Enhanced High-Temperature DC Dielectric Performance of Crosslinked Polyethylene with a Polystyrene Pinning Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. DC Dielectric Performance Measurements
2.3. Morphology and Structure Characterization
3. Results
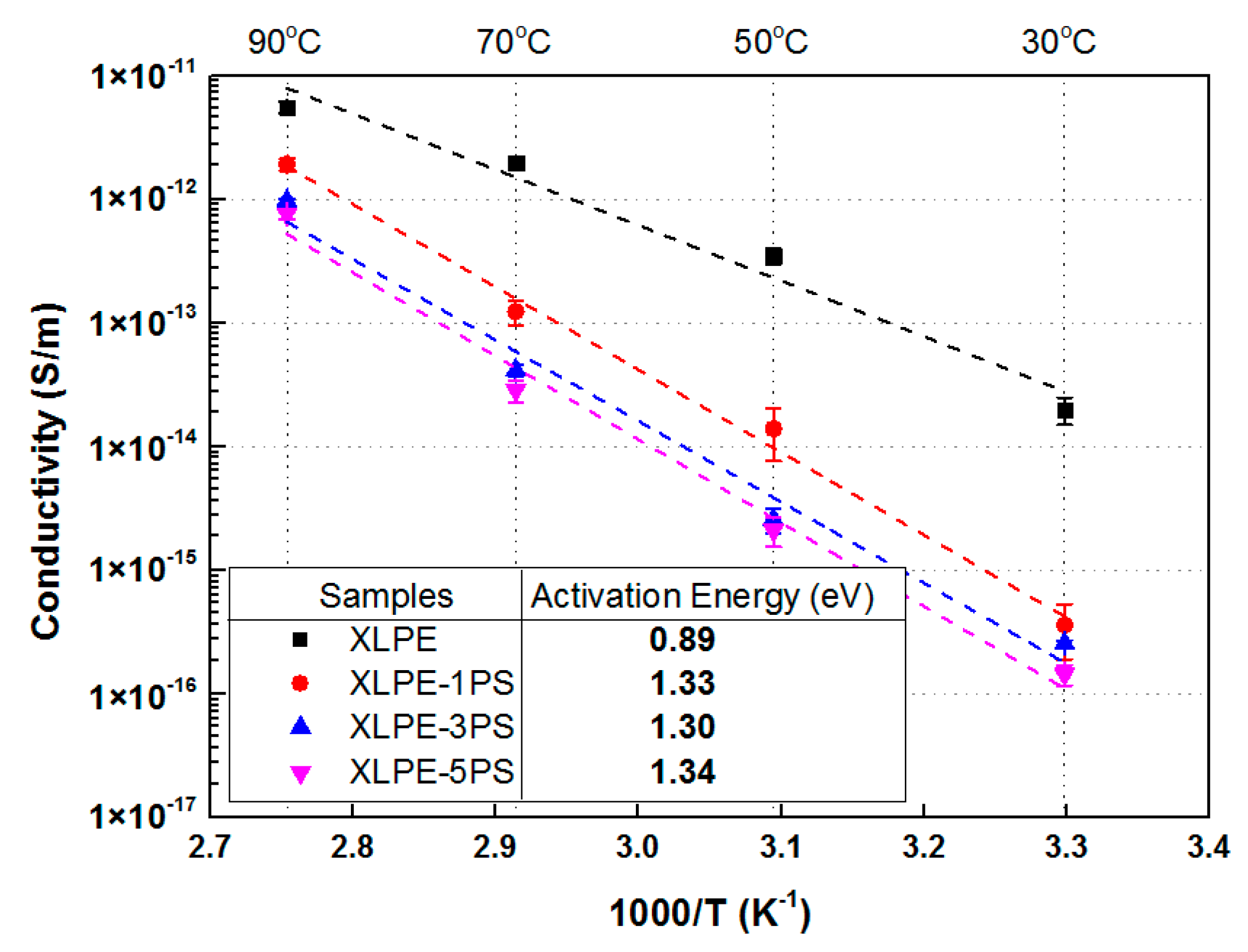
3.1. DC Conductivity Properties
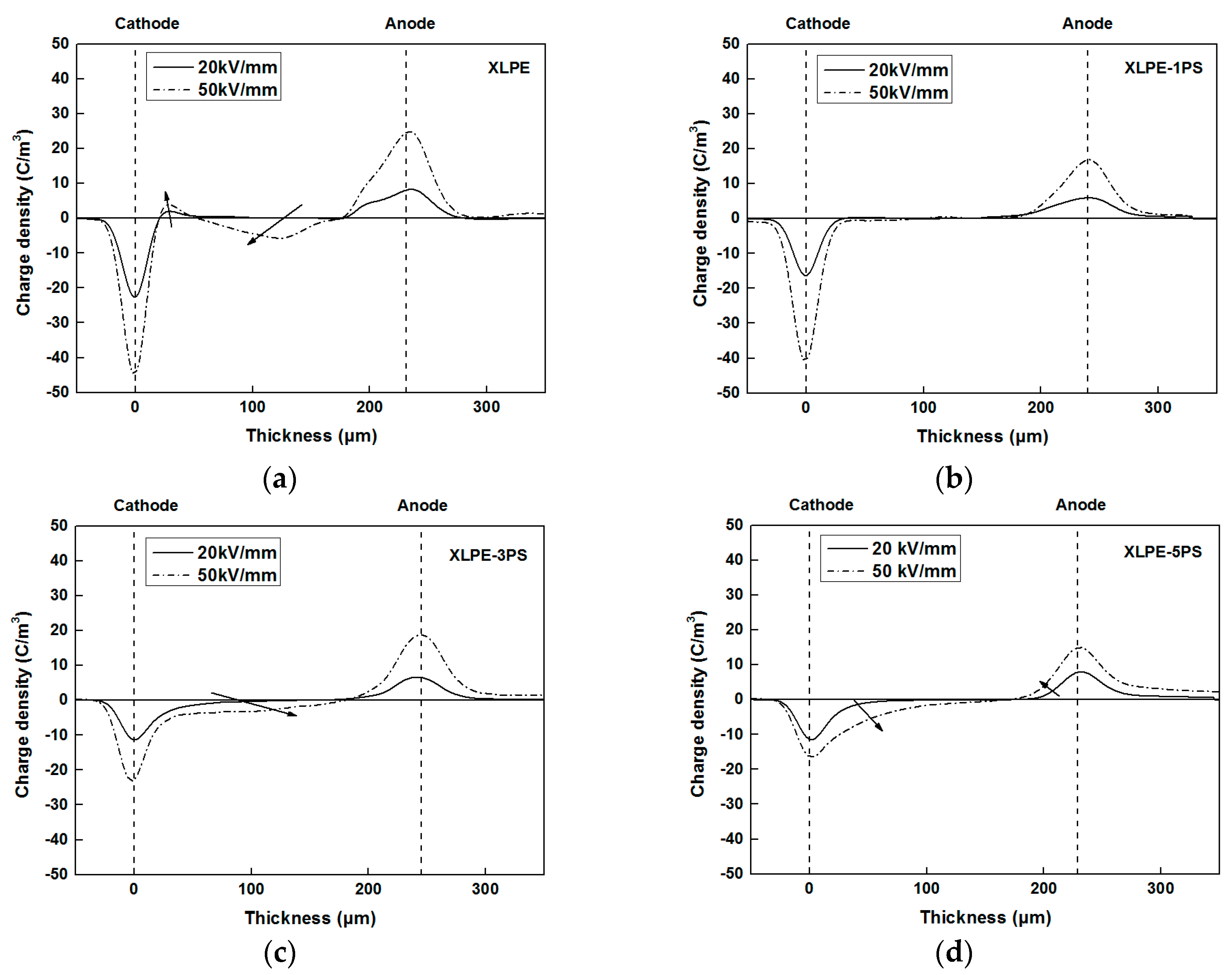
3.2. Space Charge Behaviors
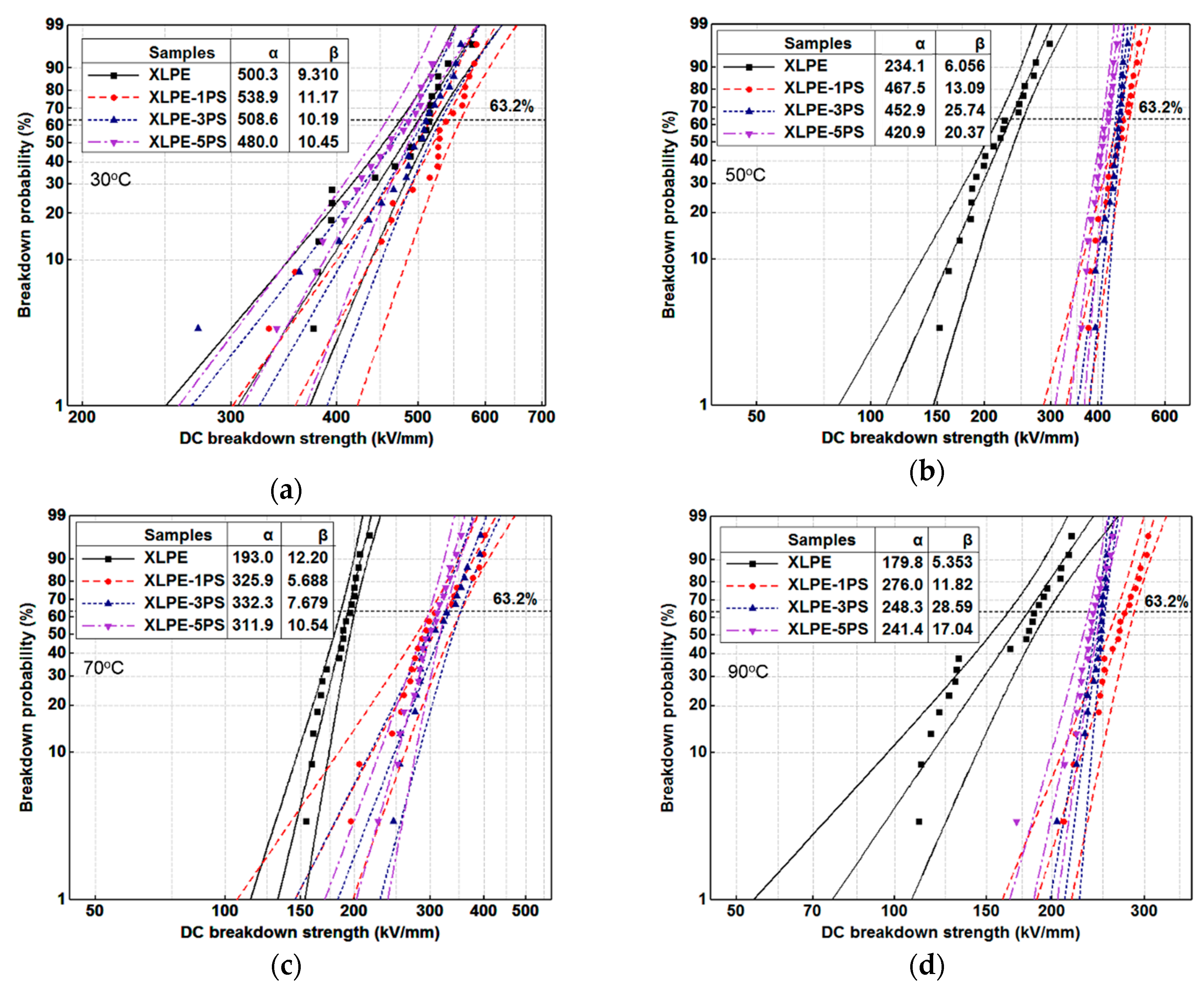
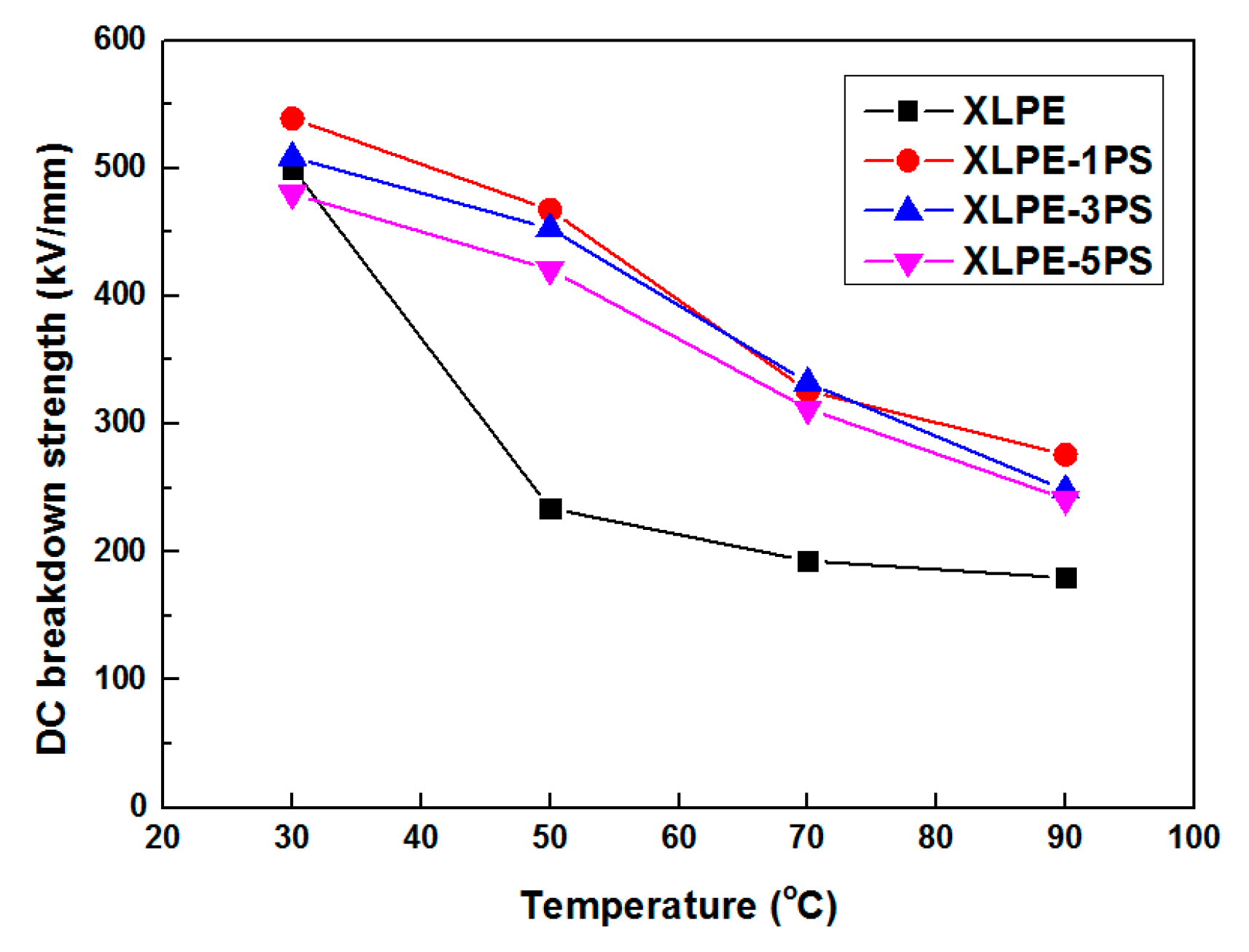
3.3. DC Breakdown Strength
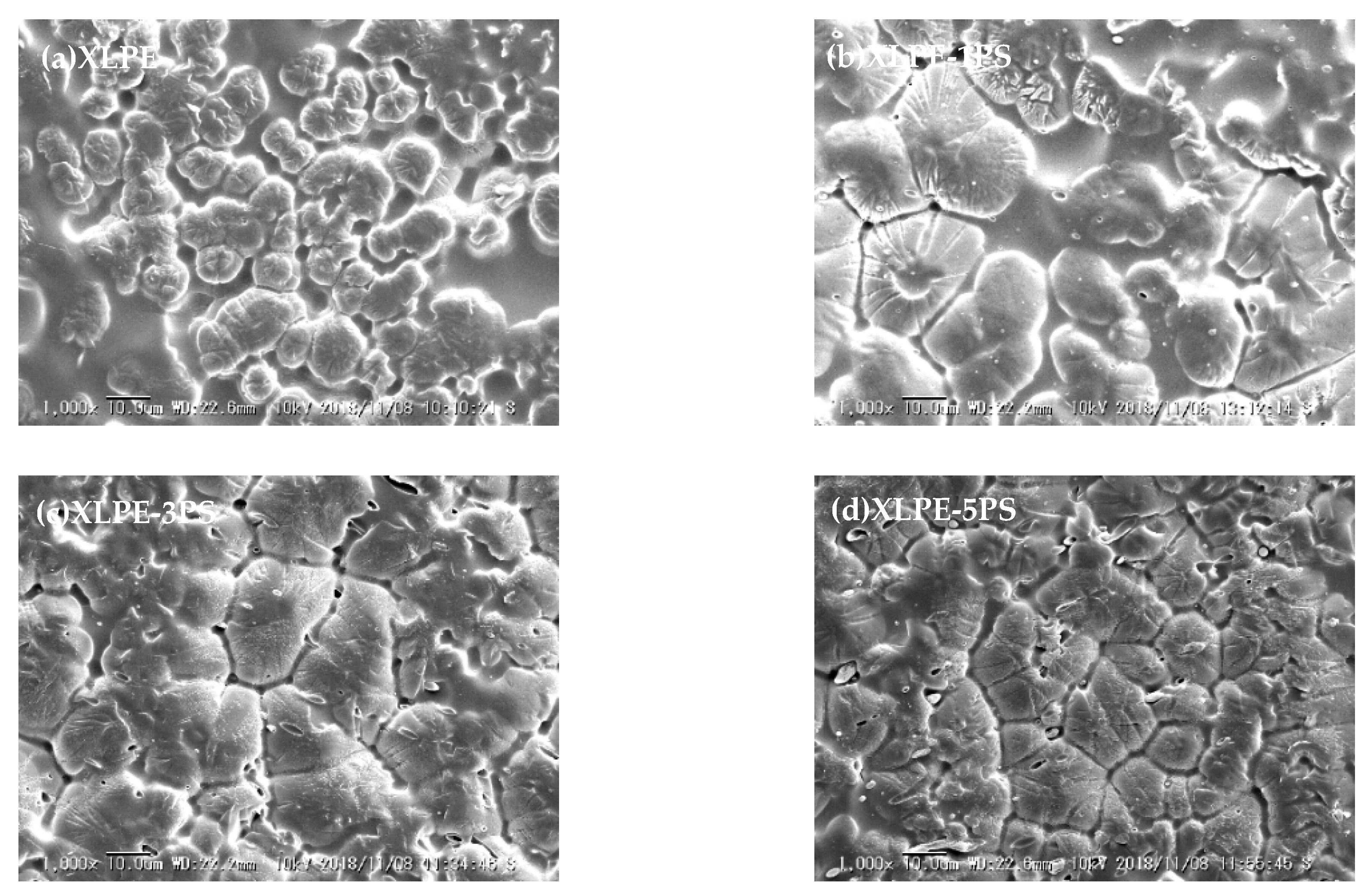
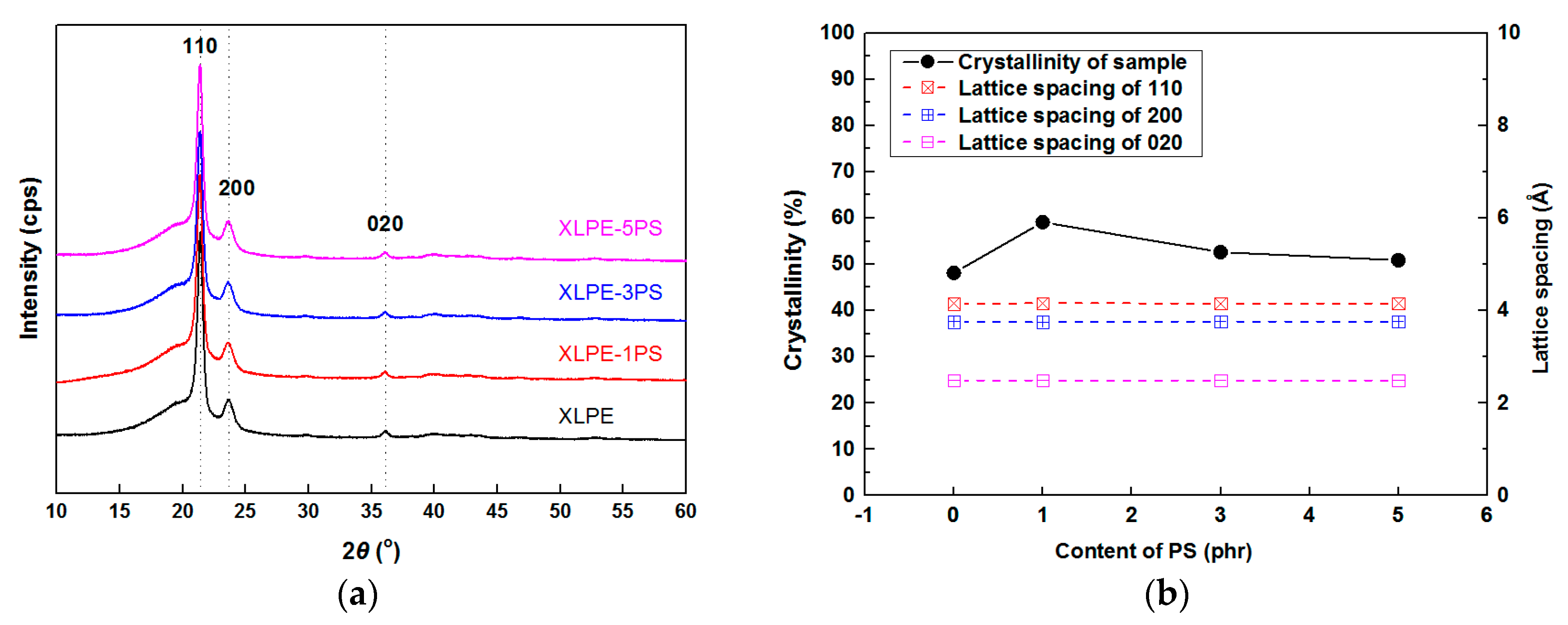
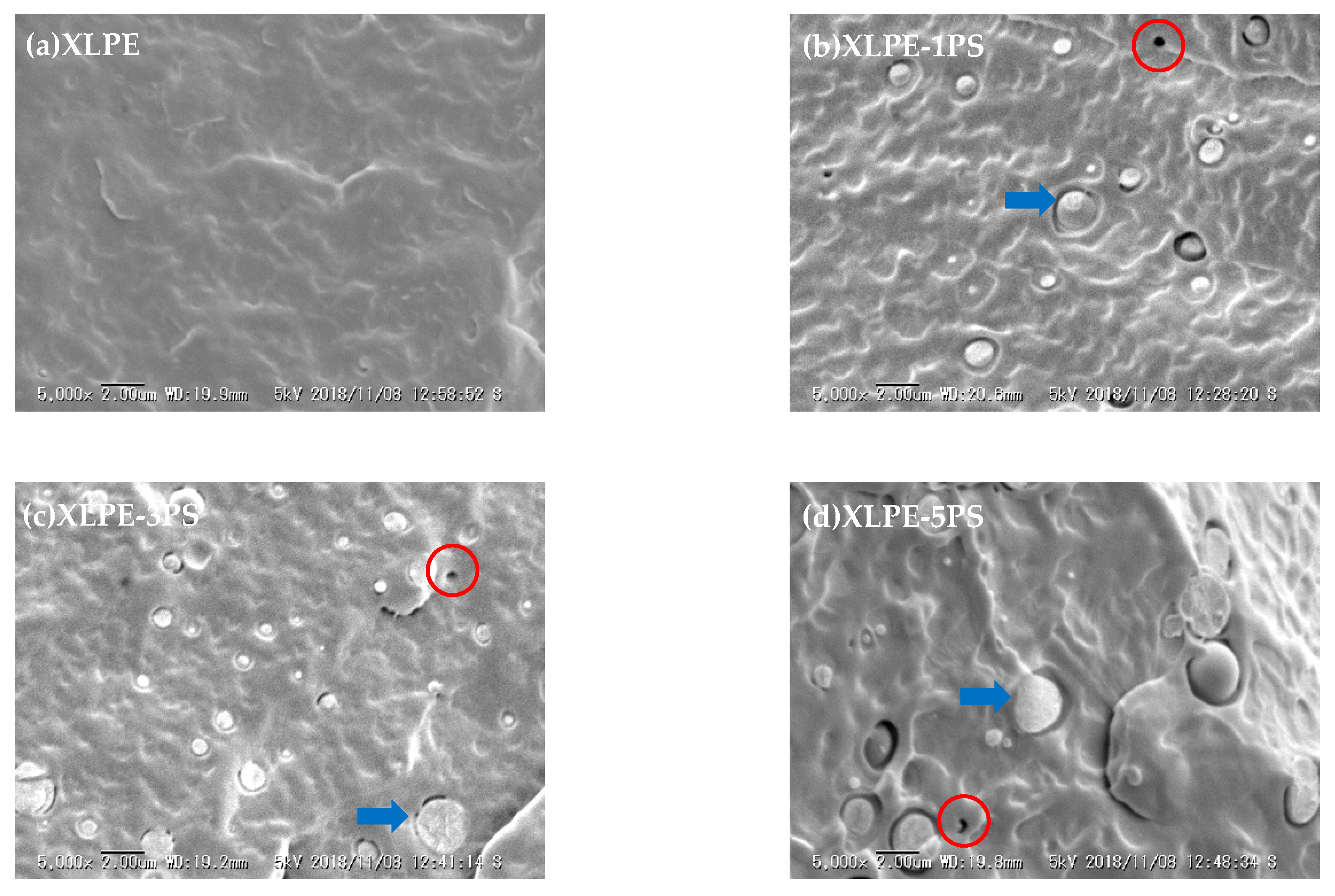
3.4. Structure Characteristics
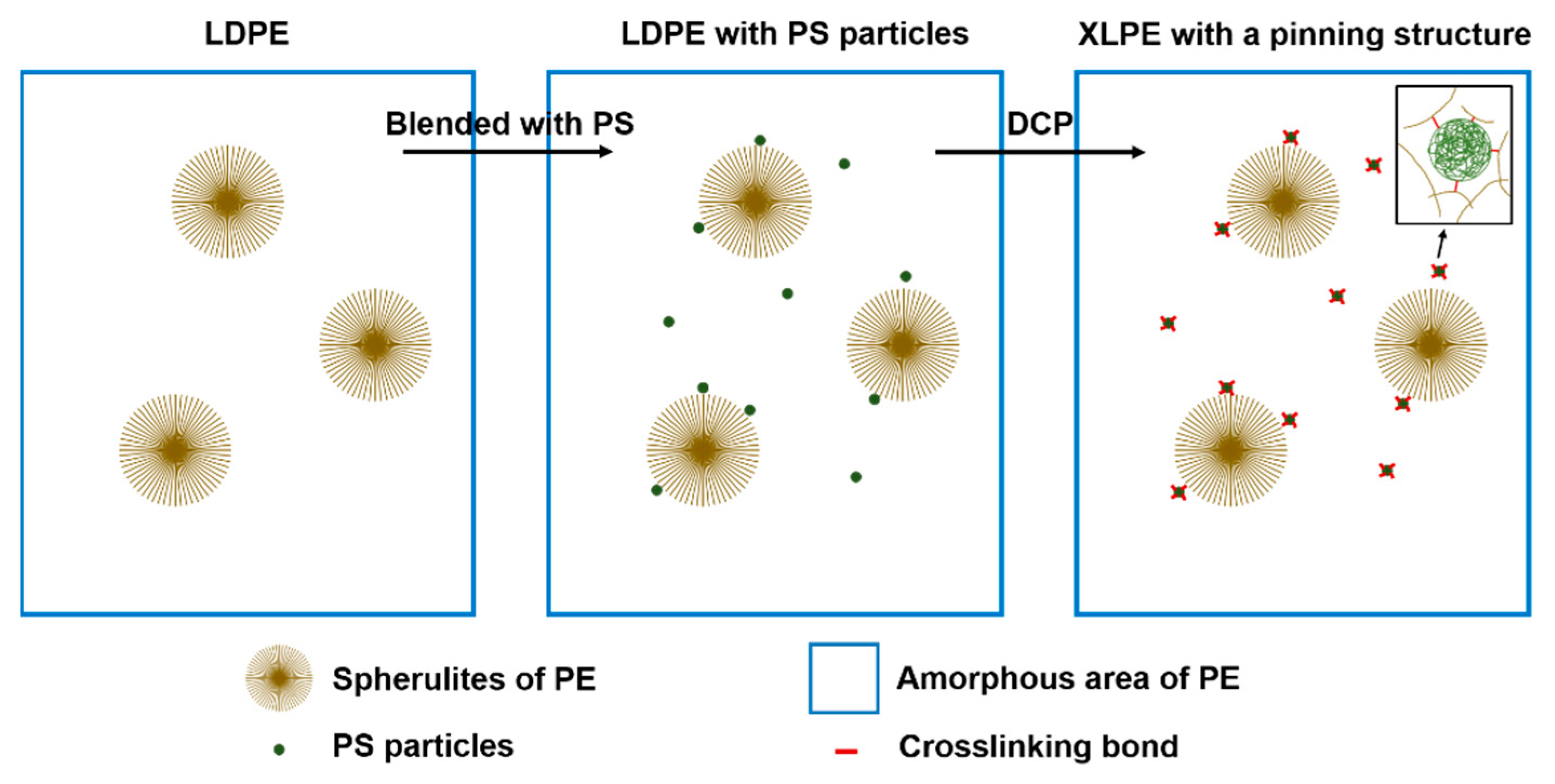
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.; Hao, M.; Xu, Z.; Vaughan, A.; Cao, J.; Wang, H. Review of high voltage direct current cables. CSEE J. Power Energy Syst. 2015, 1, 9–21. [Google Scholar] [CrossRef]
- Zhong, L.; Ren, H.; Cao, L.; Zhao, W.; Gao, J. Development of high voltage direct current extruded cables. High Volt. Eng. 2017, 43, 3473–3489. [Google Scholar]
- Jeroense, M. HVDC, the next generation of transmission: highlights with focus on extruded cable systems. IEEJ Trans. Electr. Electron. Eng. 2010, 5, 400–404. [Google Scholar] [CrossRef]
- Shang, N.; Chen, Q.; Wei, X. Preparation and dielectric properties of SiC/LSR nanocomposites for insulation of high voltage direct current cable accessories. Materials 2018, 11, 403. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, P.; Li, H.; Li, J.; Chen, Z. Improved DC performance of crosslinked polyethylene insulation depending on a higher purity. IEEE Trans. Dielectr. Electr. Insul. 2010, 5, 400–404. [Google Scholar] [CrossRef]
- Hussin, N.; Chen, G. Analysis of space charge formation in LDPE in the presence of crosslinking byproducts. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 126–133. [Google Scholar] [CrossRef]
- Montanari, G.C.; Laurent, C.; Teyssedre, G.; Campus, A.; Nilsson, U.H. From LDPE to XLPE: Investigating the change of electrical properties. Part I: Space charge, conduction and lifetime. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 438–446. [Google Scholar] [CrossRef]
- Lau, K.Y.; Vaughan, A.S.; Chen, G. Nanodielectrics: Opportunities and challenges. IEEE Electr. Insul. Mag. 2015, 31, 45–54. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Murakami, Y.; Nemoto, M.; Okuzumi, S.; Masuda, S.; Nagao, M.; Hozumi, N.; Sekiguchi, Y.; Murata, Y. DC conduction and electric breakdown of MgO/LDPE nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 33–39. [Google Scholar] [CrossRef]
- Chi, X.; Cheng, L.; Liu, W.; Zhang, X.; Li, S. Characterization of polypropylene modified by blending elastomer and nano-silica. Materials 2018, 11, 1321. [Google Scholar] [CrossRef]
- Zhou, Y.; He, J.; Hu, J.; Huang, X.; Jiang, P. Evaluation of polypropylene/polyolefin elastomer blends for potential recyclable HVDC cable insulation applications. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 673–681. [Google Scholar] [CrossRef]
- Lee, S.H.; Park, J.K.; Han, J.H.; Suh, K.S. Space charge and electrical conduction in maleic anhydride-grafted polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1995, 2, 1132–1139. [Google Scholar]
- Suh, K.S.; Lee, C.R.; Zhu, Y.; Lim, J. Electrical properties of chemically modified polyethylenes. IEEE Trans. Dielectr. Electr. Insul. 1997, 4, 681–687. [Google Scholar] [CrossRef]
- Zha, J.; Wu, Y.; Wang, S.; Wu, D.; Yan, H.; Dang, Z. Improvement of space charge suppression of polypropylene for potential application in HVDC cables. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2337–2343. [Google Scholar] [CrossRef]
- Wallgren, E.; Hult, A.; Gedde, U.W. Crosslinking of binary blends of branched polyethylene and poly(1,2-butadiene): 1. Molecular structure and kinetics. Polymer 1993, 34, 2585–2591. [Google Scholar] [CrossRef]
- Ghorbani, H.; Abbasi, A.; Jeroense, M.; Gustafsson, A.; Saltzer, M. Electrical characterization of extruded DC cable insulation—The challenge of scaling. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1465–1475. [Google Scholar] [CrossRef]
- Cao, L.; Zhong, L.; Li, Y.; Shen, Z.; Jiang, L.; Gao, J.; Chen, G. Effect of blend of polystyrene on the temperature dependence of DC breakdown characteristics of polyethylene. In Proceedings of the 2018 Condition Monitoring and Diagnosis (CMD), Perth, WA, Australia, 23–26 September 2018. [Google Scholar]
- Marzzanti, G.; Marzinotto, M. Space charge in HVDC extruded insulation: storage, effects, and measurement methods. In Extruded Cables for High Voltage Direct Current Transmission: Advance in Research and Development, 1st ed.; El-Hawary, M.E., Ed.; Wiley-IEEE Press: Piscataway, NJ, USA, 2013; pp. 107–117. [Google Scholar]
- Wang, X.; He, H.; Tu, D.; Lei, C.; Du, Q. Dielectric properties and crystalline morphology of low density polyethylene blended with metallocene catalyzed polyethylene. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 319–326. [Google Scholar] [CrossRef]
- Guo, Q.; Yang, L.; Li, X.; Li, W.; Yao, Z. The influence of nano-PS particle on structure evolution and electrical properties of PP/PS. J. Polym. Sci. Pt. B-Polym. Phys. 2018, 56, 706–717. [Google Scholar] [CrossRef]
- Mizutani, T. Space charge measurement techniques and space charge in polyethylene. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 923–933. [Google Scholar] [CrossRef]
- Suh, K.S.; Koo, J.H.; Lee, S.H.; Park, J.K.; Takada, T. Effect of sample preparation conditions and short chains on space charge formation in LDPE. IEEE Trans. Dielectr. Electr. Insul. 1996, 3, 153–160. [Google Scholar] [CrossRef]
- Fu, M.; Chen, G.; Dissado, L.A.; Fothergill, J.C. Influence of thermal treatment and residues on space charge accumulation in XLPE for DC power cable application. IEEE Trans. Dielectr. Electr. Insul. 2007, 14, 53–64. [Google Scholar] [CrossRef]
- Li, L.; Zhong, L.; Zhang, K.; Gao, J.; Xu, M. Temperature dependence of mechanical, electrical properties and crystal structure of polyethylene blends for cable insulation. Materials 2018, 11, 1922. [Google Scholar] [CrossRef] [PubMed]
- Kolesov, S.N. The influence of morphology on the electric strength of polymer insulation. IEEE Trans. Dielectr. Electr. Insul. 1980, 15, 382–388. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Lewis, T.J. Interfaces are the dominant feature of dielectrics at the nanometric level. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 739–753. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, L.; Zhong, L.; Li, Y.; Zhang, K.; Gao, J.; Chen, G. Enhanced High-Temperature DC Dielectric Performance of Crosslinked Polyethylene with a Polystyrene Pinning Structure. Materials 2019, 12, 1234. https://doi.org/10.3390/ma12081234
Cao L, Zhong L, Li Y, Zhang K, Gao J, Chen G. Enhanced High-Temperature DC Dielectric Performance of Crosslinked Polyethylene with a Polystyrene Pinning Structure. Materials. 2019; 12(8):1234. https://doi.org/10.3390/ma12081234
Chicago/Turabian StyleCao, Liang, Lisheng Zhong, Yinge Li, Kai Zhang, Jinghui Gao, and George Chen. 2019. "Enhanced High-Temperature DC Dielectric Performance of Crosslinked Polyethylene with a Polystyrene Pinning Structure" Materials 12, no. 8: 1234. https://doi.org/10.3390/ma12081234
APA StyleCao, L., Zhong, L., Li, Y., Zhang, K., Gao, J., & Chen, G. (2019). Enhanced High-Temperature DC Dielectric Performance of Crosslinked Polyethylene with a Polystyrene Pinning Structure. Materials, 12(8), 1234. https://doi.org/10.3390/ma12081234





