1. Introduction
Monolithic yttria-stabilized tetragonal zirconia (Y-TZP) is a predictable dental restorative material that exhibits a high success rate in clinical practice and is more frequently selected for an aesthetic restoration as its translucency improves [
1,
2,
3]. Compared with conventional bilayered zirconia crowns and metal-ceramic restorations, monolithic zirconia restorations have the advantage of less ceramic fracture [
4]. In addition, compared with conventional metal-ceramic restorations, monolithic zirconia crowns exhibit excellent translucency; moreover, monolithic zirconia is aesthetic because of the lack of metal exposure at the restoration margin, even when gingival recession of the abutment tooth occurs [
5,
6]. Thus, monolithic zirconia offers several advantages as an aesthetic restorative material, rendering it the first-choice material in the premolar region based on its tooth color and intensity. Furthermore, monolithic zirconia restorations are increasingly used in the anterior teeth, owing to the development of zirconia materials with high translucency [
7,
8,
9].
The toothbrushing procedure involves applying a mechanical force to the tooth surface over a prolonged period [
10]. In addition, various dentifrices have been developed for use with toothbrushes, and chemical components and abrasives of dentifrices can chemically and physically affect the surface of brushed teeth [
11,
12]. The high fluoride concentration in a dentifrice has been reported to diminish the properties of dental ceramics [
13,
14,
15]. In addition, dentifrices developed for improved tooth-whitening effects affected the optical properties of restorative ceramic materials [
12,
16]. Based on the abrasive content, toothpastes vary in their abrasion of enamel, as measured by the relative dentin abrasion (RDA) value [
10]. Investigating toothbrushing effects on shade or translucency is important. Furthermore, it is imperative to investigate whether toothbrushing may increase the surface roughness that can result in plaque accumulation and bacterial adhesion.
Studies have reported the effects of toothbrushing and dentifrices on various restorations [
12,
17,
18,
19]; however, limited studies have investigated the impact of various dentifrices on the monolithic zirconia material. Hence, this study investigated the effects of various toothpastes on the optical properties and surface properties of monolithic zirconia finished by polishing or glazing methods. In this study, the null hypothesis is that no significant change in the optical properties and surface characteristics of polished or glazed monolithic zirconia specimens occurs after the simulated toothbrushing procedure with various dentifrices.
2. Materials and Methods
2.1. Specimen Preparation
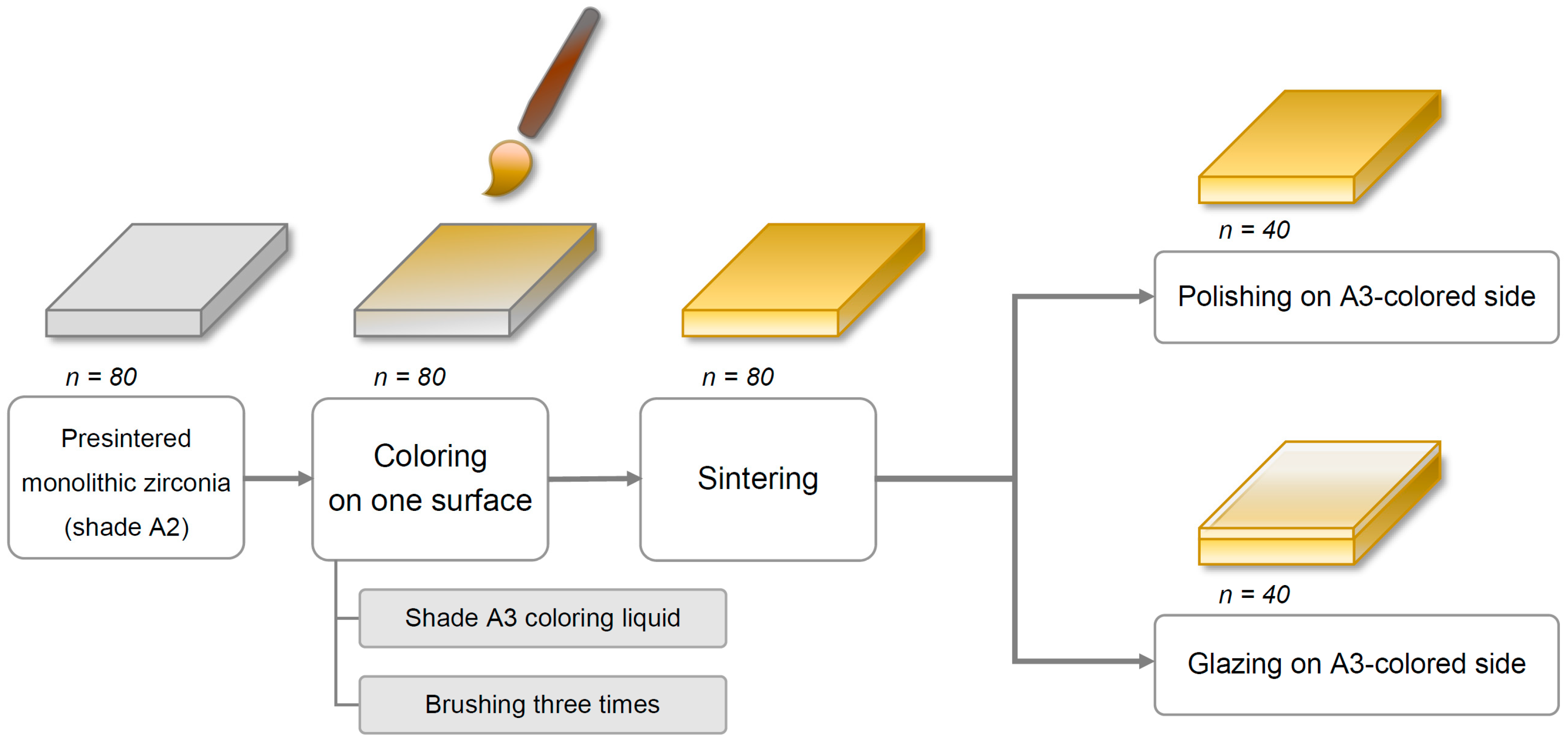
Eighty square-shaped (22.0 mm × 22.0 mm × 2.0 mm) specimens were cut from presintered blocks of monolithic Y-TZP zirconia (Rainbow Shade Block, Shade A2; Genoss, Suwon, Korea) with a low-speed diamond disc (Diamonde Blade, Samsung Clover, Seoul, Korea) under water cooling [
17]. The specimens’ thicknesses were adjusted to 2 ± 0.01 mm with a horizontal grinding machine (HRG-150; AM Technology, Asan, Korea) and were confirmed using a digital caliper (BD500-150; Bluetec, Seoul, Korea).
Coloring procedures were performed on only one side of each specimen with a metal-free coloring brush (Maedeum No. 5; Daeheung-dang, Seoul, Korea) and A3-shaded coloring liquid (Luxen CL shade A3; Dental Max, Seoul, Korea) with brushing three times to simulate the restoration coloring procedure in a dental laboratory. Then, all specimens were sintered in a furnace (PDF-1000; Dental Max, Seoul, Korea) for 10 h, including 2 h at 1530 °C as per the manufacturer’s instructions. The final dimensions of the specimens after the sintering procedure were 18.0 mm × 18.0 mm × 1.6 mm, considering approximately 20% volumetric shrinkage.
Next, all specimens were divided into two major groups based on the finishing methods—polishing (P) and glazing (G). For glazed specimens (
n = 40), the glazing material (Glaze HeraCeram; Heraeus Kulzer, Hanau, Germany) was coated on the A3 coloring liquid-applied surface of the specimens and fired in a ceramic furnace (Programat P310; Ivoclar Vivadent, AG, Schaan, Liechtenstein) as per the manufacturer’s guidelines. Of note, no extrinsic staining was performed. Next, the glazed surfaces of the square-shaped specimens were wet-ground with 320-, 1200-, and 2000-grit silicon carbide abrasive paper (C357; Paco Tech., Seoul, Korea), creating specimens with a glazed layer of 50 (±30) μm thickness. For polished specimens (
n = 40), an experienced dental laboratory technician manually polished the A3 colored surface of the specimens using a zirconia polishing set (StarGloss blue/pink/gray; Edenta AG, Hauptstrasse, Switzerland) (
Figure 1). Finally, a fiducial mark was engraved on the edge of the non-tested side of each specimen, which was used for distinction between groups.
Then, each finishing group was further categorized into the following four subgroups based on the brushing procedure and dentifrice used (n = 10/group): storage in distilled water (DW, control); brushing with a fluoride-free conventional dentifrice (C); brushing with a fluoride dentifrice (F); and brushing with a whitening dentifrice (W). Finally, based on the finishing and brushing methods, eight groups were defined as PDW, PC, PF, PW, GDW, GC, GF, and GW. Before performing tooth brushing or storage, all specimens were ultrasonically cleaned for 5 min.
2.2. Toothbrushing with a Dentifrice Slurry
All specimens were subjected to a single focal area of toothbrushing using an electric toothbrush (DB-4010; Oral-B Braun GmbH, Kronberg/Ts., Germany) with a cup-shaped toothbrush head (Precision Clean; Oral-B Braun GmbH) fixed on a customized toothbrush-holding device (
Figure 2). This electric toothbrush had oscillatory-rotating movement at a rate of 7600 strokes/min. The electric brushes were set to brush in “continuous mode” with a standardized vertical load of 2 N [
18,
19]. The vertical force was generated using orthodontic extraoral elastics 0.5-inch Extraorale Latex-Gummiringe (Dentaurum, Ispringen, Germany) and validated using a laboratory force gauge (J14002; Zeast Co., Beijing, China).
In this study, three dentifrices were used—a fluoride-free conventional dentifrice (Parodontax Classic Fluoridfrei; GlaxoSmithKline, Bühl, Germany), a fluoride dentifrice (Parodontax Fluorid; GlaxoSmithKline), and a whitening dentifrice (Crest 3d White Vivid; Procter & Gamble, Cincinnati, OH, USA). The reported RDA values for these dentifrices were 56, 56, and 233 [
11]. In addition, the fluoride concentration for these dentifrices were 0, 1400, and 1500 ppm, according to the manufacturer’s guidelines (
Table 1). Of note, an RDA value of 250 is the American Dental Association (ADA)–specified limit, and 1500 ppm fluoride ion in the dentifrice is the maximum concentration that can be purchased without a prescription in most countries. Each toothpaste was mixed with DW in a ratio of 1:4 to make a slurry along with the ISO (International Standards Organization) 11609:2017 standard (Dentistry-Toothpastes: Requirements, test methods and marking).
The total brushing time was calculated based on a brushing time of 120 s two times a day of all 28 teeth [
10,
20]. As a tooth has several surfaces to be brushed, the maximum contact time per tooth surface per day has been reported to be 5 s [
10,
21]. In addition, the simulated brushing time of 260 min for one surface of the specimen was evaluated to be equivalent to 8.5 years of tooth brushing.
Based on a typical toothbrush replacement cycle, bristles must be replaced after 45 days of use [
10]. Reportedly, brushing all 28 teeth with 72 surfaces for 45 days is equivalent to simulated toothbrushing for 270 min, assuming that one surface is being brushed for 5 s per day [
10]. Thus, in this study, the first simulated brushing was performed for 260 min (which simulated 8.5 years of toothbrushing); then, the optical properties were assessed; toothbrush heads, dentifrice slurries, and batteries were replaced; and another 260 min of brushing was performed again. Each specimen was brushed for 520 min, representing 17 years of brushing. The specimens in the DW (control) group remained submerged in DW for the same period of 520 min.
Next, 60 new electric toothbrushes and 60 new customized brush-holding devices were prepared to ensure equal experimental conditions. In addition, toothbrushing of 60 specimens was performed at the same time. After simulated brushing, all test specimens were rinsed with tap water for 30 s before all measurements.
2.3. Color and Translucency
To assess shade and translucency changes, the Commission Internationale de l’Éclairage (CIE)
L*, a*, and b* color coordinates of 80 specimens were evaluated using a dental spectrophotometer (EasyShade V, VITA Zahnfabrik, Bad Säckingen, Germany); this device has high repeatability, with intradevice intraclass correlation coefficients (ICCs) of 0.913–0.993 [
22]. In this study, each of 10 glazed zirconia specimens was measured three times to calculate the ICC and ensure the repeatability of the device used. When measuring three times, the device tip was removed from the evaluated surface of the specimen >10 cm and contacted again for other measurements to simulate a similar situation with experiments. The intradevice ICCs of the device used in this study were 1.000 for
L* and a* and 0.999 for b*. To avoid the likelihood of interdevice disagreement, all measurements were made by using only one device.
The CIE L*, a*, and b* color components of each specimen were detected over white, gray, and black polytetrafluoroethylene backgrounds (GC-3, Color calibration cards; JJC Co., Seoul, Korea) at 3 different intervals—baseline, after 260 min (simulating 8.5 years), and 520 min (simulating 17 years)—of brushing. All measurements were performed by a single trained prosthodontist (JHL) under standardized D65 light illumination (18W/D65; Philips, Santiago, Chile) of the color assessment cabinet (CAC-4, Zhengzhou Hengchen Electric Tech., Henan, China). Of note, all measurements were performed with the probe tip perpendicular to the center of the specimens. In addition, the spectrophotometer was calibrated according to the manufacturer’s instructions before each color measurement to minimize the measurement uncertainty. Furthermore, the measurements for each background of each specimen were performed four times, and the mean of four measurements was recorded for data analysis.
Measurements acquired on the gray background were used to evaluate the color difference between before and after brushing. Furthermore, CIEDE2000 color differences (Δ
E00) in each group between the baseline and simulated 8.5 years and between the baseline and simulated 17 years of toothbrushing were determined using the following formula [
23,
24]:
where Δ
L′, Δ
C′, and Δ
H′ are the differences in lightness, chroma, and hue;
SL,
SC, and
SH are weighting functions; and
RT is a rotation factor [
24]. In this study,
KL,
KC, and
KH are parametric factors set to 1.
Furthermore, the CIE
L*, a*, and b* measurements acquired on the white and black backgrounds were used to evaluate the translucency parameter (TP) by estimating the CIEDE2000 color difference (Δ
E00) between the color values obtained against white and black backgrounds at each test period [
25].
2.4. Surface Gloss
After completing the entire brushing process, the surface gloss was measured three times using a small area glossmeter (WG60; FRU, Beijing, China) at the center of each sample, and the average was recorded. In specific, all specimens were placed in a black opaque container and then covered with the glossmeter to eliminate external light exposure and hold the correct position during the examination. Notably, the glossmeter was calibrated before each measurement. The projection angle of the glossmeter was 60°, and the measurement range was from 0 (for a totally nonreflective surface) to 200 (for a totally reflective surface) gloss units (GU). The glossmeter was designed and manufactured with reference to the international standard ISO 2813.
2.5. Surface Roughness
The surface roughness was measured on each brushed surface after all interventions with simulated cycles using a Zeiss laser scanning microscope (LSM) 800 MAT confocal scanning laser system combined with a Zeiss Axio imager Z2m microscope with ZEN software (Zeiss, Jena, Germany). On the LSM 800 MAT, imaging was made using laser excitation at 405 nm with a C Epiplan-APOCHROMAT 20 × 0.7 NA. The images were acquired at three sites within the area where each sample was brushed, and the mean of three Ra values was documented. Ra is the arithmetical mean deviation, and the measurements were made with reference to the international standard ISO 4287.
2.6. X-ray Diffraction (XRD)
After completing all the brushing cycles, one randomly selected sample from each subgroup was subjected to XRD (D8 Advance; Bruker, Karlsruhe, Germany) using Cu-Kα radiation (λ = 1.5406 Å) to ascertain the crystalline phase of each zirconia specimen. The scan was performed at a step size of 0.02° with a scan rate of 2°/min in the 2-theta range between 20° and 60°.
2.7. Scanning Electron Microscopy (SEM)
One representative test specimen in each subgroup was selected for SEM (Model S-4700 SEM; Hitachi High-Technologies Co, Tokyo, Japan) examination after all interventions with simulated brushing cycles. The specimens were sputtered with platinum (Q150T Sputter Coater; Quorum Technologies Ltd., Ashford, Kent, UK) and photographed at an acceleration voltage of 15 kV at magnifications of ×1000 and ×5000.
2.8. Statistical Analysis
All statistical analyses in this study were performed using IBM SPSS Statistics (v24.0; IBM Corp., Chicago, IL, USA). Repeated-measures analysis of variance (ANOVA) was performed to analyze ΔE00 and TP (α = 0.05) with brushing time as a repeated factor and toothbrushing groups as a fixed factor. Separate analyses were conducted for each dependent variable, and a Bonferroni correction was performed. In addition, two-way ANOVA was used to determine the effects of two factors, the finishing methods and dentifrices used, on the ΔE00, TP, GU, and Ra outcome variables. The interactions between the two factors were also analyzed. In this study, the statistical significance was set at 0.05 for all analyses.
4. Discussion
This study evaluated the impact of toothbrushing on the optical properties and surface characteristics of monolithic zirconia materials. The findings rejected the null hypothesis for both optical properties and surface roughness. Statistically significant changes in color parameters were observed as toothbrushing progressed, and a decline in the surface gloss of the groups brushed with fluoride and whitening dentifrices compared with the group stored in DW was identified. In addition, the surface roughness of the glazed group brushed with a fluoride dentifrice appeared markedly rougher than the unbrushed glazed group. After toothbrushing, the glazed groups exhibited markedly higher color stability than the polished groups; however, the glazed groups exhibited less surface gloss and rougher surfaces than the polished groups.
Our findings corroborate a previous study on color change in brushed zirconia specimens. Yuan et al. [
30] reported a statistically significant shade change in extrinsically stained and glazed zirconia specimens after 15 years of simulated brushing. While the evaluated Δ
E value between the baseline and after 15-year simulated brushing was approximately 1.5, the resulting color change value was within the perceptibility tolerance of 2.6 Δ
E [
30]. Unlike the previous study that investigated extrinsically characterized zirconia (IPS shade 3), this study assessed the impact of brushing on the color change of intrinsically colored zirconia materials. As extrinsic stains can be easily damaged or removed by external trauma, such as an occlusal reduction procedure, intrinsic coloring is preferred by dentists. However, to date, no study has investigated the impact of brushing on the shade of intrinsically colored zirconia.
In this study, both polished and glazed zirconia specimens exhibited statistically significant color changes; moreover, polished specimens exhibited more color changes than glazed specimens. In addition, polished specimens became considerably darker after toothbrushing, and the color change was at the border of the perceptibility threshold. Furthermore, compared to the polished specimens, the glazed specimens revealed less shade change, which is consistent with Garza et al. [
18], who reported that after 12 years of simulated brushing, lithium disilicate specimens glazed after staining were more resistant to color change than specimens that underwent staining and glazing simultaneously. In addition, Alp et al. [
31] demonstrated that polished glass ceramics were more susceptible to staining by coffee thermocycling than glazed specimens, suggesting that the glazing layer coated over the colored specimen could play a protective role.
Regarding the surface gloss, Sen et al. [
32] reported a marked decline in the gloss of computer-aided design and computer-aided manufacturing (CAD/CAM) restorative materials after 1 year of simulated brushing. This study demonstrated that brushing with fluoride or whitening dentifrices markedly reduced the gloss of zirconia specimens. Moreover, the fluoride-free conventional dentifrice decreased the gloss; however, the difference was not statistically significant. It is possible that the fluoride content or higher RDA of the dentifrice could affect the surface characteristics of zirconia specimens. To date, several studies have demonstrated that acidic and alkaline environments could affect the optical or surface properties of ceramic specimens [
13,
14,
15]. Furthermore, dentin wear is more strongly impacted by the RDA value of dentifrices than by the stiffness of a toothbrush [
10].
In this study, the surface roughness of glazed specimens was also marginally affected by use of the fluoride dentifrice. The roughness of zirconia is crucial because it increases the contact area with moisture, which could result in low-temperature degradation [
33,
34]. In addition, a whitening dentifrice reduced the surface roughness of the polished zirconia surface, which corroborated the results from Pinelli et al. [
12]. The high RDA of a whitening dentifrice was considered to exert a polishing effect on the zirconia surface. The glazed groups revealed a rougher surface than the polished groups. Reportedly, the surface roughness threshold for bacterial colonization was 0.2 μm [
35,
36], and the roughness threshold detectable by the tongue was 0.25–0.5 μm [
37]. Although the roughness of the brushed, polished zirconia was within these thresholds, glazed zirconia after brushing exhibited higher results. Furthermore, the results of XRD exhibited no apparent evidence of phase transformation. It is possible that the several high peaks observed in the toothbrushed glazed groups imply partial wear of the glazing layer compared with GDW.
The strength of this study is that the two finishing methods of monolithic zirconia—polishing and glazing—were compared after simulated brushing. In addition, a thick glazing layer could result in errors in the intensity of contact or occlusion of the restoration when fabricated with a modeless CAD/CAM technique; thus, glazing should be selected only when needed. Moreover, brushing was simulated for 8.5 and 17 years, and the long-term effects of brushing were investigated. Previous studies have simulated shorter brushing periods of 1 to 15 years [
12,
18,
30,
32]. It is also advantageous to evaluate the number of tooth surfaces to be brushed by the scientific method and calculate the appropriate simulation time and appropriate toothbrush replacement cycle. In addition, three toothpaste formulas were compared in this study. The effects of fluoride were compared by selecting the same brand of fluoride-free toothpaste and high fluoride-containing toothpaste. In addition, a whitening toothpaste with extremely high RDA was also compared. Furthermore, substantial data on various optical and surface properties were obtained through the appropriate arrangement of measurements.
This study has some limitations. The first limitation is the in vitro design. Second, the effects of the aging of zirconia on moisture in the mouth and the fatigue of the material could not be considered because of the accumulated mastication in the clinical setting. Third, DW was used to prepare toothpaste slurry; however, the effect of the mixture of the oral saliva and toothpaste was not investigated. Finally, during the roughness measurement process through a confocal scanning laser system, the voids on the surface of the glazing layer generated in the glazing process were disturbed. Thus, additional clinical studies are warranted to overcome these limitations and validate the findings of this study.