The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Heat Treatments
2.2. Electrochemical Tests
2.3. Characterizations
3. Results and Discussion
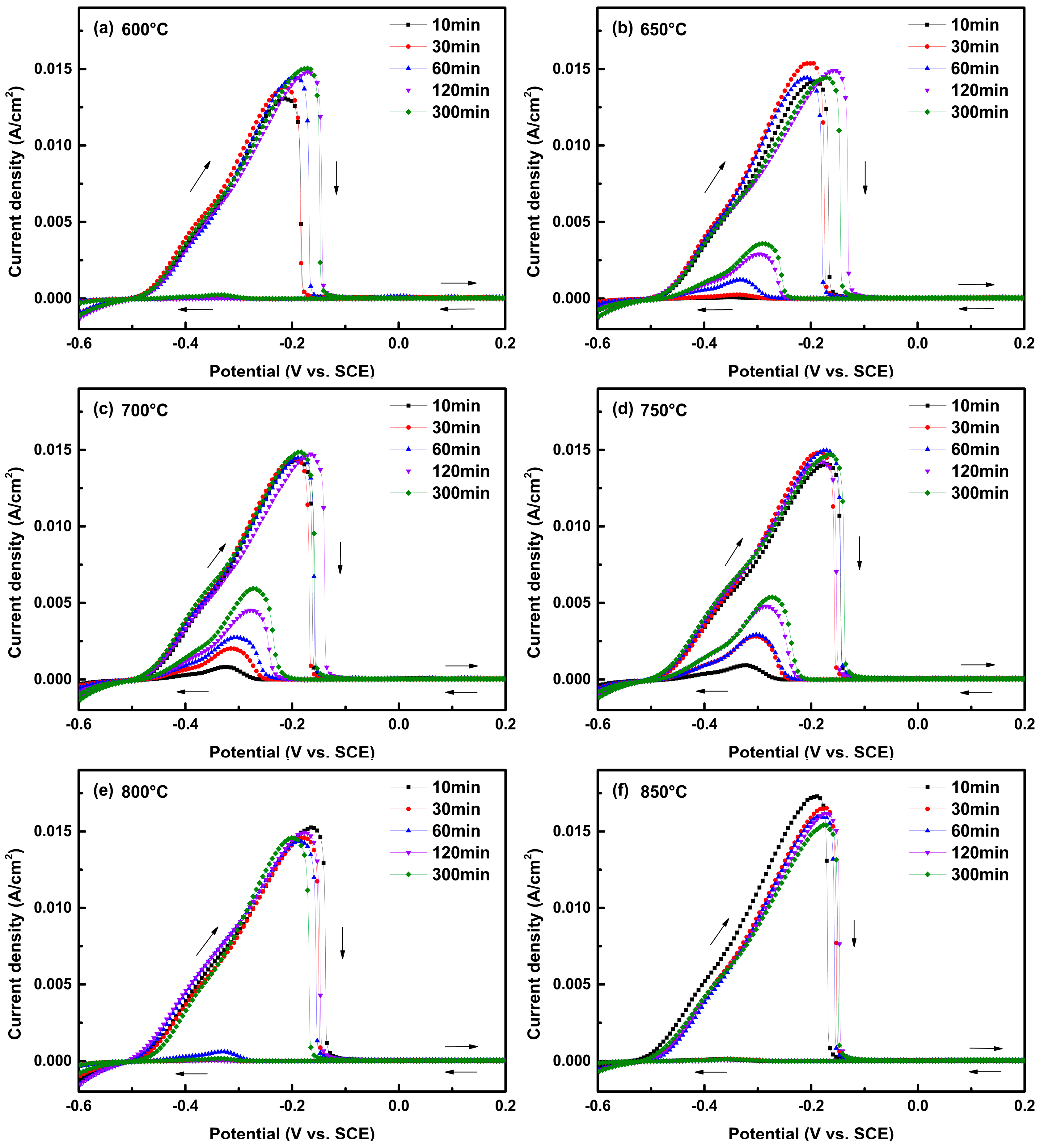
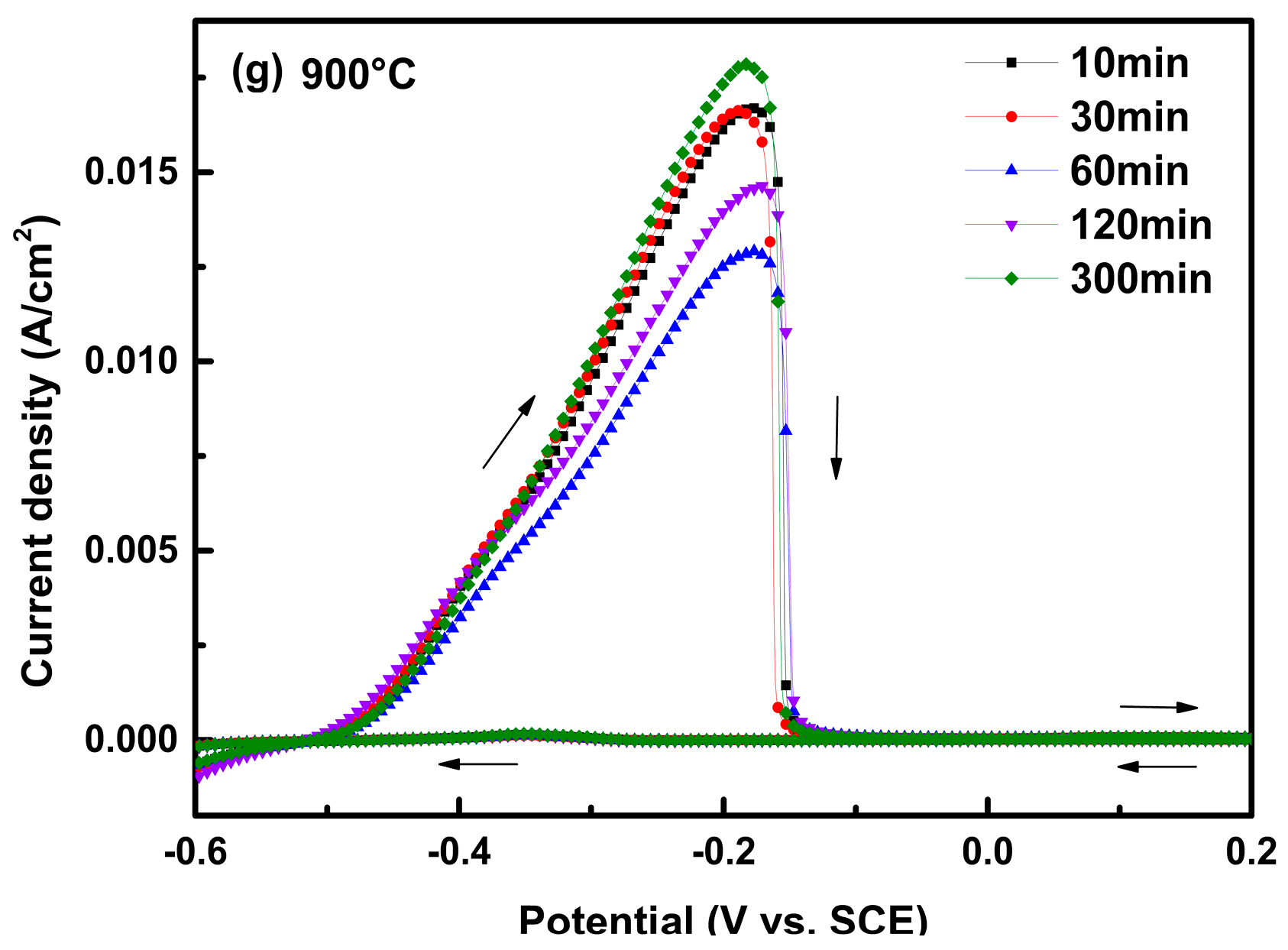
3.1. IGC Susceptibility of Cr–Mn–Ni–N–Cu Metastable Austenitic Stainless Steel in the DL-EPR Test
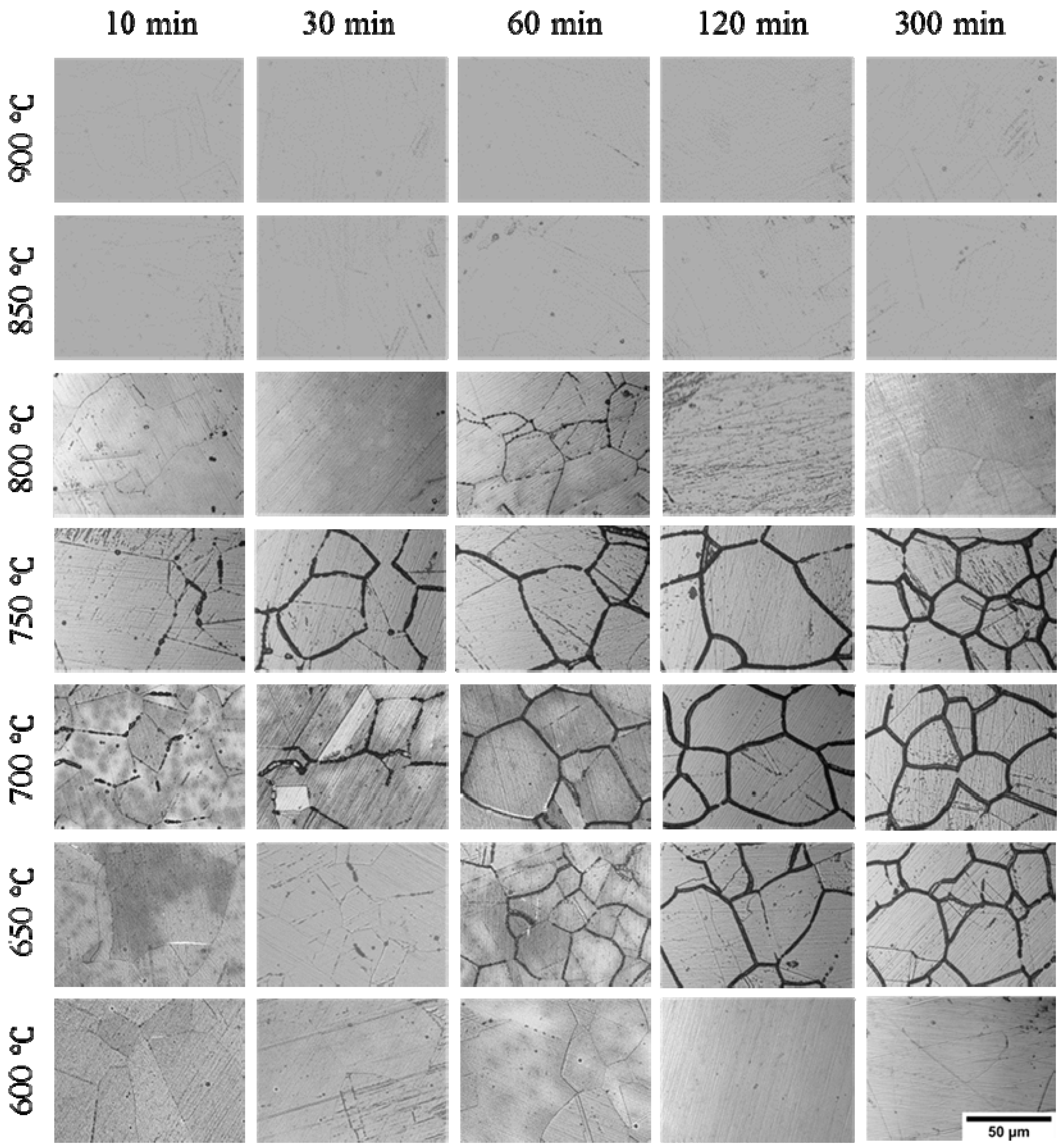
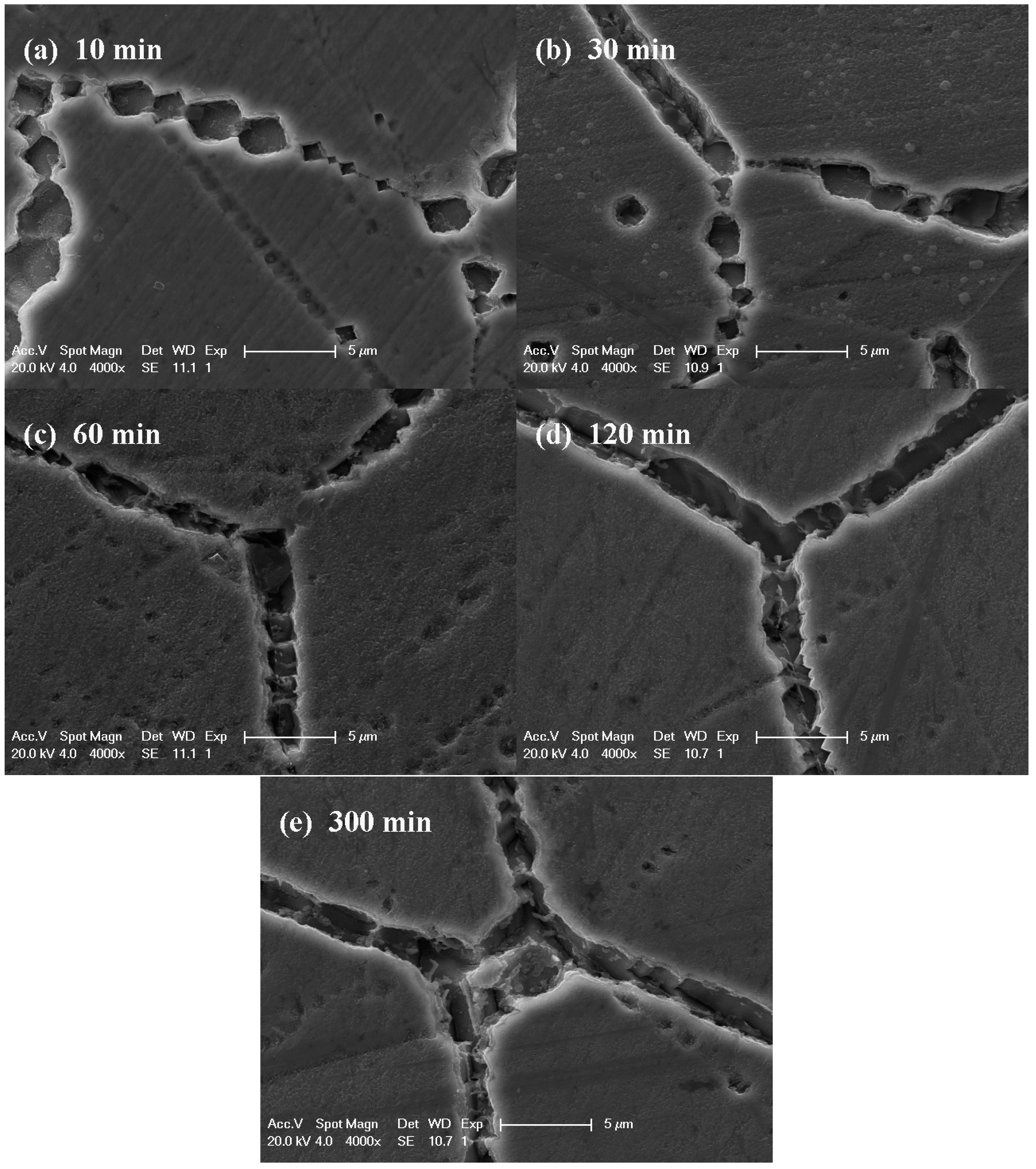
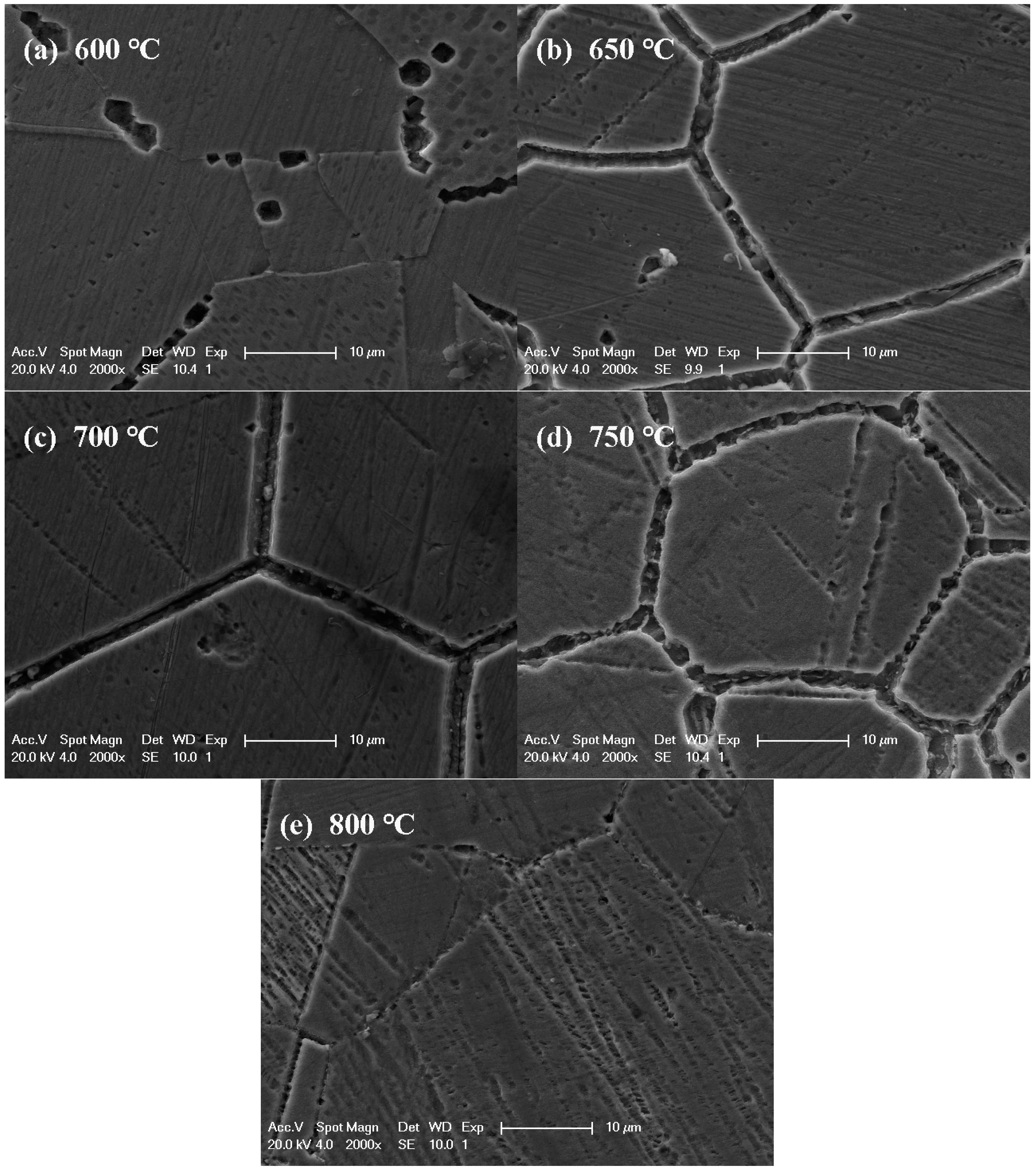
3.2. IGC Susceptibility of Cr–Mn–Ni–N–Cu Metastable Austenitic Stainless Steel in Oxalic Acid Etching
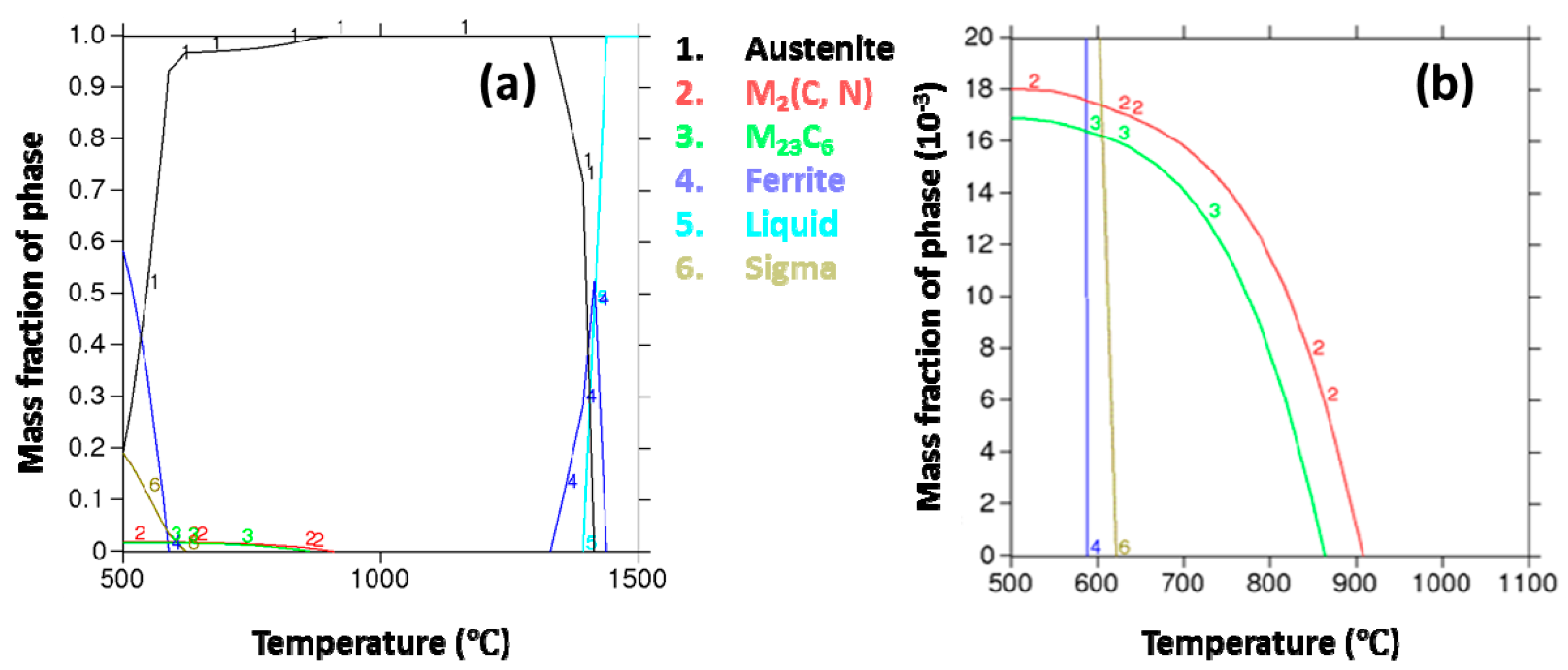
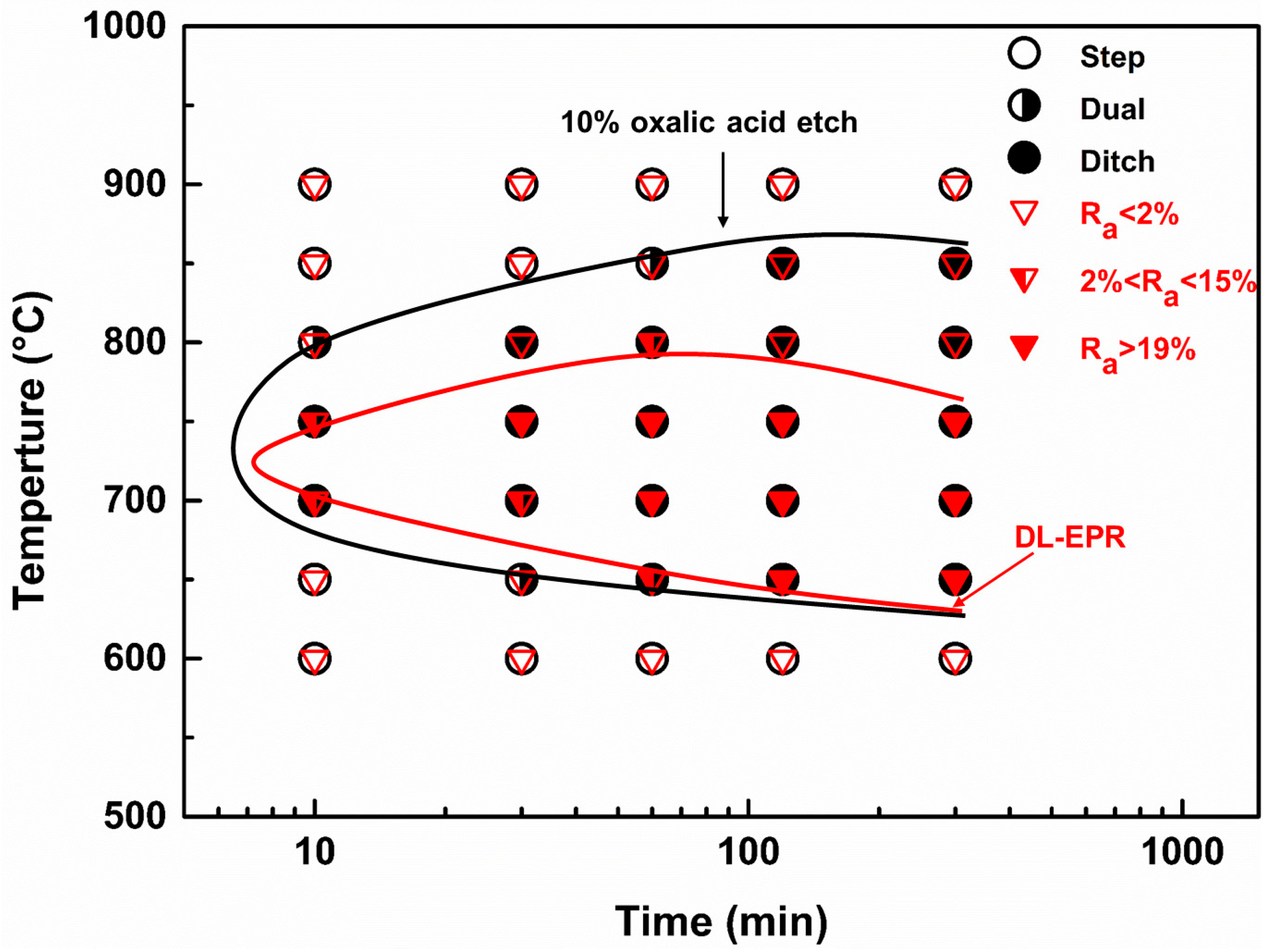
3.3. The Time-Temperature-Sensitization (TTS) Diagram for Cr–Mn–Ni–N–Cu Metastable Austenitic Stainless Steel
4. Conclusions
- (1)
- The results of DL-EPR test and oxalic acid etch for the aged Cr–Mn–Ni–N–Cu austenitic stainless steel indicated that IGC would occur for samples aged at about 650 °C to 750 °C, and the IGC susceptibility became more severe as the aging time increased. For samples aged at 800 °C, the IGC susceptibility increased first and then decreased with the increased aging time, suggesting the quick repair of chromium-depleted zones after longer aging at 800 °C.
- (2)
- The nose sensitization-temperature of the Cr–Mn–Ni–N–Cu austenitic stainless steel was about 700°C to 750 °C, suggesting that the IGC could occur in a short time at about 700 °C to 750 °C.
- (3)
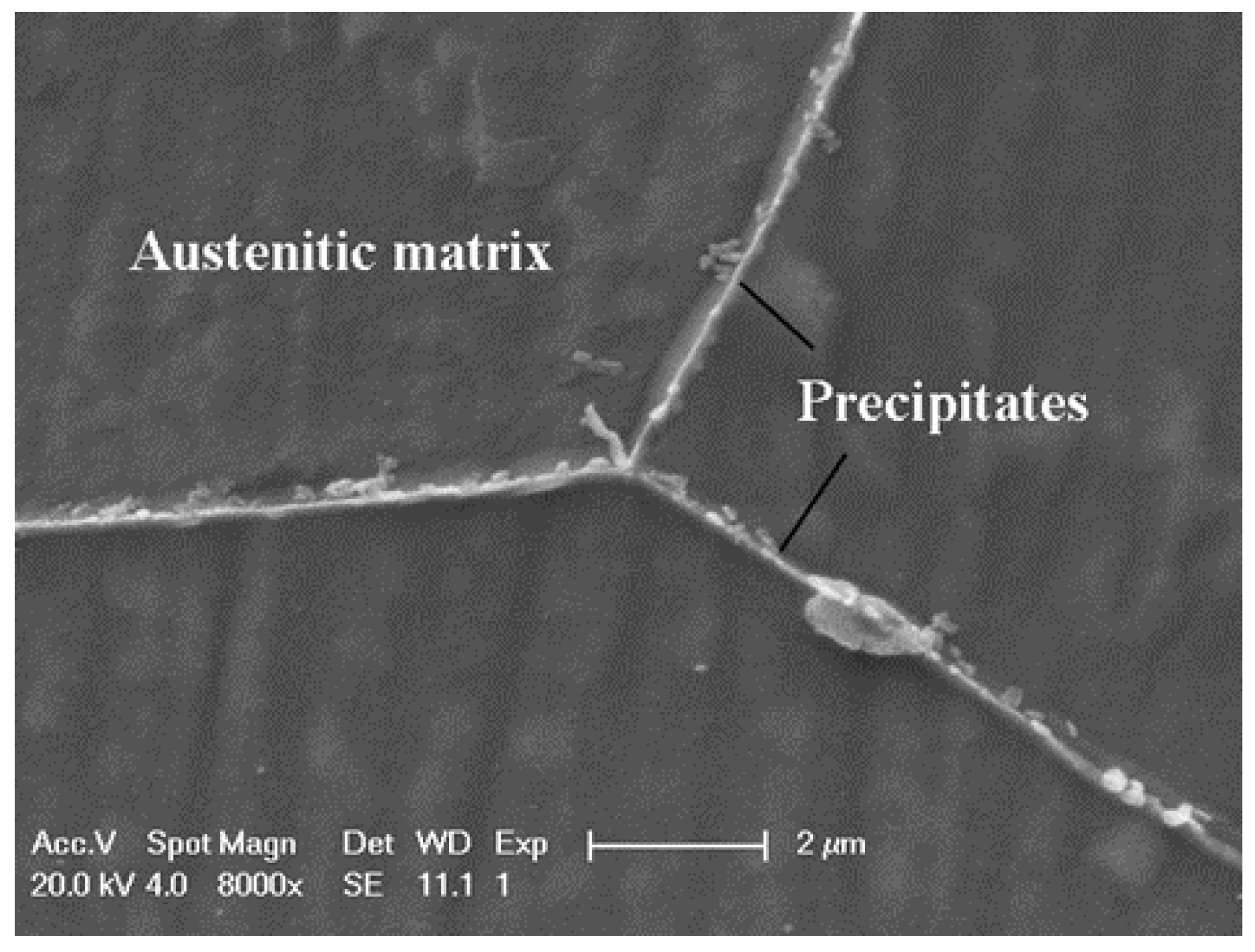
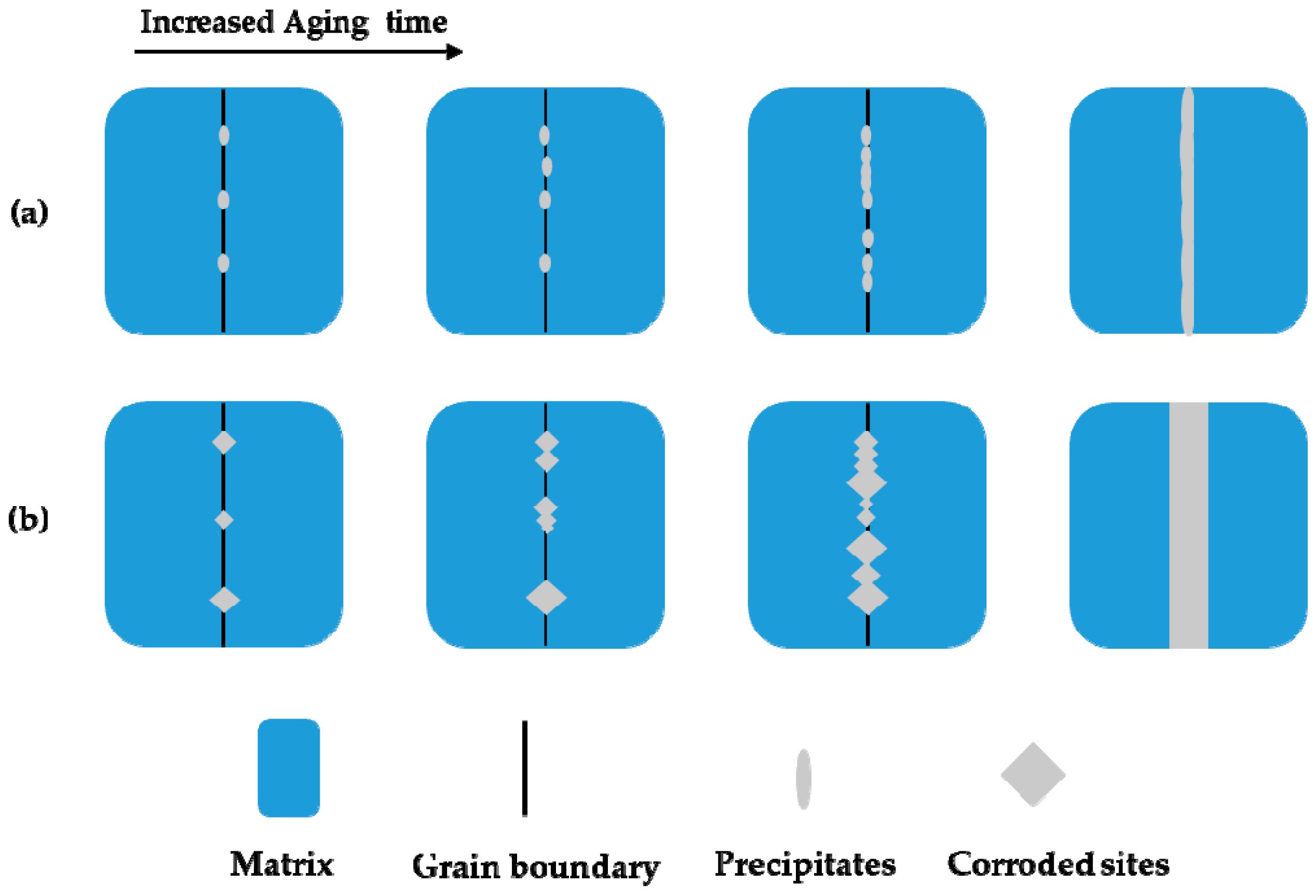
- The precipitates stood as nucleation sites for the intergranular corrosion attack of the Cr–Mn–Ni–N–Cu austenitic stainless steel. However, the growth of these corrosion sites with the extended aging time obeyed consistent crystallographic orientation along the grain boundary.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lajunen, A. Lifecycle costs and charging requirements of electric buses with different charging methods. J. Clean. Prod. 2018, 172, 56–67. [Google Scholar] [CrossRef]
- Ermolaeva, N.S.; Castro, M.B.G.; Kandachar, P.V. Materials selection for an automotive structure by integrating structural optimization with environmental impact assessment. Mater. Design 2004, 25, 689–698. [Google Scholar] [CrossRef]
- Naderi, M.; Ketabchi, M.; Abbasi, M.; Bleck, W. Analysis of microstructure and mechanical properties of different high strength carbon steels after hot stamping. J. Mater. Process. Tech. 2011, 211, 1117–1125. [Google Scholar] [CrossRef]
- Eskandari, M.; Najafizadeh, A.; Kermanpur, A.; Karimi, M. Potential application of nanocrystalline 301 austenitic stainless steel in lightweight vehicle structures. Mater. Design 2009, 30, 3869–3872. [Google Scholar] [CrossRef]
- Bleck, W.; Schael, I. Determination of crash-relevant material parameters by dynamic tensile tests. Steel Res. 2000, 71, 173–178. [Google Scholar] [CrossRef]
- Niu, G.; Wu, H.; Zhang, D.; Gong, N.; Tang, D. Heterogeneous nano/ultrafine-grained medium Mn austenitic stainless steel with high strength and ductility. Mat. Sci. Eng. A-Struct. 2018, 725, 187–195. [Google Scholar] [CrossRef]
- Hirsch, J. Recent development in aluminium for automotive applications. T. Nonferr. Metal. Soc. 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Tisza, M.; Czinege, I. Comparative study of the application of steels and aluminium in lightweight production of automotive parts. Int. J. Lightweight Mater. Manuf. 2018, 1, 229–238. [Google Scholar] [CrossRef]
- Hirsch, J.; Al-Samman, T. Superior light metals by texture engineering: Optimized aluminum and magnesium alloys for automotive applications. Acta. Mater. 2013, 61, 818–843. [Google Scholar] [CrossRef]
- Friedrich, K.; Almajid, A.A. Manufacturing aspects of advanced polymer composites for automotive applications. Appl. Compos. Mater. 2013, 20, 107–128. [Google Scholar] [CrossRef]
- Dhand, V.; Mittal, G.; Rhee, K.Y.; Park, S.; Hui, D. A short review on basalt fiber reinforced polymer composites. Compos. Part B-Eng. 2015, 73, 166–180. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Bi, H.; Gu, J.; Chen, D.; Chang, E.; Zhang, W. Martensite transformation behavior and mechanical properties of cold-rolled metastable Cr-Mn-Ni-N austenitic stainless steels. Mat. Sci. Eng. A-Struct. 2018, 724, 411–420. [Google Scholar] [CrossRef]
- Xin, J.; Song, Y.; Fang, C.; Wei, J.; Huang, C.; Wang, S. Evaluation of inter-granular corrosion susceptibility in 316LN austenitic stainless steel weldments. Fusion Eng. Des. 2018, 133, 70–76. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z.; Li, H.; Feng, H.; Zhang, B. Detection of susceptibility to intergranular corrosion of aged super austenitic stainless steel S32654 by a modified electrochemical potentiokinetic reactivation method. J. Alloy Compd. 2017, 695, 3083–3093. [Google Scholar] [CrossRef]
- Bai, G.; Lu, S.; Li, D.; Li, Y. Intergranular corrosion behavior associated with delta-ferrite transformation of Ti-modified Super304H austenitic stainless steel. Corros. Sci. 2015, 90, 347–358. [Google Scholar] [CrossRef]
- Kisko, A.; Misra, R.D.K.; Talonen, J.; Karjalainen, L.P. The influence of grain size on the strain-induced martensite formation in tensile straining of an austenitic 15Cr–9Mn–Ni–Cu stainless steel. Mat. Sci. Eng. A-Struct. 2013, 578, 408–416. [Google Scholar] [CrossRef]
- Toor, I.; Hyun, P.J.; Kwon, H.S. Development of high Mn–N duplex stainless steel for automobile structural components. Corros. Sci. 2008, 50, 404–410. [Google Scholar] [CrossRef]
- Charles, J. The new 200-series: An alternative answer to Ni surcharge? Risks of opportunities? Rev. Metall-Paris 2007, 104, 308–317. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, K.Y.; Lee, Y.D. Nitrogen-Alloyed, Metastable austenitic stainless steel for automotive structural applications. Mater. Manuf. Process 2004, 19, 51–59. [Google Scholar] [CrossRef]
- Talha, M.; Behera, C.K.; Sinha, O.P. Effect of nitrogen and cold working on structural and mechanical behavior of Ni-free nitrogen containing austenitic stainless steels for biomedical applications. Mat. Sci. Eng. C-Mater. 2015, 47, 196–203. [Google Scholar] [CrossRef]
- Xi, T.; Shahzad, M.B.; Xu, D.; Sun, Z.; Zhao, J.; Yang, C.; Qi, M.; Yang, K. Effect of copper addition on mechanical properties, corrosion resistance and antibacterial property of 316L stainless steel. Mat. Sci. Eng. C-Mater. 2017, 71, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Chen, C.; Yu, H.; Liu, F.; Yang, H.; Zhang, Z. The influence and the mechanism of the precipitate/austenite interfacial C-enrichment on the intergranular corrosion sensitivity in 310 S stainless steel. Corros. Sci. 2016, 111, 352–361. [Google Scholar] [CrossRef]
- Barla, N.A.; Ghosh, P.K.; Das, S.; Kumar, V. Simulated stress-induced sensitization study for the heat-affected zone of the 304LN stainless steel weld using a thermomechanical simulator. Metall. Mater. Trans. A 2019, 50, 1283–1293. [Google Scholar] [CrossRef]
- Fujii, T.; Tohgo, K.; Mori, Y.; Shimamura, Y. Crystallography of intergranular corrosion in sensitized austenitic stainless steel. Mater. Charact. 2018, 144, 219–226. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Sidhom, H.; Philibert, J. Prediction of chromium depleted-zone evolution during aging of Ni–Cr–Fe alloys. Acta. Mater. 2002, 50, 1383–1392. [Google Scholar] [CrossRef]
- Kaneko, K.; Fukunaga, T.; Yamada, K.; Nakada, N.; Kikuchi, M.; Saghi, Z.; Barnard, J.S.; Midgley, P.A. Formation of M23C6-type precipitates and chromium-depleted zones in austenite stainless steel. Scripta. Mater. 2011, 65, 509–512. [Google Scholar] [CrossRef]
- Sahlaoui, H.; Makhlouf, K.; Sidhom, H.; Philibert, J. Effects of ageing conditions on the precipitates evolution, chromium depletion and intergranular corrosion susceptibility of AISI 316L: Experimental and modeling results. Mat. Sci. Eng. A-Struct. 2004, 372, 98–108. [Google Scholar] [CrossRef]
- Shi, F.; Wang, L.; Cui, W.; Liu, C. Precipitation kinetics of Cr2N in high nitrogen austenitic stainless steel. J. Iron Steel Res. Int. 2008, 15, 72–77. [Google Scholar] [CrossRef]
- Wang, R.; Zheng, Z.; Zhou, Q.; Gao, Y. Effect of surface nanocrystallization on the sensitization and desensitization behavior of Super304H stainless steel. Corros. Sci. 2016, 111, 728–741. [Google Scholar] [CrossRef]
- Hong, J.; Han, D.; Tan, H.; Li, J.; Jiang, Y. Evaluation of aged duplex stainless steel UNS S32750 susceptibility to intergranular corrosion by optimized double loop electrochemical potentiokinetic reactivation method. Corros. Sci. 2013, 68, 249–255. [Google Scholar] [CrossRef]
- Sun, M.; Yang, Y.; Luo, M.; Jiang, L.; Jiang, Y.; Li, J. Investigation of susceptibility to intergranular corrosion of tin-added austenitic stainless steel. Acta Metall. Sin-Engl. 2015, 28, 1183–1189. [Google Scholar] [CrossRef][Green Version]
- Gong, J.; Jiang, Y.M.; Deng, B.; Xu, J.L.; Hu, J.P.; Li, J. Evaluation of intergranular corrosion susceptibility of UNS S31803 duplex stainless steel with an optimized double loop electrochemical potentiokinetic reactivation method. Electrochim. Acta 2010, 55, 5077–5083. [Google Scholar] [CrossRef]
- Majidi, A.P.; Streicher, M.A. The double loop reactivation method for detecting sensitization in AISI-304 stainless-steels. Corrosion 1984, 40, 584–593. [Google Scholar] [CrossRef]
- Kwok, C.T.; Lo, K.H.; Chan, W.K.; Cheng, F.T.; Man, H.C. Effect of laser surface melting on intergranular corrosion behaviour of aged austenitic and duplex stainless steels. Corros. Sci. 2011, 53, 1581–1591. [Google Scholar] [CrossRef]
- U. Kamachi Mudali, R.K.D.J. Influence of thermal aging on the intergranular corrosion resistance of types 304LN and 316LN stainless steels. Metall. Mater. Trans. A 1996, 27A, 2881–2887. [Google Scholar] [CrossRef]
- Lopez, N.; Cid, M.; Puiggali, M.; Azkarate, I.; Pelayo, A. Application of double loop electrochemical potentiodynamic reactivation test to austenitic and duplex stainless steels. Mat. Sci. Eng. A-Struct. 1997, 229, 123–128. [Google Scholar] [CrossRef]
- Muri, P.; Sousa, F.V.V.; Assis, K.S.; Rocha, A.C.; Mattos, O.R.; Margarit-Mattos, I.C.P. Experimental procedures and sensitization diagnostics of AISI304 Steel by double loop electrochemical potentiodynamic reactivation method. Electrochim. Acta 2014, 124, 183–189. [Google Scholar] [CrossRef]
- ASTM A262-15. Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Sun, J.; Sun, L.; Dai, N.; Li, J.; Jiang, Y. Investigation on ultra-pure ferritic stainless steel 436L susceptibility to intergranular corrosion using optimised double loop electrochemical potentiokinetic reactivation method. Corros. Eng. Sci. Tech. 2018, 53, 574–581. [Google Scholar] [CrossRef]
- Sourmail, T.; Too, C.H.; Bhadeshia, H.K.D.H. Sensitisation and evolution of chromium-depleted zones in Fe-Cr-Ni-C Systems. ISIJ Int. 2003, 43, 1814–1820. [Google Scholar] [CrossRef][Green Version]
- James, A.W.; Shepherd, C.M. Some effects of heat-treatment on grain-boundary chemistry and precipitation in type-316 steel. Mater. Sci. Tech-Lond 1989, 5, 333–345. [Google Scholar] [CrossRef]
- Seo, J.H.; Ryu, J.; Lee, D.N. Formation of crystallographic etch pits during AC etching of aluminum. J. Electrochem. Soc. 2003, 150, B433. [Google Scholar] [CrossRef]
- Guo, R.; Weinberg, F.; Tromans, D. Pitting corrosion of passivated zinc monocrystals. Corrosion 1995, 51, 356–366. [Google Scholar] [CrossRef]
- Cihal, V. The Role of grain boundaries in connection with intergranular corrosion. In Intergranular Corrosion of Steels and Alloys, Laird, C., Ed.; Publisher of Technical Literature: Praha, Czechoslovakia, 1984; pp. 98–99. [Google Scholar]


| Element | C | N | S | Si | Mn | P | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| wt % | 0.08 | 0.15 | 0.002 | 0.30 | 10.12 | 0.04 | 13.62 | 1.19 | 0.85 | Bal. |
| Temperature (°C) | Ra value (%) | ||||
|---|---|---|---|---|---|
| 10 min | 30 min | 60 min | 120 min | 300 min | |
| 900 | 0.70 ± 0.11 | 0.61 ± 0.14 | 0.80 ± 0.24 | 0.85 ± 0.17 | 0.75 ± 0.14 |
| 850 | 0.70 ± 0.01 | 0.76 ± 0.07 | 0.56 ± 0.10 | 0.33 ± 0.14 | 0.61 ± 0.12 |
| 800 | 0.47 ± 0.05 | 0.75 ± 0.17 | 3.55 ± 0.63 | 0.39 ± 0.08 | 1.90 ± 0.82 |
| 750 | 6.48 ± 0.05 | 19.87 ± 0.94 | 19.36 ± 0.11 | 31.30 ± 2.14 | 34.89 ± 1.64 |
| 700 | 5.97 ± 0.32 | 14.98 ± 0.95 | 20.00 ± 1.10 | 33.12 ± 2.37 | 39.97 ± 0.10 |
| 650 | 0.39 ± 0.13 | 1.68 ± 0.05 | 8.74 ± 0.27 | 19.48 ± 0.02 | 25.40 ± 0.47 |
| 600 | 0.42 ± 0.11 | 0.22 ± 0.05 | 0.22 ± 0.05 | 0.13 ± 0.04 | 1.73 ± 0.19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liu, Y.; Cheng, Y.; Li, J.; Jiang, Y. The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments. Materials 2019, 12, 1385. https://doi.org/10.3390/ma12091385
Liu G, Liu Y, Cheng Y, Li J, Jiang Y. The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments. Materials. 2019; 12(9):1385. https://doi.org/10.3390/ma12091385
Chicago/Turabian StyleLiu, Guangming, Yuanyuan Liu, Yawen Cheng, Jin Li, and Yiming Jiang. 2019. "The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments" Materials 12, no. 9: 1385. https://doi.org/10.3390/ma12091385
APA StyleLiu, G., Liu, Y., Cheng, Y., Li, J., & Jiang, Y. (2019). The Intergranular Corrosion Susceptibility of Metastable Austenitic Cr–Mn–Ni–N–Cu High-Strength Stainless Steel under Various Heat Treatments. Materials, 12(9), 1385. https://doi.org/10.3390/ma12091385




