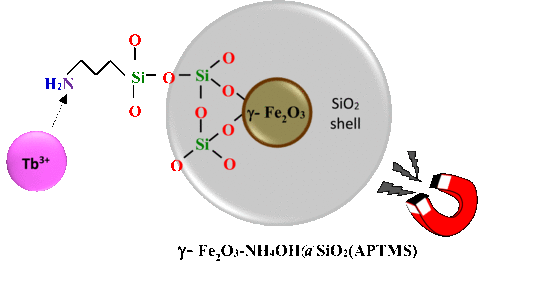
Terbium Ion Adsorption from Aqueous Solution by Using Magnetic γ-Fe2O3-NH4OH@SiO2 Nanoparticles Functionalized with Amino Groups
Abstract
:1. Introduction
2. Results and Discussion
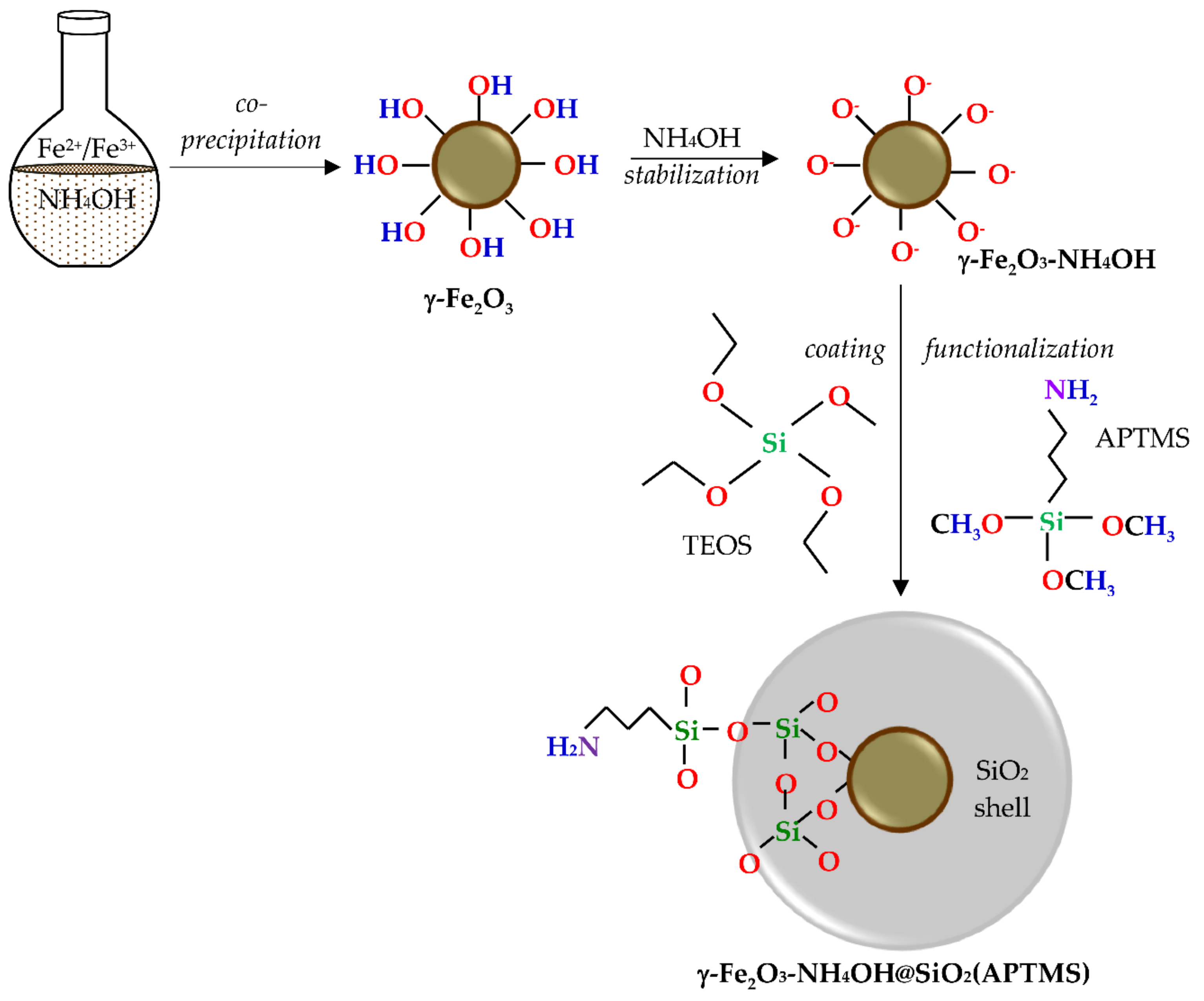
2.1. γ-Fe2O3-NH4OH@SiO2(APTMS) NPs
2.2. Characterization of γ-Fe2O3-NH4OH@SiO2(APTMS) NPs
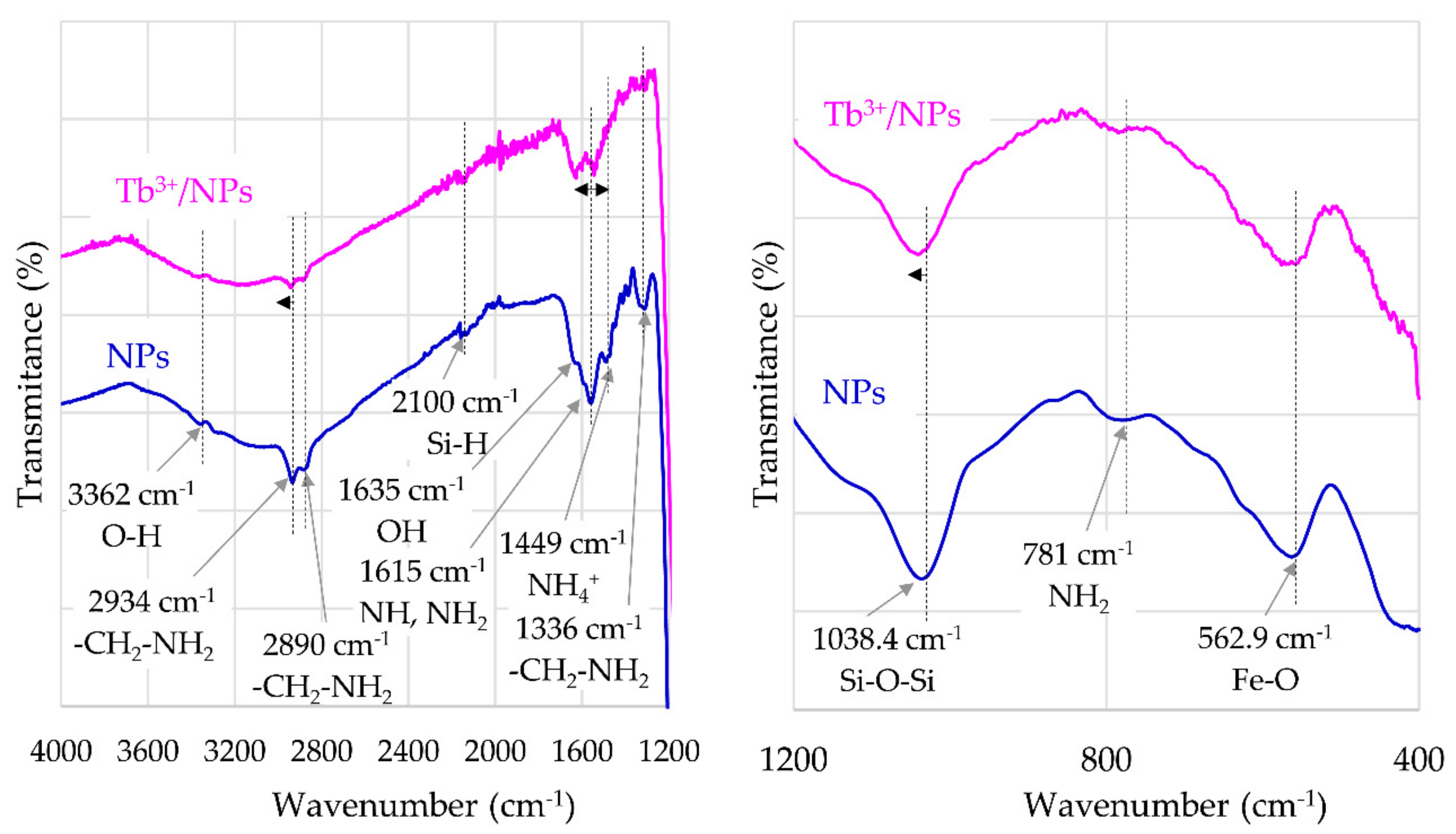
2.2.1. Structural Study
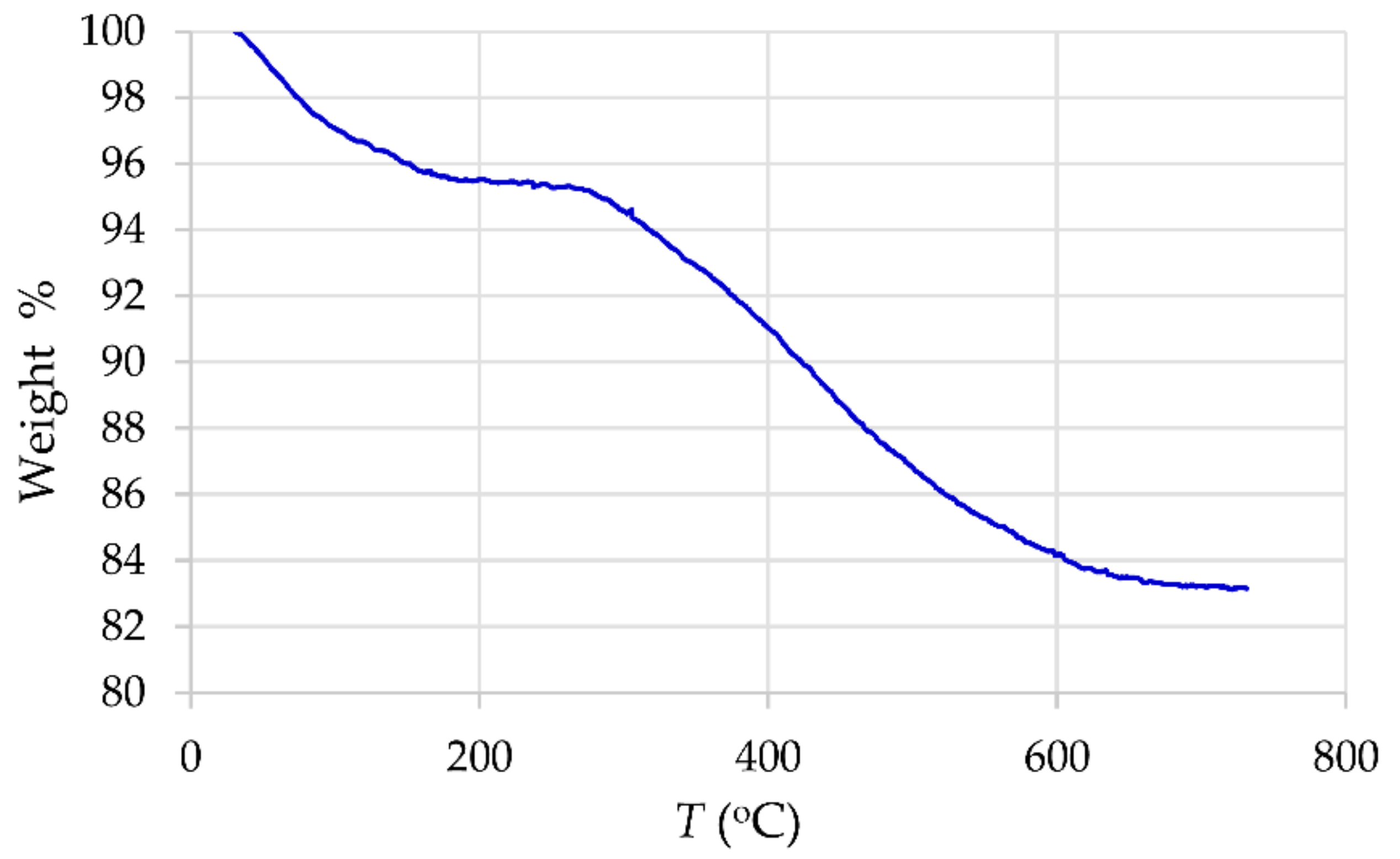
2.2.2. Thermogravimetric Analysis
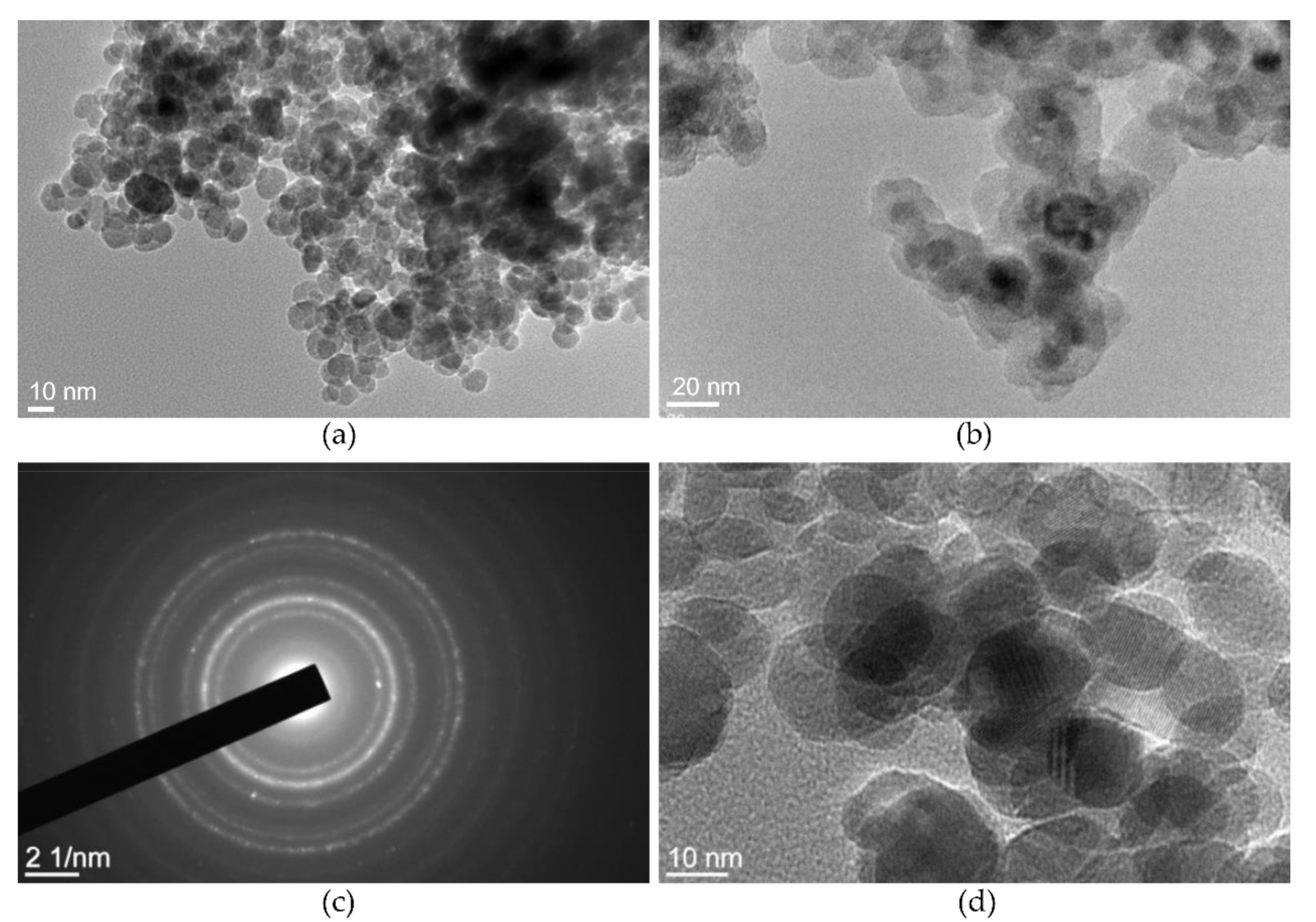
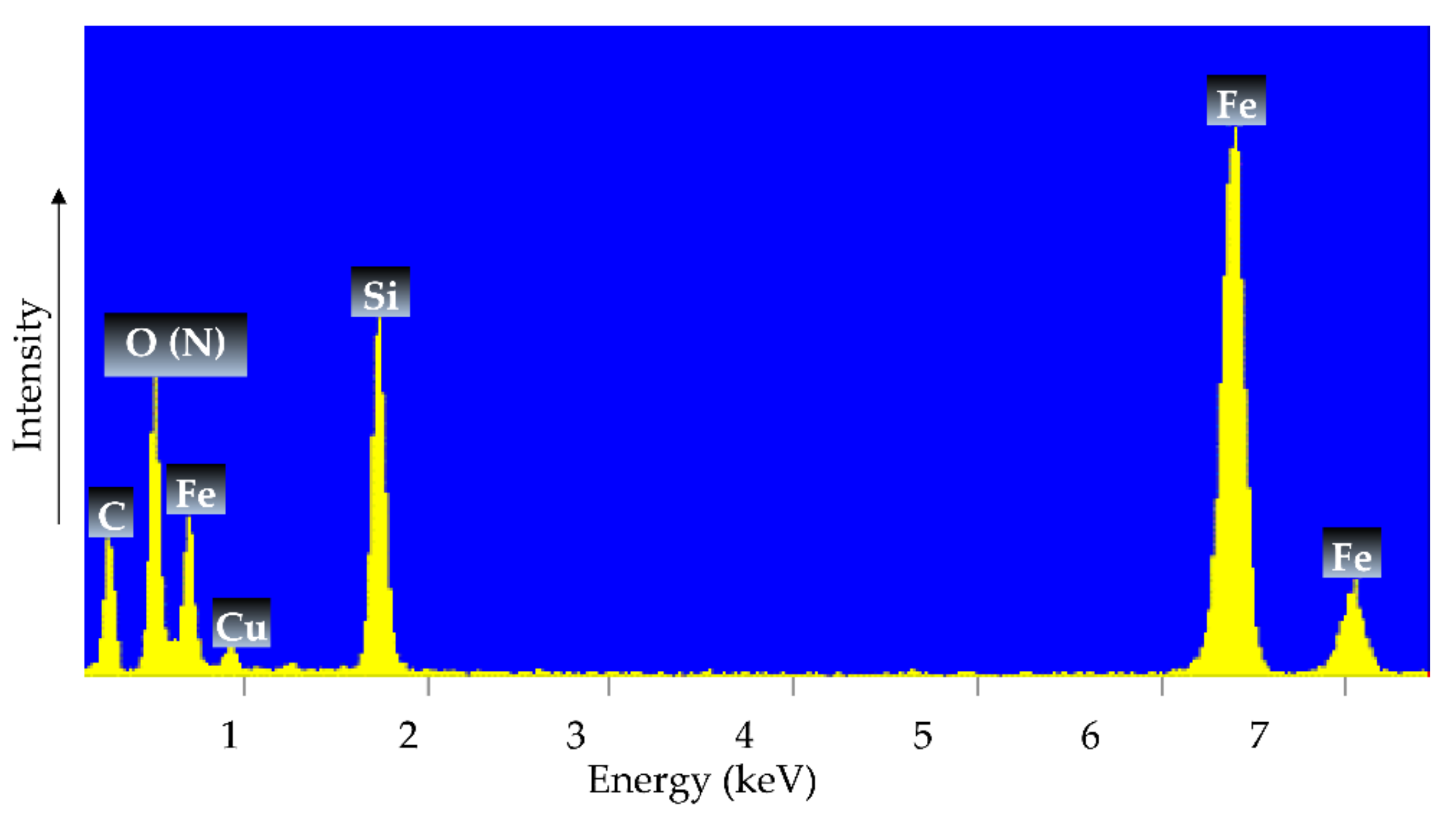
2.2.3. Morphological and Compositional Study
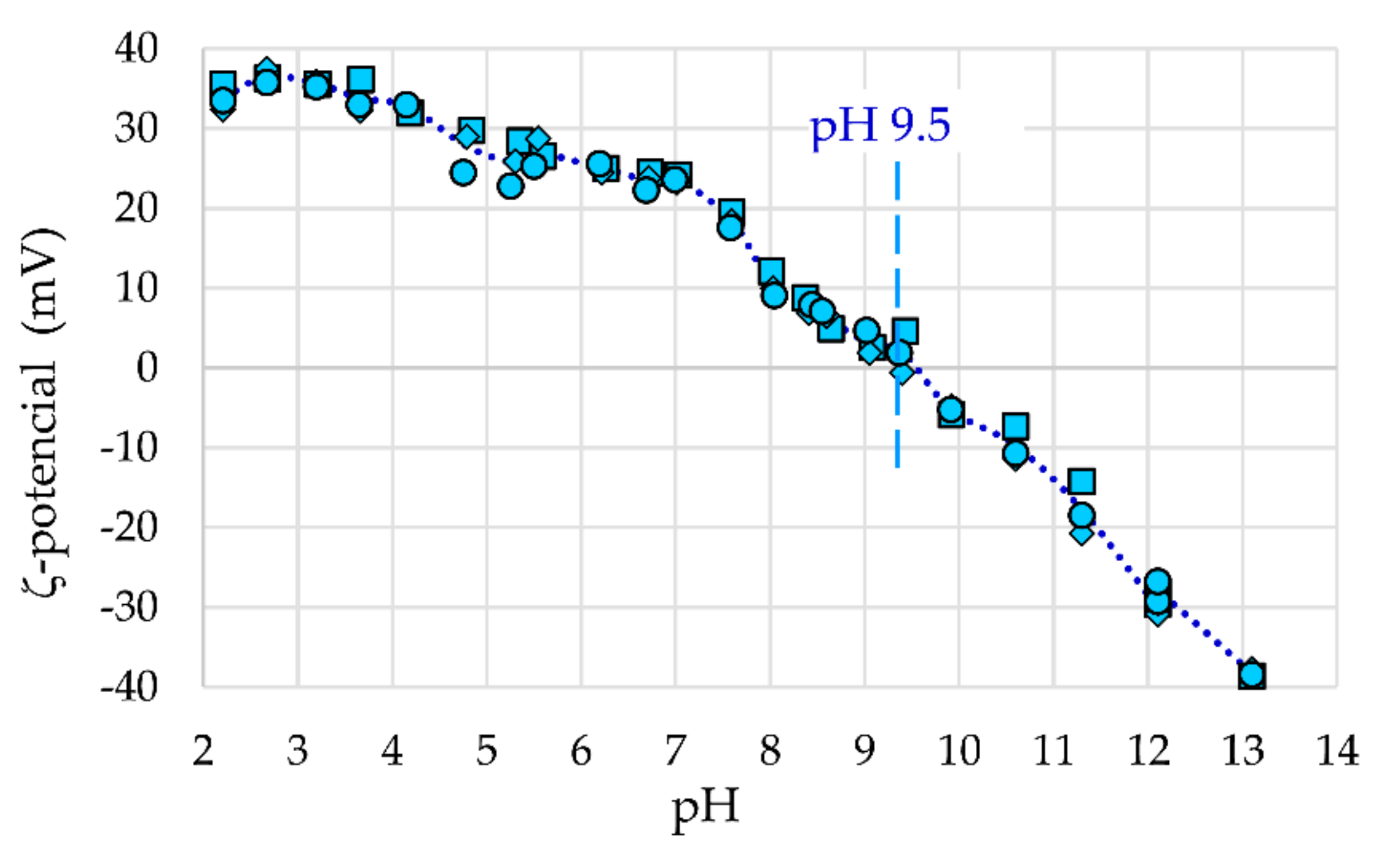
2.2.4. Surface Area and Charge Study
2.2.5. Magnetic Study
2.3. Adsorption Study
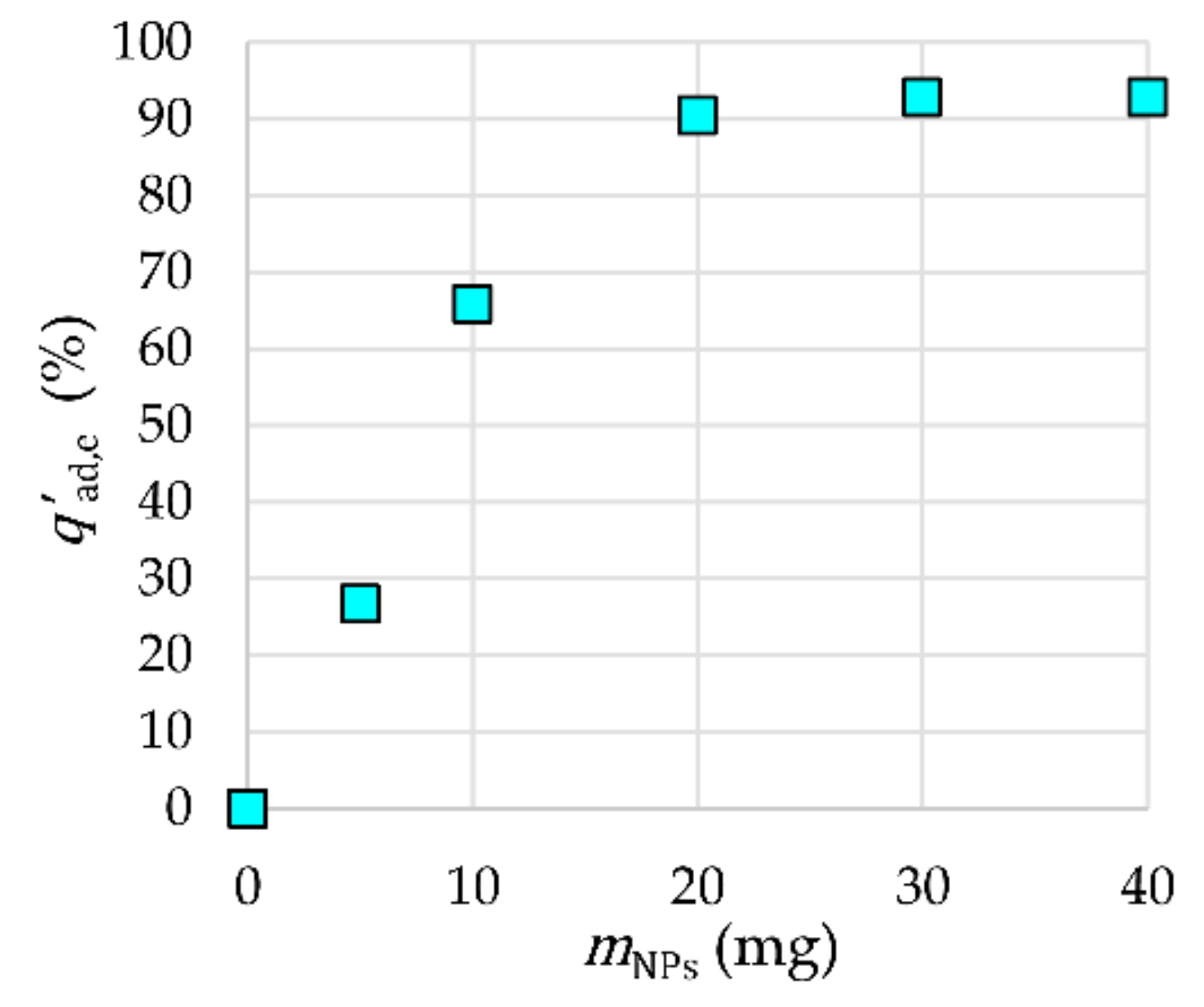
2.3.1. NP Mass Influence
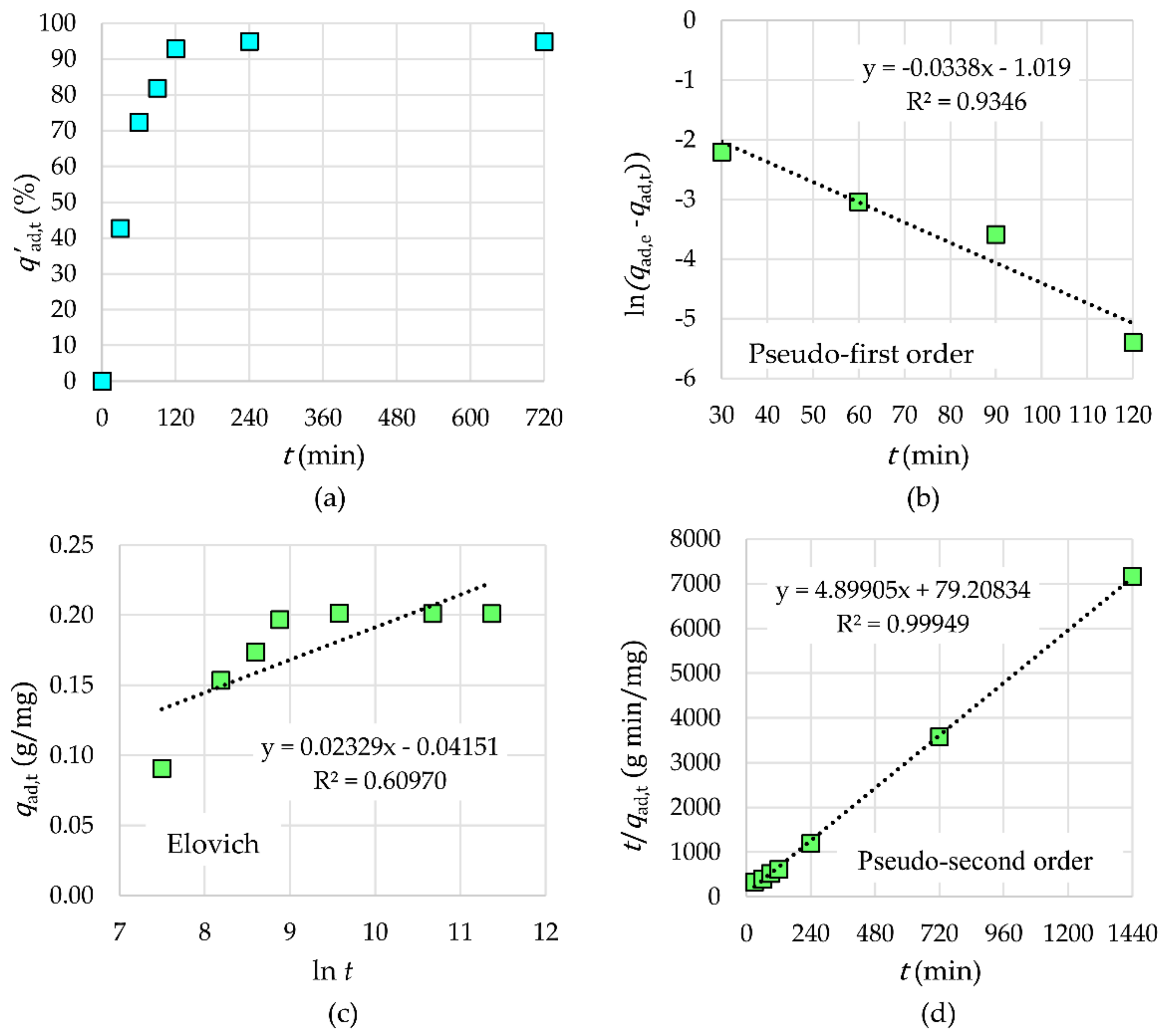
2.3.2. Kinetic Study
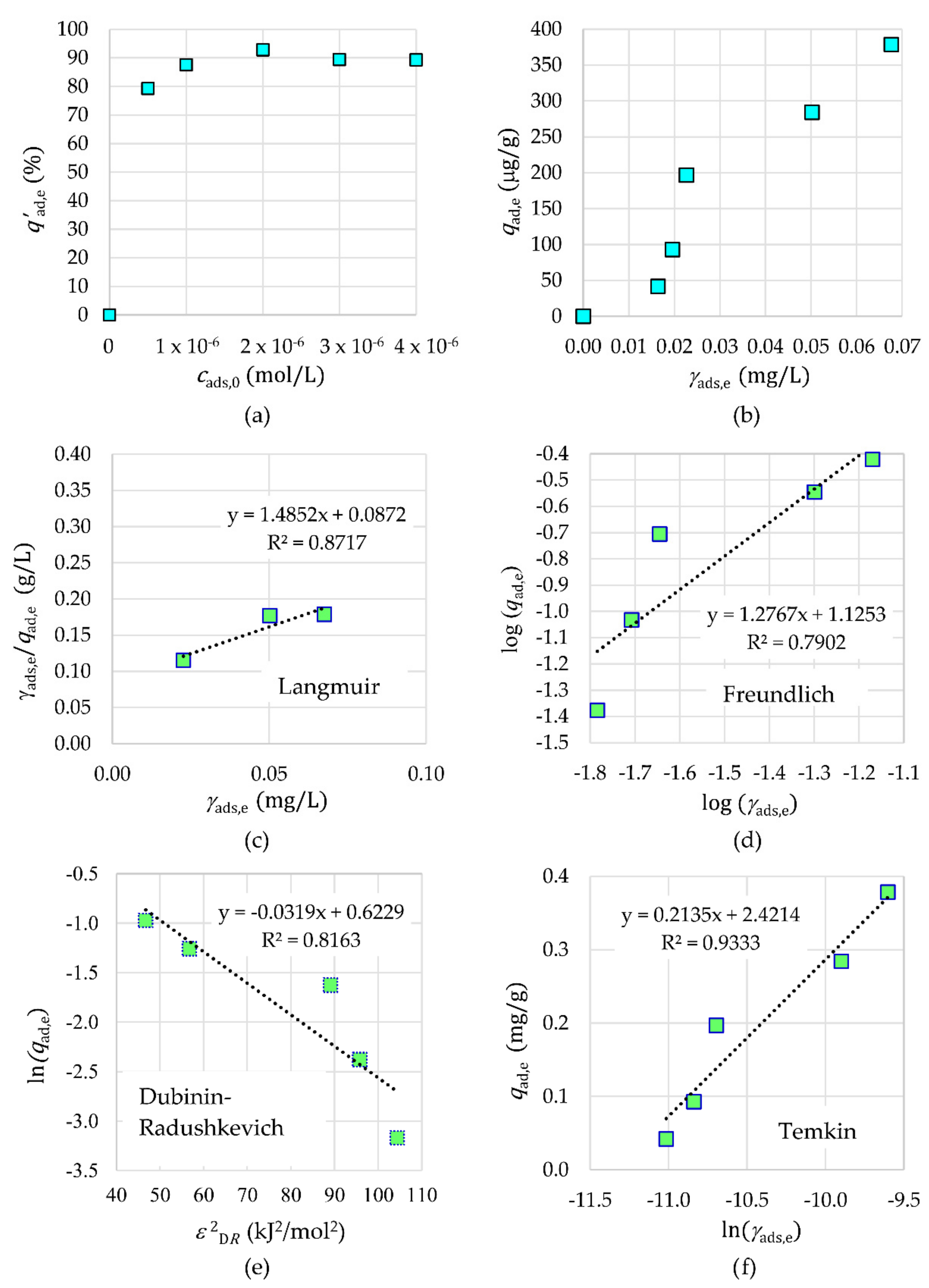
2.3.3. Adsorption Isotherms
2.3.4. Thermodynamic Study
3. Materials and Methods
3.1. Materials
3.2. Synthesis of γ-Fe2O3-NH4OH@SiO2(APTMS) NPs
3.3. Characterization and Adsorption Techniques
3.4. Procedure for Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Lima, I.B.; Leal Filho, W. Rare Earths Industry: Technological, Economic, and Environmental Implications; Elsevier Publisher: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, Mines, Magnets. Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Charalampides, G.; Vatalis, K.I.; Apostolos, B.; Ploutarch-Nikola, B. Rare earth elements: Industrial applications and economics dependency of Europe. Procedia Econ. Finance 2015, 24, 126–135. [Google Scholar] [CrossRef]
- Migaszewskia, Z.M.; Gałuszka, A. The characteristics, occurrence, and geochemical behavior of rare earth elements in the environment: A review. Crit. Rev. Env. Sci. Technol. 2015, 45, 429–471. [Google Scholar] [CrossRef]
- Golev, A.; Scott, M.; Erskine, P.D.; Ali, A.H.; Ballantyne, G.R. Rare earths supply chains: Current status, constraints and opportunities. Resour. Policy 2014, 41, 52–59. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Rare earths and the balance problem. J. Sustain. Metall. 2015, 1, 29–38. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Zhao, Y.; Zhang, C.; Wei, Y. Rare earth elements supply vs. clean energy technologies: New problems to be solve. Mineral Resour. Manag. 2016, 32, 29–44. [Google Scholar] [CrossRef]
- Shen, L.; Wu, N.; Zhong, S.; Gao, L. Overview on China’s rare earth industry restructuring and regulation reforms. J. Resour. Ecol. 2017, 8, 213–222. [Google Scholar]
- Zhang, S. On problem in rare earth exports of China and countermeasures. Int. Bus. Manag. 2013, 6, 21–25. [Google Scholar]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare earth elements: Overview of mining, mineralogy, uses, sustainability and environmental impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergorić, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical unit operations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Bhatnagar, A.; Lima, E.C. Adsorption of rare earth metals: A review of recent literature. J. Mol. Liq. 2016, 221, 954–962. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, H.; Tanaka, M. Adsorption mechanism of rare earth elements by adsorbents with diglycolamic acid ligands. Hydrometallurgy 2016, 163, 156–160. [Google Scholar] [CrossRef]
- Roosen, J.; Binnemans, K. Adsorption and chromatographic separation of rare earths with EDTA and DTPA functionalized chitosan biopolymers. J. Mater. Chem. A 2014, 2, 1530–1540. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. Adsorption properties toward trivalent rare earths by alginate beads doping with silica. Ind. Eng. Chem. Res. 2013, 52, 3453–3461. [Google Scholar] [CrossRef]
- Ramasamy, D.L.; Repo, E.; Srivastava, V.; Sillanpää, M. Chemically immobilized and physically adsorbed PAN/acetylacetone modified mesoporous silica for the recovery of rare earth elements from the waste water-comparative and optimization study. Water Res. 2017, 114, 264–276. [Google Scholar] [CrossRef]
- Ms, A.R.; Arm, A.R. Removal of heavy metals from industrial waste water by biomass based materials: A review. J. Pollut. Eff. Cont. 2017, 5, 1–13. [Google Scholar]
- Fisher, A.; Kara, D. Determination of rare earth elements in natural water samples—A review of sample separation, preconcentration and direct methodologies. Anal. Chim. Acta 2016, 935, 1–29. [Google Scholar] [CrossRef]
- Patra, S.; Roy, E.; Madhuri, R.; Sharma, P.K. Removal and recycling of precious rare earth elements from wastewater samples using imprinted magnetic ordered mesoporous carbon. ACS Sustain. Chem. Eng. 2017, 5, 6910–6923. [Google Scholar] [CrossRef]
- Zhang, H.; McDowell, R.G.; Martin, L.R.; Qiang, Y. Selective extraction of heavy and light lanthanides from aqueous solution by advanced magnetic nanosorbents. ACS Appl. Mater. Inter. 2016, 8, 9523–9531. [Google Scholar] [CrossRef]
- Jafarizadeh-Malmiri, H.; Sayyar, Z.; Anarjan, N.; Berenjian, A. Nanobiotechnology in Food: Concepts, Applications and Perspectives; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Li, X.; Zhang, F.; Zhao, D. Highly efficient lanthanide upconverting nanomaterials: Progress and challenges. Nano Today 2013, 8, 643–676. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, J.; Pan, F.; Zhou, H.; Yang, X.; Li, W.; Liu, H. O-carboxymethyl chitosan entrapped by silica: Preparation and adsorption behavior toward neodymium (III) ions. J. Chem. Technol. Biotechnol. 2013, 88, 317–325. [Google Scholar] [CrossRef]
- Ogata, T.; Narita, H.; Zanaka, M. Adsorption behavior of rare earth elements on silica gel modified with diglycol amic acid. Hydrometallurgy 2015, 152, 178–182. [Google Scholar] [CrossRef]
- Vázquez, O.F.G.; Virgen, M.R.M.; Montoya, V.H.; Gómez, R.T.; Flores, J.L.A.; Cruz, M.A.P.; Morán, M.A.M. Adsorption of heavy metals in the presence of a magnetic fields on adsorbents with different magnetic properties. Ind. Eng. Chem. Res. 2016, 55, 9323–9331. [Google Scholar] [CrossRef]
- Rajczykowski, K.; Loska, K. Stimulation of heavy metal adsorption process by using a strong magnetic field. Water Air Pollut. 2018, 229, 1–7. [Google Scholar] [CrossRef]
- Tang, S.C.N.; Yan, D.Y.S.; Lo, M.C. Sustainable wastewater treatment using microsized magnetic hydrogel with magnetic separation technology. Ind. Eng. Chem. Res. 2014, 53, 15718–15724. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite. Chem. Eng. J. 2017, 320, 151–159. [Google Scholar] [CrossRef]
- Zhu, G.; Xin, S.; Wen, Y.; Wang, Q.; Que, M.; Wang, Y. Warm white generation from a single phased phosphor Sr10[(PO4)5.5(BO4)0.5](BO2): Eu2+, Mn2+, Tb3+ for light emitting diodes. RCS Adv. 2013, 3, 9311–9318. [Google Scholar] [CrossRef]
- Nelli, I.; Kaczmarek, A.K.; Locardi, F.; Caratto, V.; Costa, G.A.; Deun, R.V. Multidoped Ln3+ gadolinium dioxycarbonates as tunable white light emitting phosphors. Dalton Trans. 2017, 46, 2785–2792. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, X.; Hua, W.; Shen, L.; Yu, X.; Wang, X. Electrospun poly(acrylic acid)/silica hydrogel nanofibers scaffold for highly efficient adsorption of lanthanide ions and its photoluminescence performance. ACS Appl. Mater. Inter. 2016, 8, 23995–24007. [Google Scholar] [CrossRef]
- Yesiller, S.U.; Eroğlu, A.E.; Shahwan, T. Removal of aqueous rare earth elements (REEs) using nano-iron based materials. J. Ind. Eng. Chem. 2013, 19, 898–907. [Google Scholar] [CrossRef]
- Wang, J. Adsorption of aqueous neodymium, europium, gadolinium, terbium, and yttrium ions onto nZVI-montmorillonite: Kinetics, thermodynamic mechanism, and the influence of coexisting ions. Environ. Sci. Pollut. Res. 2018, 25, 33521–33537. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.S.; Grandhi, S. Sorption studies of yttrium(III) ions on nano maghemite. J. Environ. Chem. Eng. 2016, 4, 4719–4730. [Google Scholar] [CrossRef]
- Čakara, D.; Fras, L.; Bračič, M.; Kleinschek, K.S. Protonation behavior of cotton fabric with irreversibly adsorbed chitosan: A potentiometric titration study. Carbohydr. Polym. 2009, 78, 36–40. [Google Scholar] [CrossRef]
- Topel, S.D.; Legaria, E.P.; Tiseanu, C.; Rocha, J.; Nedelec, J.M.; Kessler, V.G.; Seisenbaeva, G.A. Hybrid silica nanoparticles for sequestration and luminescence detection of trivalent rare-earth ions (Dy3+ and Nd3+) in solution. J. Nanopart. Res. 2014, 16, 2783. [Google Scholar] [CrossRef]
- Legaria, E.P.; Topel, S.D.; Kessler, V.G.; Seisenbaeva, G.A. Molecular insight into the selective action of a magnetically removable complexone-grafted adsorbent. Dalton Trans. 2015, 44, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.; Born, P.; Weber, A.; Kraus, T. Robust, ultrasmall organosilica nanoparticles without silica shells. J. Nanopart. Res. 2014, 16, 2462. [Google Scholar] [CrossRef]
- Sattler, K.D. Silicon Nanomaterials Sourcebook: Low-Dimensional Structures, Quantum Dots, and Nanowires; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Košak, A.; Bauman, M.; Lobnik, A. Adsorption of Mercury(II), Lead(II), Cadmium(II) and Zinc(II) from Aqueous Solutions Using Mercapto-Modified Silica Particles. Int. J. Appl. Ceram. Technol. 2015, 12, 461–472. [Google Scholar] [CrossRef]
- Khodabakhshi, M.; Bahari, A. Investigation and characterization of maghemite (γ-Fe2O3) nanoparticles and its cytotoxicity studies. Indian. J. Pharm. Educ. 2017, 51, 295–301. [Google Scholar] [CrossRef]
- Nurdin, I.; Yaacob, I.I.; Johan, M.R.; Ang, B.C. Characterization and stability monitoring of maghemite nanoparticle suspensions. Adv. Mat. Res. 2012, 576, 398–401. [Google Scholar] [CrossRef]
- Zhao, F.; Repo, E.; Song, Y.; Yin, D.; Hammouda, S.B.; Chen, L.; Kalloola, S.; Tang, J.; Tam, K.C.; Sillanpää, M. Polyethylenimine-cross-linked cellulose nanocrystals for highly efficient recovery of rare earth elements from water and a mechanism study. Green. Chem. 2017, 19, 4186–4828. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Silanpää, M. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich isotherms studies of equilibrium sorption of Zn2+ onto phosphoric acid modified rice husk. IOSR J. Appl. Chem. 2012, 3, 38–45. [Google Scholar]
- Foo, K.Y.; Hameed, B.H. Insight into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Koohaki-Mohammadpour, S.M.A.; Torab-Mostaedi, M.; Talebizadeh-Rafsanjani, A.; Naderi-Behdani, F. Adsorption isotherm, kineticm thermodynamic, and desorption studies of lanthanum and dysprosium on oxidized multiwalled carbon nanotubes. J. Disper. Sci. Technol. 2014, 35, 244–254. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Salmiati, S.; Jonbi, J.; Fulazzaky, M.A. Application of the kinetic and isotherm models for better understanding of the behavior of silver nanoparticles adsorption onto different adsorbents. J. Environ. Manag. 2018, 218, 59–70. [Google Scholar] [CrossRef]




| Tb3+/NPs | Temperature (°C) | ||
|---|---|---|---|
| 25 | 50 | 75 | |
| ΔG0 (J/mol) | −39,606 | −43,900 | −47,307 |
| ΔH0 (J/mol) | 6164 | 6164 | 6164 |
| ΔS0 (J/(mol·K)) | 154 | 154 | 154 |
| Kinetic Model | Nonlinear Form | Linear Form | Linear Plot |
|---|---|---|---|
| Pseudo-first order | |||
| Elovich | |||
| Pseudo-second order |
| Adsorption Isotherm | Nonlinear Form | Linear Form | Linear Plot |
|---|---|---|---|
| Langmuir | |||
| Freundlich | |||
| Dubinin–Radushkevich | , | ||
| Temkin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kegl, T.; Košak, A.; Lobnik, A.; Ban, I. Terbium Ion Adsorption from Aqueous Solution by Using Magnetic γ-Fe2O3-NH4OH@SiO2 Nanoparticles Functionalized with Amino Groups. Materials 2019, 12, 1294. https://doi.org/10.3390/ma12081294
Kegl T, Košak A, Lobnik A, Ban I. Terbium Ion Adsorption from Aqueous Solution by Using Magnetic γ-Fe2O3-NH4OH@SiO2 Nanoparticles Functionalized with Amino Groups. Materials. 2019; 12(8):1294. https://doi.org/10.3390/ma12081294
Chicago/Turabian StyleKegl, Tina, Aljoša Košak, Aleksandra Lobnik, and Irena Ban. 2019. "Terbium Ion Adsorption from Aqueous Solution by Using Magnetic γ-Fe2O3-NH4OH@SiO2 Nanoparticles Functionalized with Amino Groups" Materials 12, no. 8: 1294. https://doi.org/10.3390/ma12081294
APA StyleKegl, T., Košak, A., Lobnik, A., & Ban, I. (2019). Terbium Ion Adsorption from Aqueous Solution by Using Magnetic γ-Fe2O3-NH4OH@SiO2 Nanoparticles Functionalized with Amino Groups. Materials, 12(8), 1294. https://doi.org/10.3390/ma12081294