Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of the Dyes
2.1.1. 2-Butoxy-4-(Diethylamino)Benzaldehyde D5
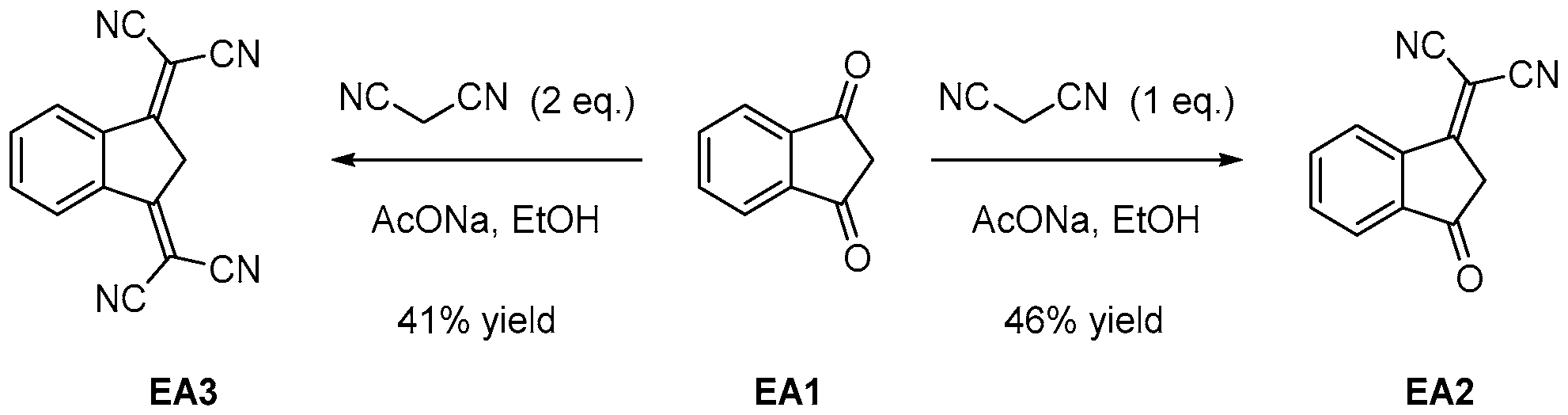
2.1.2. 1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione EA4
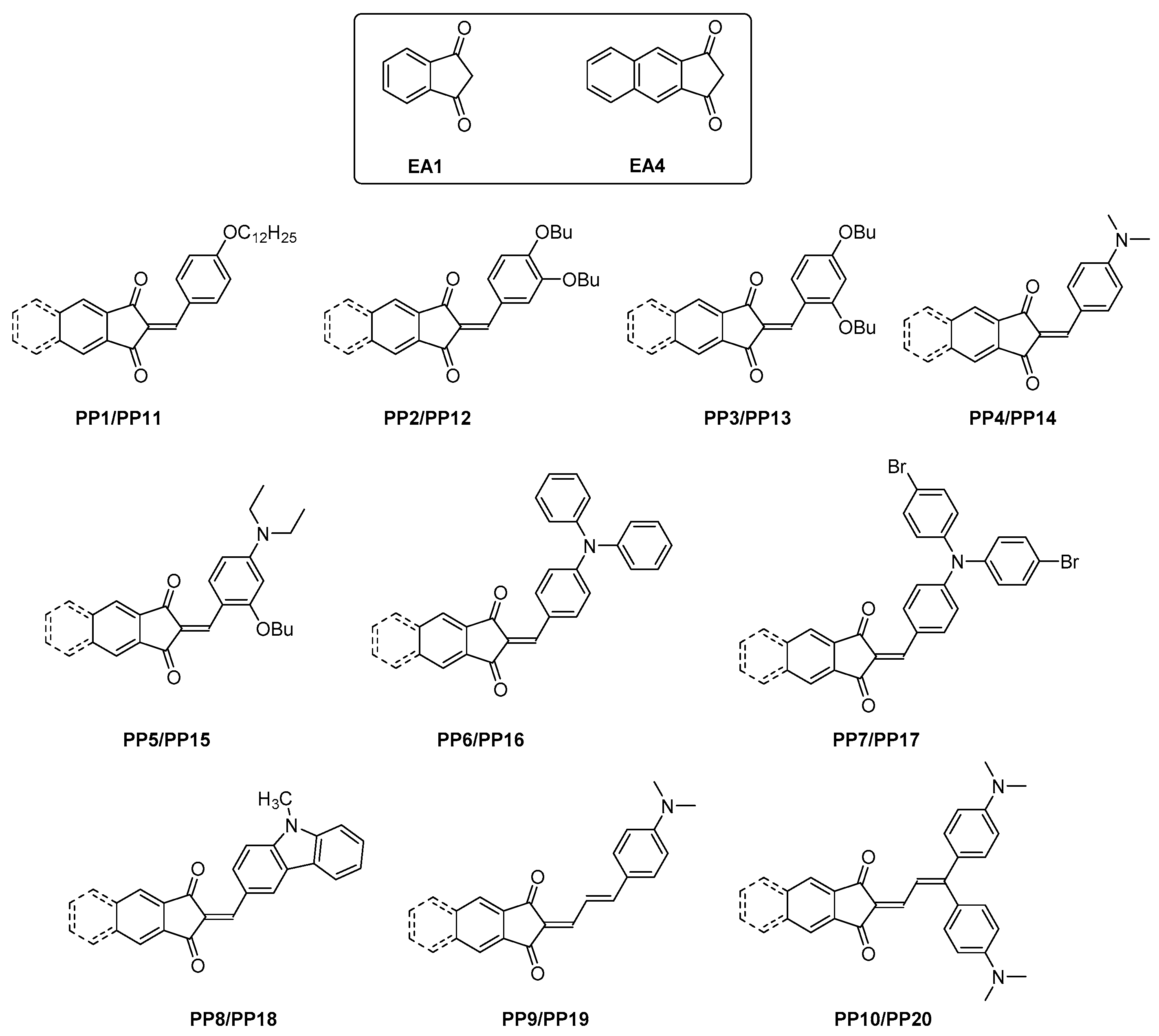
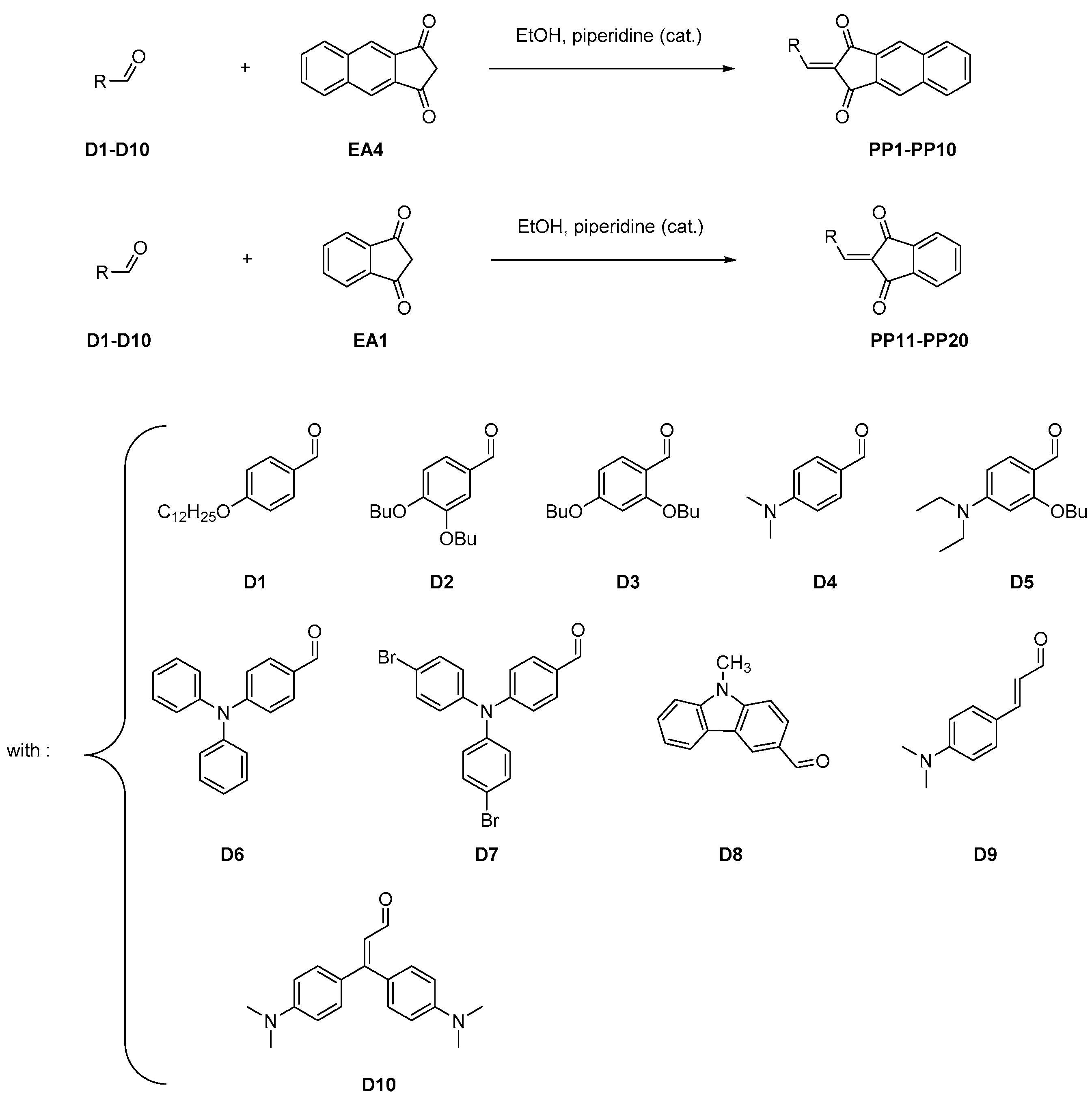
2.2. General Procedure for the Synthesis of PP1–PP10:
2.2.1. 2-(4-(Dodecyloxy)Benzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP1
2.2.2. 2-(3,4-Dibutoxybenzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP2
2.2.3. 2-(2,4-Dibutoxybenzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP3
2.2.4. 2-(4-(Dimethylamino)Benzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP4
2.2.5. 2-(2-Butoxy-4-(Diethylamino)Benzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP5
2.2.6. 2-(4-(Diphenylamino)Benzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP6
2.2.7. 2-(2-Butoxy-4-(Diethylamino)Benzylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP7
2.2.8. 2-((9-Methyl-9H-Carbazol-3-Yl)Methylene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP8
2.2.9. 2-(3-(4-(Dimethylamino)Phenyl)Allylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP9
2.2.10. 2-(3,3-Bis(4-(Dimethylamino)Phenyl)Allylidene)-1H-Cyclopenta[b]Naphthalene-1,3(2H)-Dione PP10
2.3. General Procedure for the Synthesis of PP11–PP20
2.3.1. 2-(4-(Dodecyloxy)Benzylidene)-1H-Indene-1,3(2H)-Dione PP11
2.3.2. 2-(3,4-Dibutoxybenzylidene)-1H-Indene-1,3(2H)-Dione PP12
2.3.3. 2-(2,4-Dibutoxybenzylidene)-1H-Indene-1,3(2H)-Dione PP13
2.3.4. 2-(4-(Dimethylamino)Benzylidene)-1H-Indene-1,3(2H)-Dione PP14
2.3.5. 2-(2-Butoxy-4-(Diethylamino)Benzylidene)-1H-Indene-1,3(2H)-Dione PP15
2.3.6. 2-(4-(Diphenylamino)Benzylidene)-1H-Indene-1,3(2H)-Dione PP16
2.3.7. 2-(4-(Bis(4-Bromophenyl)Amino)Benzylidene)-1H-Indene-1,3(2H)-Dione PP17
2.3.8. 2-((9-Methyl-9H-Carbazol-3-Yl)Methylene)-1H-Indene-1,3(2H)-Dione PP18
2.3.9. 2-(3-(4-(Dimethylamino)Phenyl)Allylidene)-1H-Indene-1,3(2H)-Dione PP19
2.3.10. 2-(3,3-Bis(4-(Dimethylamino)Phenyl)Allylidene)-1H-Indene-1,3(2H)-Dione PP20
3. Results and Discussion
3.1. Synthesis of the Dyes and Electron Acceptors
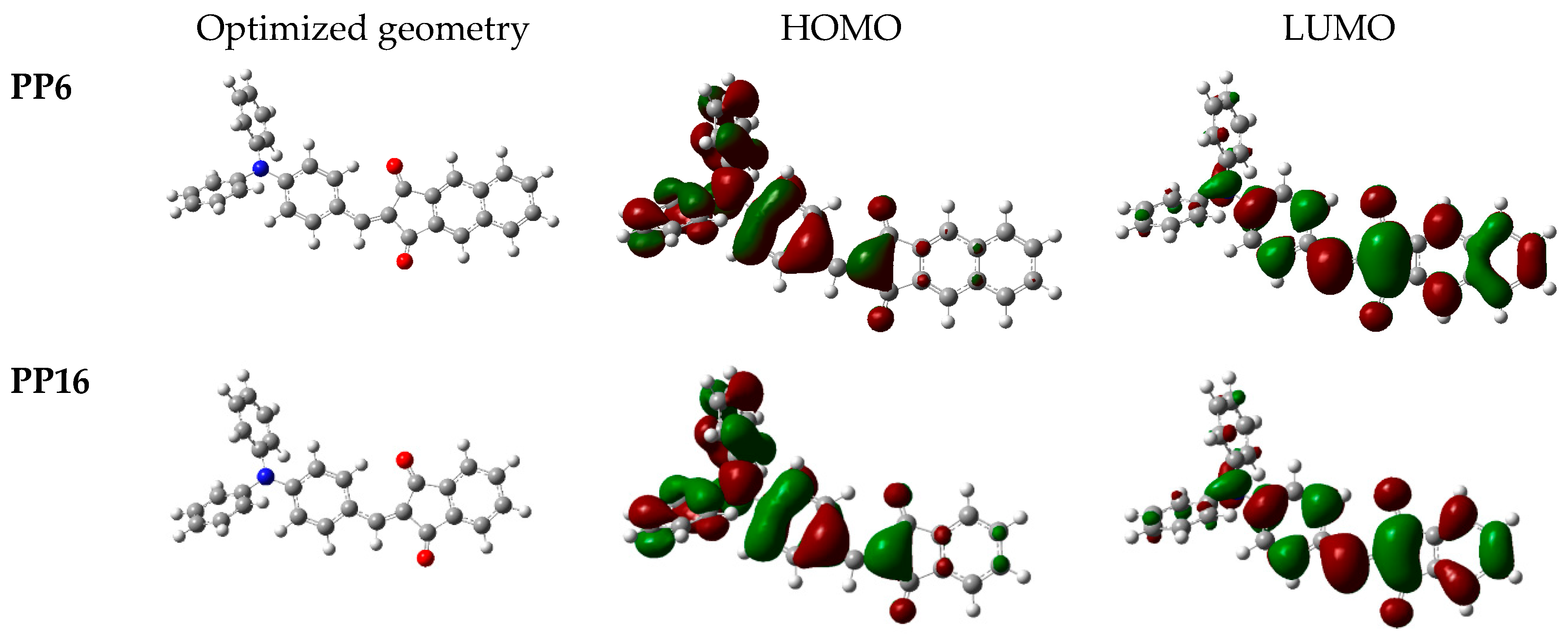
3.2. Theoretical Calculations
3.3. Optical Properties
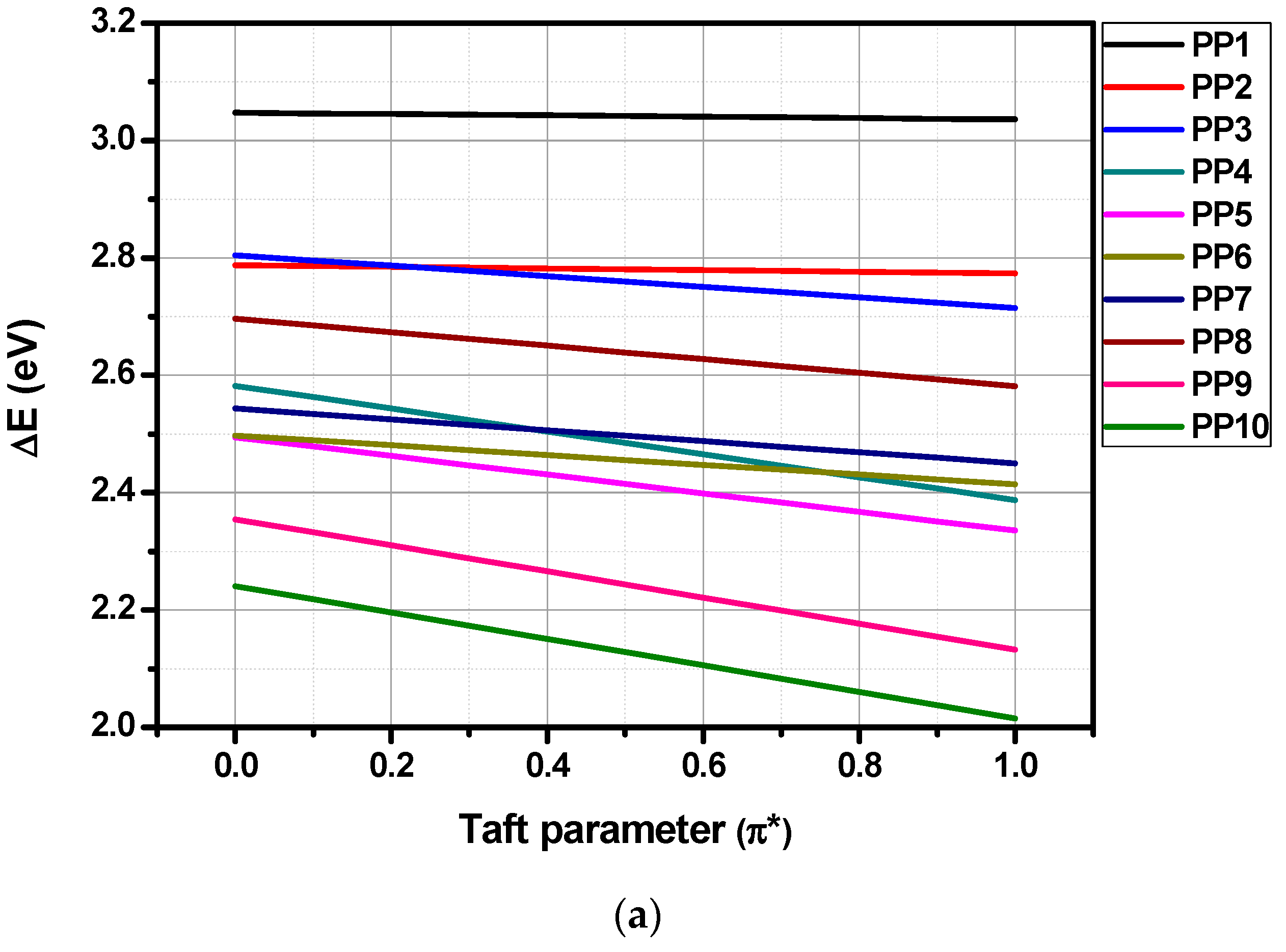
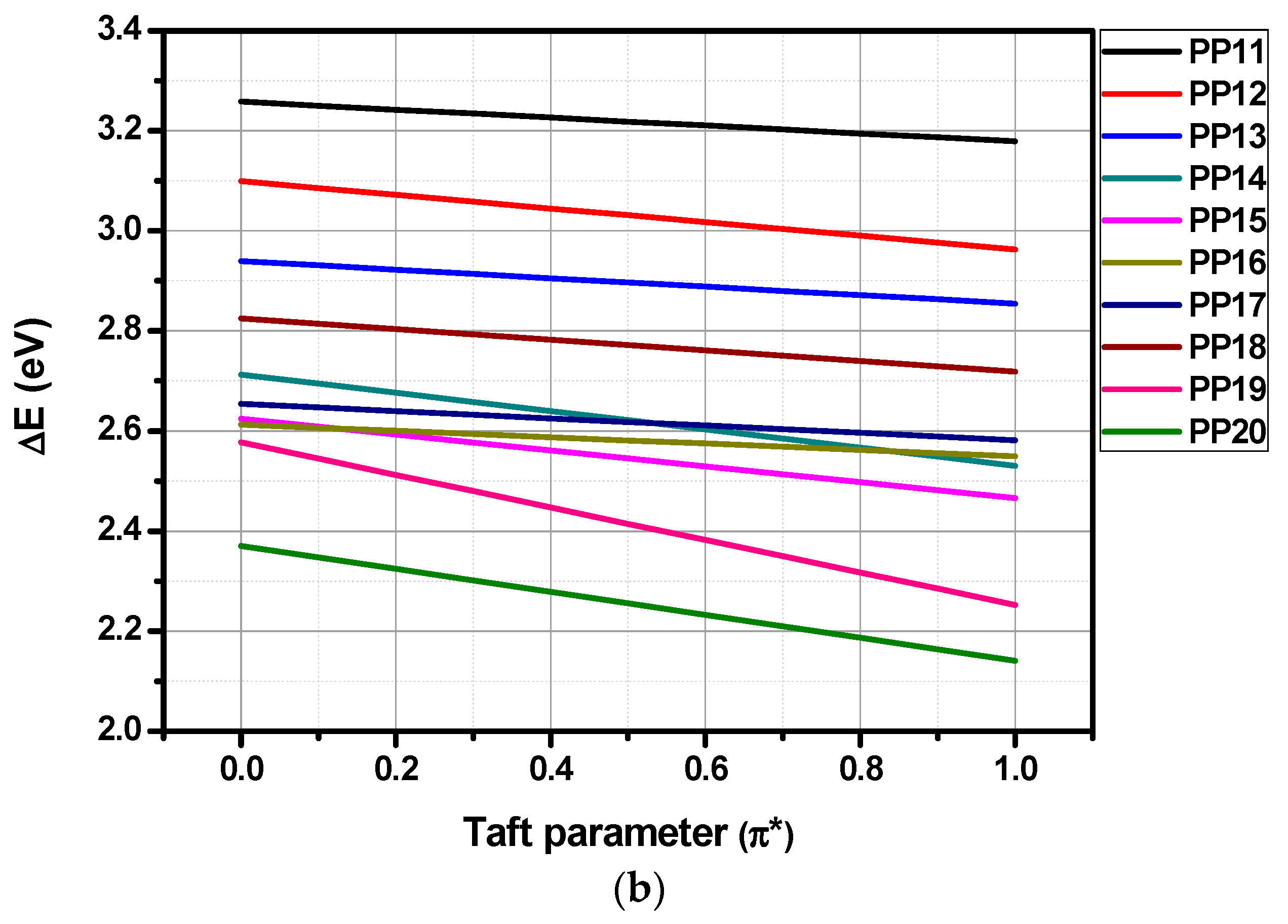
3.4. Solvatochromism
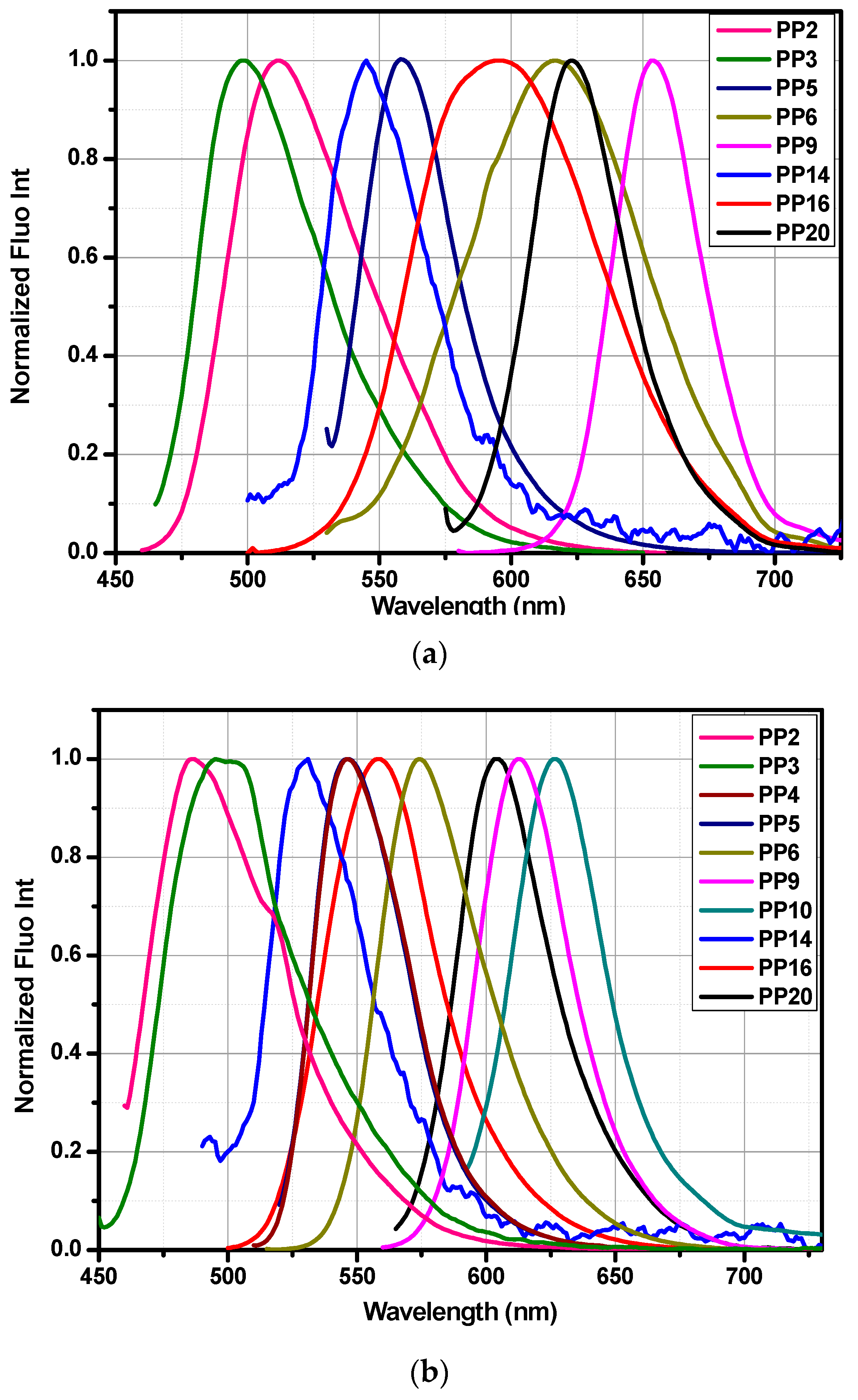
3.5. Fluorescence Spectroscopy
3.6. Electrochemical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Raimundo, J.M.; Blanchard, P.; Gallego-Planas, N.; Mercier, N.; Ledoux-Rak, I.; Hierle, R.; Roncali, J. Design and synthesis of push-pull chromophores for second-order nonlinear optics derived from rigidified thiophene-based π-conjugating spacers. J. Org. Chem. 2002, 67, 205–218. [Google Scholar] [CrossRef]
- El-Shishtawy, R.M.; Borbone, F.; Al-amshany, Z.M.; Tuzi, A.; Barsella, A.; Asiri, A.M.; Roviello, A. Thiazole azo dyes with lateral donor branch: Synthesis, structure and second order NLO properties. Dyes Pig. 2013, 96, 45–51. [Google Scholar] [CrossRef]
- Paek, S.; Lee, J.K.; Ko, J. Synthesis and photovoltaic characteristics of push–pull organic semiconductors containing an electron-rich dithienosilole bridge for solution-processed small-molecule organic solar cells. Sol. Energ. Mater. Solar Cells 2014, 120, 209–217. [Google Scholar] [CrossRef]
- Xu, S.J.; Zhou, Z.; Liu, W.; Zhang, Z.; Liu, F.; Yan, H.; Zhu, X. A twisted thieno[3,4-b]thiophene-based electron acceptor featuring a 14-π-electron indenoindene core for high-performance organic photovoltaics. Adv. Mater. 2017, 29, 1704510. [Google Scholar] [CrossRef]
- Karak, S.; Liu, F.; Russell, T.P.; Duzhko, V.V. Bulk charge carrier transport in push-pull type organic semiconductor. ACS Appl. Mater. Interfaces 2014, 6, 20904–20912. [Google Scholar] [CrossRef] [PubMed]
- Turkoglu, G.; Cinar, M.E.; Ozturk, T. Triarylborane-based materials for OLED applications. Molecules 2017, 22, 1522. [Google Scholar] [CrossRef] [PubMed]
- Archetti, G.; Abbotto, A.; Wortmann, R. Effect of polarity and structural design on molecular photorefractive properties of heteroaromatic-based push-pull dyes. Chem. Eur. J. 2006, 12, 7151–7160. [Google Scholar] [CrossRef] [PubMed]
- Ipuy, M.; Billon, C.; Micouin, G.; Samarut, J.; Andraud, C.; Bretonnière, Y. Fluorescent push–pull pH-responsive probes for ratiometric detection of intracellular pH. Org. Biomol. Chem. 2014, 12, 3641–3648. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.-H.; Xie, M.-S.; Wang, H.-X.; Niu, H.-Y.; Qu, G.-R.; Guo, H.-M. Highly selective detection of Hg2+ ion by push–pull-type purine nucleoside-based fluorescent sensor. Tetrahedron 2014, 70, 4929–4933. [Google Scholar] [CrossRef]
- Jones, R.M.; Lu, L.; Helgeson, R.; Bergstedt, T.S.; McBranch, D.W.; Whitten, D.G. Building highly sensitive dye assemblies for biosensing from molecular building blocks. Proc. Nat. Acad. Sci. USA 2001, 98, 14769–14772. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A.; Alamiry, M.A.H.; Stewart, B.; Retailleau, P. Solid-state gas sensors developed from functional difluoroboradiazaindacene dyes. Chem. Eur. J. 2009, 15, 1359–1369. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Blue-to-red light sensitive push-pull structured photoinitiators: indanedione derivatives for radical and cationic photopolymerization reactions. Macromolecules 2013, 46, 3332–3341. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. New push-pull dyes derived from Michler’s ketone for polymerization reactions upon visible lights. Macromolecules 2013, 46, 3761–3770. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push-pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm Laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Mokbel, H.; Telitel, S.; Dumur, F.; Vidal, L.; Versace, D.-L.; Tehfe, M.-A.; Graff, B.; Toufaily, J.; Fouassier, J.-P.; Gigmes, D.; Hamieh, T.; Lalevée, J. Photoinitiating systems of polymerization and in-situ incorporation of metal nanoparticles in polymer matrixes upon visible lights: push-pull malonate and malonitrile based dyes. Polym. Chem. 2013, 4, 5679–5687. [Google Scholar] [CrossRef]
- Xiao, P.; Frigoli, M.; Dumur, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Julolidine or fluorenone based push-pull dyes for polymerization upon soft polychromatic visible light or green light. Macromolecules 2014, 47, 106–112. [Google Scholar] [CrossRef]
- Bures, F. Fundamental aspects of property tuning in push–pull molecules. RSC Adv. 2014, 4, 58826–58851. [Google Scholar] [CrossRef]
- Noirbent, G.; Dumur, F. Recent advances on nitrofluorene derivatives: Versatile electron acceptors to create dyes absorbing from the visible to the near and far-infrared region. Materials 2018, 11, 2425. [Google Scholar] [CrossRef] [PubMed]
- Solanke, P.; Ruzicka, A.; Mikysek, T.; Pytela, O.; Bures, F.; Klikar, M. From linear to T-shaped indan-1,3-dione push-pull molecules: A comparative study. Helv. Chim. Acta 2018, 101, e201800090. [Google Scholar] [CrossRef]
- Solanke, P.; Bures, F.; Pytela, O.; Klikar, M.; Mikysek, T.; Mager, L.; Barsella, A.; Ruzicková, Z. T-shaped (donor-π-)2acceptor-π-donor push–pull systems based on indan-1,3-dione. Eur. J. Org. Chem. 2015, 5339–5349. [Google Scholar] [CrossRef]
- Swager, T.M.; Gutierrez, G.D. Push-pull chromophores from indan-1,3-dione. Synfacts 2014, 10, 0035. [Google Scholar]
- Durand, R.J.; Gauthier, S.; Achelle, S.; Groizard, T.; Kahlal, S.; Saillard, J.-Y.; Barsella, A.; Le Poul, N.; Le Guen, F.R. Push–pull D–π-Ru–π-A chromophores: synthesis and electrochemical, photophysical and second order nonlinear optical properties. Dalton Trans. 2018, 47, 3965–3975. [Google Scholar] [CrossRef]
- Nishiyabu, R.; Anzenbacher, P. 1,3-Indane-based chromogenic calixpyrroles with push-pull chromophores: Synthesis and anion sensing. Org. Lett. 2006, 8, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ren, H.; Yu, J.; Wang, Z.; Qian, G. An indanone-based alkoxysilane dye with second order nonlinear optical properties. Dyes Pigm. 2009, 81, 53–57. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Bryce, M.R.; Davies, S.R.; Howard, J.A.K.; Whitehead, R.; Tanner, B.K. Studies on π-acceptor molecules containing the dicyanomethylene group. X-ray crystal structure of the charge-transfer complex of tetramethyltetrathiafulvalene and 2,3-dicyano-l,4-naphthoquinone: (TMTTF)3-(DCNQ)2. J. Chem. Soc. Perkin Trans. 2 1993, 0, 313–319. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Zhou, X.; Jia, Q.-Q.; Feng, S.; Jiang, P.; Xu, X.; Ma, W.; Li, H.-B.; Bo, Z. Nonfullerene acceptors with enhanced solubility and ordered packing for high-efficiency polymer solar cells. ACS Energy Lett. 2018, 3, 1832–1839. [Google Scholar] [CrossRef]
- Sanguinet, L.; Williams, J.C.; Yang, Z.; Twieg, R.J.; Mao, G.; Singer, K.D.; Wiggers, G.; Petschek, R.G. Synthesis and characterization of new truxenones for nonlinear optical applications. Chem. Mater. 2006, 18, 4259–4269. [Google Scholar] [CrossRef]
- Sigalov, M.V.; Shainyan, B.A.; Chipanina, N.N.; Oznobikhina, L.P. Intra- and intermolecular hydrogen bonds in pyrrolylindandione derivatives and their interaction with fluoride and acetate: Possible anion sensing properties. J. Phys. Chem. A 2013, 117, 11346–11356. [Google Scholar] [CrossRef]
- Feng, H.; Qiu, N.; Wang, X.; Wang, Y.; Kan, B.; Wan, X.; Zhang, M.; Xia, A.; Li, C.; Liu, F.; Zhang, H.; Chen, Y. An A-D-A type small-molecule electron acceptor with end-extended conjugation for high performance organic solar cells. Chem. Mater. 2017, 29, 7908–7917. [Google Scholar] [CrossRef]
- Li, R.; Liu, G.; Xiao, M.; Yang, X.; Liu, X.; Wang, Z.; Ying, L.; Huang, F.; Cao, Y. Non-fullerene acceptors based on fused-ring oligomers for efficient polymer solar cells via complementary light-absorption. J. Mater. Chem. A 2017, 5, 23926–23936. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. GAUSSIAN Program; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. Condens. Matter. 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. A new mixing of Hartree–Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cances, E. The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J. Mol. Struct. THEOCHEM 1999, 464, 211–226. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comp. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Delong, W.; Lanying, W.; Yongling, W.; Shuang, S.; Juntao, F.; Xing, Z. Natural α-methylenelactam analogues: Design, synthesis and evaluation of α-alkenyl-γ and δ-lactams as potential antifungal agents against Colletotrichum orbiculare. Eur. J. Med. Chem. 2017, 130, 286–307. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, H.; Zhang, G.; Lu, Q.; Chang, J.; Dong, Y.; Shi, X.; Wei, J. facile bottom-up synthesis of coronene-based 3-fold symmetrical and highly substituted nanographenes from simple aromatics. J. Am. Chem. Soc. 2014, 136, 5057–5064. [Google Scholar] [CrossRef] [PubMed]
- Pollard, A.J.; Perkins, E.W.; Smith, N.A.; Saywell, A.; Goretzki, G.; Phillips, A.G.; Argent, S.P.; Sachdev, H.; Mueller, F.; Huefner, S.; et al. Supramolecular Assemblies Formed on an Epitaxial Graphene Superstructure. Angew. Chem. Int. Ed. 2010, 49, 1794–1799. [Google Scholar] [CrossRef]
- He, F.; Tian, L.; Tian, X.; Xu, H.; Wang, Y.; Xie, W.; Hanif, M.; Xia, J.; Shen, F.; Yang, B.; et al. diphenylamine-substituted cruciform oligo(phenylene vinylene): enhanced one- and two-photon excited fluorescence in the solid state. Adv. Funct. Mater. 2007, 17, 1551–1557. [Google Scholar] [CrossRef]
- Wang, H.; Ding, W.; Wang, G.; Pan, C.; Duan, M.; Yu, G. Tunable molecular weights of poly(triphenylamine-2,2′-bithiophene) and their effects on photovoltaic performance as sensitizers for dye-sensitized solar cells. J. Appl. Polym. Sci. 2016, 133, 44182. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dumur, F.; Garra, P.; Toufaily, J.; Hamieh, T.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. carbazole scaffold based photoinitiator/photoredox catalysts: toward new high performance photoinitiating systems and application in led projector 3d printing resins. Macromolecules 2017, 50, 2747–2758. [Google Scholar] [CrossRef]
- Malina, I.; Kampars, V.; Turovska, B.; Belyakov, S. Novel green-yellow-orange-red light emitting donor-π-acceptor type dyes based on 1,3-indandione and dimedone moieties. Dyes Pigm. 2017, 139, 820–830. [Google Scholar] [CrossRef]
- Dumur, F.; Mayer, C.R.; Dumas, E.; Miomandre, F.; Frigoli, M.; Sécheresse, F. New chelating stilbazonium-like dyes from michler’s ketone. Org. Lett. 2008, 10, 321–324. [Google Scholar] [CrossRef]
- Buckle, D.R.; Morgan, N.J.; Ross, J.W.; Smith, H.; Spicer, B.A. Antiallergic activity of 2-nitroindan-1, 3-diones. J. Med. Chem. 1973, 16, 1334–1339. [Google Scholar] [CrossRef]
- Lee, Y.; Jo, A.; Park, S.B. Rational Improvement of Molar Absorptivity Guided by Oscillator Strength: A Case Study with Furoindolizine-Based Core Skeleton. Angew. Chem. 2015, 127, 15915–15919. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.-L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, .pi.*, .alpha., and .beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic Dyes as Solvent Polarity Indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
- Lippert, E.Z. Dipolmoment und Elektronenstruktur von angeregten Molekülen. Naturforsch. 1955, 10a, 541–545. [Google Scholar] [CrossRef]
- Catalan, J. On the ET (30), π*, Py, S‘, and SPP empirical scales as descriptors of nonspecific solvent effects. J. Org. Chem. 1997, 62, 8231–8234. [Google Scholar] [CrossRef]
- Kawski, A. Zur Lösungsmittelabhängigkeit der Wellenzahl von Elektronenbanden Lumineszierender Moleküle und über die Bestimmung der Elektrischen Dipolmomente im Anregungszustand. Acta Phys. Polon. 1966, 29, 507–518. [Google Scholar]
- McRae, E.G. Theory of Solvent Effects on Molecular Electronic Spectra. Frequency Shifts. J. Phys. Chem. 1957, 61, 562–572. [Google Scholar] [CrossRef]
- Suppan, P. Solvent effects on the energy of electronic transitions: experimental observations and applications to structural problems of excited molecules. J. Chem. Soc. A 1968, 0, 3125–3133. [Google Scholar] [CrossRef]
- Bakshiev, N.G. Universal intermolecular interactions and their effect on the position of the electronic spectra of molecules in two component solutions. Opt. Spektrosk. 1964, 16, 821–832. [Google Scholar]
- Redoa, S.; Eucat, G.; Ipuy, M.; Jeanneau, E.; Gautier-Luneau, I.; Ibanez, A.; Andraud, C.; Bretonnière, Y. Tuning the solid-state emission of small push-pull dipolar dyes to the far-red through variation of the electron-acceptor group. Dyes Pigm. 2018, 156, 116–132. [Google Scholar]
- Jeong, H.; Chitumalla, R.K.; Kim, D.W.; Prabhakar Vattikuti, S.V.; Thogiti, S.; Cheruku, R.; Kim, J.H.; Jang, J.; Koyyada, G.; Jung, J.H. The comparative study of new carboxylated 1,3-indanedione sensitizers with standard cyanoacetic acid dyes using co-adsorbents in dye-sensitized solar cells. Chem. Phys. Lett. 2019, 715, 84–90. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Li, M.; Feng, H.; Ni, W.; Zhang, H.; Wan, X.; Chen, Y. Efficient carbazole-based small-molecule organic solar cells with an improved fill factor. RSC Adv. 2018, 8, 4867–4871. [Google Scholar] [CrossRef]
- Cao, D.; Peng, J.; Hong, Y.; Fang, X.; Wang, L.; Meier, H. Enhanced performance of the dye-sensitized solar cells with phenothiazine-based dyes containing double D−A branches. Org. Lett. 2011, 13, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhao, F.; Zhang, Q.; Lau, T.-K.; Li, T.; Liu, K.; Ling, Q.; Wang, C.; Lu, X.; You, W.; Zhan, X. Fused nonacyclic electron acceptors for efficient polymer solar cells. J. Am. Chem. Soc. 2017, 139, 1336–1343. [Google Scholar] [CrossRef]
- Guerlin, A.; Dumur, F.; Dumas, E.; Miomandre, F.; Wantz, G.; Mayer, C.R. Tunable optical properties of chromophores derived from oligo(para-phenylene vinylene). Org. Lett. 2010, 12, 2382–2385. [Google Scholar] [CrossRef]
- Pommerehne, J.; Vestweber, H.; Guss, W.; Mahrt, R.F.; Bässler, H.; Porsch, M.; Daub, J. Efficient two layer leds on a polymer blend basis. Adv. Mater. 1995, 7, 551–554. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]

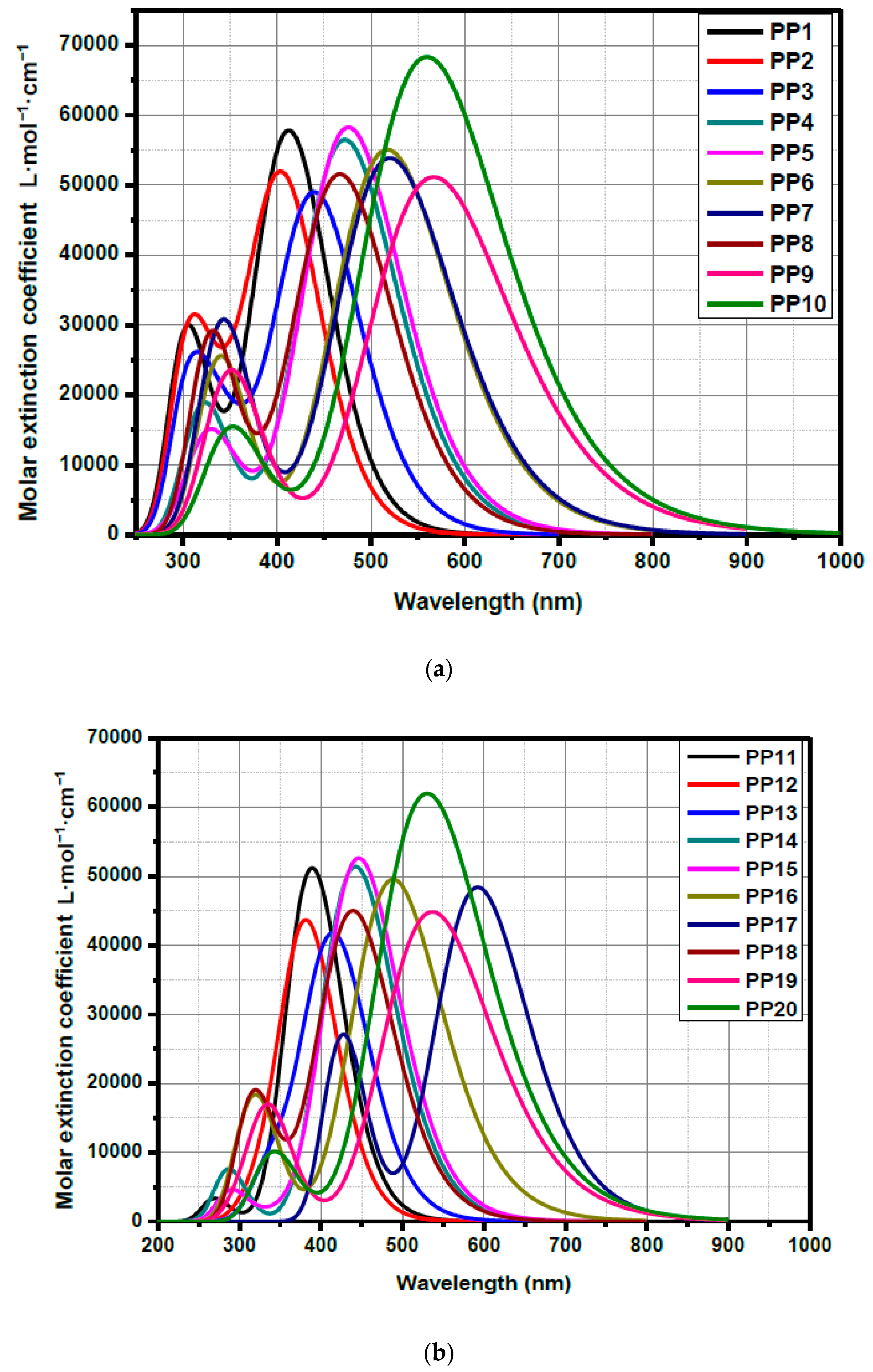
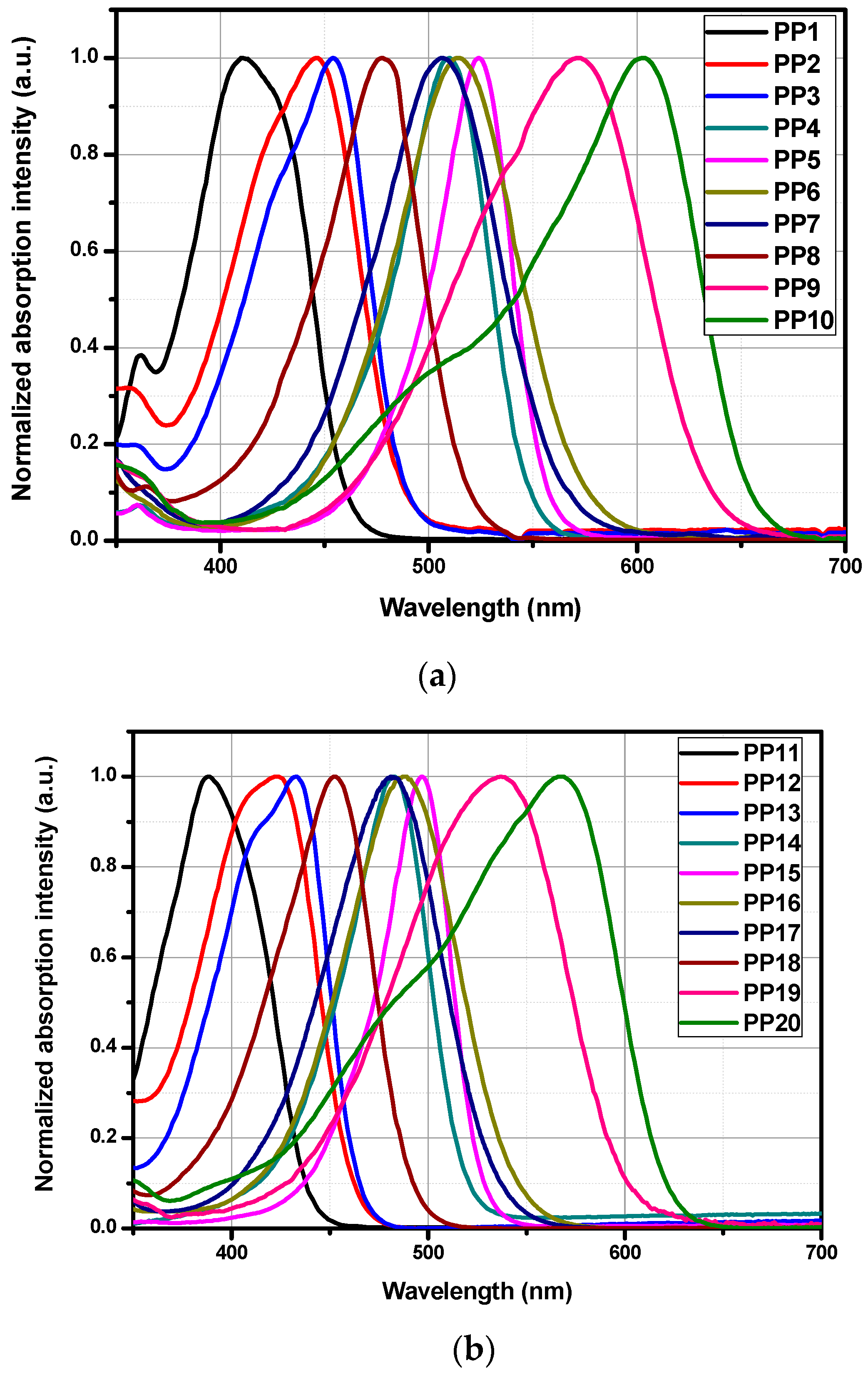
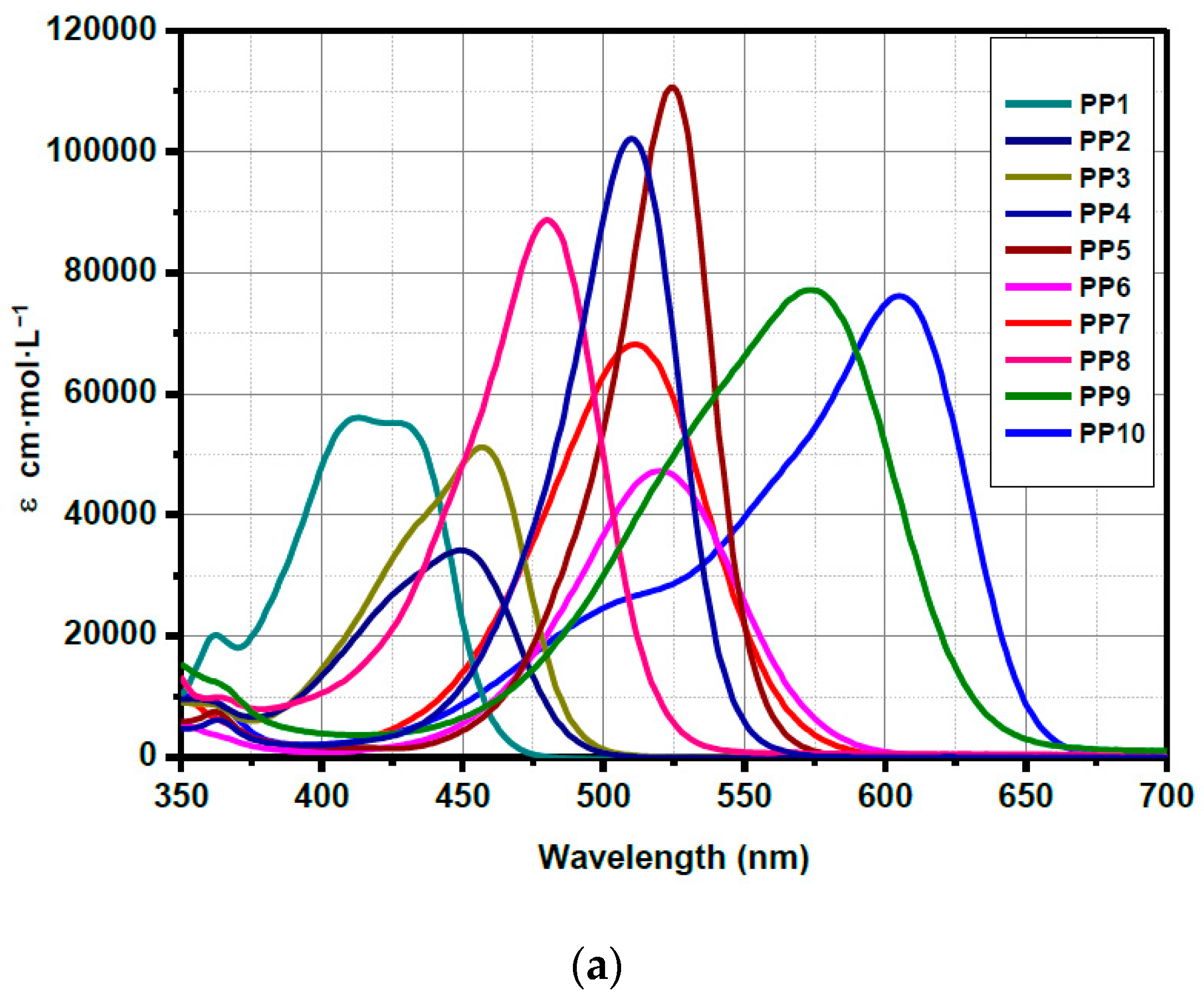
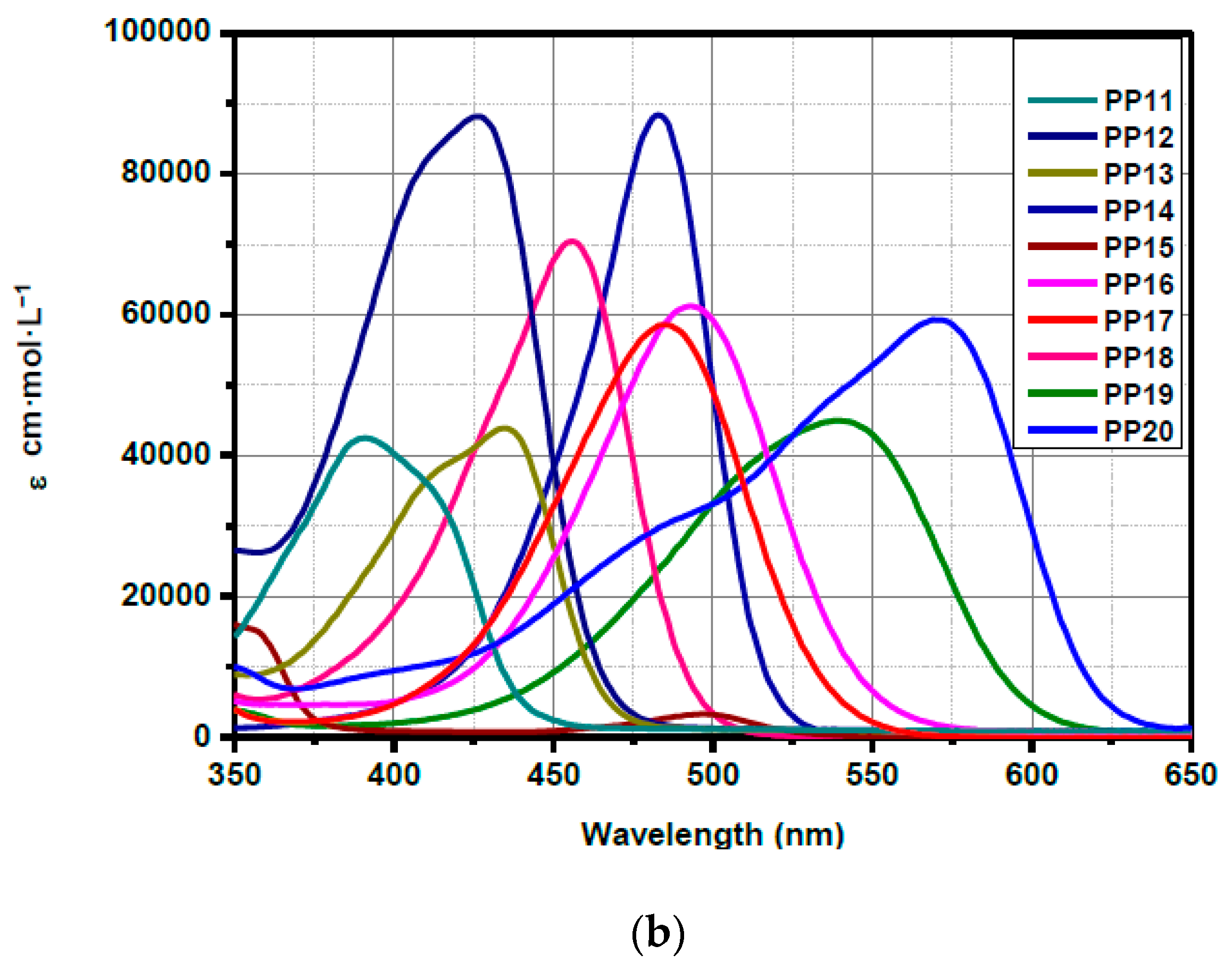


| Compounds | PP1 | PP2 | PP3 | PP4 | PP5 | PP6 | PP7 | PP8 | PP9 | PP10 |
| Reaction yields (%) | 88 | 84 | 88 | 74 | 94 | 89 | 92 | 85 | 84 | 88 |
| Compounds | PP11 | PP12 | PP13 | PP14 | PP15 | PP16 | PP17 | PP18 | PP19 | PP20 |
| Reaction yields (%) | 74 | 85 | 75 | 82 | 92 | 87 | 81 | 78 | 89 | 85 |
| Compounds | EHOMO (eV) | ELUMO (eV) | λmax (nm) | Transitions |
|---|---|---|---|---|
| PP1 | −6.279 | −2.865 | 414 | HOMO->LUMO (95%) |
| PP2 | −6.468 | −2.983 | 406 | HOMO->LUMO (72%) HOMO-1->LUMO (22%) HOMO-2->LUMO (5%) |
| PP3 | −5.974 | −2.691 | 441 | HOMO->LUMO (98%) |
| PP4 | −5.717 | −2.668 | 473 | HOMO->LUMO (99%) |
| PP5 | −5.522 | −2.489 | 477 | HOMO->LUMO (99%) |
| PP6 | −5.633 | −2.836 | 518 | HOMO->LUMO (99%) |
| PP7 | −5.826 | −3.003 | 520 | HOMO->LUMO (99%) |
| PP8 | −5.953 | −2.816 | 471 | HOMO->LUMO (99%) |
| PP9 | −5.554 | −2.874 | 567 | HOMO->LUMO (100%) |
| PP10 | −5.262 | −2.62 | 574 | HOMO->LUMO (90%) HOMO-1->LUMO (10%) |
| PP11 | −6.3 | −2.71 | 390 | HOMO->LUMO (97%) |
| PP12 | −6.498 | −2.842 | 385 | HOMO->LUMO (94%) HOMO-1->LUMO (4%) |
| PP13 | −5.982 | −2.526 | 417 | HOMO->LUMO (97%) |
| PP14 | −5.715 | −2.498 | 444 | HOMO->LUMO (95%) HOMO->L+1 (4%) |
| PP15 | −5.513 | −2.309 | 448 | HOMO->LUMO (95%) HOMO->LUMO+1 (4%) |
| PP16 | −5.635 | −2.694 | 490 | HOMO->LUMO (99%) |
| PP17 | −5.835 | −2.878 | 493 | HOMO->LUMO (99%) |
| PP18 | −5.962 | −2.656 | 443 | HOMO->LUMO (98%) |
| PP19 | −5.555 | −2.748 | 537 | HOMO->LUMO (100%) |
| PP20 | −5.253 | −2.489 | 545 | HOMO->LUMO (85%) HOMO-1->LUMO (15%) |
| Compounds | π*1 | PP12 | PP22 | PP32 | PP42 | PP52 | PP62 | PP72 | PP82 | PP92 | PP102 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| diethyl ether | 0.27 | 403 | 442 | 445 | 490 | 505 | 499 | 489 | 464 | 534 | 569 |
| toluene | 0.54 | 405 | 450 | 441 | 501 | 512 | 509 | 501 | 473 | 553 | 582 |
| chloroform | 0.78 | 412 | 450 | 457 | 520 | 524 | 520 | 510 | 480 | 574 | 606 |
| THF | 0.58 | 405 | 444 | 450 | 502 | 517 | 504 | 497 | 471 | 555 | 587 |
| 1,4-dioxane | 0.55 | 402 | 443 | 446 | 498 | 512 | 503 | 496 | 468 | 545 | 581 |
| acetone | 0.71 | 405 | 441 | 449 | 505 | 520 | 501 | 496 | 471 | 562 | 595 |
| dichloromethane | 0.82 | 411 | 448 | 456 | 510 | 524 | 514 | 505 | 476 | 571 | 603 |
| DMSO | 1.00 | 414 | 447 | 458 | 522 | 534 | 512 | 506 | 482 | 590 | 620 |
| heptane | -0.08 | 410 | 445 | 443 | 479 | 497 | 497 | 486 | 461 | 529 | 551 |
| acetonitrile | 0.75 | 404 | 439 | 450 | 507 | 523 | 505 | 496 | 471 | 565 | 597 |
| dimethylformamide | 0.87 | 409 | 447 | 454 | 514 | 528 | 509 | 503 | 478 | 579 | 609 |
| ethyl acetate | 0.54 | 404 | 444 | 447 | 498 | 513 | 500 | 491 | 468 | 546 | 579 |
| p-xylene | 0.43 | 406 | 448 | 448 | 496 | 509 | 504 | 497 | 470 | 547 | 580 |
| 1,2-dichloroethane | 0.81 | 410 | 447 | 454 | 509 | 524 | 515 | 504 | 474 | 567 | 603 |
| dimethylacetamide | 0.88 | 410 | 447 | 456 | 516 | 529 | 508 | 502 | 477 | 577 | 608 |
| diglyme | 0.64 | 406 | 445 | 450 | 506 | 518 | 504 | 497 | 474 | 567 | 593 |
| cyclohexane | 0.00 | 412 | 446 | 445 | 482 | 499 | 502 | 492 | 462 | 532 | 556 |
| triethylamine | 0.14 | 439 | 454 | 450 | 486 | 501 | 503 | 507 | 463 | 533 | 559 |
| hexane | -0.08 | 409 | 444 | 442 | 477 | 495 | 497 | 488 | 457 | 525 | 551 |
| anisole | 0.73 | 413 | 450 | 454 | 508 | 520 | 514 | 504 | 476 | 567 | 599 |
| pentane | -0.09 | 408 | 442 | 440 | 476 | 495 | 495 | 485 | 458 | 524 | 548 |
| nitrobenzene | 1.01 | 433 | 452 | 461 | 518 | 532 | 519 | 509 | 484 | 585 | 613 |
| diethyl carbonate | 0.45 | 402 | 444 | 447 | 495 | 510 | 500 | 493 | 466 | 542 | 575 |
| Compounds | π*1 | PP113 | PP123 | PP133 | PP143 | PP153 | PP163 | PP173 | PP183 | PP193 | PP203 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| diethyl ether | 0.27 | 384 | 404 | 424 | 462 | 480 | 474 | 467 | 442 | 502 | 531 |
| toluene | 0.54 | 388 | 408 | 429 | 474 | 486 | 485 | 476 | 451 | 520 | 552 |
| chloroform | 0.78 | 391 | 416 | 436 | 483 | 497 | 493 | 485 | 456 | 539 | 571 |
| THF | 0.58 | 387 | 411 | 428 | 476 | 490 | 479 | 472 | 448 | 518 | 553 |
| 1,4-dioxane | 0.55 | 385 | 406 | 426 | 471 | 486 | 474 | 471 | 444 | 512 | 549 |
| acetone | 0.71 | 385 | 410 | 429 | 479 | 493 | 477 | 471 | 447 | 526 | 555 |
| dichloromethane | 0.82 | 388 | 417 | 433 | 482 | 497 | 489 | 482 | 452 | 537 | 567 |
| DMSO | 1.00 | 391 | 425 | 435 | 492 | 506 | 484 | 481 | 458 | 554 | 584 |
| heptane | −0.08 | 379 | 400 | 422 | 457 | 473 | 476 | 468 | 439 | 478 | 523 |
| acetonitrile | 0.75 | 383 | 410 | 428 | 480 | 494 | 477 | 471 | 446 | 528 | 556 |
| DMF | 0.87 | 388 | 415 | 433 | 488 | 501 | 482 | 479 | 453 | 541 | 579 |
| ethyl acetate | 0.54 | 385 | 409 | 426 | 474 | 486 | 474 | 470 | 447 | 520 | 544 |
| p-xylene | 0.43 | 388 | 407 | 429 | 471 | 484 | 481 | 474 | 449 | 522 | 548 |
| 1,2-dichloroethane | 0.81 | 388 | 421 | 432 | 481 | 496 | 487 | 480 | 453 | 536 | 567 |
| dimethylacetamide | 0.88 | 388 | 412 | 432 | 489 | 500 | 485 | 478 | 454 | 543 | 573 |
| diglyme | 0.64 | 386 | 412 | 429 | 479 | 490 | 480 | 472 | 450 | 524 | 559 |
| cyclohexane | 0.00 | 382 | 402 | 423 | 459 | 474 | 478 | 471 | 441 | 481 | 527 |
| triethylamine | 0.14 | 384 | 403 | 424 | 461 | 476 | 478 | 471 | 443 | 489 | 530 |
| hexane | −0.08 | 378 | 400 | 422 | 455 | 471 | 474 | 467 | 437 | 476 | 521 |
| anisole | 0.73 | 389 | 415 | 432 | 481 | 495 | 487 | 482 | 454 | 530 | 570 |
| pentane | −0.09 | 378 | 399 | 420 | 454 | 470 | 474 | 465 | 436 | 474 | 520 |
| nitrobenzene | 1.01 | n.d.2 | n.d. 2 | 437 | 491 | 504 | 493 | 482 | 459 | 552 | 582 |
| diethyl carbonate | 0.45 | 384 | 405 | 426 | 471 | 484 | 475 | 470 | 445 | 510 | 541 |
| Dichloromethane | ||||||||||
| Compounds | PP1 | PP2 | PP3 | PP4 | PP5 | PP6 | PP7 | PP8 | PP9 | PP10 |
| excitation (nm) | 411 | 448 | 456 | 510 | 524 | 524 | 505 | 476 | 571 | 603 |
| emission (nm) | - | 512 | 499 | - | 558 | 617 | - | - | 654 | - |
| Stokes shift (nm) | - | 64 | 43 | - | 34 | 103 | - | - | - | - |
| Compounds | PP11 | PP12 | PP13 | PP14 | PP15 | PP16 | PP17 | PP18 | PP19 | PP20 |
| excitation (nm) | 388 | 417 | 433 | 482 | 497 | 489 | 482 | 452 | 537 | 567 |
| emission (nm) | - | - | - | 545 | - | 596 | - | - | - | 623 |
| Stokes shift (nm) | - | - | - | 63 | - | 107 | - | - | - | 56 |
| Toluene | ||||||||||
| Compounds | PP1 | PP2 | PP3 | PP4 | PP5 | PP6 | PP7 | PP8 | PP9 | PP10 |
| excitation (nm) | 405 | 450 | 441 | 501 | 512 | 509 | 501 | 473 | 553 | 582 |
| emission (nm) | - | 487 | 495 | 546 | 546 | 574 | - | - | 613 | 626 |
| Stokes shift (nm) | - | 37 | 54 | 45 | 34 | 65 | - | - | 60 | 44 |
| Compounds | PP11 | PP12 | PP13 | PP14 | PP15 | PP16 | PP17 | PP18 | PP19 | PP20 |
| excitation (nm) | 388 | 408 | 429 | 474 | 486 | 485 | 476 | 451 | 520 | 552 |
| emission (nm) | - | 467 | - | 530 | - | 558 | - | - | - | 604 |
| Stokes shift (nm) | - | 59 | - | 56 | - | 73 | - | - | - | 52 |
| Compounds | Ered (V/Fc) | EOx (V/Fc) | EHOMO (eV) | ELUMO (eV) | ΔEel (eV)1 | ΔEopt (eV)2 |
|---|---|---|---|---|---|---|
| PP1 | −1.30 | 1.19 | −5.99 | −3.50 | 2.49 | 3.07 |
| PP2 | −1.26 | 1.18 | −5.98 | −3.54 | 2.44 | 2.82 |
| PP3 | −1.36 | 1.15 | −5.95 | −3.44 | 2.51 | 2.76 |
| PP4 | −1.33 | 0.68 | −5.48 | −3.47 | 2.01 | 2.45 |
| PP5 | −1.40 | 0.63 | −5.43 | −3.40 | 2.03 | 2.37 |
| PP6 | −1.32 | 0.79 | −5.59 | −3.48 | 2.11 | 2.46 |
| PP7 | −1.26 | 0.84 | −5.64 | −3.54 | 2.10 | 2.50 |
| PP8 | −1.38 | 0.95 | −5.75 | −3.42 | 2.33 | 2.63 |
| PP9 | −1.22 | 0.49 | −5.29 | −3.58 | 1.71 | 2.19 |
| PP10 | −1.30 | 0.41 | −5.21 | −3.50 | 1.71 | 2.08 |
| PP11 | −1.46 | 1.19 | −5.99 | −3.34 | 2.65 | 3.24 |
| PP12 | −1.44 | 1.18 | −5.98 | −3.36 | 2.62 | 3.02 |
| PP13 | −1.50 | 1.23 | −6.03 | −3.30 | 2.73 | 2.90 |
| PP14 | −1.56 | 0.65 | −5.45 | −3.24 | 2.21 | 2.58 |
| PP15 | −1.30 | 0.54 | −5.34 | −3.50 | 1.84 | 2.51 |
| PP16 | −1.44 | 0.77 | −5.57 | −3.36 | 2.21 | 2.60 |
| PP17 | −1.29 | 0.85 | −5.65 | −3.51 | 2.14 | 2.63 |
| PP18 | −1.52 | 0.94 | −5.74 | −3.28 | 2.46 | 2.78 |
| PP19 | −1.30 | 0.49 | −5.29 | −3.50 | 1.79 | 2.35 |
| PP20 | −1.30 | 0.36 | −5.16 | −3.50 | 1.66 | 2.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigot, C.; Noirbent, G.; Bui, T.-T.; Péralta, S.; Gigmes, D.; Nechab, M.; Dumur, F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials 2019, 12, 1342. https://doi.org/10.3390/ma12081342
Pigot C, Noirbent G, Bui T-T, Péralta S, Gigmes D, Nechab M, Dumur F. Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials. 2019; 12(8):1342. https://doi.org/10.3390/ma12081342
Chicago/Turabian StylePigot, Corentin, Guillaume Noirbent, Thanh-Tuân Bui, Sébastien Péralta, Didier Gigmes, Malek Nechab, and Frédéric Dumur. 2019. "Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications" Materials 12, no. 8: 1342. https://doi.org/10.3390/ma12081342
APA StylePigot, C., Noirbent, G., Bui, T.-T., Péralta, S., Gigmes, D., Nechab, M., & Dumur, F. (2019). Push-Pull Chromophores Based on the Naphthalene Scaffold: Potential Candidates for Optoelectronic Applications. Materials, 12(8), 1342. https://doi.org/10.3390/ma12081342









