Controlling the Composition and Magnetic Properties of Nano-SrFe12O19 Powder Synthesized from Oily Cold Mill Sludge by the Citrate Precursor Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
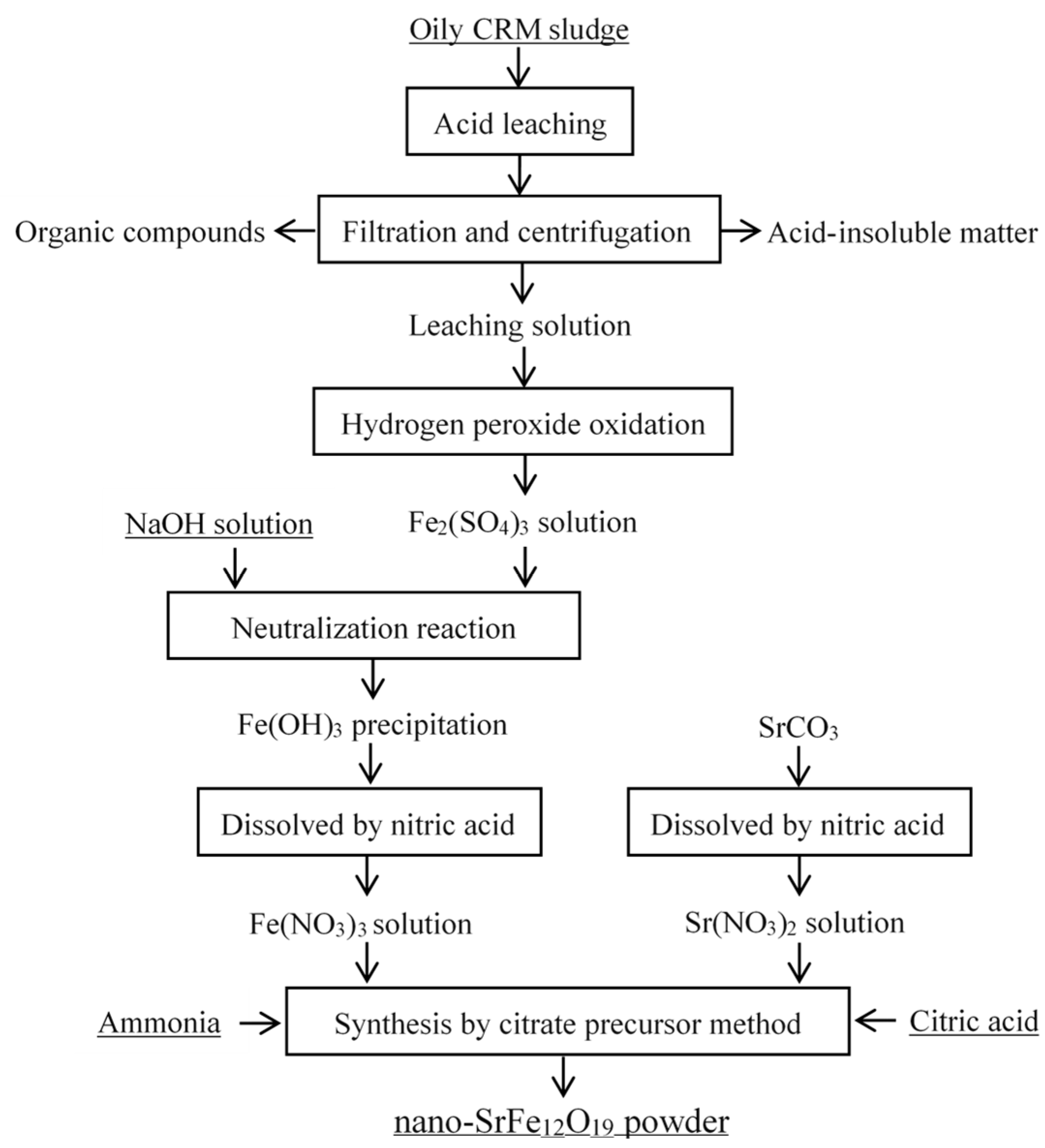
2.2. Treatment of Oily CRM Sludge
2.3. Preparation of Strontium Ferrites
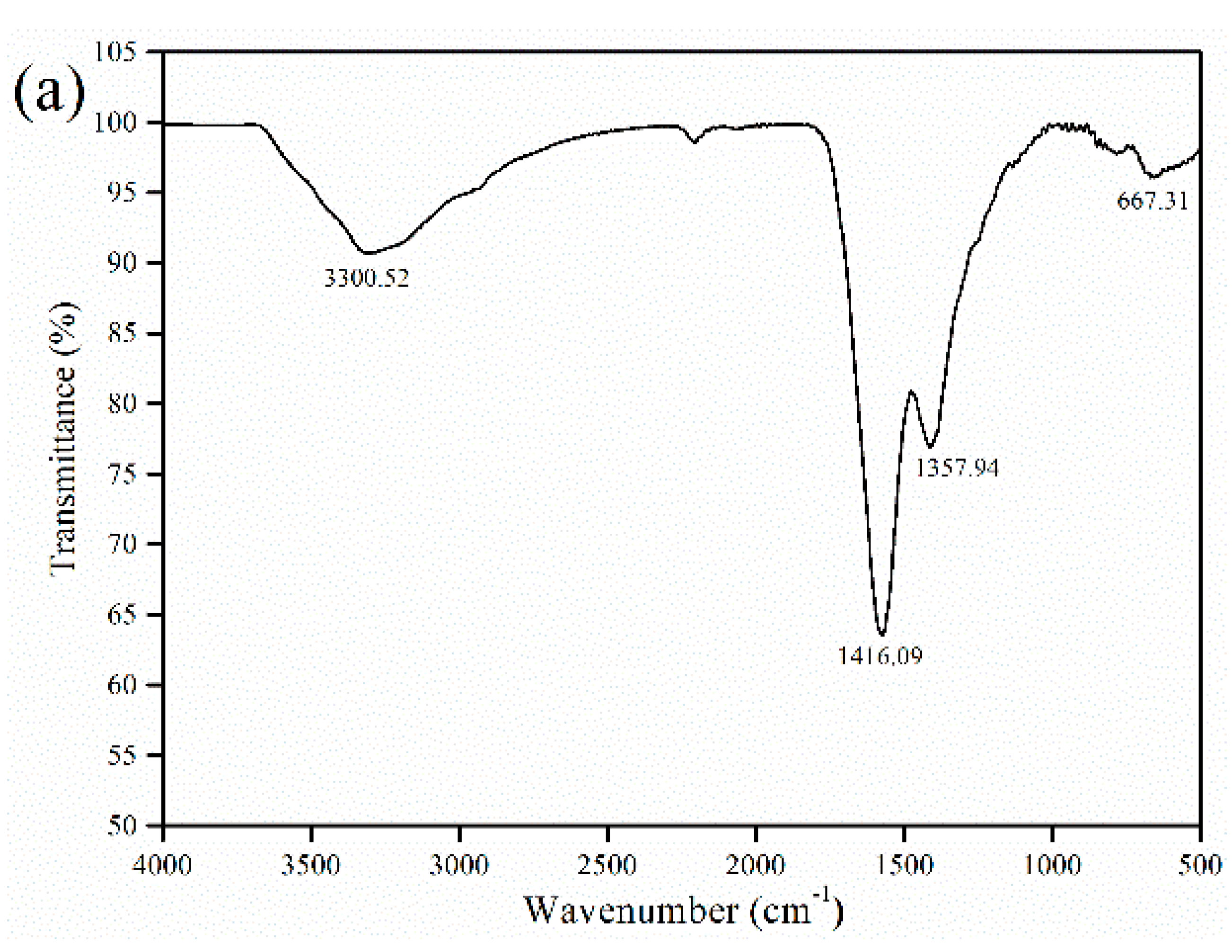
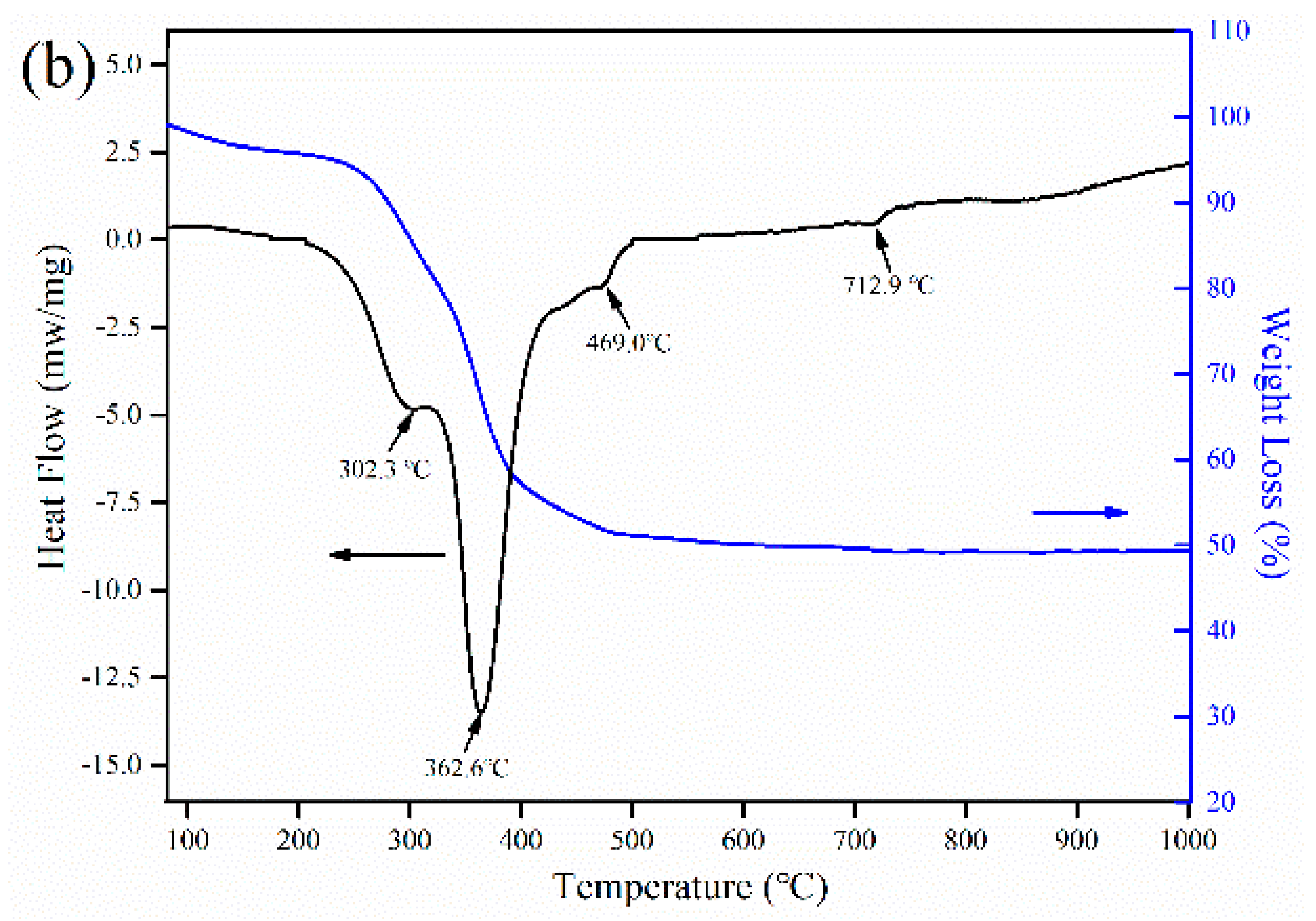
2.4. Characterization
3. Results and Discussion
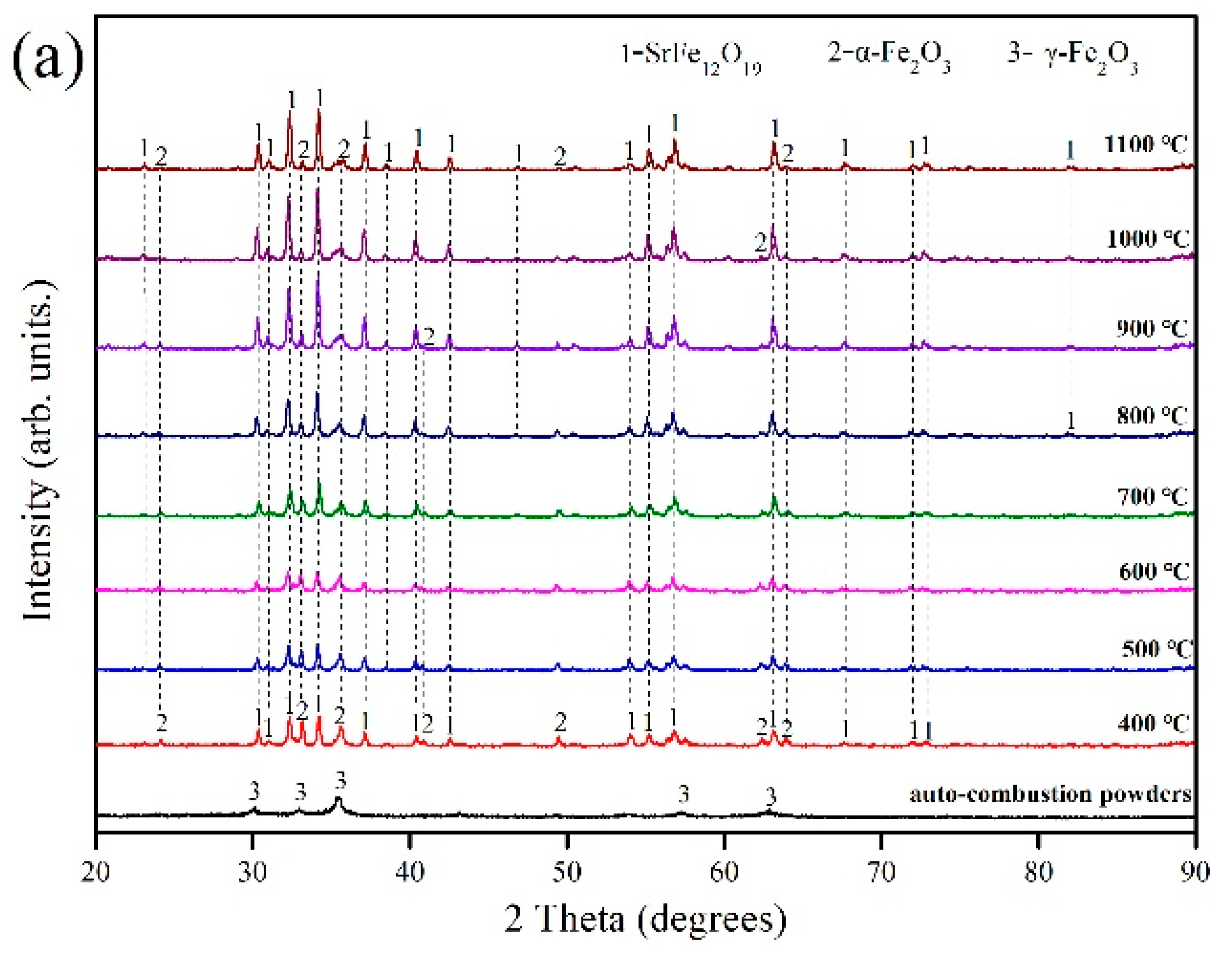
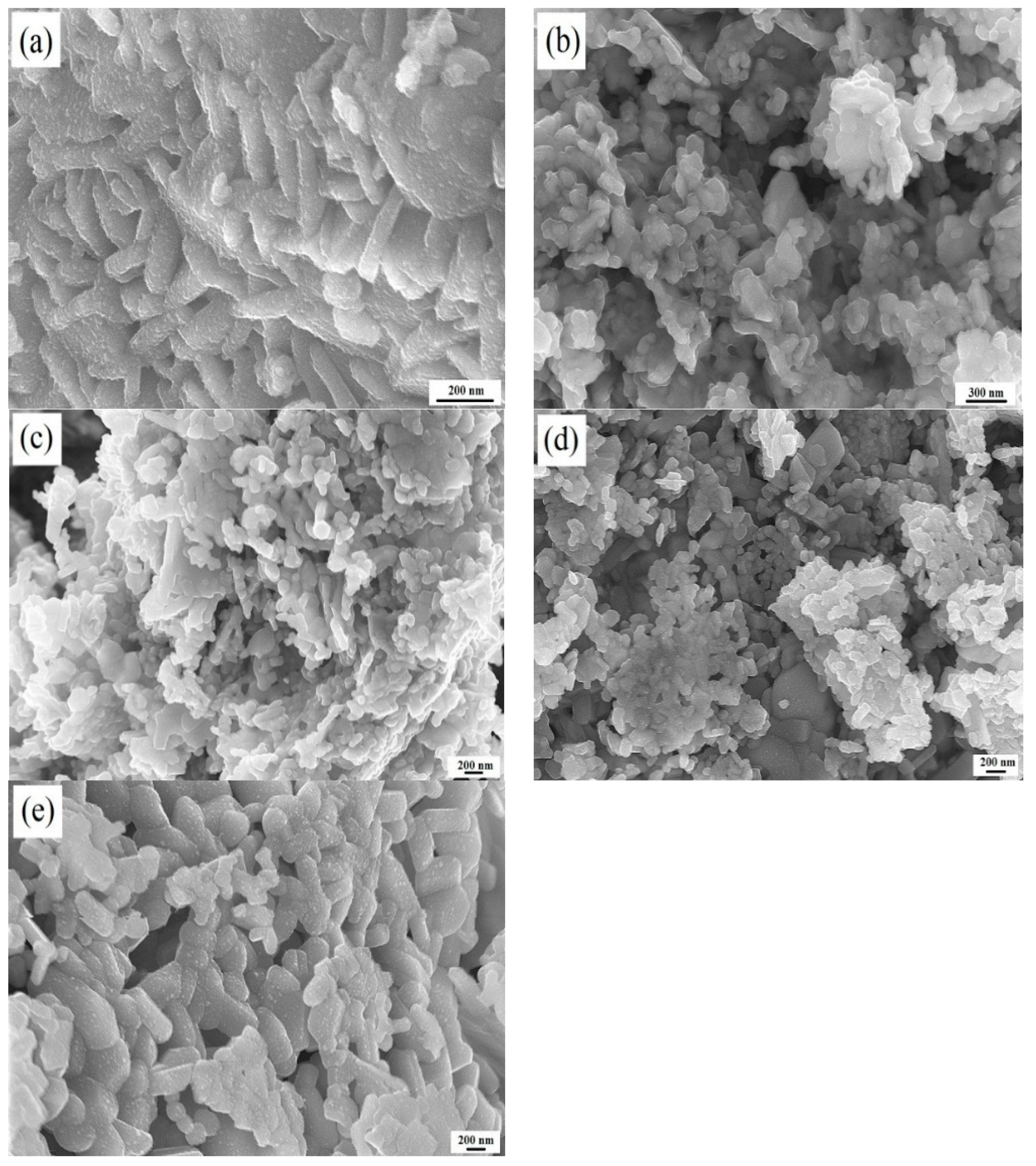
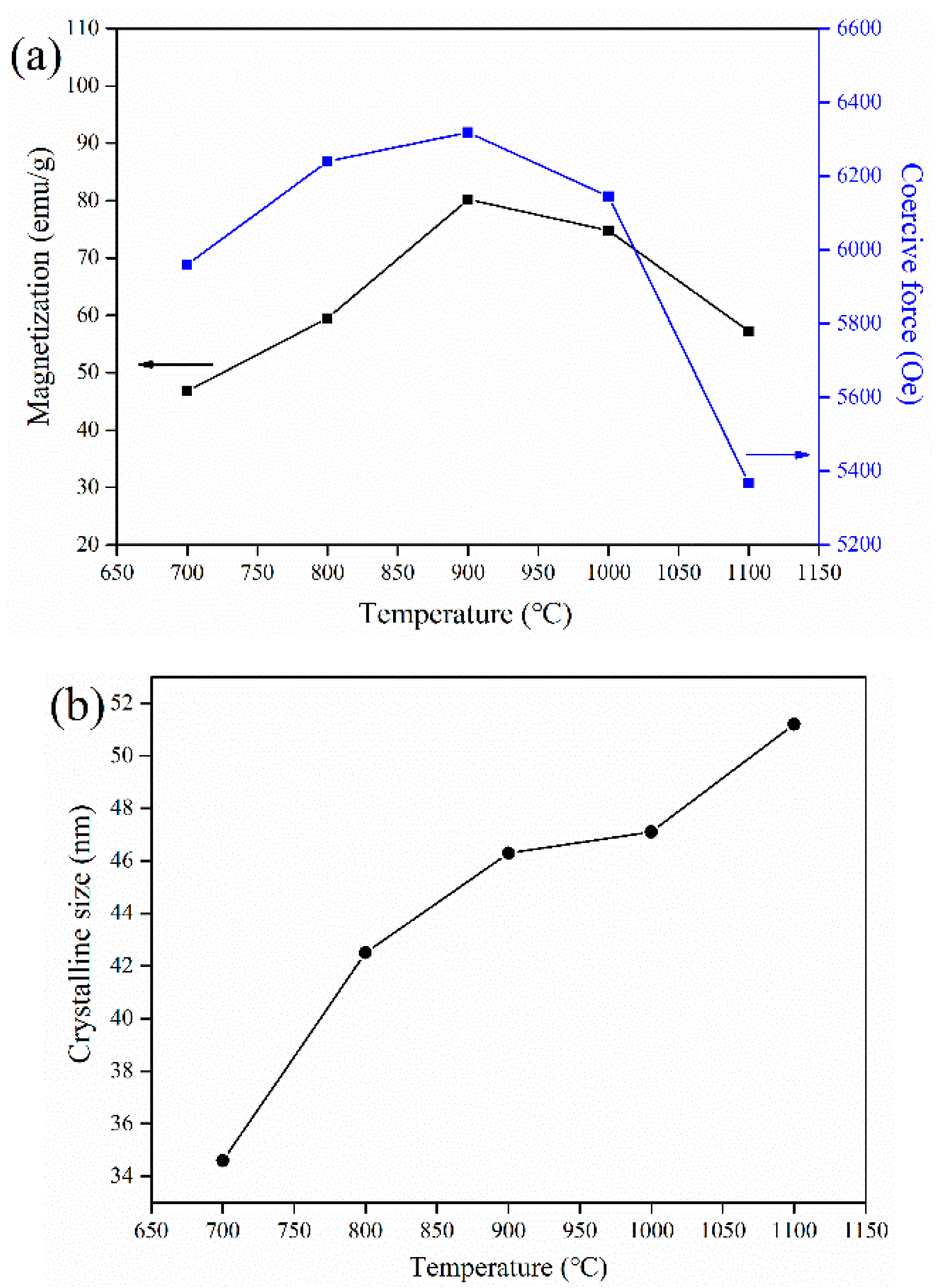
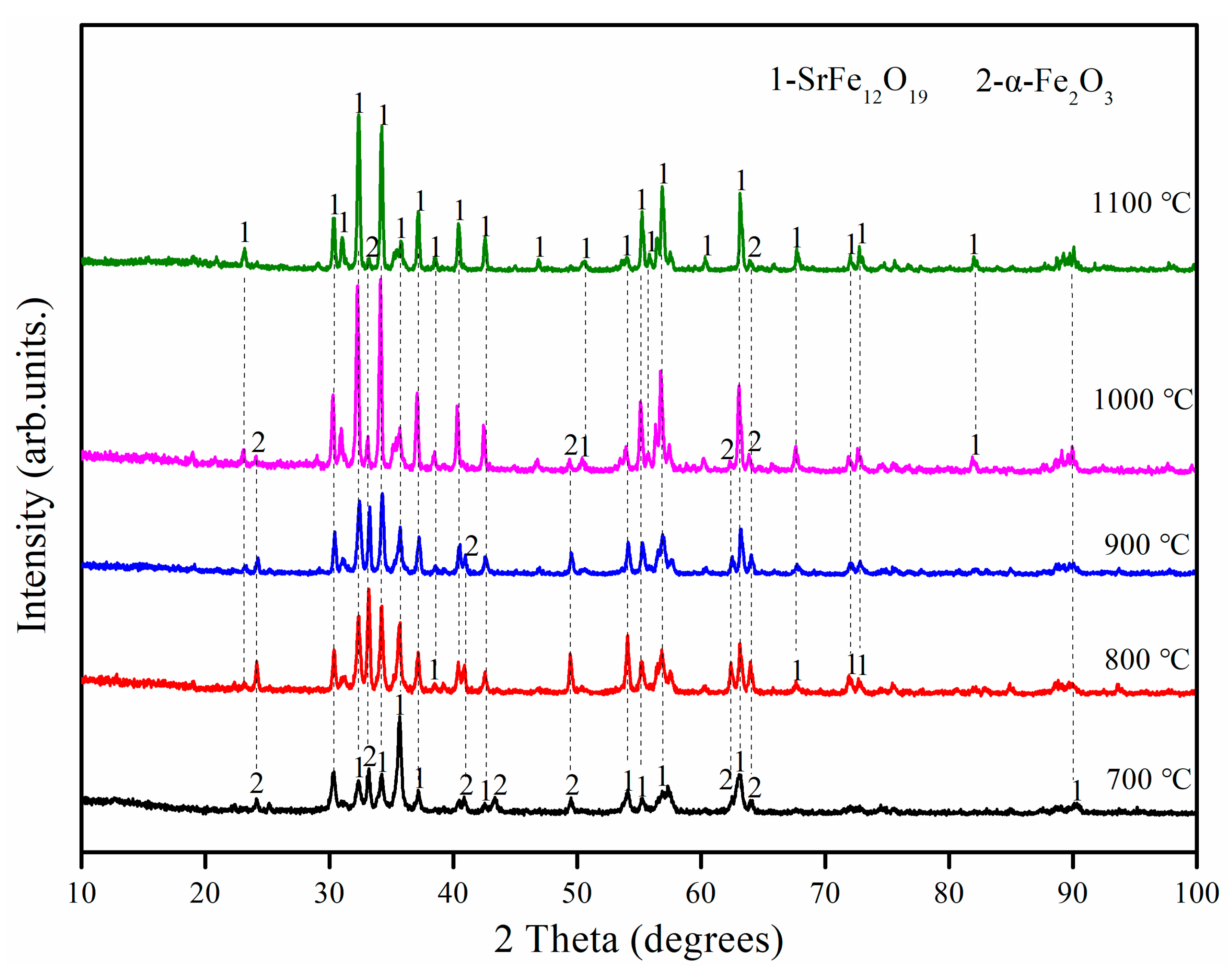
3.1. Effect of Annealing Temperature
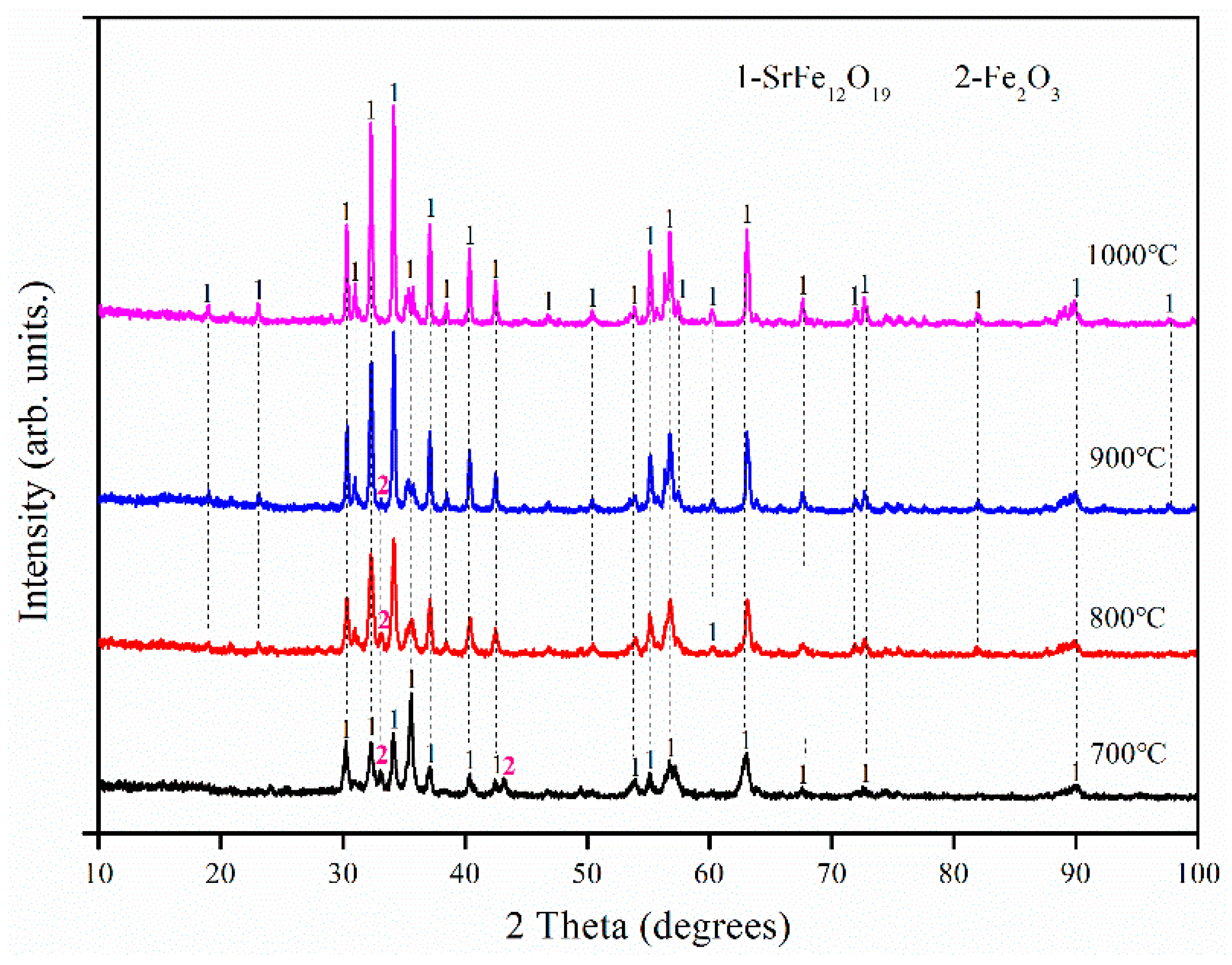
3.2. Effect of Fe/Sr Molar Ratio
3.3. Comparison of Magnetic Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ramezanzaeh, G.; Ghasemi, A.; Mozaffarinia, R.; Alizadeh, A. Electromagnetic wave reflection loss and magnetic properties of M-type SrFe12−x(Mn0.5Sn0.5)xO19 hexagonal ferrite nanoparticles in the Ku microwave band. Ceram. Int. 2017, 43, 10231–10238. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Zhang, L.T.; Abbas, W.; Murtaza, G. Synthesis and characterizations of Al-Sm substituted Ba-Sr M-type hexagonal ferrite nanoparticles via sol–gel route. Ceram. Int. 2018, 44, 18678–18685. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Kostishyn, V.G.; Panina, L.V.; Korovushkin, V.V.; Turchenko, V.A.; Thakur, P.; Thakur, A.; Yang, Y.; Vinnik, D.A.; Yakovenko, E.S. Control of electromagnetic properties in substituted M-type hexagonal ferrites. J. Alloys Compd. 2018, 754, 247–256. [Google Scholar] [CrossRef]
- Ueda, H.; Tanioku, Y.; Michioka, C.; Yoshimura, K. Magnetocrystalline anisotropy of La- and Co-substituted M-type strontium ferrites: Role of Co2+ and Fe2+. Phys. Rev. B 2017, 95, 224421. [Google Scholar] [CrossRef]
- Hessien, M.M.; Rashad, M.M.; El-Barawy, K. Controlling the composition and magnetic properties of strontium hexaferrite synthesized by co-precipitation method. J. Magn. Magn. Mater. 2008, 320, 336–343. [Google Scholar] [CrossRef]
- Das, T.; Nicholas, J.D.; Qi, Y. Long-range charge transfer and oxygen vacancy interactions in strontium ferrite. J. Mater. Chem. A 2017, 5, 4493–4506. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhao, L.; Wang, P.H.; Guo, L.; Yu, J.H. Low material density and high microwave-absorption performance of hollow strontium ferrite nanofibers prepared via coaxial electrospinning. J. Alloys Compd. 2016, 687, 541–547. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, X.S.; Feng, S.J.; Rehman, K.M.U.; Li, M.L.; Zhang, C.; Li, H.H.; Meng, X.Y. Microstructure and magnetic properties of M-type strontium hexagonal ferrites with Y-Co substitution. J. Magn. Magn. Mater. 2017, 436, 126–129. [Google Scholar] [CrossRef]
- Spaoletova, N.; Kushnir, S.; Ahn, K.; An, S.Y.; Choi, M.; Kim, J.Y.; Choi, C.; Wi, S. M-Zn (M = Sb, V, and Nb) Substituted Strontium Hexaferrites with Enhaced Saturation Magnetization for Permanent Magnet Applications. J. Magn. 2016, 21, 315–321. [Google Scholar] [CrossRef]
- Liu, J.R.; Hong, R.Y.; Feng, W.G.; Badami, D.; Wang, Y.Q. Large-scale production of strontium ferrite by molten-salt-assisted coprecipitation. Powder Technol. 2014, 262, 142–149. [Google Scholar] [CrossRef]
- Anis-ur-Rehman, M.; Asghar, G. Variation in structural and dielectric properties of co-precipitated nanoparticles strontium ferrites due to value of pH. J. Alloys Compd. 2011, 509, 435–439. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Li, L.; Qi, S. Preparation and excellent microwave absorption properties of silver/strontium ferrite/graphite nanosheet composites via sol–gel method. J. Mater. Sci.: Mater. Electron. 2016, 27, 10045–10051. [Google Scholar] [CrossRef]
- Xia, A.; Zuo, C.; Chen, L.; Jin, C.; Lv, Y. Hexagonal SrFe12O19 ferrites: Hydrothermal synthesis and their sintering properties. J. Magn. Magn. Mater. 2013, 332, 186–191. [Google Scholar] [CrossRef]
- Lei, C.; Tang, S.; Du, Y. Synthesis of aligned La3+-substituted Sr-ferrites via molten salt assisted sintering and their magnetic properties. Ceram. Int. 2016, 42, 15511–15516. [Google Scholar] [CrossRef]
- Li, Y.; Bao, D.; Wang, Z.; Ye, H.; Kong, B. Synthesis of Ca2+ doped SrLa-ferrite powder through molten salt assisted calcination process. J. Alloys Compd. 2018, 765, 201–206. [Google Scholar]
- Alamolhoda, S.; Mirkazemi, S.M.; Ghiami, Z.; Niyaifar, M. Structure and magnetic properties of Zr-Mn substituted strontium hexaferrite Sr(Zr,Mn)xFe12−2xO19 nanoparticles synthesized by sol–gel auto-combustion method. Bull. Mater. Sci. 2016, 39, 1311–1318. [Google Scholar] [CrossRef]
- Masoudpanah, S.M.; Seyyed Ebrahimi, S.A. Effect of citric acid content on the structural and magnetic properties of SrFe12O19 thin films. Thin Solid Films 2011, 520, 199–203. [Google Scholar] [CrossRef]
- Kaur, P.; Chawla, S.K.; Meena, S.S.; Yusuf, S.M.; Bindra Narang, S. Synthesis of Co-Zr doped nanocrystalline strontium hexaferrites by sol–gel auto-combustion route using sucrose as fuel and study of their structural, magnetic and electrical properties. Ceram. Int. 2016, 42, 14475–14489. [Google Scholar] [CrossRef]
- Thakur, A.; Singh, R.R.; Barman, P.B. Structural and magnetic properties of La3+ substituted strontium hexaferrite nanoparticles prepared by citrate precursor method. J. Magn. Magn. Mater. 2013, 326, 35–40. [Google Scholar] [CrossRef]
- Brightlin, B.C.; Balamurugan, S.; Arun, T. Microstructural and magnetic features of SrFe12O19 materials synthesized from different fuels by sol–gel auto-combustion method. J. Supercond. Novel Magn. 2017, 30, 1427–1437. [Google Scholar] [CrossRef]
- Roohani, E.; Arabi, H.; Sarhaddi, R.; Sudkhah, S.; Shabani, A. Effect of annealing temperature on structural and magnetic properties of strontium hexaferrite nanoparticles synthesized by sol–gel auto-combustion method. Int. J. Mod. Phys. B 2015, 29, 1550190. [Google Scholar] [CrossRef]
- Chawla, S.K.; Kaur, P.; Mudsainiyan, R.K.; Meena, S.S.; Yusuf, S.M. Effect of Fuel on the Synthesis, Structural, and Magnetic Properties of M-Type Hexagonal SrFe12O19 Nanoparticles. J. Supercond. Novel Magn. 2015, 28, 1589–1599. [Google Scholar] [CrossRef]
- Durmus, Z.; Sozeri, H.; Toprak, M.S.; Baykal, A. Effect of Fuel on the Synthesis and Properties of Poly(methyl methacrylate) Modified SrFe12O19 Nanoparticles. J. Supercond. Novel Magn. 2012, 25, 1957–1963. [Google Scholar] [CrossRef]
- Roohani, E.; Arabi, H.; Sarhaddi, R.; Sudkhah, S. M-Type Strontium Hexaferrite Nanoparticles Prepared by Sol–gel Auto-combustion Method: The Role of Co Substitution in Structural, Morphological, and Magnetic Properties. J. Supercond. Novel Magn. 2017, 30, 1599–1608. [Google Scholar] [CrossRef]
- Hessien, M.M.; Rashad, M.M.; Hassan, M.S.; El-Barawy, K. Synthesis and magnetic properties of strontium hexaferrite from celestite ore. J. Alloys Compd. 2009, 476, 373–378. [Google Scholar] [CrossRef]
- Xie, T.; Xu, L.; Liu, C.; Ding, S.; Yang, J.; Wu, W. Synthesis and adsorption properties of high specific surface area strontium ferrite from Industrial Strontium Residue. Vacuum 2013, 93, 71–78. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, S.G.; Tian, J.J.; Pan, D.A.; Zhu, H.X. Strontium ferrite powders prepared from oily cold rolling mill sludge by solid-state reaction method. Rare Met. 2013, 32, 518–523. [Google Scholar] [CrossRef]
- Hu, P.; Pan, D.A.; Zhang, S.G.; Tian, J.J.; Volinsky, A.A. Mn-Zn soft magnetic ferrite nanoparticles synthesized from spent alkaline Zn-Mn batteries. J. Alloys Compd. 2011, 509, 3991–3994. [Google Scholar] [CrossRef]
- Winburn, R.S.; Grier, D.G.; Mccarthy, G.J.; Peterson, R.B. Rietveld quantitative X-ray diffraction analysis of NIST fly ash standard reference materials. Powder Diffr. 2000, 15, 163–172. [Google Scholar] [CrossRef]
- Gordani, G.R.; Ghasemi, A.; Saidi, A. Enhanced magnetic properties of substituted Sr-hexaferrite nanoparticles synthesized by co-precipitation method. Ceram. Int. 2014, 40, 4945–4952. [Google Scholar] [CrossRef]
- Chen, W.; Wu, W.W.; Zhou, C.; Zhou, S.F.; Li, M.Y.; Ning, Y. Structural and magnetic properties evolution of Co-Nd substituted M-type hexagonal strontium ferrites synthesized by ball-milling-assisted ceramic process. J. Electron. Mater. 2017, 47, 2110–2119. [Google Scholar] [CrossRef]
- Grewall, J.K.; Kaur, M. Effect of core-shell reversal on the structural, magnetic and adsorptive properties of Fe2O3-GO nanocomposites. Ceram. Int. 2017, 43, 16611–16621. [Google Scholar] [CrossRef]
- Loan, T.T.; Nga, T.T.V.; Duong, N.P.; Soontaranon, S.; Hien, T.D. Influence of Structure and Oxidation State on Magnetic Properties of Sr1−xLaxFe12−xCoxO19 Nanoparticles Prepared by Sol–gel Combustion Method. J. Electron. Mater. 2017, 46, 3396–3405. [Google Scholar] [CrossRef]
- Jean, M.; Nachbaur, V.; Bran, J.; Le Breton, J.M. Synthesis and characterization of SrFe12O19 powder obtained by hydrothermal process. J. Alloys Compd. 2010, 496, 306–312. [Google Scholar] [CrossRef]
- Wang, Z.; Zhong, L.; Lv, J.; Zheng, H.Q.Y.; Fang, Y.; Jin, M.; Xu, J. Microwave-assisted synthesis of SrFe12O19 hexaferrites. J. Magn. Magn. Mater. 2010, 322, 2782–2785. [Google Scholar]

| Component | Content (wt %) |
|---|---|
| Fe | 70.6 |
| Ni | 0.049 |
| Mn | 0.18 |
| Cr | 0.065 |
| Si | 0.058 |
| V | 0.024 |
| Oil and moisture | 18.2 |
| Other | 10.82 |
| Annealing Temperature (°C) | Phase Content | Crystalline Size (nm) | Magnetic Properties | ||
|---|---|---|---|---|---|
| Ms (emu/g) | Mr (emu/g) | Hc (Oe) | |||
| 700 | 73% SrFe12O19 27% α-Fe2O3 | 29.6 | 40.8 ± 0.1 | 21.3 ± 0.1 | 854 ± 70 |
| 800 | 80% SrFe12O19 20% α-Fe2O3 | 30.2 | 42.7 ± 0.1 | 21.8 ± 0.1 | 4770 ± 50 |
| 900 | 86% SrFe12O19 14% α-Fe2O3 | 34.6 | 46.9 ± 0.1 | 24.2 ± 0.1 | 5260 ± 50 |
| 1000 | 92% SrFe12O19 8% α-Fe2O3 | 44.6 | 59.8 ± 0.1 | 31.1 ± 0.1 | 5080 ± 40 |
| Annealing Temperature (°C) | Phase Content | Crystalline Size (nm) | Magnetic Properties | ||
|---|---|---|---|---|---|
| Ms (emu/g) | Mr (emu/g) | Hc (Oe) | |||
| 700 | 85% SrFe12O19 15% α-Fe2O3 | 45.5 | 45.5 ± 0.1 | 21.3 ± 0.1 | 1170.1 ± 60 |
| 800 | 90% SrFe12O19 10% α-Fe2O3 | 50.1 | 50.1 ± 0.1 | 26.6 ± 0.1 | 5737.9 ± 30 |
| 900 | 93% SrFe12O19 7% α-Fe2O3 | 58.1 | 58.1 ± 0.1 | 31.1 ± 0.1 | 6437.8 ± 20 |
| 1000 | 100% SrFe12O19 | 74.2 | 67.5 ± 0.1 | 36.1 ± 0.1 | 6176.0 ± 20 |
| Sample | Synthetic Method | Magnetic Properties | ||
|---|---|---|---|---|
| Ms (emu/g) | Mr (emu/g) | Hc (Oe) | ||
| SrFe12O19@900 | CPM | 80.2 | 39.8 | 6318 |
| SrFe11.6O19@1000 | CPM | 67.5 | 36.1 | 6176 |
| SrFe12O19 powder [20] | MA-SGM | 54.8 | 29.5 | 5261 |
| Sr0.9La0.1Fe11.9Co0.1O19 powder [20] | SGM | 73 | 36 | 7700 |
| Sr0.85Nd0.15Fe12O19 powder [21] | CPM | 63 | 35.15 | 6885 |
| SrFe12O19 nanoribbons [22] | SAE | 67.9 | 37.3 | 7310 |
| SrFe12O19 powder [23] | SGM | 59.3 | 34.9 | 6725 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Zhang, S.; Steenari, B.-M.; Ekberg, C. Controlling the Composition and Magnetic Properties of Nano-SrFe12O19 Powder Synthesized from Oily Cold Mill Sludge by the Citrate Precursor Method. Materials 2019, 12, 1250. https://doi.org/10.3390/ma12081250
Liu B, Zhang S, Steenari B-M, Ekberg C. Controlling the Composition and Magnetic Properties of Nano-SrFe12O19 Powder Synthesized from Oily Cold Mill Sludge by the Citrate Precursor Method. Materials. 2019; 12(8):1250. https://doi.org/10.3390/ma12081250
Chicago/Turabian StyleLiu, Bo, Shengen Zhang, Britt-Marie Steenari, and Christian Ekberg. 2019. "Controlling the Composition and Magnetic Properties of Nano-SrFe12O19 Powder Synthesized from Oily Cold Mill Sludge by the Citrate Precursor Method" Materials 12, no. 8: 1250. https://doi.org/10.3390/ma12081250
APA StyleLiu, B., Zhang, S., Steenari, B.-M., & Ekberg, C. (2019). Controlling the Composition and Magnetic Properties of Nano-SrFe12O19 Powder Synthesized from Oily Cold Mill Sludge by the Citrate Precursor Method. Materials, 12(8), 1250. https://doi.org/10.3390/ma12081250






