Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Pure TiO2 and Ce-doped TiO2 Nanostructures
2.2. Characterization
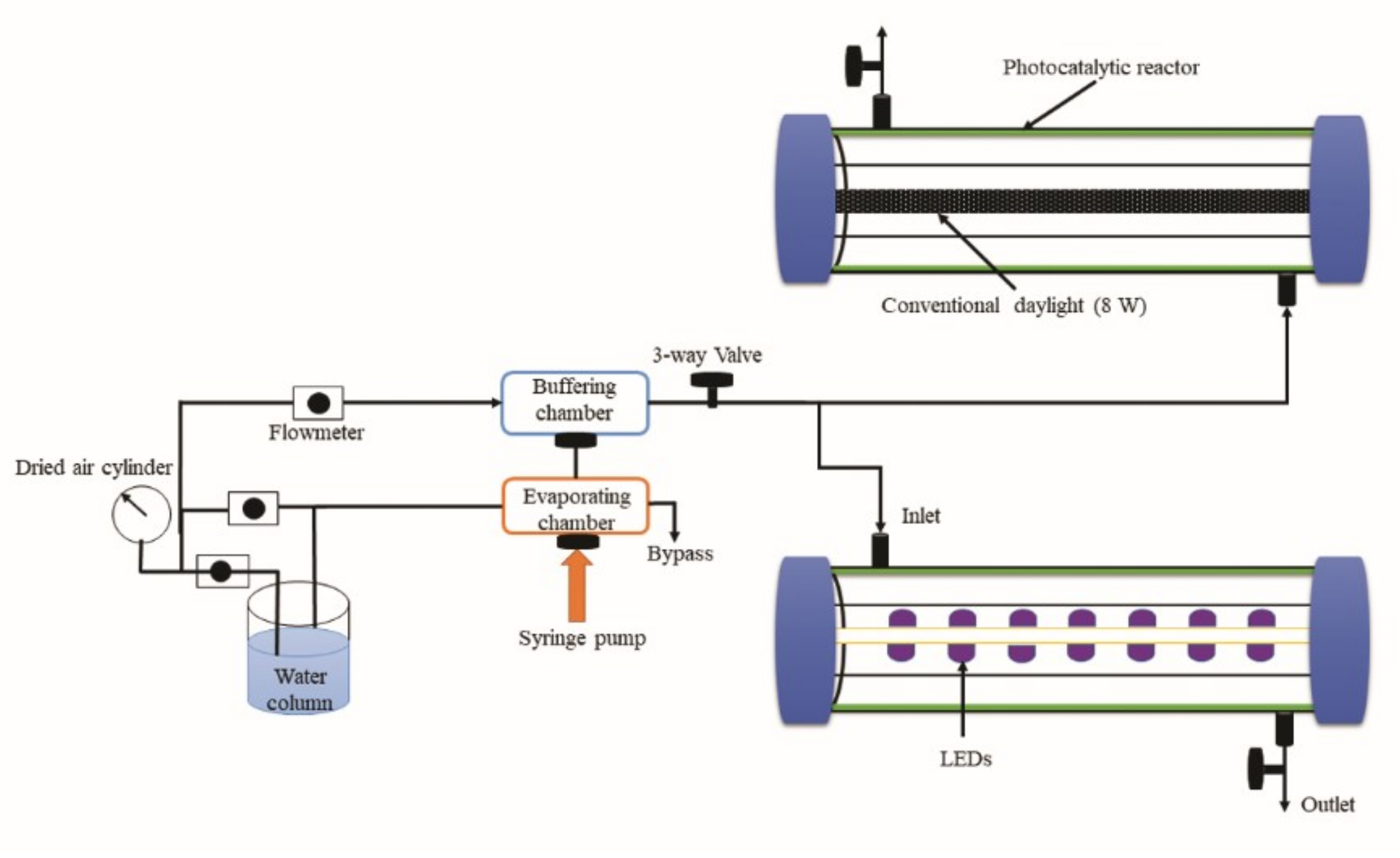
2.3. Photocatalytic Reactor System and Degradation of Target Compounds
3. Results and Discussion
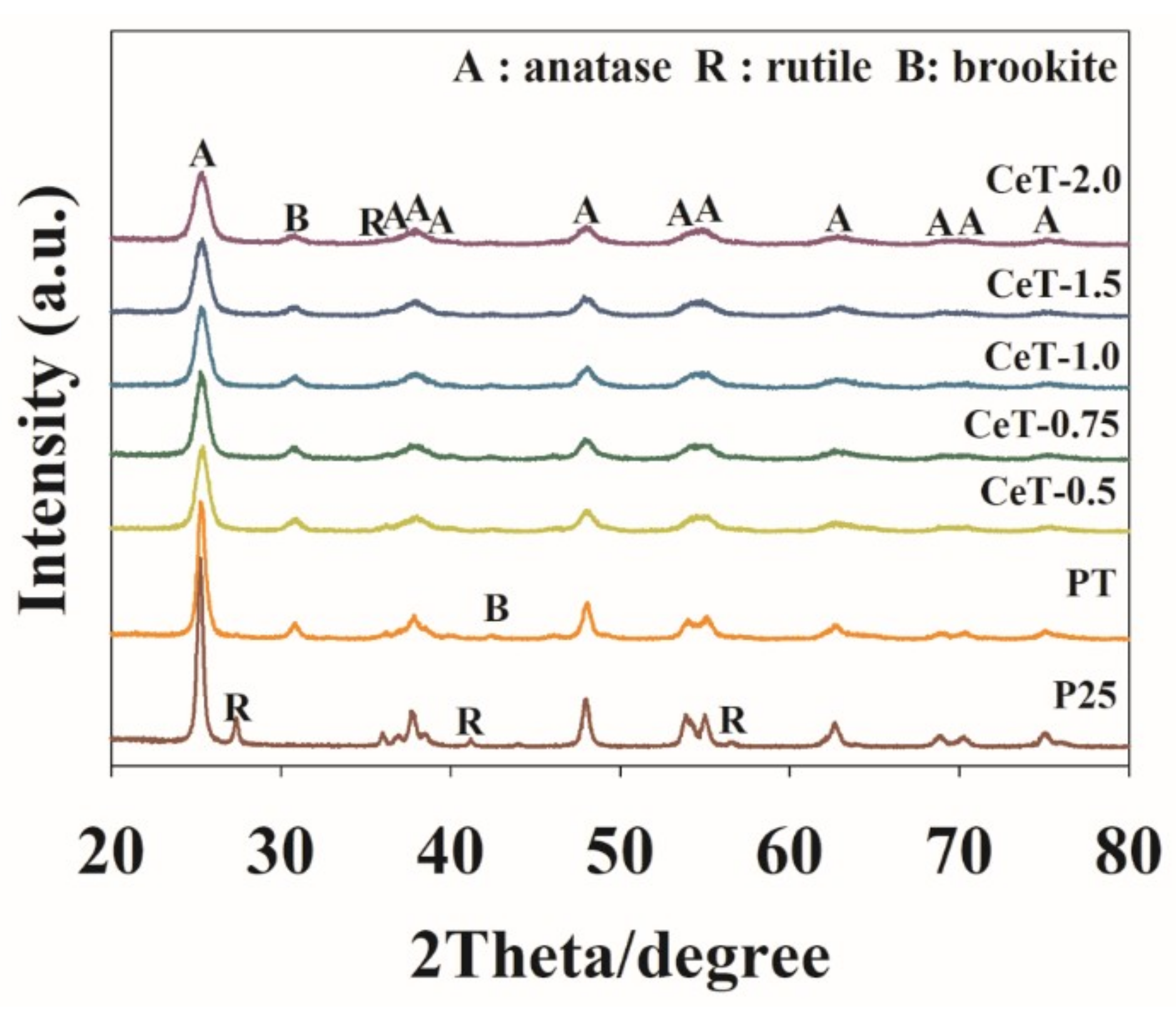
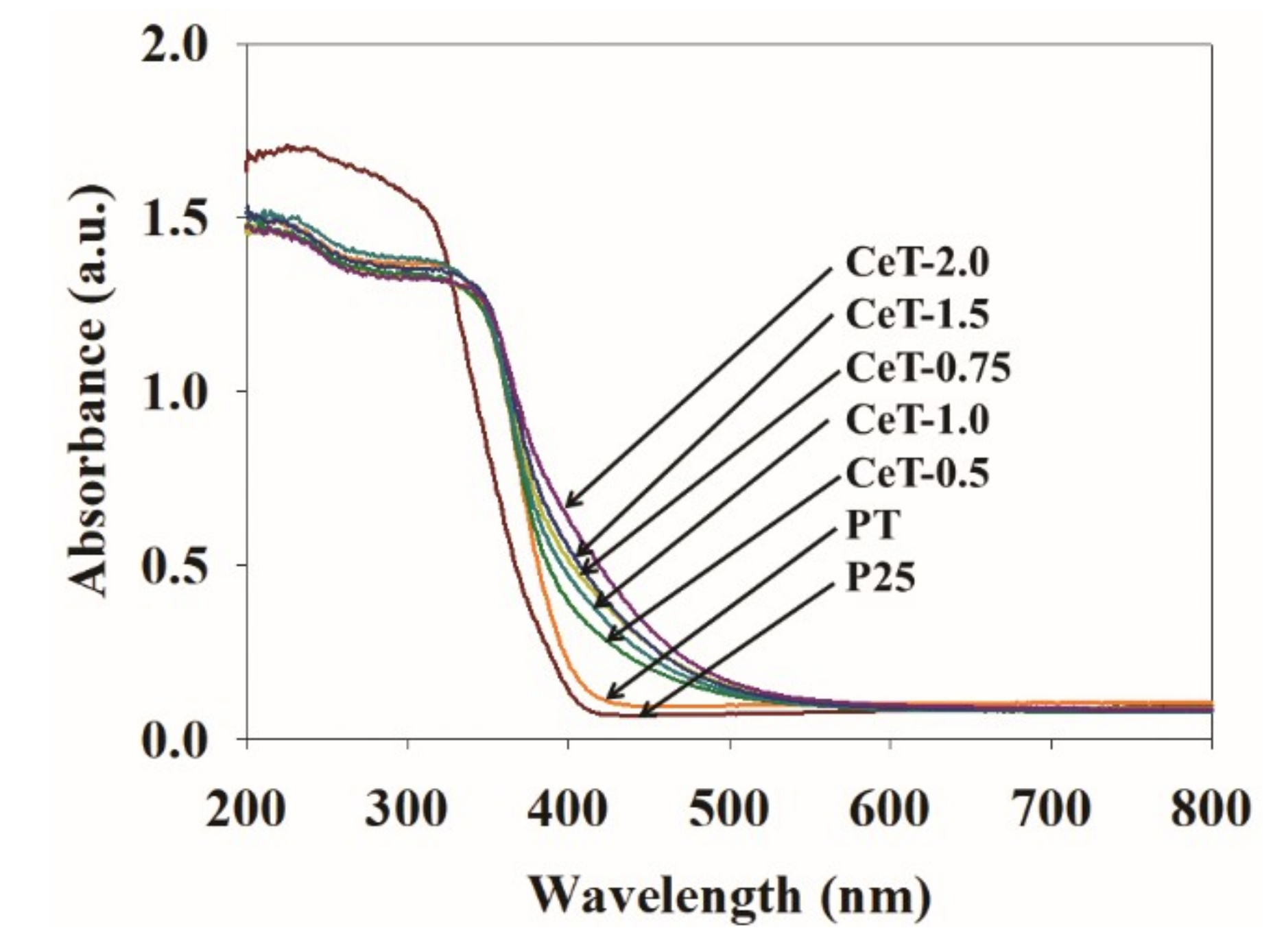
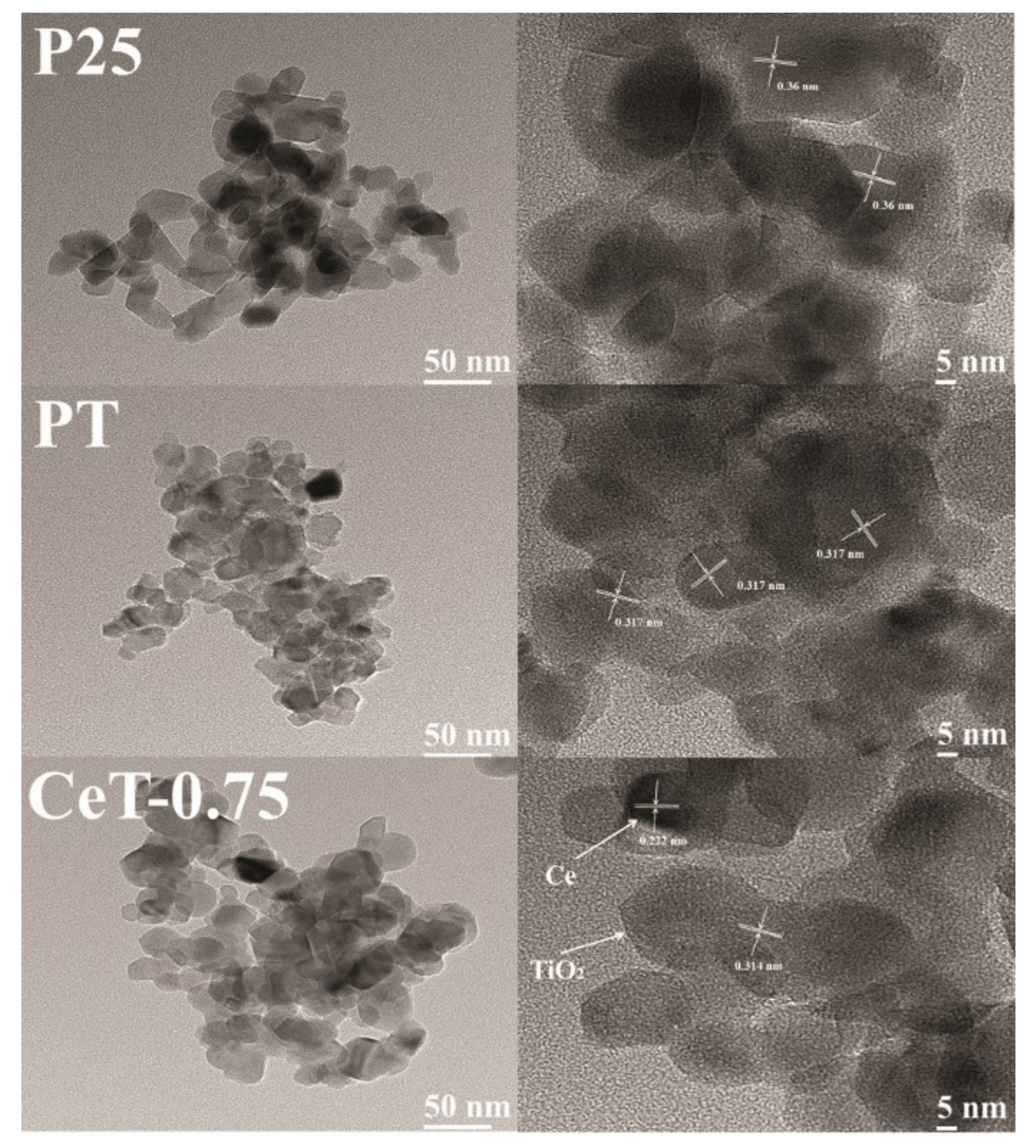
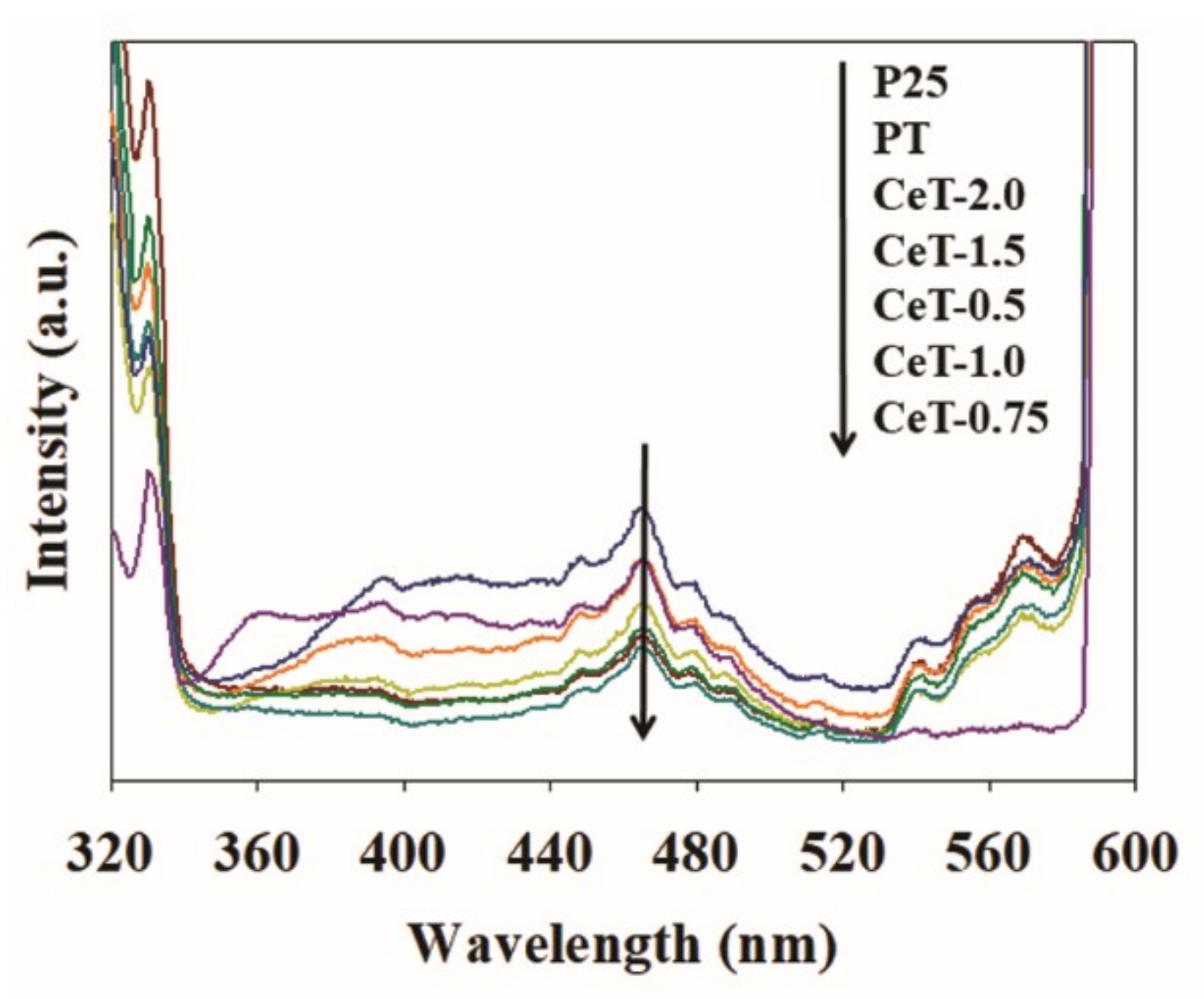
3.1. Characterization of the Fabricated Catalysts
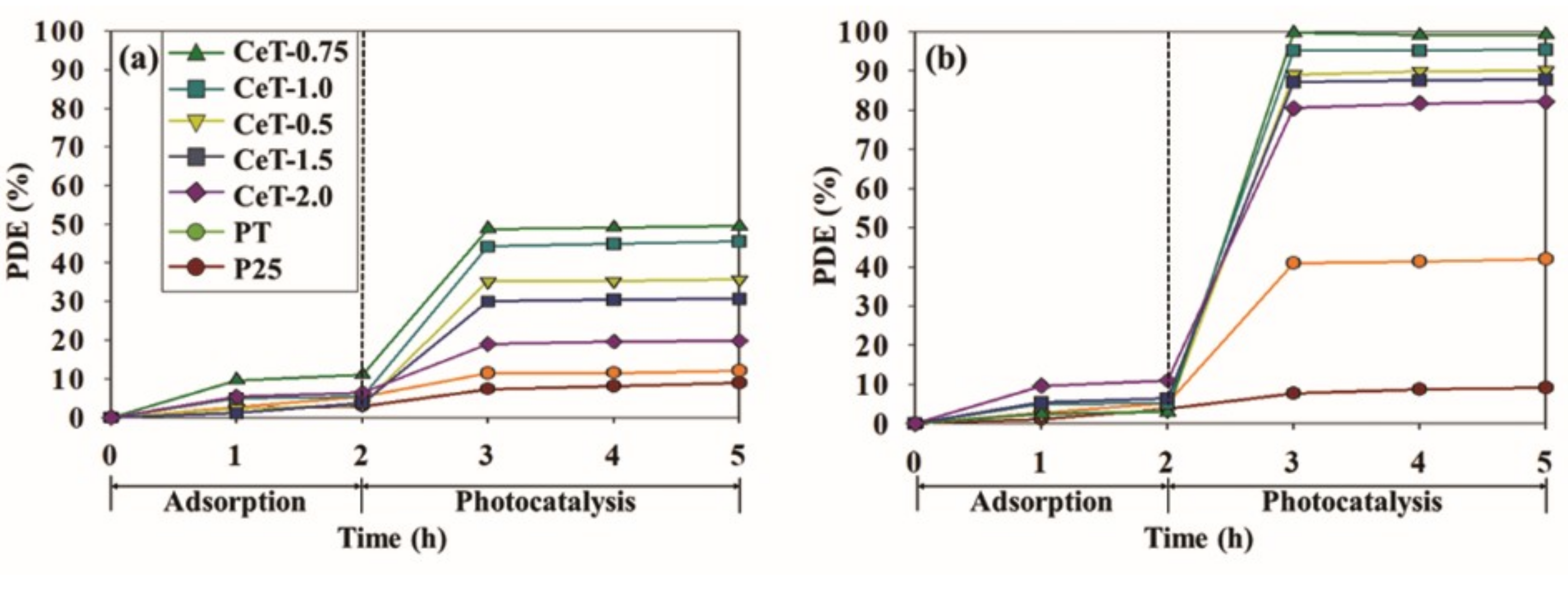
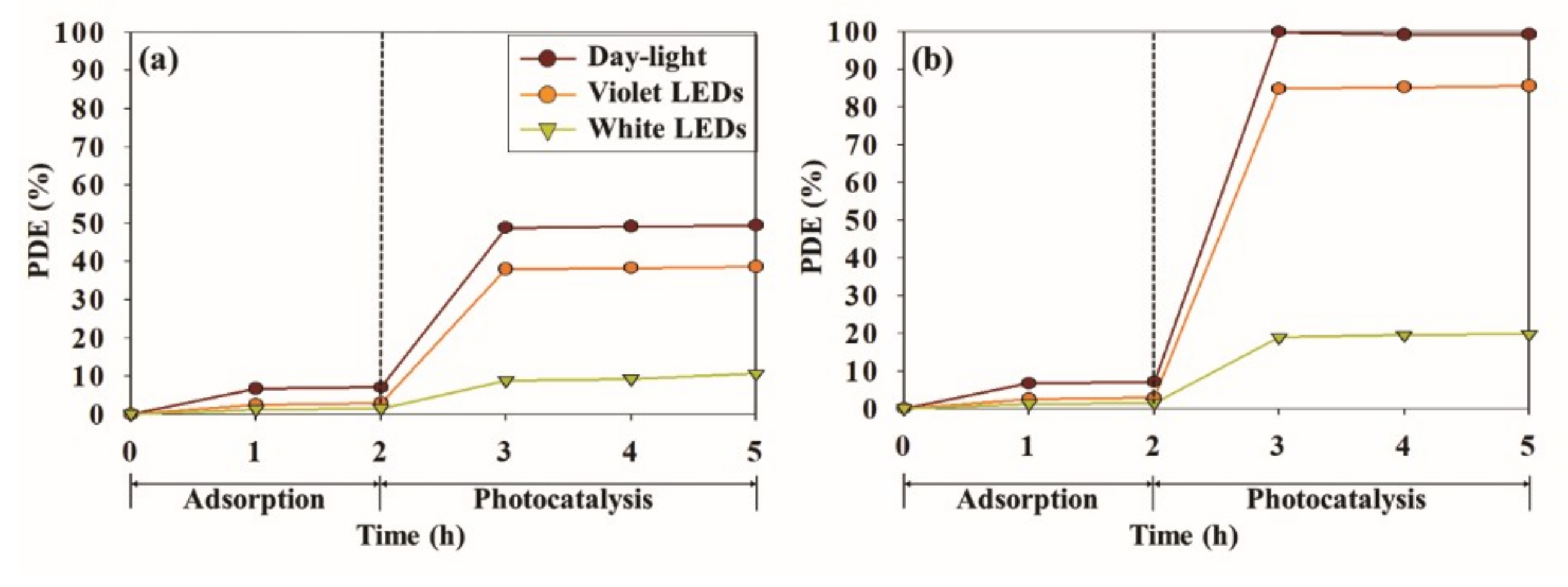
3.2. Photocatalytic Performace
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shin, S.H.; Jo, W.K. Longitudinal variations in indoor VOC concentrations after moving into new apartments and indoor source characterization. Environ. Sci. Pollut. Res. 2013, 20, 3696–3707. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Borrull, F.; Marcé, R.M. Chronic risk assessment of exposure to volatile organic compounds in the atmosphere near the largest Mediterranean industrial site. Environ. Int. 2012, 39, 200–209. [Google Scholar] [CrossRef]
- Shin, H.T.; Yu, C.L.; Wu, M.T.; Liu, C.S.; Tsai, C.H.; Hung, D.Z.; Wu, C.S.; Kuo, H.W. Subclinical abnormalities in workers with continuous low-level toluene exposure. Toxicol. Ind. Health 2011, 27, 691–699. [Google Scholar]
- Jiménez-Garza, O.; Márquez-Gamiño, S.; Albores, A.; Caudillo-Cisneros, C.; Carrieri, M.; Bartolucci, G.B.; Manno, M. CYP2E1 phenotype in Mexican workers occupationally exposed to low levels of toluene. Toxicol. Lett. 2012, 210, 254–263. [Google Scholar] [CrossRef]
- Kandyala, R.; Raghavendra, S.P.; Rajasekharan, S.T. Xylene: An overview of its health hazards and preventive measures. J. Oral Maxillofac. Pathol. 2010, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, H.; Sleiman, M.; Sullivan, D.P.; Jacquiod, C.; Sablayrolles, J.; Molins, L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B 2012, 128, 159–170. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C 2012, 3, 169–189. [Google Scholar] [CrossRef]
- Lai, W.W.P.; Lin, H.H.H.; Lin, A.Y.C. TiO2 photocatalytic degradation and transformation of oxazaphosphorine drugs in an aqueous environment. J. Hazard. Mater. 2015, 287, 133–141. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Yeung, K.L.; Maira, A.J.; Stolz, J.; Hung, E.; Ho, N.K.C.H.; Wei, A.C.; Soria, J.; Chao, K.J.; Yue, P.L. Ensemble effects in nanostructured TiO2 used in the gas-phase photooxidation of trichloroethylene. J. Phys. Chem. B 2002, 106, 4608–4616. [Google Scholar] [CrossRef]
- Yu, T.; Tan, X.; Zhao, L.; Yin, Y.; Chen, P.; Wei, J. Characterization, activity and kinetics of a visible light driven photocatalyst: Cerium and nitrogen co-doped TiO2 nanoparticles. Chem. Eng. J. 2010, 157, 86–92. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, G.; Sun, Z.; Zheng, S.; Frost, R.L. A comparative study about the influence of metal ions (Ce, La and V) doping on the solar-light-induced photodegradation toward rhodamine B. J. Environ. Chem. Eng. 2015, 3, 1444–1451. [Google Scholar] [CrossRef]
- Liu, J.; Han, R.; Zhao, Y.; Wang, H.; Lu, W.; Yu, T.; Zhang, Y. Enhanced photoactivity of V–N codoped TiO2 derived from a two-step hydrothermal procedure for the degradation of PCP–Na under visible light irradiation. J. Phys. Chem. C 2011, 115, 4507–4515. [Google Scholar] [CrossRef]
- Jing, L.Q.; Sun, X.J.; Xin, B.F.; Wang, B.; Cai, W.; Fu, H. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar]
- Sukharev, V.; Kershaw, R. Concerning the role of oxygen in photocatalytic decomposition of salicylic acid in water. J. Photochem. Photobiol. A 1996, 98, 165–169. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Docio, C.M.Á.; Ramírez, V.Z.; Lozano, J.F.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]
- Jiang, D.; Xu, Y.; Hou, B.; Wu, D.; Sun, Y. Synthesis of visible light-activated TiO2 photocatalyst via surface organic modification. J. Solid State Chem. 2007, 180, 1787–1791. [Google Scholar] [CrossRef]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Zhang, X.W.; Lei, L.C. One step preparation of visible-light responsive Fe-TiO2 coating photocatalysts by MOCVD. Mater. Lett. 2008, 62, 895–897. [Google Scholar] [CrossRef]
- Zhu, J.F.; Chen, F.; Zhang, J.L.; Chen, H.J.; Anpo, M. Fe3+-TiO2 photocatalysis prepared by combining sol-gel method with hydrothermal treatment and their characterization. J. Photochem. Photobiol. A 2006, 180, 196–204. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, K.S.; Kim, Y.S.; Chung, Y.C.; Kim, S.J. Photocatalytic activity of Ni 8 wt.%-doped TiO2 photocatalyst synthesized by mechanical alloying under visible light. J. Am. Ceram. Soc. 2006, 89, 515–518. [Google Scholar]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Chen, F.; He, D.; Anpo, M. Preparation and photocatalytic properties of Fe3+-doped Ag@TiO2 core–shell nanoparticles. J. Colloid Interface Sci. 2008, 323, 182–186. [Google Scholar] [CrossRef]
- Murakami, N.; Ono, A.; Nakamura, M.; Tsubota, T. Development of a visible-light-responsive rutile rod by site-selective modification of iron (III) ion on {111} exposed crystal faces. Appl. Catal. B 2010, 97, 115–119. [Google Scholar] [CrossRef]
- Chang, S.-M.; Liu, W. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B 2014, 156–157, 466–475. [Google Scholar] [CrossRef]
- Venieri, D.; Fraggedaki, A.; Kostadima, M.; Chatzisymeon, E.; Binas, V.; Zachopoulos, A.; Kiriakidis, G.; Mantzavinos, D. Solar light and metal-doped TiO2 to eliminate water-transmitted bacterial pathogens: Photocatalyst characterization and disinfection performance. Appl. Catal. B 2014, 154–155, 93–101. [Google Scholar] [CrossRef]
- Yan, N.; Zhu, Z.; Zhang, J.; Zhao, Z.; Liu, Q. Preparation and properties of Ce-doped TiO2 photocatalyst. Mater. Res. Bull. 2012, 47, 1869–1873. [Google Scholar] [CrossRef]
- Ranjit, K.T.; Willner, I.; Bossmann, S.H.; Braun, A.M. Lanthanide oxide doped titanium dioxide photocatalysts: Effective photocatalysts for the enhanced degradation of salicylic acid and t-cinnamic acid. J. Catal. 2001, 204, 305–313. [Google Scholar] [CrossRef]
- Chai, Y.; Ding, H.; Zhang, Z.; Xian, Y.; Pan, Z.; Jin, L. Study on photocatalytic oxidation for determination of the low chemical oxygen demand using a nano-TiO2-Ce(SO4)2 coexisted system. Talanta 2006, 68, 610–615. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Hou, M.F.; Cheah, K.W.; Choy, W.C.H. Enhanced photocatalytic activity of Ce3+–TiO2 for 2-mercaptobenzothiazole degradation in aqueous suspension for odour control. Appl. Catal. A 2005, 285, 181–189. [Google Scholar] [CrossRef]
- Rapsonmanikis, A.; Apostolopoulou, A.; Stathatos, E.; Lianos, P. Cerium-modified TiO2 nanocrystalline films for visible light photocatalytic activity. J. Photochem. Photobiol. A 2014, 280, 46–53. [Google Scholar] [CrossRef]
- Prasa, K.; Pinjari, D.V.; Pandit, A.B.; Mhaske, S.T. Phase transformation of nanostructured titanium dioxide from anatase-to-rutile via combined ultrasound assisted sol-gel technique. Ultrason. Sonochem. 2010, 17, 409–415. [Google Scholar] [CrossRef]
- Cheng, H.; Ma, J.; Qi, L. Hydrothermal preparation of uniform nanosize rutile and anatase particles. Chem. Mater. 1995, 7, 663–671. [Google Scholar] [CrossRef]
- Oseghe, E.O.; Ofomaja, A.E. Facile microwave synthesis of pine cone derived C-doped TiO2 for the photodegradation of tetracycline hydrochloride under visible-LED light. J. Environ. Manag. 2018, 223, 860–867. [Google Scholar] [CrossRef]
- Neppolian, B.; Wang, Q.; Jung, H.; Choi, H. Ultrasonic-assisted sol-gel method of preparation of TiO2 nano-particles: Characterization, properties and 4-chlorophenol removal application. Ultrason. Sonochem. 2008, 15, 649–658. [Google Scholar] [CrossRef]
- Anandan, S.; Wu, J.J. Ultrasound assisted synthesis of TiO2-WO3 heterostructures for the catalytic degradation of Tergitol (NP-9) in water. Ultrason. Sonochem. 2014, 21, 1284–1288. [Google Scholar] [CrossRef]
- Yadav, R.S.; Mishra, P.; Pandey, A.C. Growth mechanism and optical property of ZnO nanoparticles synthesized by sonochemical method. Ultrason. Sonochem. 2008, 15, 863–868. [Google Scholar] [CrossRef]
- Feng, H.; Yu, L.E.; Zhang, M.-H. Ultrasonic synthesis and photocatalytic performance of metal-ions doped TiO2 catalysts under solar light irradiation. Mater. Res. Bull. 2013, 48, 672–681. [Google Scholar] [CrossRef]
- Deosarkar, M.P.; Pawar, S.M.; Bhanvase, B.A. In situ sonochemical synthesis of Fe3O4–graphene nanocomposite for lithium rechargeable batteries. Chem. Eng. Process. 2014, 83, 49–55. [Google Scholar] [CrossRef]
- Gedanken, A. Using sonochemistry for the fabrication of nanomaterials. Ultrason. Sonochem. 2004, 11, 47–55. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, W.-K. Three-dimensional TiO2 structures incorporated with tungsten oxide for treatment of toxic aromatic volatile compounds. Catalysts 2017, 7, 97. [Google Scholar] [CrossRef]
- Venkatachalam, N.; Palanichamy, M.; Murugesan, V. Sol–gel preparation and characterization of alkaline earth metal doped nano TiO2: Efficient photocatalytic degradation of 4-chlorophenol. J. Mol. Catal. A 2007, 273, 177–185. [Google Scholar] [CrossRef]
- Xia, J.; Jiang, D.; Chen, M.; Li, D.; Zhu, J.; Lu, X.; Yan, C. Preparation and characterization of monodisperse Ce-doped TiO2 microspheres with visible light photocatalytic activity. Colloids Surf. A 2010, 372, 107–114. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Leret, P.; Álvarez-Docio, C.M.; del Campo, A.; Fernández, J.F. Enhancement of UV absorption behavior in ZnO–TiO2 composites. Bol. Soc. Esp. Cerám. V. 2016, 55, 55–62. [Google Scholar] [CrossRef]
- Lee, J.Y.; Jo, W.-K. Simplified sonochemical preparation of titania embedded with selected metals for purification of benzene and toluene. Ultrason. Sonochem. 2016, 28, 250–256. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Viswananthan, B. Effect of surface area, pore volume and particle size of P25 titania on the phase transformation of anatase to rutile. Indian J. Chem. 2009, 48, 1378–1382. [Google Scholar]
- Papadimitriou, V.C.; Stefanopoulos, V.G.; Romanias, M.N.; Papagiannakopoulos, P.; Sambani, K.; Tudose, V.; Kiriakidis, G. Determination of photo-catalytic activity of un-doped and Mn-doped TiO2 anatase powders on acetaldehyde under UV and visible light. Thin Solid Films 2011, 520, 1195–1201. [Google Scholar] [CrossRef]
- Laokiat, L.; Khemthong, P.; Grisdanurak, N.; Sreearunothai, P.; Pattanasiriwisawa, W.; Klysubun, W. Photocatalytic degradation of benzene, toluene, ethylbenzene, and xylene (BTEX) using transition metal-doped titanium dioxide immobilized on fiberglass cloth. Korean J. Chem. Eng. 2012, 29, 377–383. [Google Scholar] [CrossRef]
- Chun, H.H.; Lee, J.Y.; Jo, W.K. Photocatalysis of low-concentration gaseous organic pollutants over electrospun iron-doped titanium dioxide nanofibers. Solid State Sci. 2013, 25, 103–109. [Google Scholar] [CrossRef]
- Luiza, D.d.B.; Andersen, S.L.F.; Berger, C.; José, H.J.; Moreira, R.d.F.P.M. Photocatalytic reduction of nitrate ions in water over metal-modified TiO2. J. Photochem. Photobiol. A 2012, 246, 36–44. [Google Scholar] [CrossRef]
- Wang, S.; Meng, K.K.; Zhao, L.; Jiang, Q.; Lian, J.S. Superhydrophilic Cu-doped TiO2 thin film for solar-driven photocatalysis. Ceram. Int. 2014, 40, 5107–5110. [Google Scholar] [CrossRef]
- Sood, S.; Umar, A.; Mehta, S.K.; Kansal, S.K. Highly effective Fe-doped TiO2 nanoparticles photocatalysts for visible-light driven photocatalytic degradation of toxic organic compounds. J. Colloid Interface Sci. 2015, 450, 213–223. [Google Scholar] [CrossRef]
- Hu, S.; Li, F.; Fan, Z.; Chang, C.-C. Enhanced photocatalytic activity and stability of nano-scaled TiO2 co-doped with N and Fe. Appl. Surf. Sci. 2011, 258, 182–188. [Google Scholar] [CrossRef]
- Kim, T.-H.; Rodríguez-González, V.; Gyawali, G.; Cho, S.-H.; Sekino, T.; Lee, S.-W. Synthesis of solar light responsive Fe, N co-doped TiO2 photocatalyst by sonochemical method. Catal. Today 2013, 212, 75–80. [Google Scholar] [CrossRef]
- Singh, R.; Pal, B. Study of excited charge carrier’s lifetime for the observed photoluminescence and photocatalytic activity of CdS nanostructures of different shapes. J. Mole. Catal. A 2013, 371, 77–85. [Google Scholar] [CrossRef]


| Photocatalyst | SBET (m2 g−1) | Total Pore Volume (cm3 g−1) | Particle Size (nm) | Crystallite Size of Anatase (nm) | Band Gap, Eg (eV) |
|---|---|---|---|---|---|
| P25 | 58.1 | 0.198 | 24.6 | 24.2 | 3.20 |
| PT | 63.5 | 0.212 | 22.5 | 16.4 | 3.11 |
| CeT-0.5 | 96.7 | 0.312 | 14.8 | 12.4 | 3.07 |
| CeT-0.75 | 108.6 | 0.314 | 13.2 | 10.9 | 3.03 |
| CeT-1.0 | 104.7 | 0.312 | 13.6 | 11.7 | 3.00 |
| CeT-1.5 | 94.5 | 0.309 | 15.1 | 12.7 | 2.97 |
| CeT-2.0 | 93.5 | 0.310 | 15.3 | 12.8 | 2.89 |
| Kinetic Model | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Target Compound | Toluene | o-Xylene | Toluene | o-Xylene | |||||
| Parameter | k1 (h−1) | R2 | k1 (h−1) | R2 | k2 (L μg−1 h−1) | R2 | k2 (L μg−1 h−1) | R2 | |
| Adsorption step | P25 | 0.0147 | 0.8524 | 0.0189 | 0.9461 | 0.0396 | 0.8537 | 0.0443 | 0.9442 |
| PT | 0.0272 | 1.0000 | 0.0272 | 1.0000 | 0.0742 | 1.0000 | 0.0645 | 1.0000 | |
| CeT-0.5 | 0.0168 | 0.9525 | 0.0168 | 0.9525 | 0.0453 | 0.9542 | 0.0393 | 0.9542 | |
| CeT-0.75 | 0.0583 | 0.8484 | 0.0147 | 0.8524 | 0.1639 | 0.8532 | 0.0344 | 0.8537 | |
| CeT-1.0 | 0.0272 | 0.7934 | 0.0272 | 0.7934 | 0.0742 | 0.7945 | 0.0645 | 0.7945 | |
| CeT-1.5 | 0.0189 | 0.9461 | 0.0331 | 0.8669 | 0.0510 | 0.9442 | 0.0788 | 0.8700 | |
| CeT-2.0 | 0.0331 | 0.8669 | 0.0583 | 0.8484 | 0.0907 | 0.8700 | 0.1424 | 0.8532 | |
| Photocatalytic degradation step | P25 | 0.0326 | 0.9447 | 0.0338 | 0.9167 | 0.0905 | 0.9466 | 0.0816 | 0.9178 |
| PT | 0.0453 | 0.9159 | 0.1937 | 0.9045 | 0.1280 | 0.9173 | 0.5925 | 0.9078 | |
| CeT-0.5 | 0.1573 | 0.9003 | 0.8274 | 0.8998 | 0.5241 | 0.9020 | 7.5242 | 0.9101 | |
| CeT-0.75 | 0.2429 | 0.8975 | 1.7160 | 0.9057 | 0.9227 | 0.8992 | 99.755 | 0.9475 | |
| CeT-1.0 | 0.2160 | 0.9024 | 1.0981 | 0.8966 | 0.7871 | 0.9055 | 16.990 | 0.9046 | |
| CeT-1.5 | 0.1304 | 0.8950 | 0.7508 | 0.8949 | 0.4173 | 0.8954 | 5.9113 | 0.8978 | |
| CeT-2.0 | 0.0786 | 0.8989 | 0.6144 | 0.9014 | 0.2333 | 0.8995 | 3.7749 | 0.9103 | |
| Dopant | Doped TiO2 Preparation Method | Target Compounds | Initial Pollutant Concentration (ppm) | Light Sources | Photocatalytic Degradation Efficiency (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Doped TiO2 | Pure TiO2 | ||||||
| Fe | Solvothermal method | Toluene | 1000–1200 | Visible light | 48 | 20 | [47] |
| W | 54 | ||||||
| V | 69 | ||||||
| Fe | Electrospinning method | Benzene, Toluene, Ethylbenzene, and o-Xylene | 0.1 | Visible light | 68 (toluene) and 91 (o-xylene) | 35 (toluene) and 58 (o-xylene) | [48] |
| Ce | Sonochemical method | Toluene and o-Xylene | 0.1 | Visible light | 49 (toluene) and 99.4 (o-xylene) | 11.6 (toluene) and 41.4 (o-xylene) | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Choi, J.-H. Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene. Materials 2019, 12, 1265. https://doi.org/10.3390/ma12081265
Lee JY, Choi J-H. Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene. Materials. 2019; 12(8):1265. https://doi.org/10.3390/ma12081265
Chicago/Turabian StyleLee, Joon Yeob, and Jeong-Hak Choi. 2019. "Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene" Materials 12, no. 8: 1265. https://doi.org/10.3390/ma12081265
APA StyleLee, J. Y., & Choi, J.-H. (2019). Sonochemical Synthesis of Ce-doped TiO2 Nanostructure: A Visible-Light-Driven Photocatalyst for Degradation of Toluene and O-Xylene. Materials, 12(8), 1265. https://doi.org/10.3390/ma12081265




