustible Natural Gas Supplied and Transported through Gas Pipelines; STO Gazprom 089-2010: Moscow, Russia, 2011. [Google Scholar]
- Pismen, M.K. Production of Hydrogen in Oil Industry; Khimiya: Moscow, Russia, 1976; p. 208. [Google Scholar]
- Bargin, O.V.; Liberman, A.L. Hydrocarbons Incremented by Metal Catalysts; Khimiya: Moscow, Russia, 1981; p. 264. [Google Scholar]
- Nechaev, Y.S. Physical Complex Problems of Aging, Embrittlement and Destruction of Metallic Materials of Hydrogen Power Engineering and Gas Mains. Phys. Usp. 2008, 178, 709–726. [Google Scholar]
- Galchenko, V.F. Methanotrophic Bacteria; GEOS: Moscow, Russia, 2001; p. 500. [Google Scholar]
| Substances | CH4 | C | H2 |
|---|---|---|---|
| Standard Gibbs free energy, ΔG0, kJ/mole | 50 | 0 | 0 |
| Standard enthalpy of formation, ΔH0, kJ/mole | 74.6 | 0 | 0 |
| Standard entropy of formation, ΔS0, kJ/mole | 186.19 | 5.7 | 130.5 |
| Substance | CH4 | C2H6 | C3H8 | C4H10 | CO2 | H2S |
|---|---|---|---|---|---|---|
| Weight content, % | 97 | 1.5 | 0.5 | 10−2 | 10−3 | 10−5 |
| Treatment | Phases | Phase Composition, vol.% | Lattice Parameters | Microstress |
|---|---|---|---|---|
| Before | BCC α-Fe phase | 100 | a = 2.8697 | 0.001065 |
| After | BCC α-Fe phase | 100 | a = 2.8692 | 0.000425 |
| Chemical Elements | Weight Content, % (Outer Surface) | Weight Content, % (Inner Surface) |
|---|---|---|
| C | 0.078 | 0.072 |
| Si | 0.321 | 0.352 |
| Mn | 1.331 | 1.266 |
| S | 0.002 | 0.003 |
| Fe | 98.268 | 98.307 |
| Weight Content, wt.% | Outer Surface | Bulk | Inner Surface |
|---|---|---|---|
| H content | 0.00069 | 0.00067 | 0.00065 |
| Direct measurement error | 0.00005 | 0.00004 | 0.00003 |
| Chemical Elements | Weight Content, % (Outer Surface) | Weight Content, % (Inner Surface) |
|---|---|---|
| C | 0.075 | 0.071 |
| Si | 0.318 | 0.339 |
| Mn | 1.315 | 1.331 |
| S | 0.004 | 0.004 |
| Fe | 98.288 | 98.255 |
| Weight Content, wt.% | Outer Surface | Bulk | Inner Surface |
|---|---|---|---|
| H content | 0.00020 | 0.00083 | 0.00086 |
| Direct measurement error | 0.00005 | 0.00006 | 0.00004 |
| Before Treatment | After Treatment | |
|---|---|---|
| Proportional limit strength ± 20, MPa | 352 | 296 |
| Elastic limit strength ± 20, MPa | 352 | 296 |
| Yield strength ± 20, MPa | 352 | 300 |
| Tensile strength ± 20, MPa | 494 | 462 |
| Maximum percent elongation ± 0.1, % | 32 | 27 |
| Resilience KCV, J/cm2 | Initial State | State after Treatment |
|---|---|---|
| KCV ± 20 | 355 | 345 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titov, A.I.; Lun-Fu, A.V.; Gayvaronskiy, A.V.; Bubenchikov, M.A.; Bubenchikov, A.M.; Lider, A.M.; Syrtanov, M.S.; Kudiiarov, V.N. Hydrogen Accumulation and Distribution in Pipeline Steel in Intensified Corrosion Conditions. Materials 2019, 12, 1409. https://doi.org/10.3390/ma12091409
Titov AI, Lun-Fu AV, Gayvaronskiy AV, Bubenchikov MA, Bubenchikov AM, Lider AM, Syrtanov MS, Kudiiarov VN. Hydrogen Accumulation and Distribution in Pipeline Steel in Intensified Corrosion Conditions. Materials. 2019; 12(9):1409. https://doi.org/10.3390/ma12091409
Chicago/Turabian StyleTitov, Anatolii I., Aleksandr V. Lun-Fu, Aleksandr V. Gayvaronskiy, Mikhail A. Bubenchikov, Aleksei M. Bubenchikov, Andrey M. Lider, Maxim S. Syrtanov, and Viktor N. Kudiiarov. 2019. "Hydrogen Accumulation and Distribution in Pipeline Steel in Intensified Corrosion Conditions" Materials 12, no. 9: 1409. https://doi.org/10.3390/ma12091409
APA StyleTitov, A. I., Lun-Fu, A. V., Gayvaronskiy, A. V., Bubenchikov, M. A., Bubenchikov, A. M., Lider, A. M., Syrtanov, M. S., & Kudiiarov, V. N. (2019). Hydrogen Accumulation and Distribution in Pipeline Steel in Intensified Corrosion Conditions. Materials, 12(9), 1409. https://doi.org/10.3390/ma12091409






