In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation
Abstract
1. Introduction
2. Materials and Methods
2.1. Abutment Material Surface Morphology, Roughness and Wettability Analysis
2.2. Cell Culture
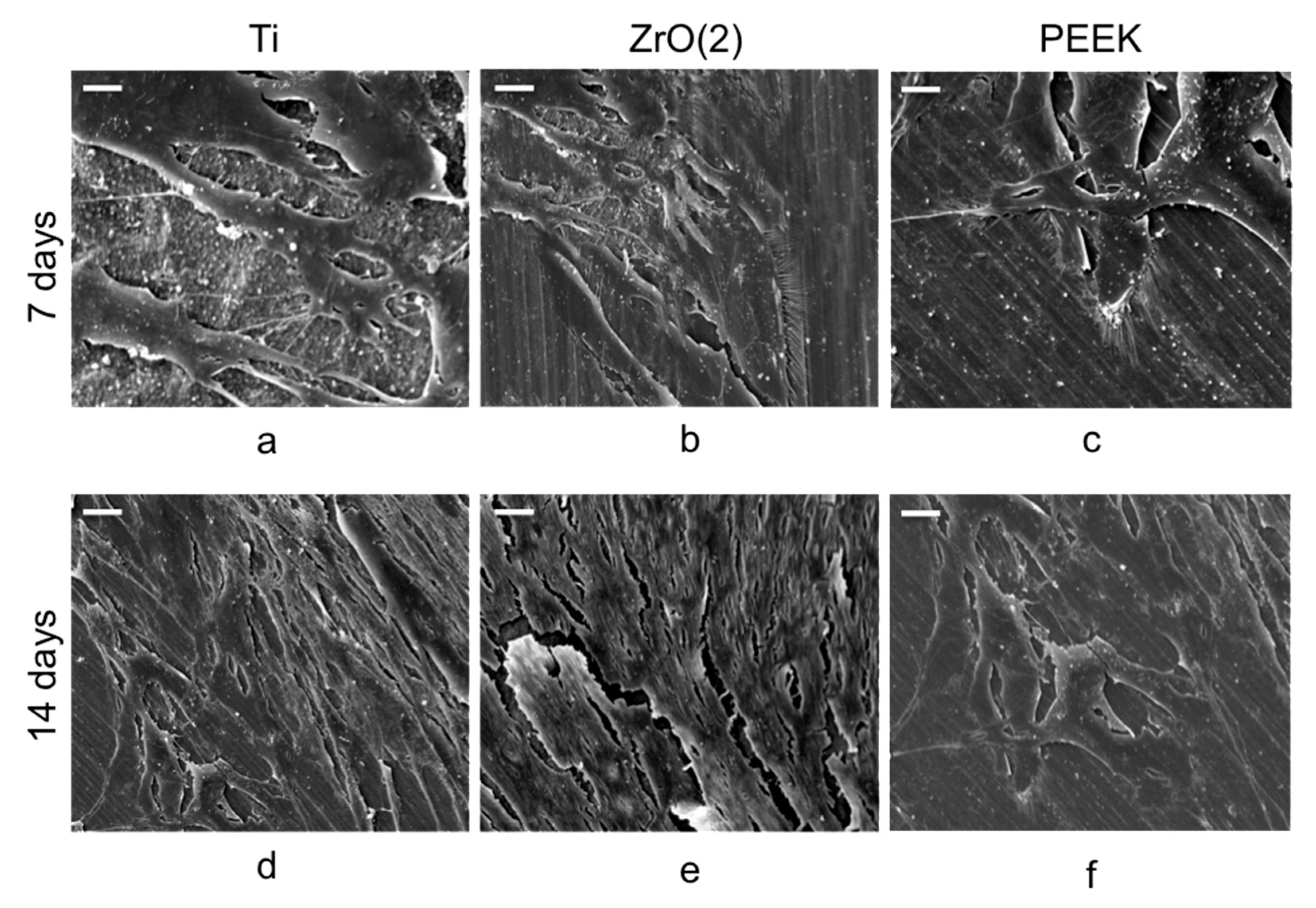
2.3. Cell Attachment and Morphology
2.4. Cell Viability and Proliferation
2.5. Cell Migration
2.6. Statistical Analysis
3. Results
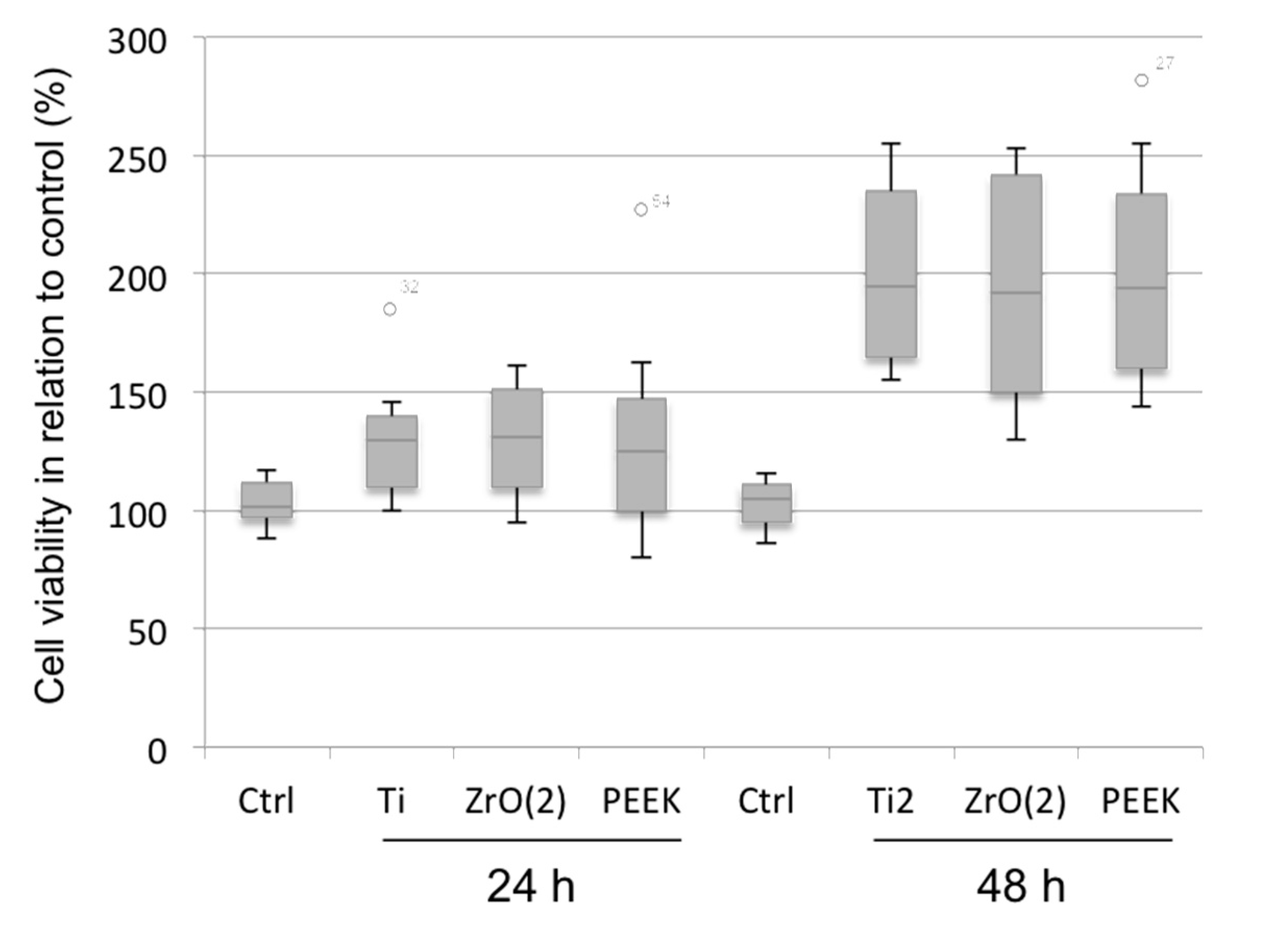
3.1. Cell Viability and Morphology Analysis
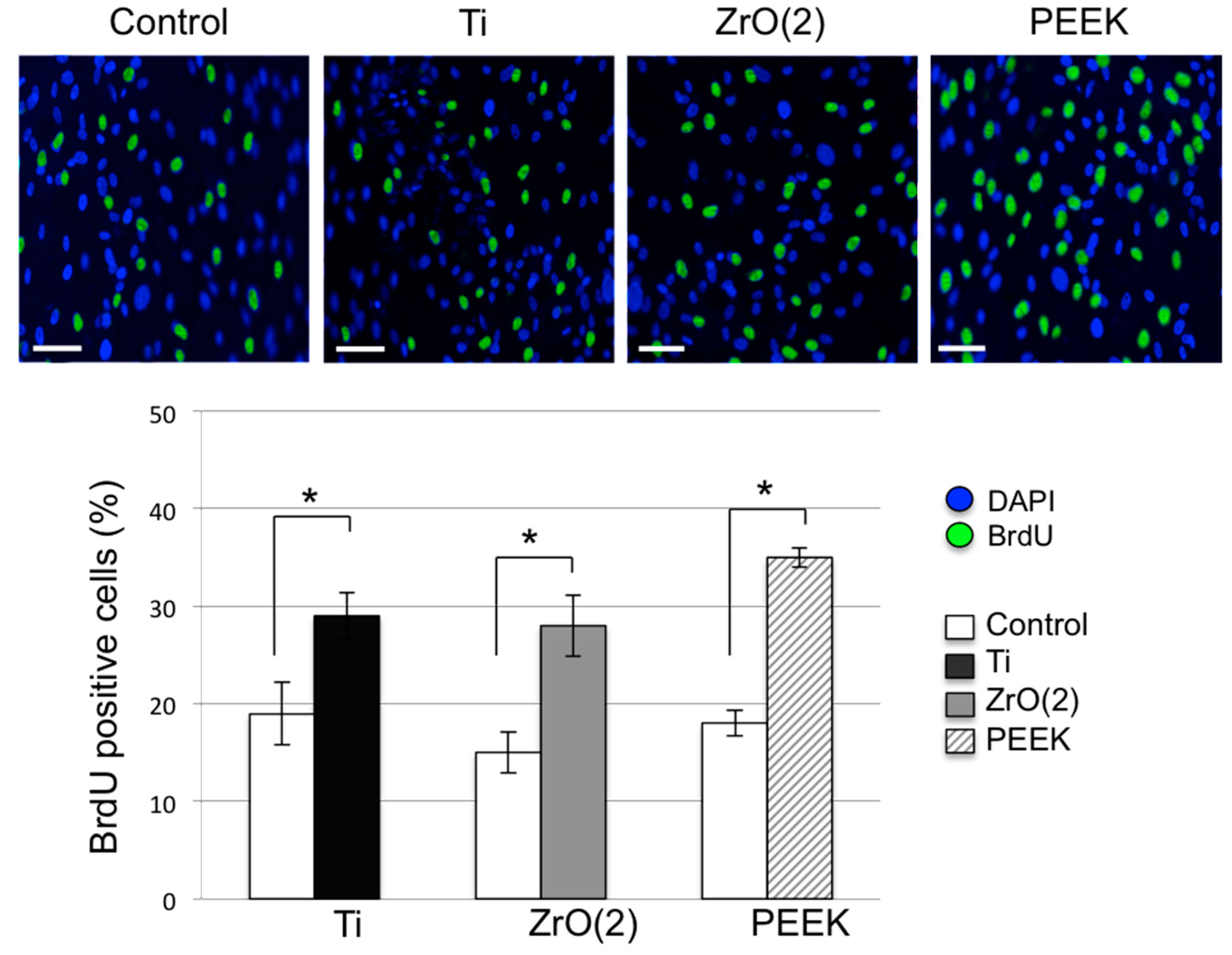
3.2. Cell Proliferation and Migration
3.3. Abutment Material Surface Characterization
4. Discussion
5. Conclusions
- -
- Modified PEEK surface may augment biocompatibility by having a positive impact on viability, adhesion, migration and proliferation of human gingival epithelial keratinocytes as compared to titanium and zirconia.
- -
- Accumulation of knowledge on surface abutment effect on surrounding epithelial tissue might provide new insights into the development of future novel nanostructured and biocompatible implants abutments.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PEEK | polyetheretherketone |
| CAD/CAM | Computer-Aided-Design/Computer-Aided-Manufacturing |
| HGEK | human gingival epithelial keratinocytes cultured |
| Ti | titanium |
| ZrO2 | zirconia |
References
- Abrahamsson, I.; Berglundh, T.; Glantz, P.O.; Lindhe, J. The mucosal attachment at different abutments. An experimental study in dogs. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Abrahamsson, I.; Berglundh, T.; Lindhe, J. Soft tissue reactions to plaque formation at implant abutments with different surface topography. An experimental study in dogs. J. Clin. Periodontol. 2002, 29, 456–461. [Google Scholar] [CrossRef]
- D’Ercole, S.; Tripodi, D.; Marzo, G.; Bernardi, S.; Continenza, M.A.; Piattelli, A.; Iaculli, F.; Mummolo, S. Microleakage of bacteria in different implant-abutment assemblies: an in vitro study. J. Appl. Biomater. Funct Mater. 2015, 13, 174–180. [Google Scholar] [CrossRef]
- Pae, A.; Lee, H.; Kim, H.S.; Kwon, Y.D.; Woo, Y.H. Attachment and growth behaviour of human gingival fibroblasts on titanium and zirconia ceramic surfaces. Biomed. Mater. 2009, 4, 025005. [Google Scholar] [CrossRef] [PubMed]
- Grossner-Schreiber, B.; Herzog, M.; Hedderich, J.; Duck, A.; Hannig, M.; Griepentrog, M. Focal adhesion contact formation by fibroblasts cultured on surface-modified dental implants: an in vitro study. Clin. Oral Implants Res. 2006, 17, 736–745. [Google Scholar] [CrossRef]
- Kasaj, A.; Reichert, C.; Gotz, H.; Rohrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 14, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, C.; Cimasoni, G. Functional characteristics of gingival and periodontal ligament fibroblasts. J. Dent. Res. 1996, 75, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thomsen, P. The soft tissue barrier at implants and teeth. Clin. Oral Implants Res. 1991, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Dorkhan, M.; Yücel-Lindberg, T.; Hall, J.; Svensäter, G.; Davies, J.R. Adherence of human oral keratinocytes and gingival fibroblasts to nano-structured titanium surfaces. BMC Oral Health 2014, 21, 14–75. [Google Scholar] [CrossRef]
- Gould, T.R.; Brunette, D.M.; Westbury, L. The attachment mechanism of epithelial cells to titanium in vitro. J. Periodontal. Res. 1981, 16, 611–616. [Google Scholar] [CrossRef]
- Lauer, G.; Wiedmann-Al-Ahmad, M.; Otten, J.E.; Hubner, U.; Schmelzeisen, R.; Schilli, W. The titanium surface texture effects adherence and growth of human gingival keratinocytes and human maxillar osteoblast-like cells in vitro. Biomaterials 2001, 22, 2799–2809. [Google Scholar] [CrossRef]
- Gupta, A.; Dhanraj, M.; Sivagami, G. Status of surface treatment in endosseous implant: a literary overview. Indian. J. Dent. Res. 2010, 21, 433–438. [Google Scholar] [CrossRef]
- Fillies, T.; Wiesmann, H.P.; Sommer, D.; Joos, U.; Meyer, U. Osteoblast reaction on SLA and microgrooved implant surfaces. MKG 2005, 9, 24–28. [Google Scholar]
- Keller, J.C. Physical and biological characteristics of implant materials. Adv. Dent. Res. 1999, 13, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Pabst, A.M.; Walter, C.; Grassmann, L.; Weyhrauch, M.; Brüllmann, D.D.; Ziebart, T.; Scheller, H.; Lehmann, K.M. Influence of CAD/CAM all-ceramic materials on cell viability, migration ability and adenylate kinase release of human gingival fibroblasts and oral keratinocytes. Clin. Oral Investig. 2014, 18, 1111–1118. [Google Scholar] [CrossRef]
- Mitsias, M.; Koutayas, S.O.; Wolfart, S.; Kern, M. Influence of zirconia abutment preparation on the fracture strength of single implant lithium disilicate crowns after chewing simulation. Clin. Oral Implants Res. 2014, 25, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, T.; Schnell, A.; Walter, C.; Kämmerer, P.W.; Pabst, A.; Lehmann, K.M.; Ziebart, J.; Klein, M.O.; Al-Nawas, B. Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces. Clin. Oral Investig. 2013, 17, 301–309. [Google Scholar] [CrossRef]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Liao, K. Performance characterization and modeling of a composite hip prosthesis. Exp. Tech. 1994, 18, 33–38. [Google Scholar] [CrossRef]
- Corvelli, A.A.; Biermann, P.J.; Roberts, J.C. Design, analysis and fabrication of a composite segmental bone replacement implant. J. Adv. Mater. 1997, 28, 2–8. [Google Scholar]
- Dhawan, U.; Pan, H.A.; Lee, C.H.; Chu, Y.H.; Huang, G.S.; Lin, Y.R.; Chen, W.L. Spatial Control of Cell-Nanosurface Interactions by Tantalum Oxide Nanodots for Improved Implant Geometry. PLoS ONE 2016, 11, e0158425. [Google Scholar] [CrossRef]
- Zhu, W.; Teel, G.; O’Brien, C.M.; Zhuang, T.; Keidar, M.; Zhang, L.G. Enhanced human bone marrow mesenchymal stem cell functions on cathodic arc plasma-treated titanium. Int. J. Nanomed. 2015, 10, 7385–7396. [Google Scholar]
- Cicciù, M.; Fiorillo, L.; Herford, A.S.; Crimi, S.; Bianchi, A.; D’Amico, C.; Laino, L.; Cervino, G. Bioactive Titanium Surfaces: Interactions of Eukaryotic and Prokaryotic Cells of Nano Devices Applied to Dental Practice. Biomedicines 2019, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, D.J.; Springer, G.S.; Goodman, S.B. Composite implant for bone replacement. J. Compos. Mater. 1997, 31, 1593–1632. [Google Scholar] [CrossRef]
- Neumann, E.A.; Villar, C.C.; Franca, M.F. Fracture resistance of abutment screws made of titanium, polyetheretherketone, and carbon fiber-reinforced polyetheretherketone. Braz. Oral Res. 2014, 28, 1–5. [Google Scholar] [CrossRef]
- Hahnel, S.; Wieser, A.; Lang, R.; Rosentritt, M. Biofilm formation on the surface of modern implant abutment materials. Clin. Oral Implants Res. 2015, 26, 1297–1301. [Google Scholar] [CrossRef] [PubMed]
- Santing, H.J.; Meijer, H.J.; Raghoebar, G.M.; Özcan, M. Fracture strength and failure mode of maxillary implant-supported provisional single crowns: a comparison of composite resin crowns fabricated directly over PEEK abut- ments and solid titanium abutments. Clin. Implant Dent. Relat. Res. 2012, 14, 882–889. [Google Scholar] [CrossRef]
- Tetelman, E.D.; Babbush, C.A. A new transitional abutment for immediate aesthetics and function. Implant Dent. 2008, 17, 51–58. [Google Scholar] [CrossRef]
- Schwitalla, A.; Muller, W.D. PEEK dental implants: A review of the literature. J. Oral Implant 2013, 39, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Ramenzoni, L.L.; Weber, F.E.; Attin, T.; Schmidlin, P.R. Cerium chloride application promotes wound healing and cell proliferation in human foreskin fibroblasts. Materials 2017, 10, 573. [Google Scholar] [CrossRef]
- Wenz, L.; Merritt, K.; Brown, S.; Moet, A.; Steffee, A. In vitro biocompatibility of polyetheretherketone and polysulfone composites. J. Biomed. Mater. Res. 1990, 24, 207–215. [Google Scholar] [CrossRef]
- Kumar, T.A.; Jei, J.B.; Muthukumar, B. Comparison of osteogenic potential of poly-ether-ether-ketone with titanium-coated poly-ether-ether-ketone and titanium-blended poly-ether-ether-ketone: An in vitro study. J. Indian Prosthodont. Soc. 2017, 17, 167–174. [Google Scholar] [CrossRef]
- Van Brakel, R.; Cune, M.S.; Van Winkelhoff, A.J.; De Putter, C.; Verhoeven, J.W.; Van Der Reijden, W. Early bacterial colonization and soft tissue health around zirconia and titanium abutments: An in vivo study in man. Clin. Oral Implants Res. 2011, 22, 571–577. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, A.; Polimeni, A.; Di Iorio, D.; Carinci, F. Bacterial adhesion on commercially pure titanium and anatase-coated titanium healing screws: An in vivo human study. J. Periodontol. 2010, 81, 1466–1471. [Google Scholar] [CrossRef]
- Gatewood, R.R.; Cobb, C.M.; Killoy, W.J. Microbial colonization on natural tooth structure compared with smooth and plasma-sprayed dental implant surfaces. Clin. Oral Implants Res. 1993, 4, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Wassmann, T.; Kreis, S.; Behr, M.; Buergers, R. The influence of surface texture and wettability on initial bacterial adhesion on titanium and zirconium oxide dental implants. Int. J. Implant Dent. 2017, 3, 32. [Google Scholar] [CrossRef]
- Rochford, E.T.; Poulsson, A.H.; Salavarrieta Varela, J.; Lezuo, P.; Richards, R.G.; Moriarty, T.F. Bacterial adhesion to orthopaedic implant materials and a novel oxygen plasma modified PEEK surface. Colloids Surf. B 2014, 113, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.C.; Smolinski, S.; Blake, D.M.; Huang, Z.; Wolfrum, E.J.; Jacoby, W.A. Bactericidal activity of photocatalytic TiO(2) reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Ma, R.; Tang, T. Current strategies to improve the bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef]
- Koutouzis, T.; Richardson, J.; Lundgren, T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J. Oral Implantol. 2011, 37, 174–182. [Google Scholar] [CrossRef]
- Sturz, C.R.; Faber, F.J.; Scheer, M.; Rothamel, D.; Neugebauer, J. Effects of various chair-side surface treatment methods on dental restorative materials with respect to contact angles and surface roughness. Dent. Mater. J. 2015, 34, 796–813. [Google Scholar] [CrossRef]
- Katzer, A.; Marquardt, H.; Westendorf, J.; Wening, J.V.; Von Foerster, G. Polyetheretherketone-cytotoxicity and mutagenicity in vitro. Biomaterials 2002, 23, 1749–1759. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Cheng, M.H.W.; Tang, S.M.; Yu, S.C.; Liao, K.; Tan, C.T.; Khor, K.A.; Cheang, P. Tensile properties, tension-tension fatigue and biological response of polyetheretherketone-hydroxyapatite composites for load bearing orthopedic implants. Biomaterials 2003, 24, 2245–2250. [Google Scholar] [CrossRef]
- Suska, F.; Omar, O.; Emanuelsson, L.; Taylor, M.; Gruner, P.; Kinbrum, A.; Hunt, D.; Hunt, T.; Taylor, A.; Palmquist, A. Enhancement of CRFPEEK osseointegration by plasma-sprayed hydroxyapatite: A rabbit model. J. Biomater. Appl. 2014, 29, 234–242. [Google Scholar] [CrossRef]
- Ha, S.W.; Mayer, J.; Koch, B.; Wintermant, E. Plasma sprayed hydroxyapatite coating on carbon fibre reinforced thermoplastic composite materials. J. Mater. Sci. Mater. Med. 1994, 5, 481–484. [Google Scholar] [CrossRef]
- Rahmitasari, F.; Ishida, Y.; Kurahashi, K.; Matsuda, T.; Watanabe, M.; Ichikawa, T. PEEK with Reinforced Materials and Modifications for Dental Implant Applications. Dent. J. 2017, 5, 35. [Google Scholar] [CrossRef]
- Qahtani, M.S.A.A.; Wu, Y.; Spintzyk, S.; Krieg, P.; Killinger, A.; Schweizer, E.; Stephan, I.; Scheideler, L.; Geis-Gerstorfer, J.; Rupp, F. UV-A and UV-C light induced hydrophilization of dental implants. Dent. Mater. 2015, 31, 157–167. [Google Scholar] [CrossRef]
- Huang, R.; Shao, P.; Burns, C.; Feng, X. Sulfonation of poly(ether ether ketone) (PEEK): Kinetic study and characterization. J. Appl. Polym. Sci. 2001, 82, 2651–2660. [Google Scholar] [CrossRef]
- Nieminen, T.; Kallela, I.; Wuolijoki, E.; Kainulainen, H.; Hiidenheimo, I.; Rantala, I. Amorphous and crystalline polyetheretherketone: Mechanical properties and tissue reactions during a 3-year follow-up. J. Biomed. Mater. Res. 2008, 84, 377–383. [Google Scholar] [CrossRef]
- Rupp, F.; Gittens, R.A.; Scheideler, L.; Marmur, A.; Boyan, B.D.; Schwartz, Z.; Geis-Gerstorfer, J. A review on the wettability of dental implant surfaces I: Theoretical and experimental aspects. Acta Biomater. 2014, 10, 2894–2906. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.I.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Xu, A.; Liu, X.; Gao, X.; Deng, F.; Deng, Y.; Wei, S. Enhancement of osteogenesis on micro/nano-topographical carbon fiber-reinforced polyetheretherketone-nanohydroxyapatite biocomposite. Mater. Sci. Eng. C 2015, 48, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.W.; Kirch, M.; Birchler, F.; Eckert, K.L.; Mayer, J.; Wintermantel, E.; Sittig, C.; Pfund-Klingenfuss, I.; Textor, M.; Spencer, N.D.; Guecheva, M.; Vonmont, H. Surface activation of polyetheretherketone (PEEK) and formation of calcium phosphate coatings by precipitation. Mater. Sci. Mater. Med. 1997, 8, 683–690. [Google Scholar] [CrossRef]
- Awaja, F.; Zhang, S.; James, N.; McKenzie, D.R. Enhanced autohesive bonding of polyetheretherketone (PEEK) for biomedical applications using a methane/oxygen plasma treatment. Plasma Process Polym. 2010, 7, 1010–1021. [Google Scholar] [CrossRef]
- Awaja, F.; Bax, D.V.; Zhang, S.; James, N.; McKenzie, D.R. Cell adhesion to PEEK treated by plasma immersion ion implantation and deposition for active medical implants. Plasma Proc. Polym. 2012, 9, 355–362. [Google Scholar] [CrossRef]
- Waser-Althaus, J.; Salamon, A.; Waser, M.; Padeste, C.; Kreutzer, M.; Pieles, U.; Müller, B.; Peters, K. Differentiation of human mesenchymal stem cells on plasma-treated polyetheretherketone. J. Mater. Sci. Mater. Med. 2014, 25, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Brydone, A.S.; Morrison, D.S.S.; Stormonth-Darling, J.; Meek, R.D.M.; Tanner, K.E.; Gadegaard, N. Design and fabrication of a 3D nanopatterned PEEK implant for cortical bone regeneration in a rabbit model. Eur. Cells Mater. 2012, 24, 39. [Google Scholar]

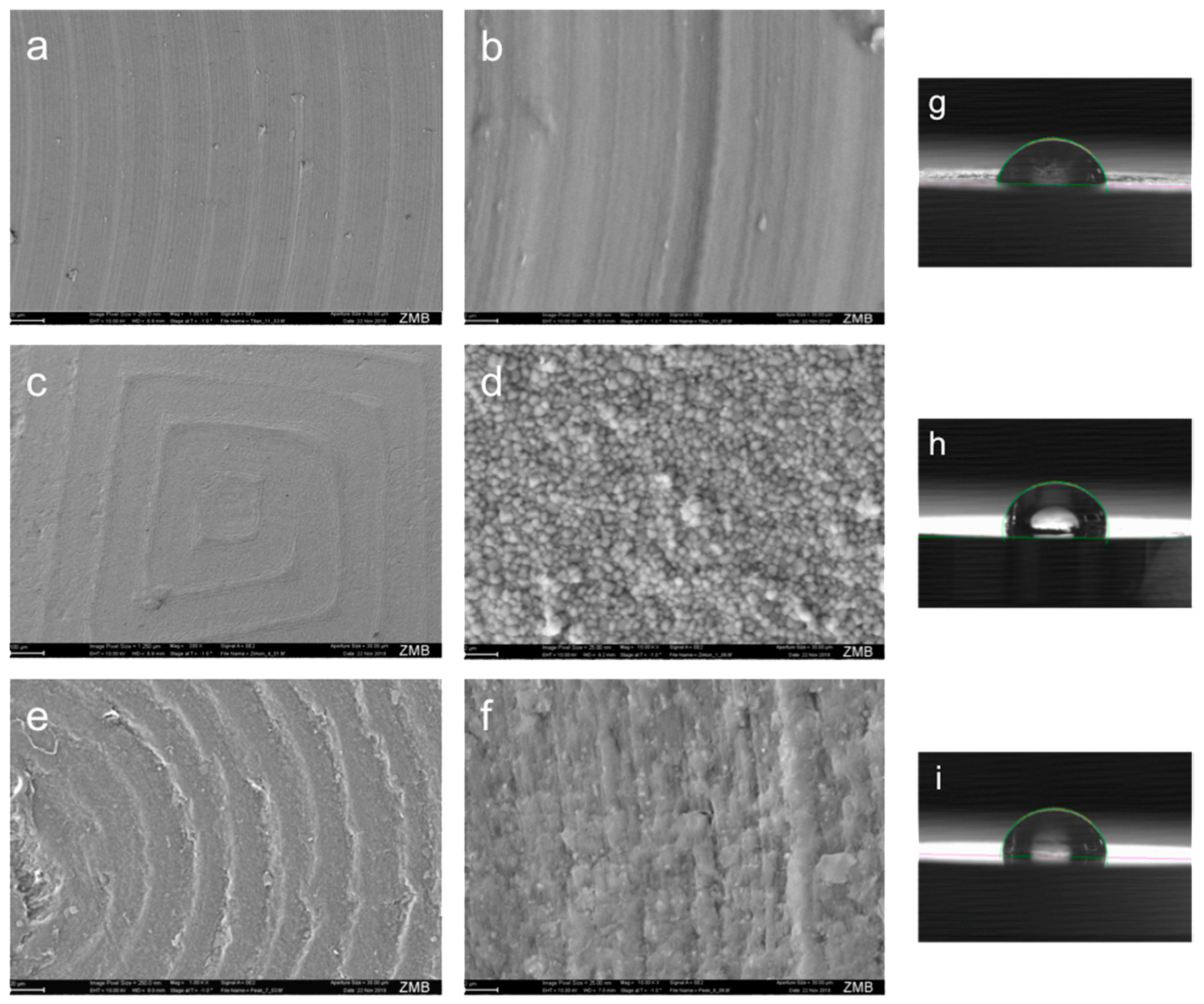
| Abutment Material | Roughness | Ra [μm] | Wettability | [°] |
|---|---|---|---|---|
| Ti | Smooth | 0.086 ± 0.006 | Hydrophilic | 72.3 ± 5.4 |
| ZrO2 | Rough | 1.152 ± 0.186 | Hydrophobic | 98.2 ± 8.6 |
| PEEK | Medium | 0.827 ± 0.012 | Hydrophobic | 90.3 ± 7.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramenzoni, L.L.; Attin, T.; Schmidlin, P.R. In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation. Materials 2019, 12, 1401. https://doi.org/10.3390/ma12091401
Ramenzoni LL, Attin T, Schmidlin PR. In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation. Materials. 2019; 12(9):1401. https://doi.org/10.3390/ma12091401
Chicago/Turabian StyleRamenzoni, Liza L., Thomas Attin, and Patrick R. Schmidlin. 2019. "In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation" Materials 12, no. 9: 1401. https://doi.org/10.3390/ma12091401
APA StyleRamenzoni, L. L., Attin, T., & Schmidlin, P. R. (2019). In Vitro Effect of Modified Polyetheretherketone (PEEK) Implant Abutments on Human Gingival Epithelial Keratinocytes Migration and Proliferation. Materials, 12(9), 1401. https://doi.org/10.3390/ma12091401






