Seed-Assisted Synthesis of Graphene Films on Insulating Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
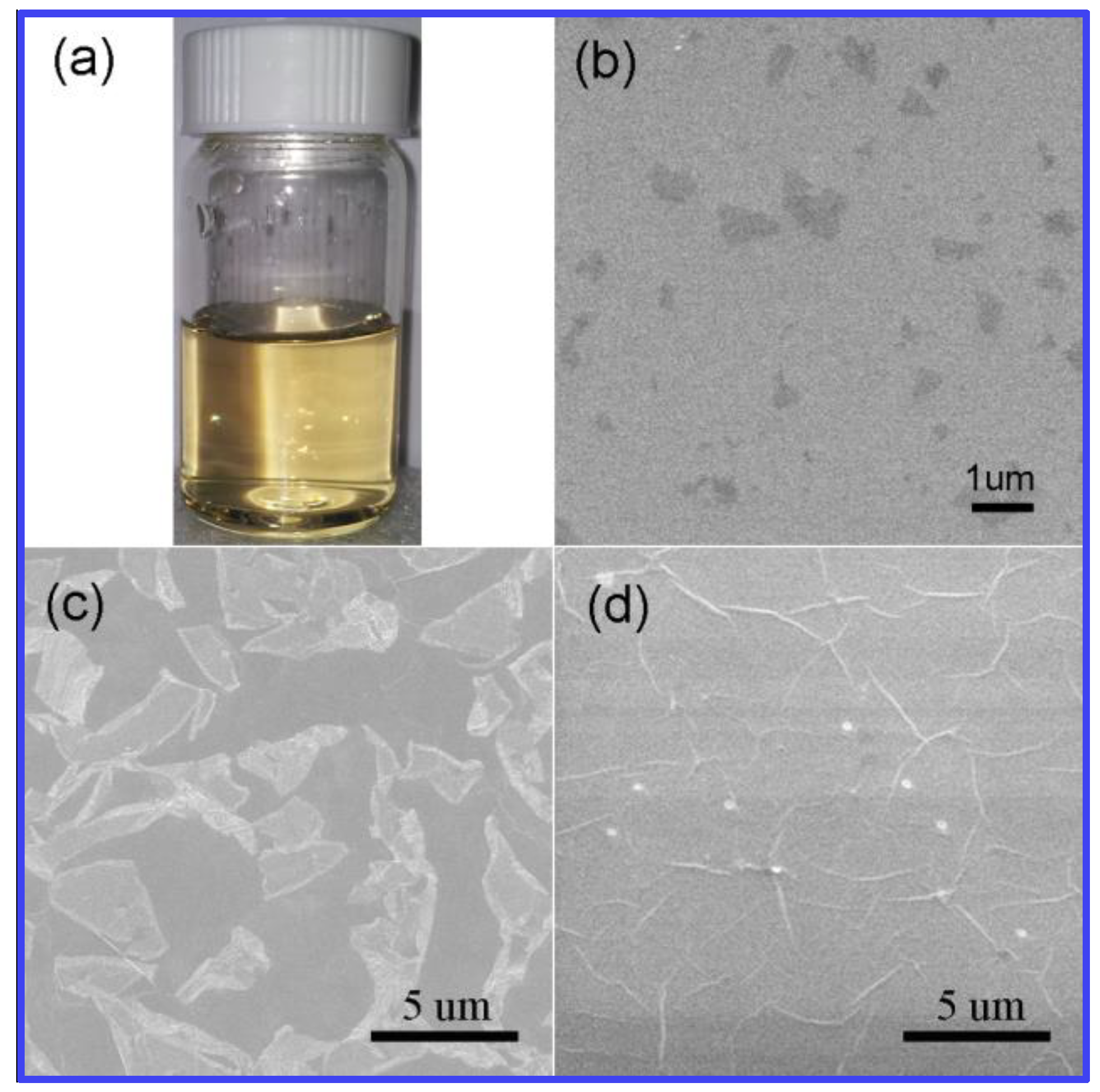
2.2. Preparation of GO Seeds
2.3. Preparation of PVA Aqueous Solution
2.4. Preparation of PVA Aqueous Solution
2.5. Characterizations
3. Results and Discussion
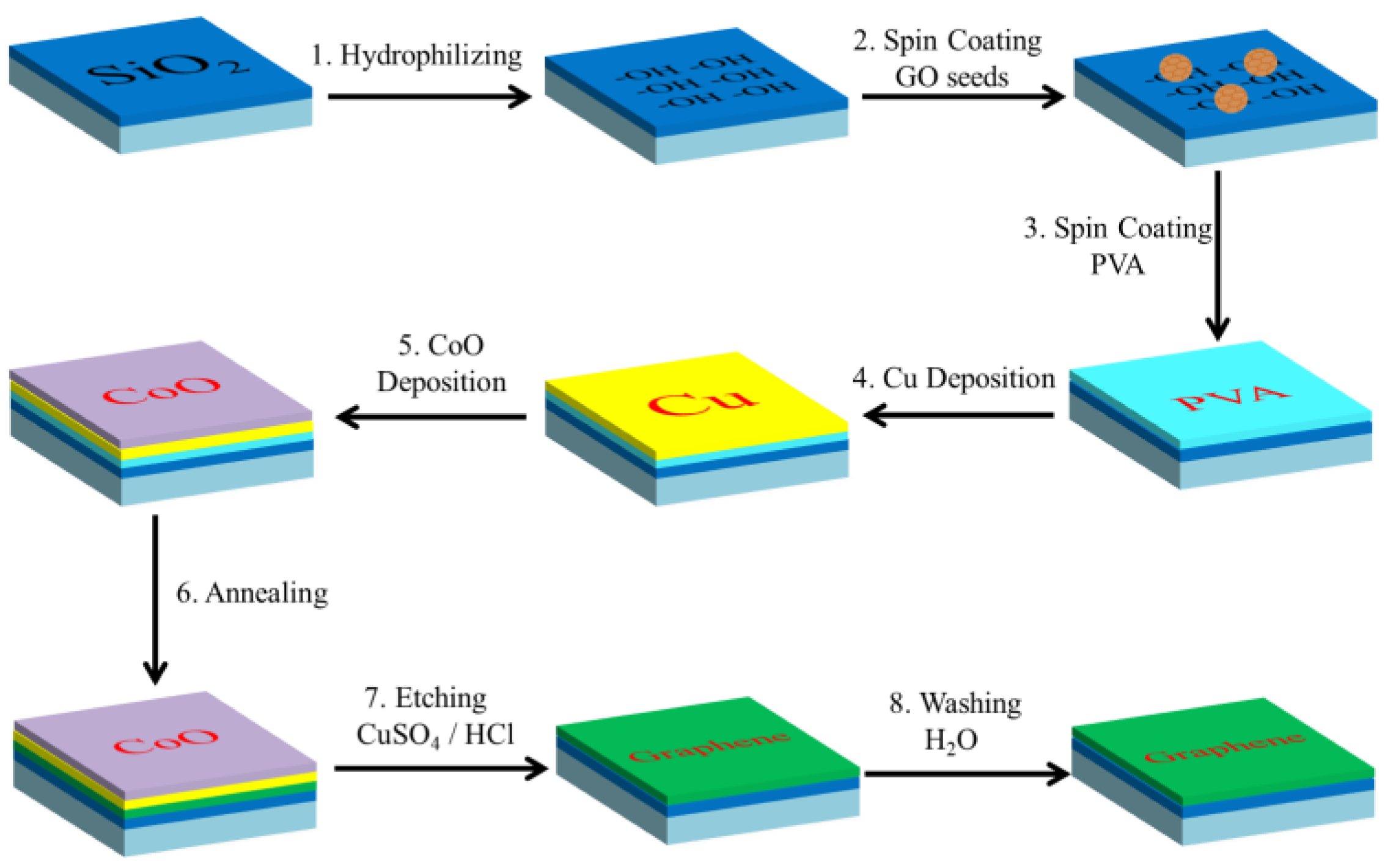
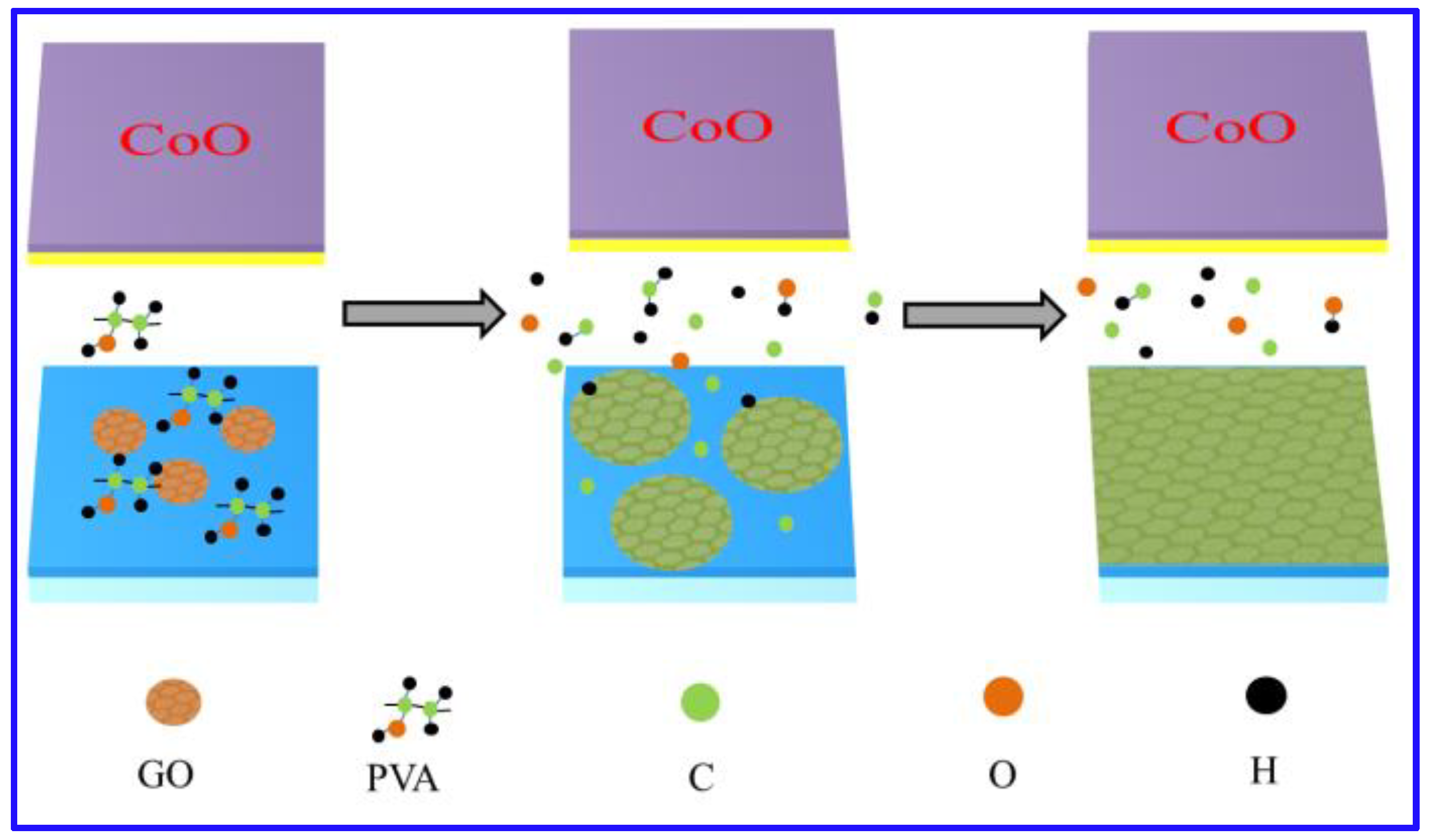
3.1. Synthetic Process of Graphene
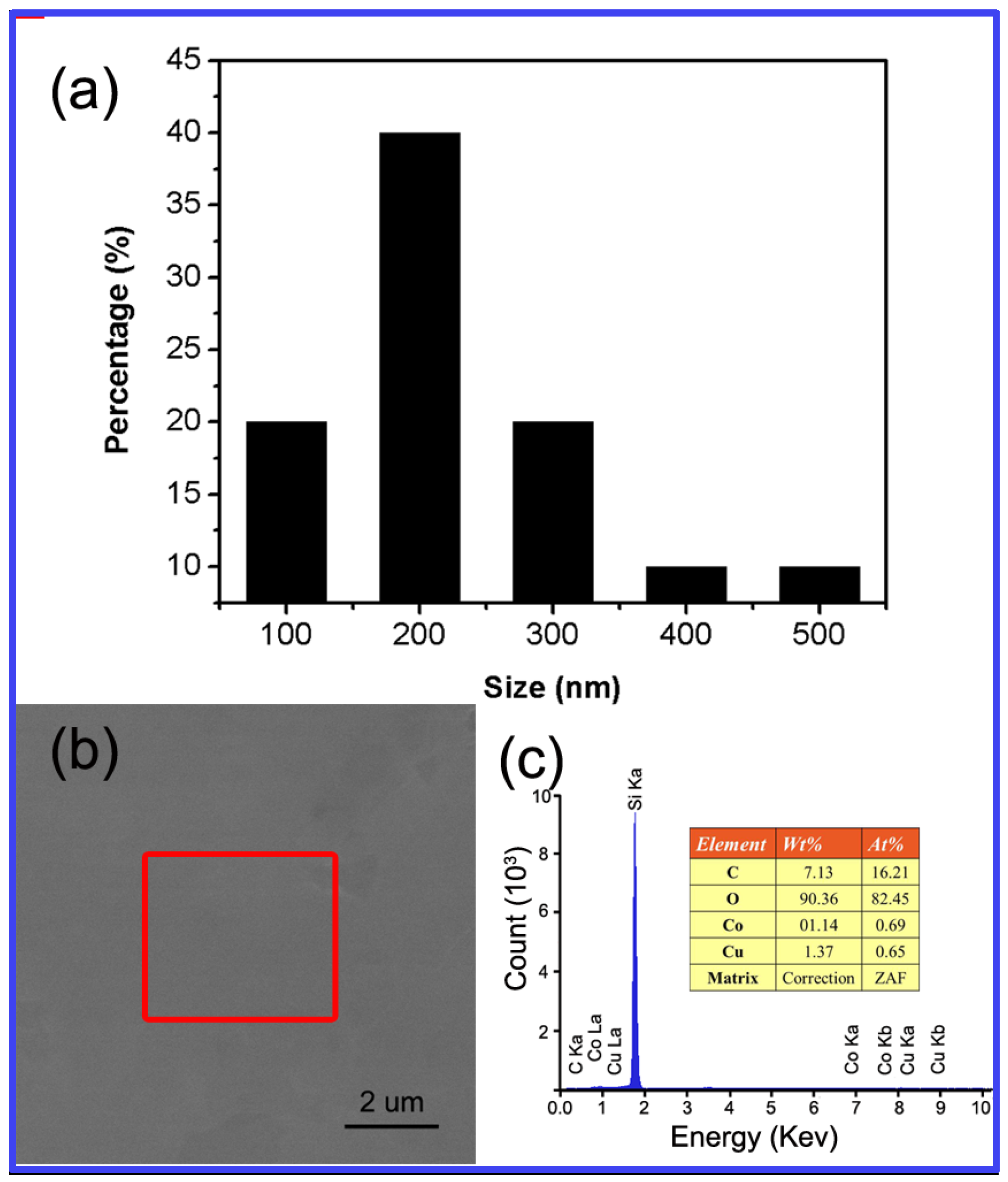
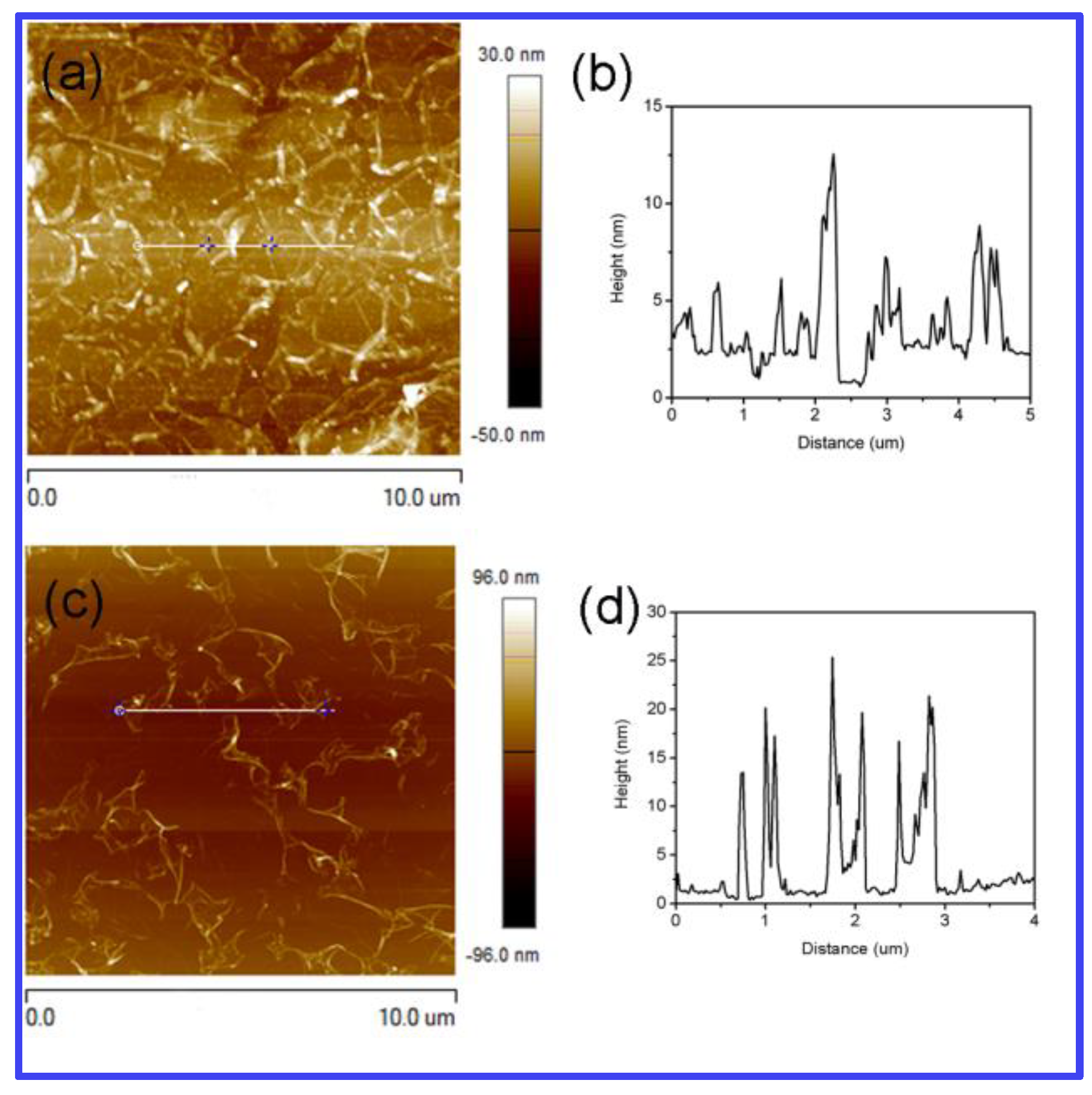
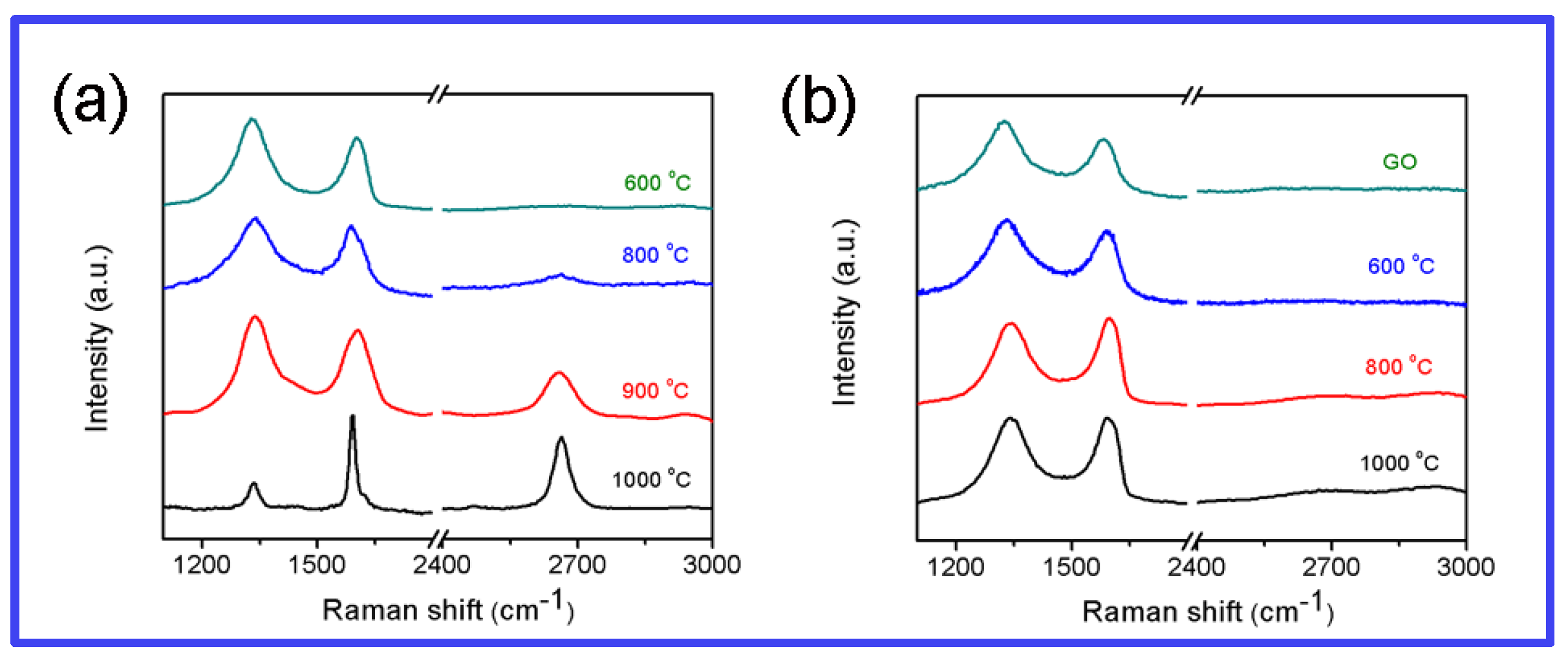
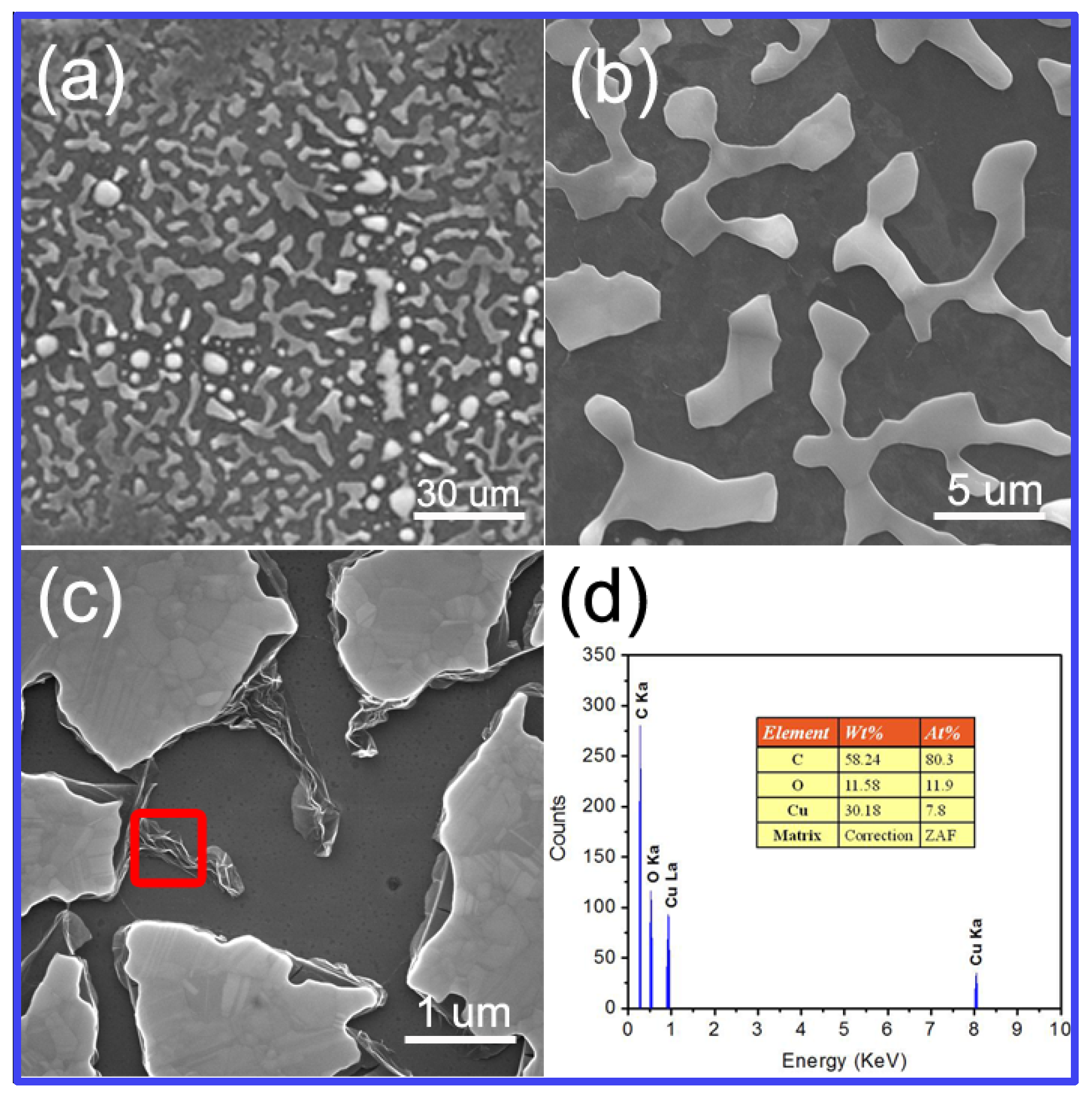
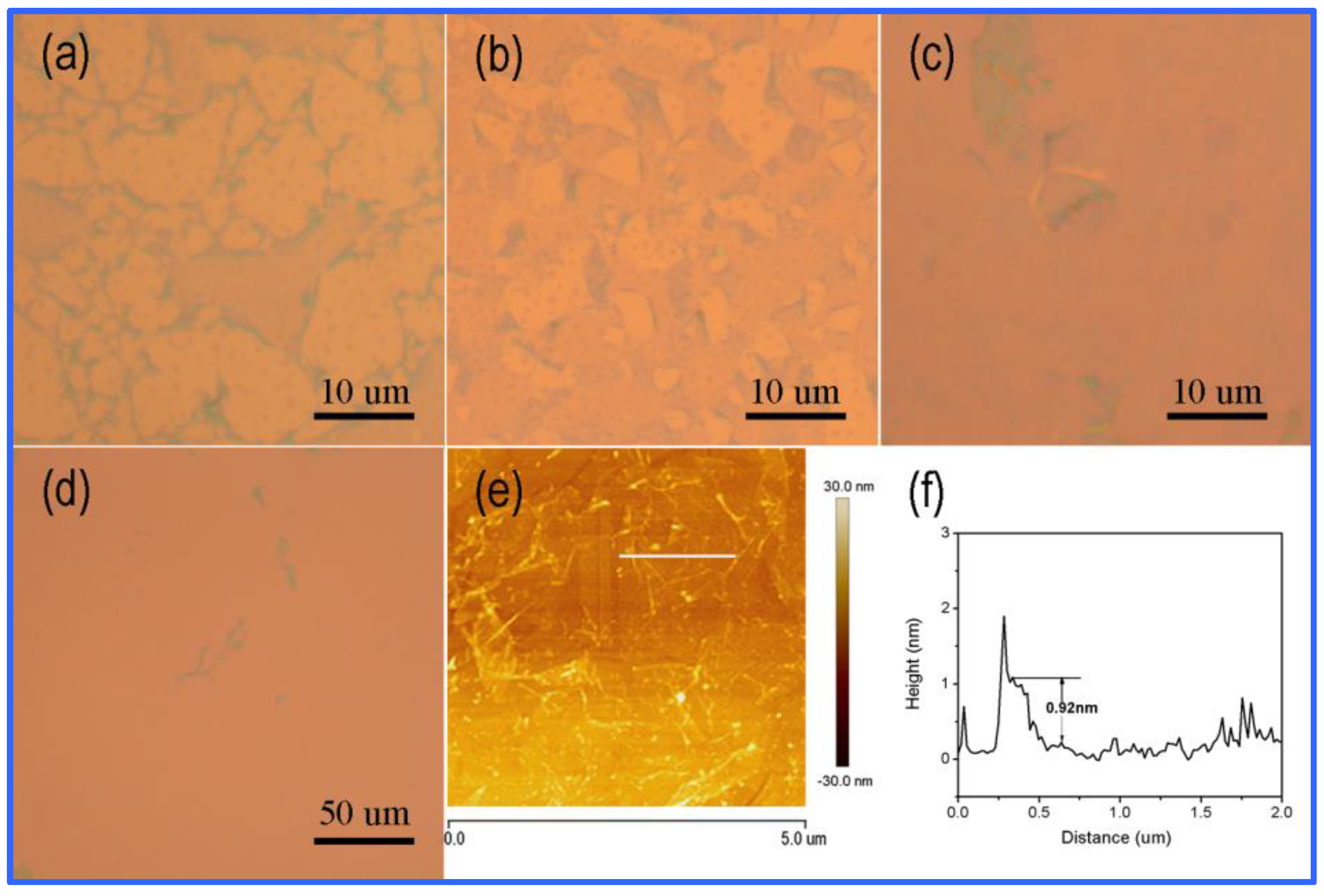
3.2. Structural and Morphological Investigations
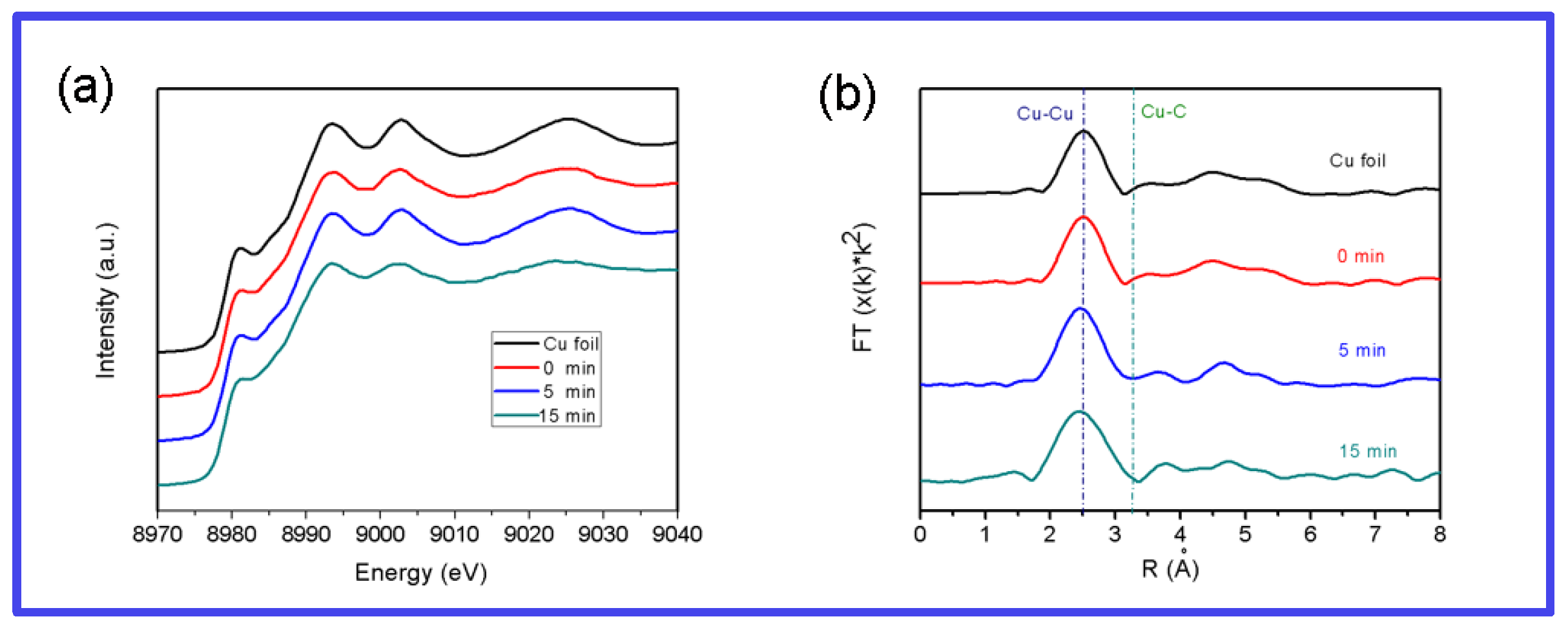
3.3. Mechanism of Graphene Growth and Ultraviolet-Visible Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bejagam, K.K.; Singh, S.; Deshmukh, S.A. Nanoparticle activated and directed assembly of graphene into a nanoscroll. Carbon 2018, 134, 43–52. [Google Scholar] [CrossRef]
- Zhang, H.; Chhowalla, M.; Liu, Z.F. 2D nanomaterials: Graphene and transition metal dichalcogenides. Chem. Soc. Rev. 2018, 47, 3015–3017. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, R.C.; Suter, J.L.; Coveney, P.V. Graphene-graphene interactions: Friction, superlubricity, and exfoliation. Adv. Mater. 2018, 30, 1705791. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Peng, D.; Jing, Q.; Niu, J.; Cheng, X.; Feng, Z.; Wu, X. Green and effective removal of aqueous graphene oxide under UV-light irradiation. Nanomaterials 2018, 8, 654. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.Q.; Tang, J.J.; Sun, J.; Yan, C. High efficient reduction of graphene oxide via nascent hydrogen at room temperature. Materials 2018, 11, 340. [Google Scholar] [CrossRef]
- Zhuo, Q.; Gao, J.; Peng, M.; Bai, L.; Deng, J.; Xia, Y.; Ma, Y.; Zhong, J.; Sun, X. Large-scale synthesis of graphene by the reduction of graphene oxide at room temperature using metal nanoparticles as catalyst. Carbon 2013, 52, 559–564. [Google Scholar] [CrossRef]
- Zhuo, Q.; Zhang, Y.; Du, Q.; Yan, C. Facile reduction of graphene oxide at room temperature by ammonia borane via salting out effect. J. Colloid Interface Sci. 2015, 457, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Shang, T.; Yang, W.; Yang, H.; Lin, S.; Jia, X.; Cai, Q.; Yang, X. Effectively exerting the reinforcement of dopamine reduced graphene oxide on epoxy-based composites via strengthened interfacial bonding. ACS Appl. Mater. Inter. 2016, 8, 13037–13050. [Google Scholar] [CrossRef]
- Zhuo, Q.; Ma, Y.; Gao, J.; Zhang, P.; Xia, Y.; Tian, Y.; Sun, X.; Zhong, J.; Sun, X. Facile synthesis of graphene/metal nanoparticle composites via self-catalysis reduction at room temperature. Inorg. Chem. 2013, 52, 3141–3147. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Chen, M.M.; Zhang, Y.; Zhao, W.X.; Wang, C.Y. A biomass-derived nitrogen-doped porous carbon for high-energy supercapacitor. Carbon 2018, 140, 404–412. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Sun, J.; Hu, D.; Xiao, C.; Zhuo, Q.Q.; Wang, J.J.; Qin, C.X.; Dai, L.X. Large-sized graphene oxide/modified tourmaline nanoparticle aerogel with stable honeycomb-like structure for high-efficiency PM2.5 capture. J. Mater. Chem. A 2018, 6, 16139–16148. [Google Scholar] [CrossRef]
- Li, X.; Colombo, L.; Ruoff, R.S. Synthesis of graphene films on copper foils by chemical vapor deposition. Adv. Mater. 2016, 28, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Fan, Y.; Rong, Y.; Porter, B.; Lau, C.S.; Zhou, Y.; He, Z.; Wang, S.; Bhaskaran, H.; Warner, J.H. Doping graphene transistors using vertical stacked monolayer ws2 heterostructures grown by chemical vapor deposition. ACS Appl. Mater. Inter. 2016, 8, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.-Q.; Wang, Q.; Zhang, Y.-P.; Zhang, D.; Li, Q.-L.; Gao, C.-H.; Sun, Y.-Q.; Ding, L.; Sun, Q.-J.; Wang, S.-D.; et al. Transfer-free synthesis of doped and patterned graphene films. ACS Nano 2015, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-F.; Wu, F.; Wang, C.; Zhang, P.-X.; Hu, H.-Y.; Xie, N.; Pan, M.; Zeng, Z.; Deng, S.; Wu, M.; et al. One-step chemical vapor deposition synthesis of 3d n-doped carbon nanotube/n-doped graphene hybrid material on nickel foam. Nanomaterials 2018, 8, 700. [Google Scholar] [CrossRef]
- Du, Q.C.; Zhou, Y.; Pan, X.W.; Zhang, J.X.; Zhuo, Q.Q.; Chen, S.T.; Chen, G.M.; Liu, T.F.; Xu, F.; Yan, C. A graphene-melamine-sponge for efficient and recyclable dye adsorption. RSC Adv. 2016, 6, 54589–54596. [Google Scholar] [CrossRef]
- Wu, C.C.; Wang, X.; Zhuo, Q.Q.; Sun, J.; Qin, C.X.; Wang, J.J.; Dai, L.X. A facile continuous wet-spinning of graphene oxide fibers from aqueous solutions at high pH with the introduction of ammonia. Carbon 2018, 138, 292–299. [Google Scholar] [CrossRef]
- Mondelli, P.; Gupta, B.; Betti, M.G.; Mariani, C.; Duffin, J.L.; Motta, N. High quality epitaxial graphene by hydrogen-etching of 3C-SiC(111) thin-film on Si(111). Nanotechnology 2017, 28, 115601. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Q.; Ye, P.D.; Capano, M.A.; Xuan, Y.; Sui, Y.; Qi, M.; Cooper, J.A.; Shen, T.; Pandey, D.; Prakash, G.; et al. Top-gated graphene field-effect-transistors formed by decomposition of SiC. Appl. Phys. Lett. 2008, 92, 092102. [Google Scholar] [CrossRef]
- Hao, Y.; Bharathi, M.S.; Wang, L.; Liu, Y.; Chen, H.; Nie, S.; Wang, X.; Chou, H.; Tan, C.; Fallahazad, B.; et al. The role of surface oxygen in the growth of large single-crystal graphene on copper. Science 2013, 342, 720–723. [Google Scholar] [CrossRef]
- Politano, A.; Radovic, I.; Borka, D.; Miskovic, Z.L.; Yu, H.K.; Farias, D.; Chiarello, G. Dispersion and damping of the interband pi plasmon in graphene grown on Cu(111) foils. Carbon 2017, 114, 70–76. [Google Scholar] [CrossRef]
- Sha, J.; Salvatierra, R.V.; Dong, P.; Li, Y.; Lee, S.-K.; Wang, T.; Zhang, C.; Zhang, J.; Ji, Y.; Ajayan, P.M.; et al. Three-dimensional rebar graphene. ACS Appl. Mater. Inter. 2017, 9, 7376–7384. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Feng, S.; Wei, X.; Shen, J.; Lu, W.; Shi, H.; Tao, K.; Lu, S.; Sun, T.; Yu, L.; et al. Facile synthesis of 3D graphene flowers for ultrasensitive and highly reversible gas sensing. Adv. Funct. Mater. 2016, 26, 7462–7469. [Google Scholar] [CrossRef]
- Ramabadran, U.; Ryan, G.; Zhou, X.; Farhat, S.; Manciu, F.; Tong, Y.G.; Ayler, R.; Garner, G. Reduced graphene oxide on nickel foam for supercapacitor electrodes. Materials 2017, 10, 1295. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Ren, W.; Xu, H.; Jin, L.; Wang, Z.; Ma, T.; Ma, L.-P.; Zhang, Z.; Fu, Q.; Peng, L.-M.; et al. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef]
- Li, X.S.; Cai, W.W.; An, J.H.; Kim, S.; Nah, J.; Yang, D.X.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical delamination of CVD-grown graphene film: Toward the recyclable use of copper catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, H.; Wang, H.S.; Sun, Q.; Zhang, X.; Cong, C.; Xie, H.; Liu, X.; Zhou, X.; Huang, F.; et al. Silane-catalysed fast growth of large single-crystalline graphene on hexagonal boron nitride. Nat. Commun. 2015, 6, 6499. [Google Scholar] [CrossRef]
- Weber, N.-E.; Binder, A.; Kettner, M.; Hirth, S.; Weitz, R.T.; Tomovic, Z. Metal-free synthesis of nanocrystalline graphene on insulating substrates by carbon dioxide-assisted chemical vapor deposition. Carbon 2017, 112, 201–207. [Google Scholar] [CrossRef]
- Vishwakarma, R.; Rosmi, M.S.; Takahashi, K.; Wakamatsu, Y.; Yaakob, Y.; Araby, M.I.; Kalita, G.; Kitazawa, M.; Tanemura, M. Transfer free graphene growth on SiO2 substrate at 250 degrees C. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Chauhan, N.; Palaninathan, V.; Raveendran, S.; Poulose, A.C.; Nakajima, Y.; Hasumura, T.; Uchida, T.; Hanajiri, T.; Maekawa, T.; Kumar, D.S. N-2-plasma-assisted one-step alignment and patterning of graphene oxide on a SiO2/Si substrate via the langmuir-blodgett technique. Adv. Mater. Inter. 2015, 2, 1400515. [Google Scholar] [CrossRef]
- Hwang, J.; Kim, M.; Campbell, D.; Alsalman, H.A.; Kwak, J.Y.; Shivaraman, S.; Woll, A.R.; Singh, A.K.; Hennig, R.G.; Gorantla, S.; et al. Van der Waals epitaxial growth of graphene on sapphire by chemical vapor deposition without a metal catalyst. ACS Nano 2013, 7, 385–395. [Google Scholar] [CrossRef]
- Song, H.J.; Son, M.; Park, C.; Lim, H.; Levendorf, M.P.; Tsen, A.W.; Park, J.; Choi, H.C. Large scale metal-free synthesis of graphene on sapphire and transfer-free device fabrication. Nanoscale 2012, 4, 3050–3054. [Google Scholar] [CrossRef]
- Munoz, R.; Munuera, C.; Martinez, J.I.; Azpeitia, J.; Gomez-Aleixandre, C.; Garcia-Hernandez, M. Low temperature metal free growth of graphene on insulating substrates by plasma assisted chemical vapor deposition. 2D Mater. 2017, 4, 015009. [Google Scholar] [CrossRef]
- Wu, J.B.; Agrawal, M.; Becerril, H.A.; Bao, Z.N.; Liu, Z.F.; Chen, Y.S.; Peumans, P. Organic light-emitting diodes on solution-processed graphene transparent electrodes. ACS Nano 2010, 4, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Greber, T.; Osterwalder, J. Some like it flat: Decoupled h-BN monolayer substrates for aligned graphene growth. ACS Nano 2016, 10, 11187–11195. [Google Scholar] [CrossRef]
- Lin, T.; Liu, Z.; Zhou, M.; Bi, H.; Zhang, K.; Huang, F.; Wan, D.; Zhong, Y. Rapid microwave synthesis of graphene directly on h-bn with excellent heat dissipation performance. ACS Appl. Mater. Interfaces 2014, 6, 3088–3092. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, J. Graphene as transparent electrodes: fabrication and new emerging applications. Small 2016, 12, 1400–1419. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Chen, K.; Liu, D.; Chen, J.; Miao, Q.; Xu, J. High-quality large-area graphene from dehydrogenated polycyclic aromatic hydrocarbons. Chem. Mater. 2012, 24, 3906–3915. [Google Scholar] [CrossRef]
- Ji, H.; Hao, Y.; Ren, Y.; Charlton, M.; Lee, W.H.; Wu, Q.; Li, H.; Zhu, Y.; Wu, Y.; Piner, R.; et al. Graphene growth using a solid carbon feedstock and hydrogen. ACS Nano 2011, 5, 7656–7661. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.-J.; Lim, H.; Shin, G.-Y.; Han, T.-H.; Oh, S.H.; Ahn, J.-H.; Choi, H.C.; Lee, T.-W. Graphenes converted from polymers. J. Phys. Chem. Lett. 2011, 2, 493–497. [Google Scholar] [CrossRef]
- Geng, D.; Wang, H.; Yu, G. Graphene single crystals: Size and morphology engineering. Adv. Mater. 2015, 27, 2821–2837. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Lu, Y.; Han, C.; Niu, T.; Chen, W.; Wee, A.T.S. Critical crystal growth of graphene on dielectric substrates at low temperature for electronic devices. Angew. Chem. Int. Ed. 2013, 52, 14121–14126. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Lin, W.; Huang, Z.; Zhang, L.; Li, H.; Chen, X.; Cai, W.; Ruoff, R.S.; Chen, S. Controllable seeding of single crystal graphene islands from graphene oxide flakes. Carbon 2014, 79, 406–412. [Google Scholar] [CrossRef]
- Zheng, Y.; Su, C.; Lu, J.; Loh, K.P. Room-temperature ice growth on graphite seeded by nano-graphene oxide. Angew. Chem. Int. Ed. 2013, 52, 8708–8712. [Google Scholar] [CrossRef]
- Yu, Q.; Jauregui, L.A.; Wu, W.; Colby, R.; Tian, J.; Su, Z.; Cao, H.; Liu, Z.; Pandey, D.; Wei, D.; et al. Control and characterization of individual grains and grain boundaries in graphene grown by chemical vapour deposition. Nat. Mater. 2011, 10, 443–449. [Google Scholar] [CrossRef]
- Wu, W.; Jauregui, L.A.; Su, Z.; Liu, Z.; Bao, J.; Chen, Y.P.; Yu, Q. Growth of single crystal graphene arrays by locally controlling nucleation on polycrystalline cu using chemical vapor deposition. Adv. Mater. 2011, 23, 4898–4903. [Google Scholar] [CrossRef]
- Wu, T.; Ding, G.; Shen, H.; Wang, H.; Sun, L.; Zhu, Y.; Jiang, D.; Xie, X. Continuous graphene films synthesized at low temperatures by introducing coronene as nucleation seeds. Nanoscale 2013, 5, 5456–5461. [Google Scholar] [CrossRef]
- Kalita, G.; Hirano, R.; Ayhan, M.E.; Tanemura, M. Fabrication of a Schottky junction diode with direct growth graphene on silicon by a solid phase reaction. J. Phys. D Appl. Phys. 2013, 46, 455103. [Google Scholar] [CrossRef]
- Peng, Z.; Yan, Z.; Sun, Z.; Tour, J.M. Direct growth of bilayer graphene on sio2 substrates by carbon diffusion through nickel. ACS Nano 2011, 5, 8241–8247. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Wang, Y.; Yu, T.; Shen, Z. Raman spectroscopy and imaging of graphene. Nano Res. 2008, 1, 273–291. [Google Scholar] [CrossRef]
- Ismach, A.; Druzgalski, C.; Penwell, S.; Schwartzberg, A.; Zheng, M.; Javey, A.; Bokor, J.; Zhang, Y. Direct chemical vapor deposition of graphene on dielectric surfaces. Nano Lett. 2010, 10, 1542. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.H.; Wang, H.M.; Kasim, J.; Fan, H.M.; Yu, T.; Wu, Y.H.; Feng, Y.P.; Shen, Z.X. Graphene thickness determination using reflection and contrast spectroscopy. Nano Lett. 2007, 7, 2758. [Google Scholar] [CrossRef]
- Mak, K.F.; Sfeir, M.Y.; Wu, Y.; Lui, C.H.; Misewich, J.A.; Heinz, T.F. Measurement of the optical conductivity of graphene. Phys. Rev. Lett. 2008, 101, 196405. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M.; Takano, S.; Hasegawa, K.; Noda, S. Direct synthesis of few- and multi-layer graphene films on dielectric substrates by “etching-precipitation” method. Carbon 2015, 82, 254–263. [Google Scholar] [CrossRef]
- Xu, S.; Man, B.; Jiang, S.; Yue, W.; Yang, C.; Liu, M.; Chen, C.; Zhang, C. Direct growth of graphene on quartz substrates for label-free detection of adenosine triphosphate. Nanotechnology 2014, 25, 165702. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-J.; Choi, W.M.; Yoon, S.-M.; Han, G.H.; Woo, Y.S.; Kim, E.S.; Chae, S.J.; Li, X.-S.; Benayad, A.; Duong Dinh, L.; et al. Transfer-free growth of few-layer graphene by self-assembled monolayers. Adv. Mater. 2011, 23, 4392–4397. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, Y.; Wang, X.; Li, D.; Wang, F.; Xie, S. Direct growth of nanocrystalline graphene/graphite transparent electrodes on Si/SiO2 for metal-free schottky junction photodetectors. Adv. Funct. Mater. 2014, 24, 835–840. [Google Scholar] [CrossRef]
- Yen, W.-C.; Chen, Y.-Z.; Yeh, C.-H.; He, J.-H.; Chiu, P.-W.; Chueh, Y.-L. Direct growth of self-crystallized graphene and graphite nanoballs with Ni vapor-assisted growth: From controllable growth to material characterization. Sci. Rep. 2014, 4, 4739. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, T.; Song, X.; Zhao, Y.; Lin, Y.; Wang, H.; Ma, D.; Chen, Y.; Xiang, W.; Wing, J.; et al. Direct growth of high-quality graphene on high-kappa dielectric srtio3 substrates. J. Am. Chem. Soc. 2014, 136, 6574–6577. [Google Scholar] [CrossRef] [PubMed]
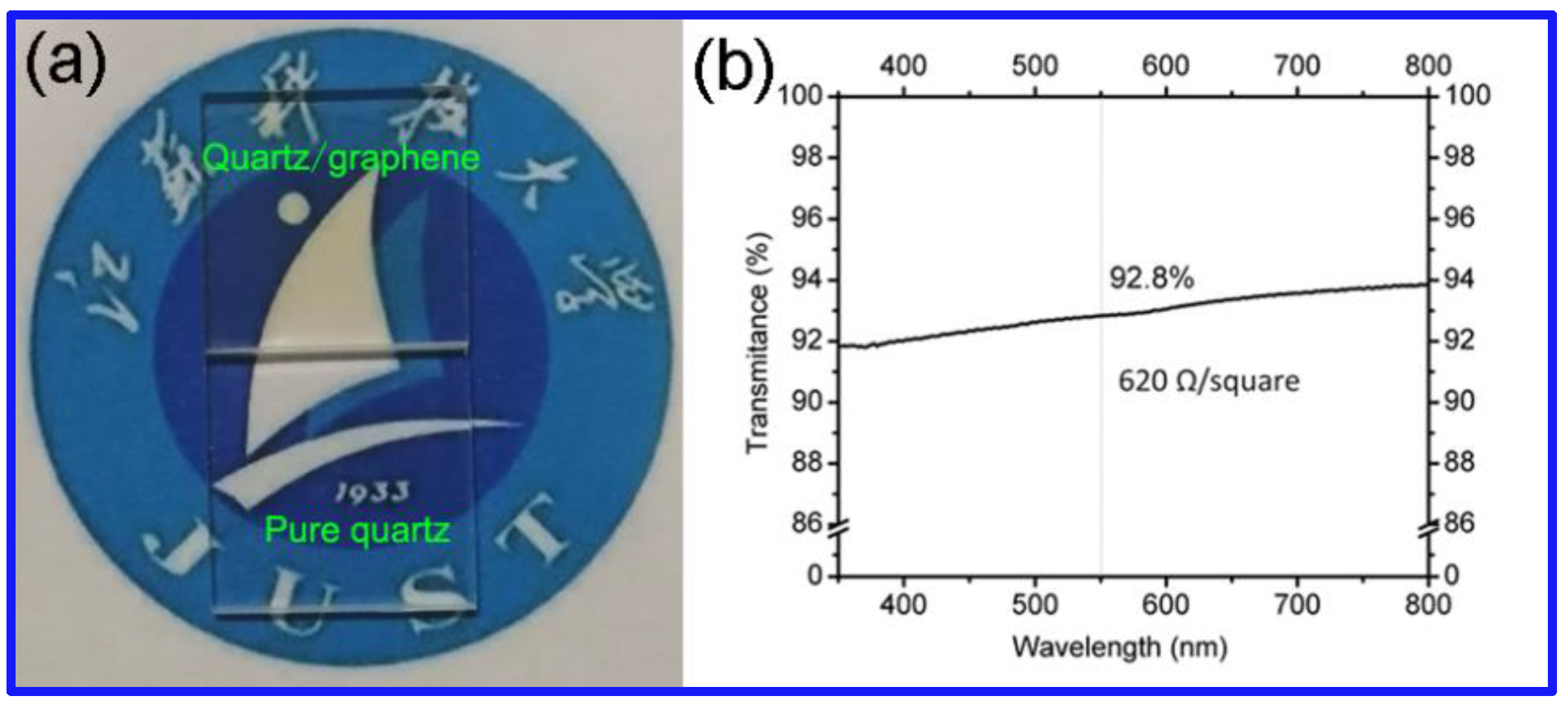
| Substrate | Size(mm) | Raman (ID/IG) | Transmittance (%) | Square Resistance (KΩ/sq) | Ref. |
|---|---|---|---|---|---|
| Quartz | Limit by substrate | ~1 | 92.0 | 0.9 | [56] |
| Quartz | ~200 | ~0.2 | 82.0 | 0.27 | [57] |
| SiO2 | Limit by substrate | <0.2 | N/A | N/A | [58] |
| Quartz | 10 | ~1 | 84.0 | 30 | [59] |
| Quartz | N/A | ~2 | ~70 | 0.6 | [60] |
| SrTiO3 | Limit by substrate | ~1 | 96.4 | 0.95 | [61] |
| Quartz | Limit by substrate | 0.31 | 92.6 | 0.62 | this paper |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuo, Q.; Mao, Y.; Lu, S.; Cui, B.; Yu, L.; Tang, J.; Sun, J.; Yan, C. Seed-Assisted Synthesis of Graphene Films on Insulating Substrate. Materials 2019, 12, 1376. https://doi.org/10.3390/ma12091376
Zhuo Q, Mao Y, Lu S, Cui B, Yu L, Tang J, Sun J, Yan C. Seed-Assisted Synthesis of Graphene Films on Insulating Substrate. Materials. 2019; 12(9):1376. https://doi.org/10.3390/ma12091376
Chicago/Turabian StyleZhuo, Qiqi, Yipeng Mao, Suwei Lu, Bolu Cui, Li Yu, Jijun Tang, Jun Sun, and Chao Yan. 2019. "Seed-Assisted Synthesis of Graphene Films on Insulating Substrate" Materials 12, no. 9: 1376. https://doi.org/10.3390/ma12091376
APA StyleZhuo, Q., Mao, Y., Lu, S., Cui, B., Yu, L., Tang, J., Sun, J., & Yan, C. (2019). Seed-Assisted Synthesis of Graphene Films on Insulating Substrate. Materials, 12(9), 1376. https://doi.org/10.3390/ma12091376






