Microstructure and Mechanical Properties of Nanocrystalline Al-Zn-Mg-Cu Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering
Abstract
:1. Introduction
2. Materials and Methods
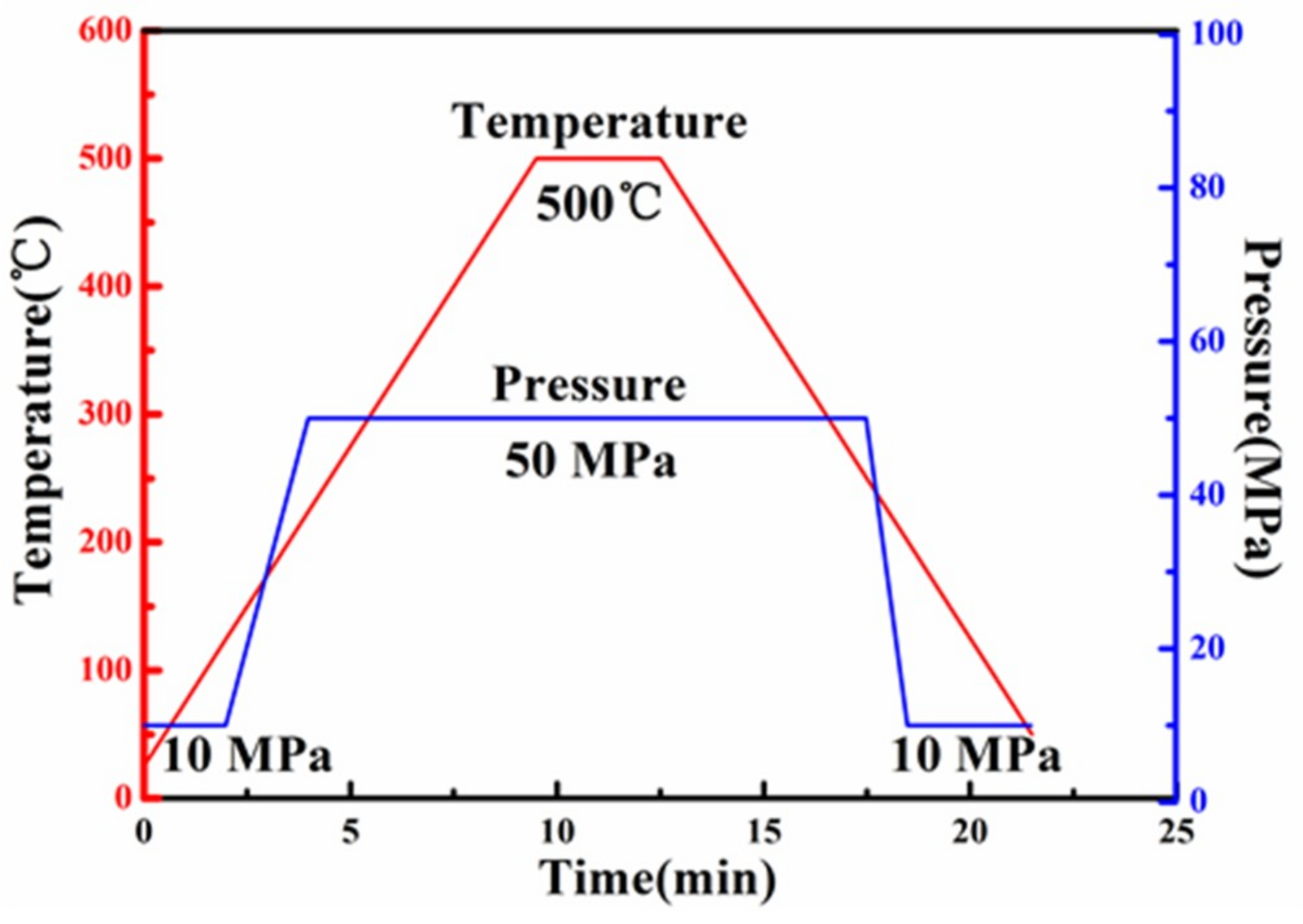
2.1. Materials Preparation
2.2. Material Characterization and Performance Testing
3. Results and Discussion
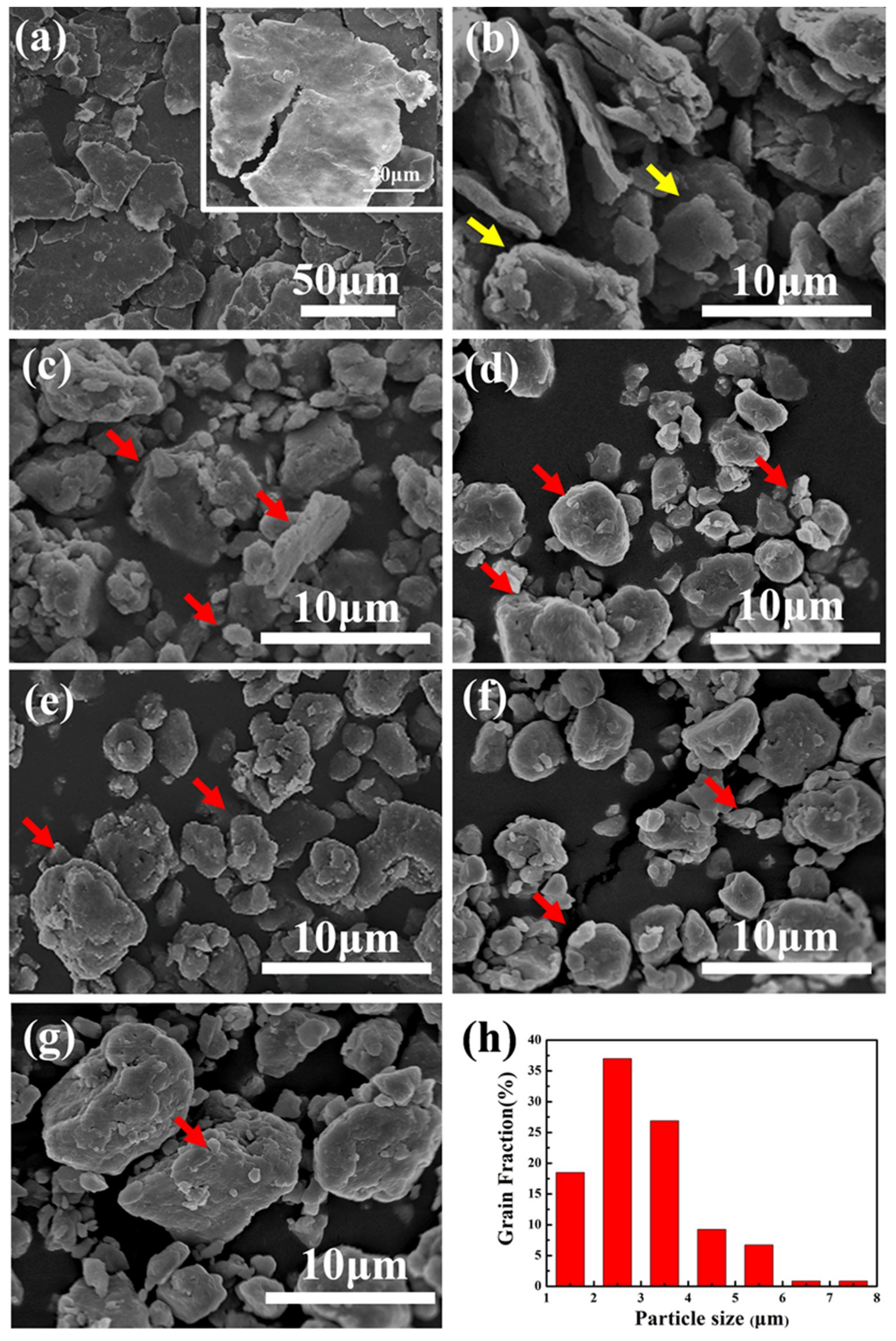
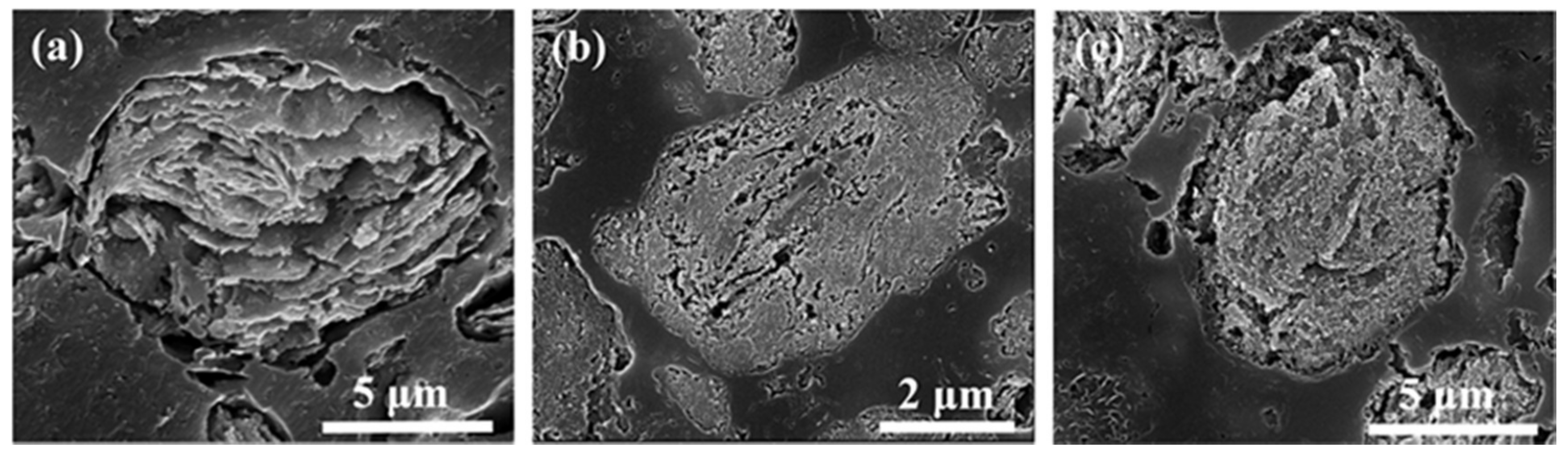
3.1. Morphology and Grain Size of Milled Powders
3.2. Microstructure of SPS Samples
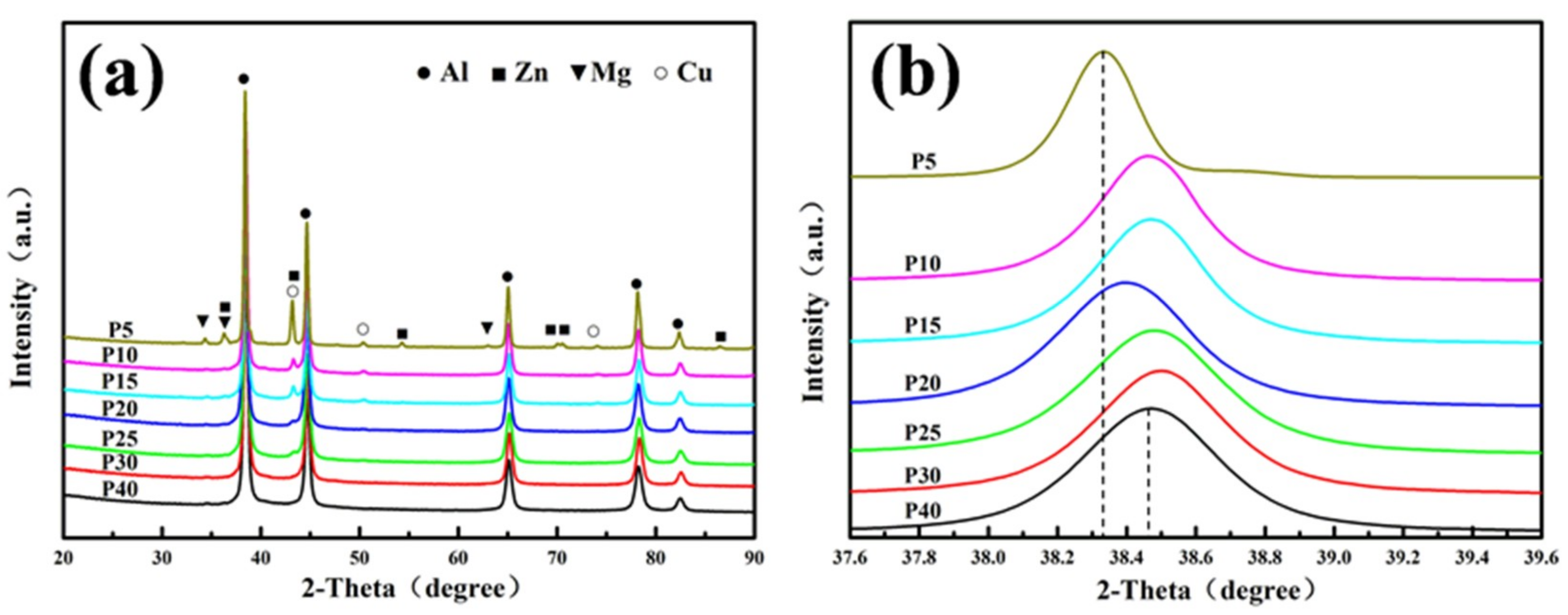
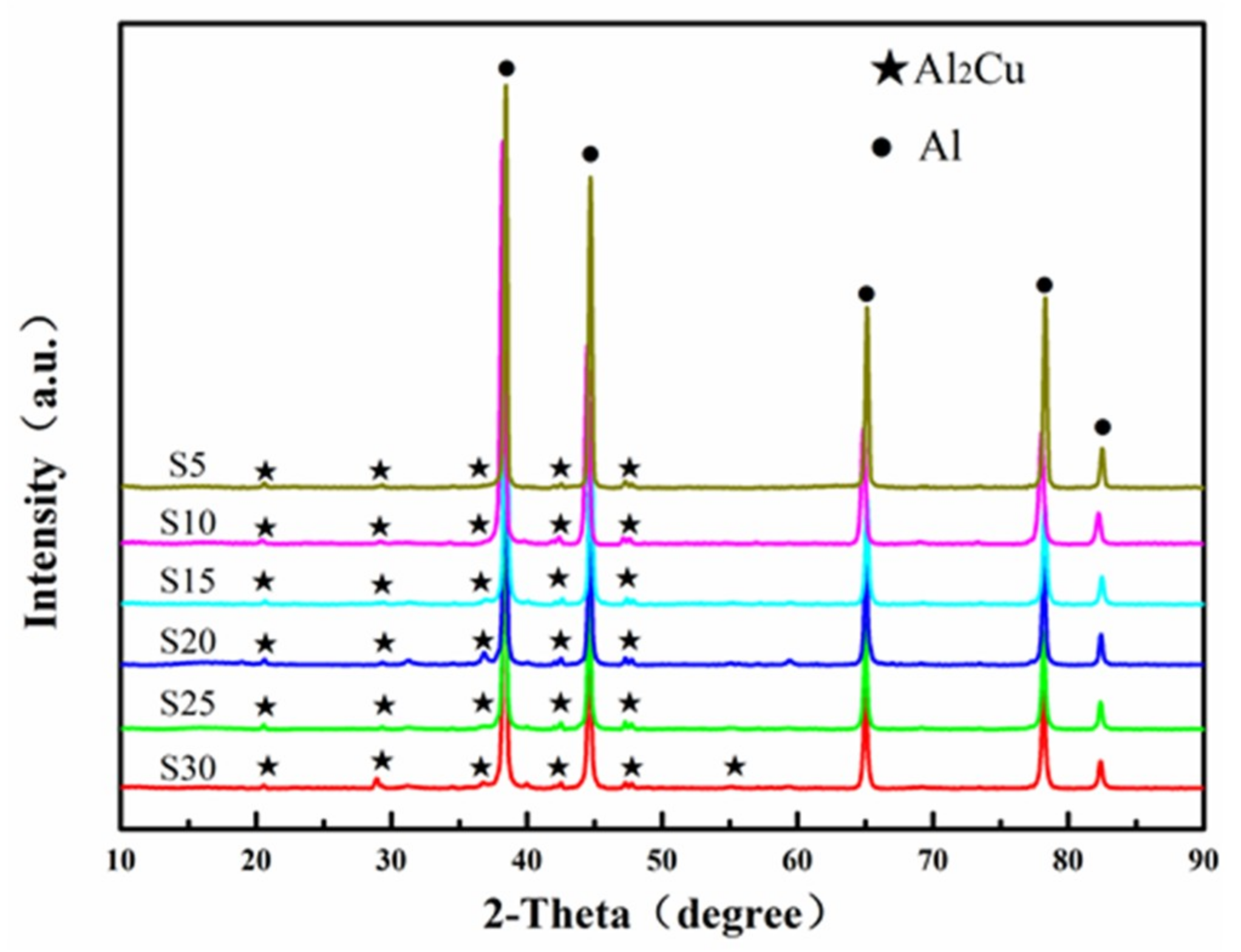
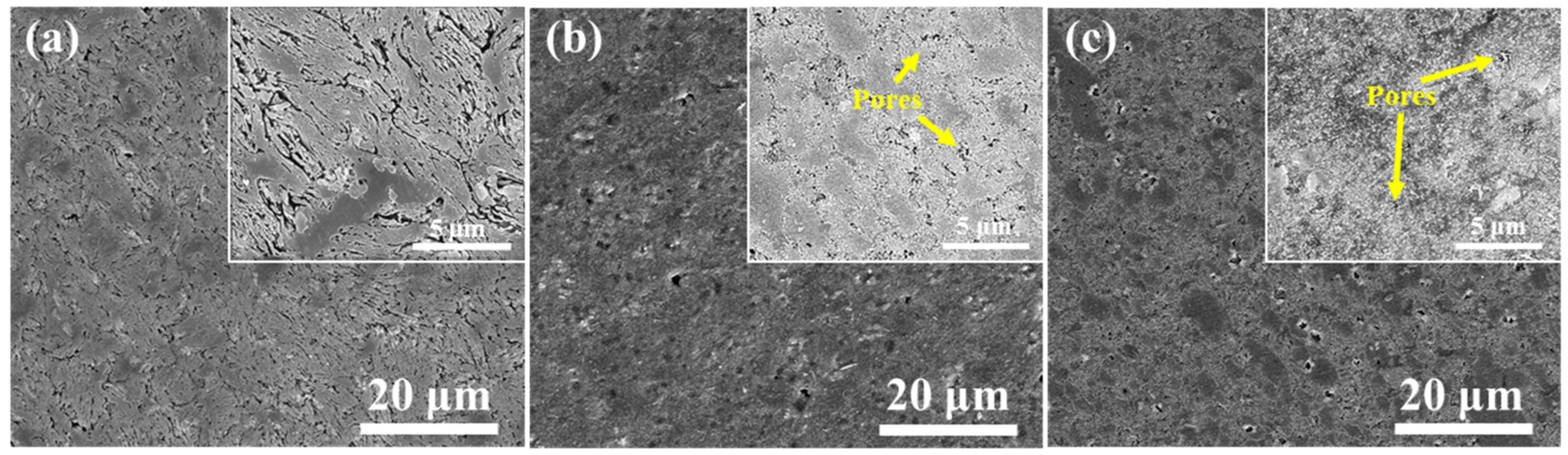
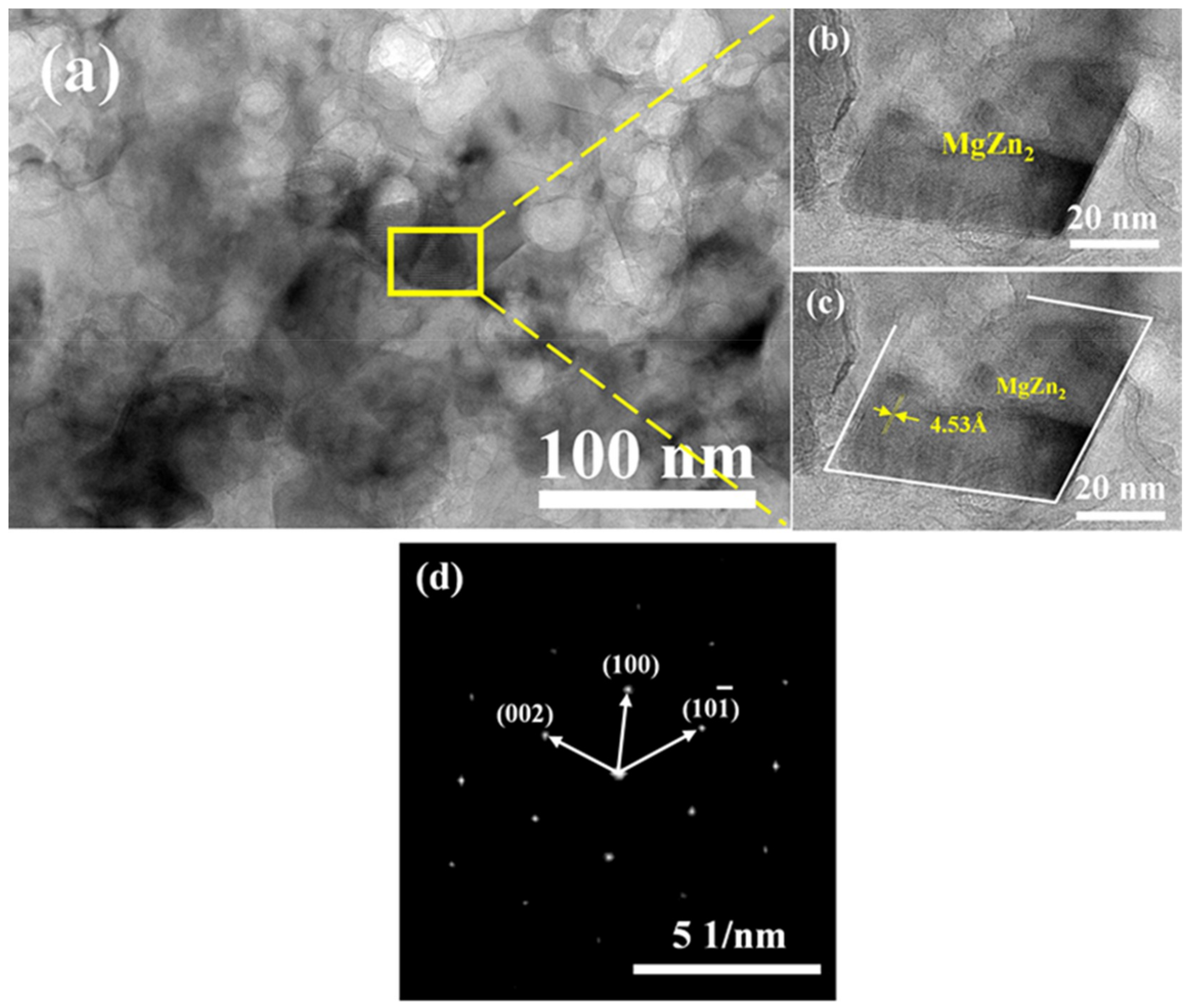
3.2.1. Microstructure of SPS Samples
3.2.2. Thermodynamic of Second Phase Formation
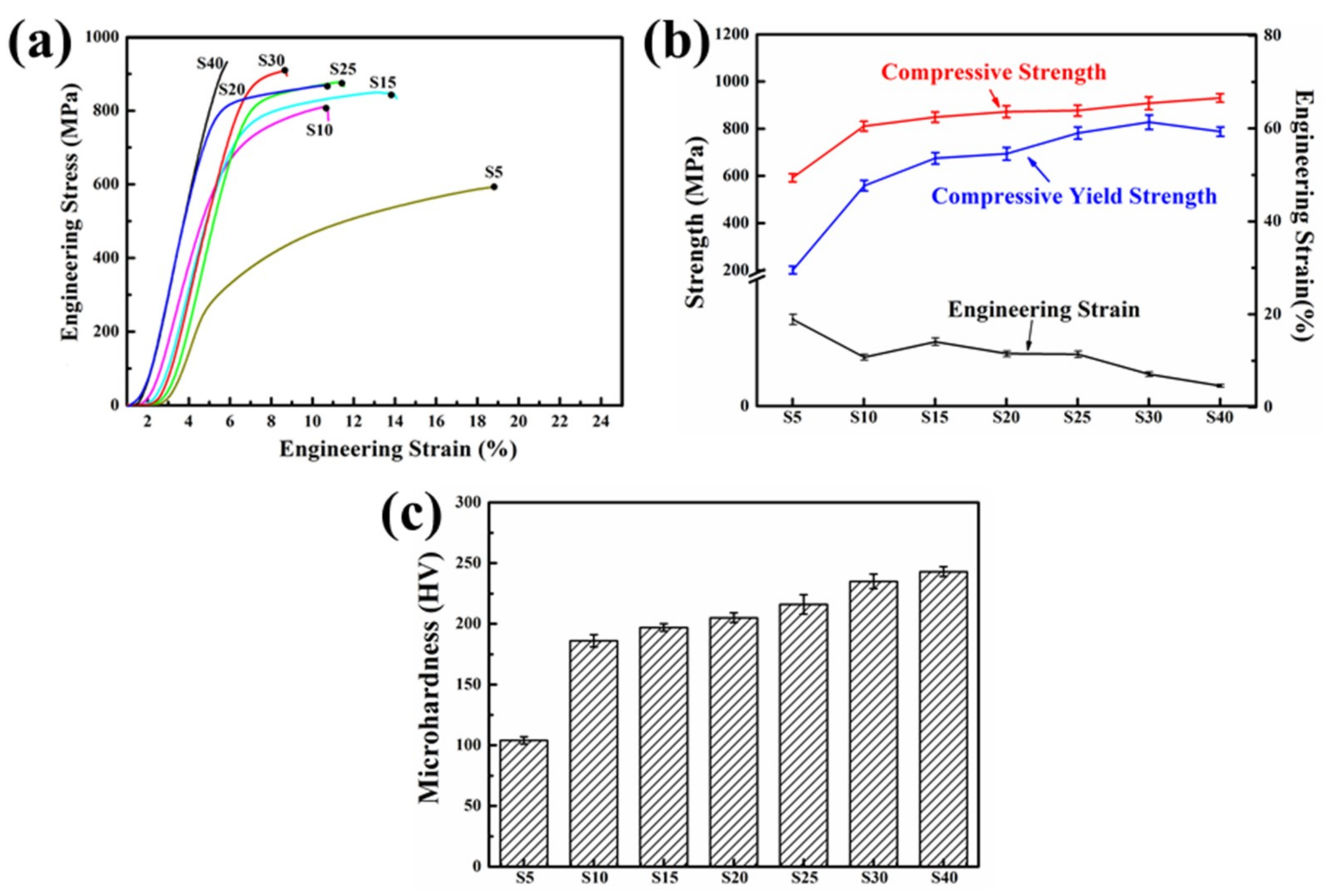
3.2.3. Mechanical Properties of SPS Samples
3.3. Strengthening Mechanism
3.3.1. Grain Boundary Strengthening
3.3.2. Dislocation Strengthening
3.3.3. Solid-Solution Strengthening
3.3.4. Orowan Strengthening
4. Conclusions
- (1)
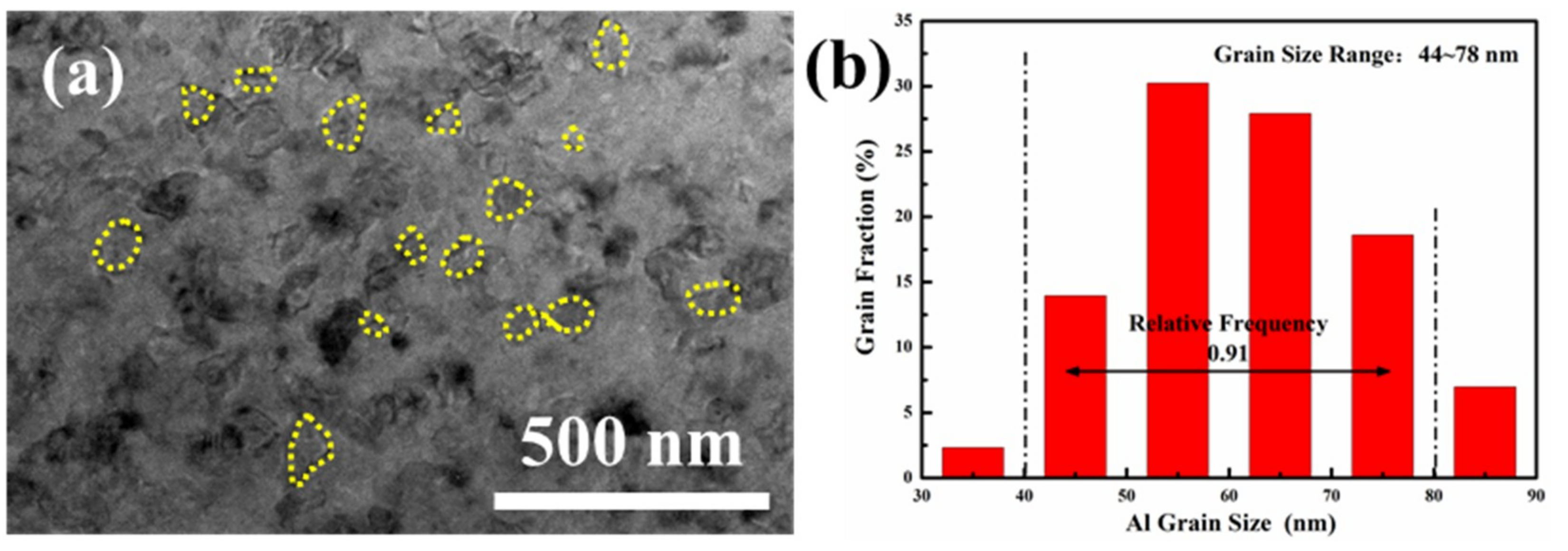
- With the extension of the milling time, the Zn, Mg and Cu alloy elements are gradually dissolved in α-Al. When the ball milling time is 30 h, complete solid solution is achieved, and no intermetallic phase is formed. The ball-milled Al powder undergoes flattening, crushing and welding processes. When the ball milling time is 30 h, the powder particle size is 2–5 μm. The α-Al grain size was 23.2 nm. The lattice distortion was 0.156% causing by the solid solution of the alloying elements.
- (2)
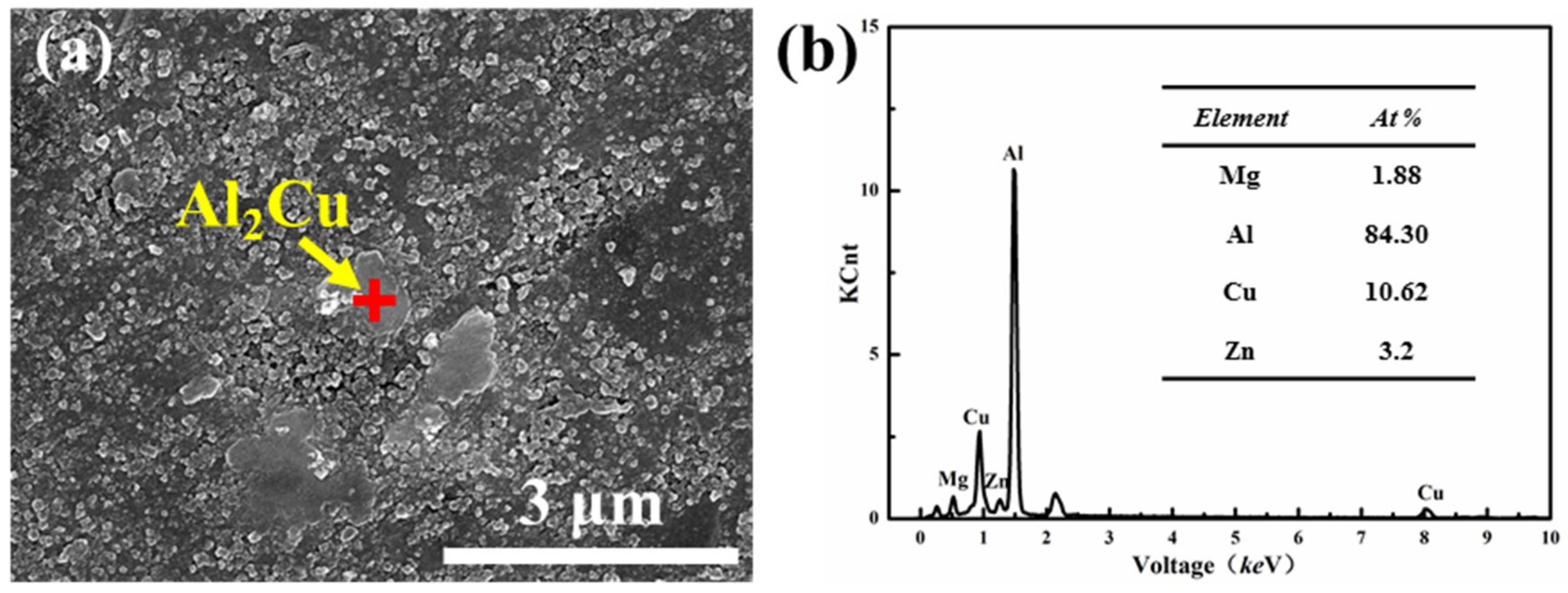
- The grain growth of the ball milled powder occurred during the SPS sintering process. The grain size after ball milling for 30 h was increased by 53.5 nm from the powder 23.2 nm. Due to the desolvation of the supersaturated solid solution, micro-sized Al2Cu is precipitated at the α-Al grain boundary, and nano-size MgZn2 is precipitated in the α-Al crystal.
- (3)
- With the increase in ball milling duration, the compressive strength, yield strength and Vickers microhardness of the sintered samples increased gradually but the engineering strain decreased. The compressive strength, yield strength, Vickers microhardness and engineering strain after ball milling for 30 h were ~908 MPa, ~853 MPa, ~235 HV and ~8.1%, respectively.
- (4)
- In the nanocrystalline bulk Al-7Zn-2.5Mg-2.5Cu alloy prepared by the MA-SPS method, the high strength of the nanocrystalline bulk Al-7Zn-2.5Mg-2.5Cu alloy was attributed to fine-grained strengthening, while the dislocation strengthening and Orowan strengthening due to the precipitated second phase particles are the next.
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Starke, E.A.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Ju, X.; Zhang, F.; Chen, Z.; Ji, G.; Wang, M.; Wu, Y.; Zhong, S.; Wang, H. Microstructure of Multi-Pass Friction-Stir-Processed Al-Zn-Mg-Cu Alloys Reinforced by Nano-Sized TiB2 Particles and the Effect of T6 Heat Treatment. Metals 2017, 7, 530. [Google Scholar] [CrossRef]
- Mohanty, P.S.; Gruzleski, J.E. Mechanism of grain refinement in aluminium. Acta Mater. 1995, 43, 2001–2012. [Google Scholar] [CrossRef]
- Li, X.; Cai, Q.; Zhao, B.; Xiao, Y.; Li, B. Effect of nano TiN/Ti refiner addition content on the microstructure and properties of as-cast Al-Zn-Mg-Cu alloy. J. Alloys Compd. 2016, 675, 201–210. [Google Scholar] [CrossRef]
- Wang, F.; Xiong, B.; Zhang, Y.; Liu, H.; Li, Z.; Liu, Q. Microstructure and mechanical properties of spray-deposited Al–Zn–Mg–Cu alloy processed through hot rolling and heat treatment. Mater. Sci. Eng. A 2009, 518, 144–149. [Google Scholar] [CrossRef]
- Kusui, J.; Fujii, K.; Yokoe, K.; Yokota, T.; Osamura, K.; Kubota, O.; Okuda, H. Development of super-high strength Al-Zn-Mg-Cu P/M alloys. Mater. Sci. Forum 1996, 217–222, 1823–1828. [Google Scholar] [CrossRef]
- Harding, D.M.; Donaldson, W.I.; Hexemer, L.R., Jr.; Bishop, P.D. Effects of Post-Sinter Processing on an Al-Zn-Mg-Cu Powder Metallurgy Alloy. Metals 2017, 7, 370. [Google Scholar] [CrossRef]
- Liu, Z.F.; Zhang, Z.H.; Korznikov, A.V.; Lu, J.F.; Korznikova, G.; Wang, F.C. A novel and rapid route for synthesizing nanocrystalline aluminum. Mater. Sci. Eng. A 2014, 615, 320–323. [Google Scholar] [CrossRef]
- Lityńska-Dobrzyńska, L.; Dutkiewicz, J.; Maziarz, W.; Góral, A. The effect of zirconium addition on microstructure and properties of ball milled and hot compacted powder of Al-12wt% Zn-3wt% Mg-1.5wt% Cu alloy. J. Alloys Compd. 2011, 509, S304–S308. [Google Scholar] [CrossRef]
- Azimi, A.; Shokuhfar, A.; Zolriasatein, A. Nanostructured Al-Zn-Mg-Cu-Zr alloy prepared by mechanical alloying followed by hot pressing. Mater. Sci. Eng. A 2014, 595, 124–130. [Google Scholar] [CrossRef]
- Anas, N.S.; Ramakrishna, M.; Dash, R.K.; Rao, T.N.; Vijay, R. Influence of process control agents on microstructure and mechanical properties of Al alloy produced by mechanical alloying. Mater. Sci. Eng. A 2019, 751, 171–182. [Google Scholar] [CrossRef]
- Fadeeva, V.I.; Leonov, A.V. Formation of Al-Fe Supersaturated Solid Solution by Mechanical Alloying. Mater. Sci. Forum 1992, 88–90, 481–488. [Google Scholar] [CrossRef]
- Sheibani, S.; Heshmati-Manesh, S.; Ataie, A. Structural investigation on nano-crystalline Cu-Cr supersaturated solid solution prepared by mechanical alloying. J. Alloys Compd. 2010, 495, 59–62. [Google Scholar] [CrossRef]
- Balima, F.; Largeteau, A. Phase transformation of alumina induced by high pressure spark plasma sintering (HP-SPS). Scr. Mater. 2019, 158, 20–23. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kukeya, S.; Ohfuji, H. Microstructure and room and high temperature mechanical properties of ultrafine structured Al-5 wt%Y2O3 and Al-5 wt%La2O3 nanocomposites fabricated by mechanical alloying and hot pressing. Mater. Sci. Eng. A 2019, 748, 428–433. [Google Scholar] [CrossRef]
- Yan, L.; Yan, B. Fe-Si/MnZn(Fe2O4)2 Core-shell Composites with Excellent Magnetic Properties by Mechanical Milling and Spark Plasma Sintering (SPS). Metals 2018, 8, 553. [Google Scholar] [CrossRef]
- Xie, G.; Ohashi, O.; Yoshioka, T.; Song, M.; Mitsuishi, K.; Yasuda, H.; Furuya, K.; Noda, T. Effect of Interface Behavior between Particles on Properties of Pure Al Powder Compacts by Spark Plasma Sintering. Mater. Trans. 2001, 42, 1846–1849. [Google Scholar] [CrossRef]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Aqeeli, N.A.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark plasma sintering of metals and metal matrix nanocomposites: A Review. J. Nanomater. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Liu, D.; Xiong, Y.; Topping, T.D.; Zhou, Y.; Haines, C.; Paras, J.; Martin, D.; Kapoor, D.; Schoenung, J.M.; Lavernia, E.J. Spark Plasma Sintering of Cryomilled Nanocrystalline Al Alloy—Part II: Influence of Processing Conditions on Densification and Properties. Metall. Mater. Trans. A 2012, 43, 340–350. [Google Scholar] [CrossRef]
- Chua, A.S.; Brochu, M.; Bishop, D.P. Spark plasma sintering of prealloyed aluminium powders. Powder Metall. 2015, 58, 51–60. [Google Scholar] [CrossRef]
- Gu, J.; Gu, S.; Xue, L.; Wu, S.; Yan, Y. Microstructure and mechanical properties of in-situ Al13Fe4/Al composites prepared by mechanical alloying and spark plasma sintering. Mater. Sci. Eng. A 2012, 558, 684–691. [Google Scholar] [CrossRef]
- Kong, Q.; Lian, L.; Liu, Y.; Zhang, J. Fabrication and Characterization of Nanocrystalline Al-Cu Alloy by Spark Plasma Sintering. Mater. Manuf. Process. 2014, 29, 1232–1236. [Google Scholar] [CrossRef]
- Park, K.; Park, J.; Kwon, H. Effect of intermetallic compound on the Al-Mg composite materials fabricated by mechanical ball milling and spark plasma sintering. J. Alloys Compd. 2018, 739, 311–318. [Google Scholar] [CrossRef]
- Carreño-Gallardo, C.; Estrada-Guel, I.; López-Meléndez, C.; Ledezma-Sillas, E.; Castañeda-Balderas, R.; Pérez-Bustamante, R.; Herrera-Ramírez, M.J. B4C Particles Reinforced Al2024 Composites via Mechanical Milling. Metals 2018, 8, 647. [Google Scholar] [CrossRef]
- Yuan, H.X.; Wang, Y.Q.; Shi, Y.J.; Gardner, L. Stub column tests on stainless steel built-up sections. Thin Wall Struct. 2014, 83, 103–114. [Google Scholar] [CrossRef]
- Pan, D.; Li, L.; Wu, Y.; Liu, T.; Yu, H. Characteristics and properties of glass-ceramics using lead fuming slag. J. Clean. Prod. 2018, 175, 251–256. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Velasco, F.; Robert, M.H.; Torralba, J.M. Effect of mechanical alloying on the morphology, microstructure and properties of aluminium matrix composite powders. Mater. Sci. Eng. A 2003, 342, 131–143. [Google Scholar] [CrossRef]
- Sivasankaran, S.; Sivaprasad, K.; Narayanasamy, R.; Iyer, V.K. An investigation on flowability and compressibility of AA 6061100−x-xwt.% TiO2 micro and nanocomposite powder prepared by blending and mechanical alloying. Powder Technol. 2010, 201, 70–82. [Google Scholar] [CrossRef]
- Bhaduri, A.; Gopinathan, V.; Ramakrishnan, P.; Miodownik, A.P. Microstructural changes in a mechanically alloyed Al-6.2Zn-2.5Mg-1.7Cu alloy (7010) with and without particulate SiC reinforcement. Metall. Mater. Trans. A 1996, 27, 3718–3726. [Google Scholar] [CrossRef]
- Iqbal, M.; Shaikh, M.A.; Akhter, J.I.; Ahmad, M.; Akhtar, M.; Moughal, M.J. Synthesis by ball milling and characterization of Al-Zn alloys. J. Mater. Sci. 2004, 39, 4255–4262. [Google Scholar] [CrossRef]
- Fogagnolo, J.B.; Amador, D.; Ruiz-Navas, E.M.; Torralba, J.M. Solid solution in Al–4.5wt% Cu produced by mechanical alloying. Mater. Sci. Eng. A 2006, 433, 45–49. [Google Scholar] [CrossRef]
- Chen, H.B.; Tao, K.; Yang, B.; Zhang, J.S. Nanostructured Al-Zn-Mg-Cu alloy synthesized by cryomilling and spark plasma sintering. Trans. Nonferr. Met. Soc. China 2009, 19, 1110–1115. [Google Scholar] [CrossRef]
- Villars, P. Al-Cu Binary Phase Diagram 0-2.7 at.% Cu. Available online: https://materials.springer.com/isp/phase-diagram/docs/c_0102006 (accessed on 15 March 2019).
- Villars, P. Al-Zn Binary Phase Diagram 0-45 at.% Zn. Available online: https://materials.springer.com/isp/phase-diagram/docs/c_0906809 (accessed on 15 March 2019).
- The Mg-Al binary phase diagram: Datasheet from MSI Eureka in SpringerMaterials. Available online: https://materials.springer.com/msi/phase-diagram/docs/sm_msi_r_10_012175_01_full_LnkDia0 (accessed on 15 March 2019).
- Sivasankaran, S.; Sivaprasad, K.; Narayanasamy, R.; Satyanarayana, P.V. X-ray peak broadening analysis of AA 6061100−x−xwt.% Al2O3 nanocomposite prepared by mechanical alloying. Mater. Charact. 2011, 62, 661–672. [Google Scholar] [CrossRef]
- Suryanarayana, C. Mechanical alloying and milling. Prog. Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- Medvedeva, N.I.; Gornostyrev, Y.N.; Novikov, D.L.; Mryasov, O.N.; Freeman, A.J. Ternary site preference energies, size misfits and solid solution hardening in NiAl and FeAl. Acta Mater. 1998, 46, 3433–3442. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Zeng, S.; Cheng, N.; Chen, T. Intermetallic phase formation and evolution during homogenization and solution in Al-Zn-Mg-Cu alloys. Sci. China Technol. Sci. 2013, 56, 2827–2838. [Google Scholar] [CrossRef]
- Li, C.M.; Zeng, S.M.; Chen, Z.Q.; Cheng, N.P.; Chen, T.X. First-principles calculations of elastic and thermodynamic properties of the four main intermetallic phases in Al-Zn-Mg-Cu alloys. Comp. Mater. Sci. 2014, 93, 210–220. [Google Scholar] [CrossRef]
- Queudet, H.; Lemonnier, S.; Barraud, E.; Guyon, J.; Ghanbaja, J.; Allain, N.; Gaffet, E. One-step consolidation and precipitation hardening of an ultrafine-grained Al-Zn-Mg alloy powder by Spark Plasma Sintering. Mater. Sci. Eng. A 2017, 685, 227–234. [Google Scholar] [CrossRef]
- Queudet, H.; Lemonnier, S.; Barraud, E.; Ghanbaja, J.; Allain, N.; Gaffet, E. Effect of heat treatments on the microstructure of an ultrafine-grained Al-Zn-Mg alloy produced by powder metallurgy. Mater. Sci. Eng. A 2017, 685, 71–78. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al-Zn-Mg-Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Li, B.; Sun, F.; Cai, Q.; Cheng, J.; Zhao, B. Effect of TiN nanoparticles on microstructure and properties of Al2024-TiN nanocomposite by high energy milling and spark plasma sintering. J. Alloys Compd. 2017, 726, 638–650. [Google Scholar] [CrossRef]
- Miller, W.S.; Humphreys, F.J. Strengthening mechanisms in particulate metal matrix composites. Scr. Mater. 1991, 25, 33–38. [Google Scholar] [CrossRef]
- Kendig, K.L.; Miracle, D.B. Strengthening mechanisms of an Al-Mg-Sc-Zr alloy. Acta Mater. 2002, 50, 4165–4175. [Google Scholar] [CrossRef]
- Li, C.L.; Mei, Q.S.; Li, J.Y.; Chen, F.; Ma, Y.; Mei, X.M. Hall-Petch relations and strengthening of Al-ZnO composites in view of grain size relative to interparticle spacing. Scr. Mater. 2018, 153, 27–30. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Chai, L.; Wang, H.; Chen, Z.; Xiang, Z.; Jin, T. Microstructure and mechanical properties of an in-situ TiB2/Al-Zn-Mg-Cu-Zr composite fabricated by Melt-SHS process. Mater. Sci. Eng. A 2018, 720, 60–68. [Google Scholar] [CrossRef]
- Huskins, E.L.; Cao, B.; Ramesh, K.T. Strengthening mechanisms in an Al-Mg alloy. Mater. Sci. Eng. A 2010, 527, 1292–1298. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liao, X.Z.; Jin, Z.; Valiev, R.Z.; Zhu, Y.T. Microstructures and mechanical properties of ultrafine grained 7075 Al alloy processed by ECAP and their evolutions during annealing. Acta Mater. 2004, 52, 4589–4599. [Google Scholar] [CrossRef]
- Ma, K.; Wen, H.; Hu, T.; Topping, T.D.; Isheim, D.; Seidman, D.N.; Lavernia, E.J.; Schoenung, J.M. Mechanical behavior and strengthening mechanisms in ultrafine grain precipitation-strengthened aluminum alloy. Acta Mater. 2014, 62, 141–155. [Google Scholar] [CrossRef]
- Xia, P.; Liu, Z.; Bai, S.; Lu, L.; Gao, L. Enhanced fatigue crack propagation resistance in a superhigh strength Al-Zn-Mg-Cu alloy by modifying RRA treatment. Mater. Charact. 2016, 118, 438–445. [Google Scholar] [CrossRef]


| Elements | Al | Zn | Mg | Cu |
|---|---|---|---|---|
| Mesh size (μm) | 38–75 | 4–23 | 75–150 | 3–20 |
| Purity (%) | 99.5 | 99.99 | 99 | 99.99 |
| Sample | Grain Size d (nm) | Micro Strain ε (%) |
|---|---|---|
| P5 | 58.9 ± 3.9 | 0.053 ± 0.0083 |
| P10 | 49.1 ± 1.9 | 0.138 ± 0.0069 |
| P15 | 51.1 ± 2.4 | 0.177 ± 0.0076 |
| P20 | 28.9 ± 0.6 | 0.135 ± 0.0075 |
| P25 | 28.0 ± 0.7 | 0.164 ± 0.0074 |
| P30 | 22.3 ± 0.6 | 0.156 ± 0.0072 |
| P40 | 23.0 ± 0.5 | 0.138 ± 0.0084 |
| Sample | S20 | S25 | S30 | S40 |
|---|---|---|---|---|
| Grain size d (nm) | 73.5 ± 3.3 | 58.5 ± 2.1 | 53.5 ± 2.0 | 60.5 ± 2.2 |
| Micro strain ε (%) | 0.03 ± 0.006 | 0.05 ± 0.005 | 0.075 ± 0.0056 | 0.085 ± 0.0045 |
| Phase | MgZn2 (η) | Al2CuMg (S) | Al2Cu (θ) |
|---|---|---|---|
| ΔH (kJ/mol) | −10.36 | −6.645 | −3.86 |
| Eb (kJ/mol) | 142.06 | 319.7 | 358.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, J.; Cai, Q.; Zhao, B.; Yang, S.; Chen, F.; Li, B. Microstructure and Mechanical Properties of Nanocrystalline Al-Zn-Mg-Cu Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials 2019, 12, 1255. https://doi.org/10.3390/ma12081255
Cheng J, Cai Q, Zhao B, Yang S, Chen F, Li B. Microstructure and Mechanical Properties of Nanocrystalline Al-Zn-Mg-Cu Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials. 2019; 12(8):1255. https://doi.org/10.3390/ma12081255
Chicago/Turabian StyleCheng, Jingfan, Qizhou Cai, Bingyi Zhao, Songfeng Yang, Fei Chen, and Bing Li. 2019. "Microstructure and Mechanical Properties of Nanocrystalline Al-Zn-Mg-Cu Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering" Materials 12, no. 8: 1255. https://doi.org/10.3390/ma12081255
APA StyleCheng, J., Cai, Q., Zhao, B., Yang, S., Chen, F., & Li, B. (2019). Microstructure and Mechanical Properties of Nanocrystalline Al-Zn-Mg-Cu Alloy Prepared by Mechanical Alloying and Spark Plasma Sintering. Materials, 12(8), 1255. https://doi.org/10.3390/ma12081255




