Technology to Produce High-Purity Anhydrous Rubidium Perrhenate on an Industrial Scale
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Rubidium Perrhenate by Ion Exchange
2.2.1. Static Condition
Sorption Stage
Elution Stage
2.2.2. Dynamic Test
2.2.3. Crystalization and Purification Test
2.3. Characterization
3. Results and Discussion
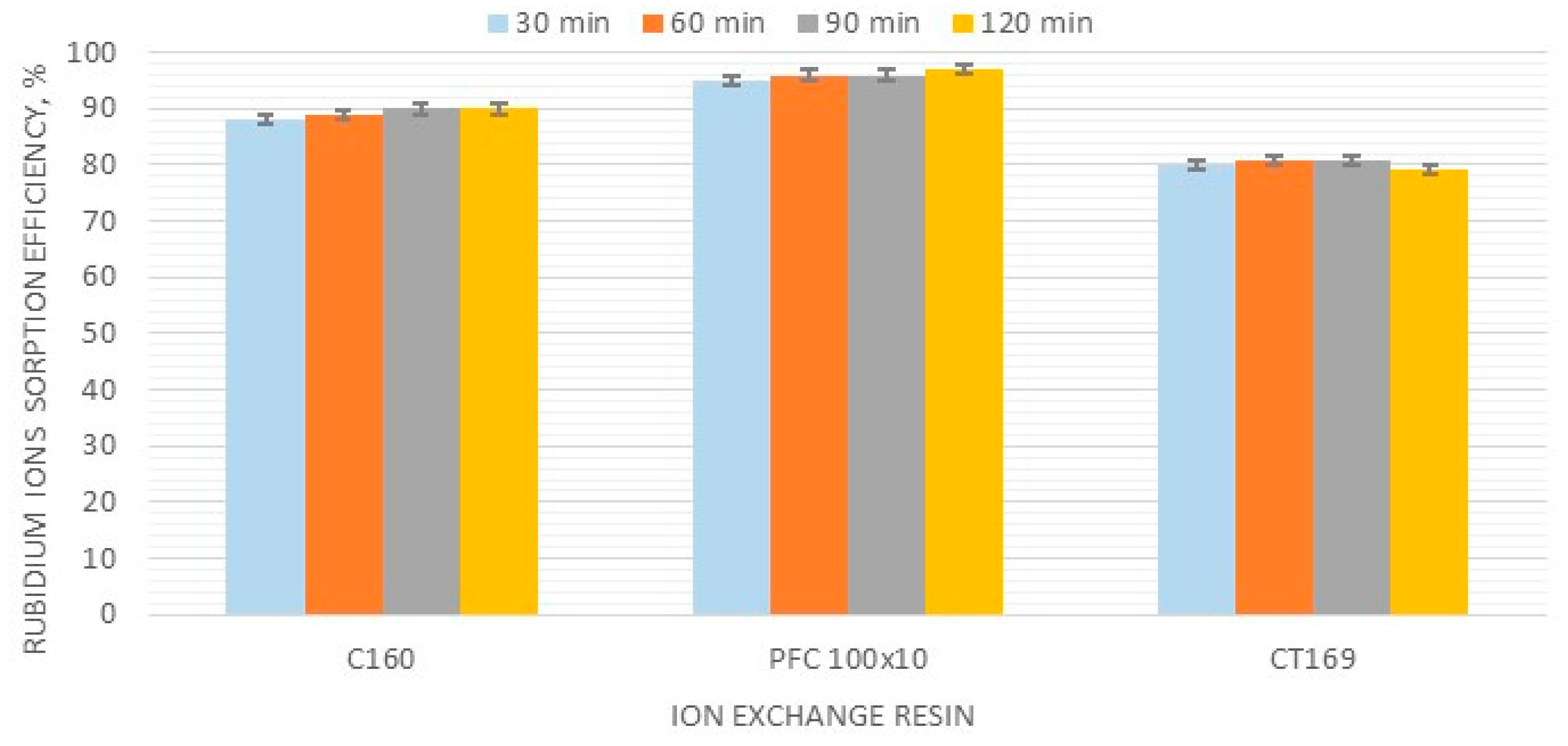
3.1. Selection of Ion-Exchange Resin—Sorption Stage
3.2. Effect of Temperature on the Rubidium Sorption Efficiency
3.3. Effect of Contact Time on Rubidium Sorption Efficiency
3.4. Effect of the Rubidium Concentration on Rubidium Sorption Efficiency
3.5. Selection of Ion-Exchange Resin—Elution Stage
3.6. Effect of Contact Time on the Rubidium Elution Efficiency
3.7. Effect of Temperature on the Rubidium Elution Efficiency
3.8. Influence of the Rhenium-To-Rubidium Ratio on Rubidium Elution Efficiency
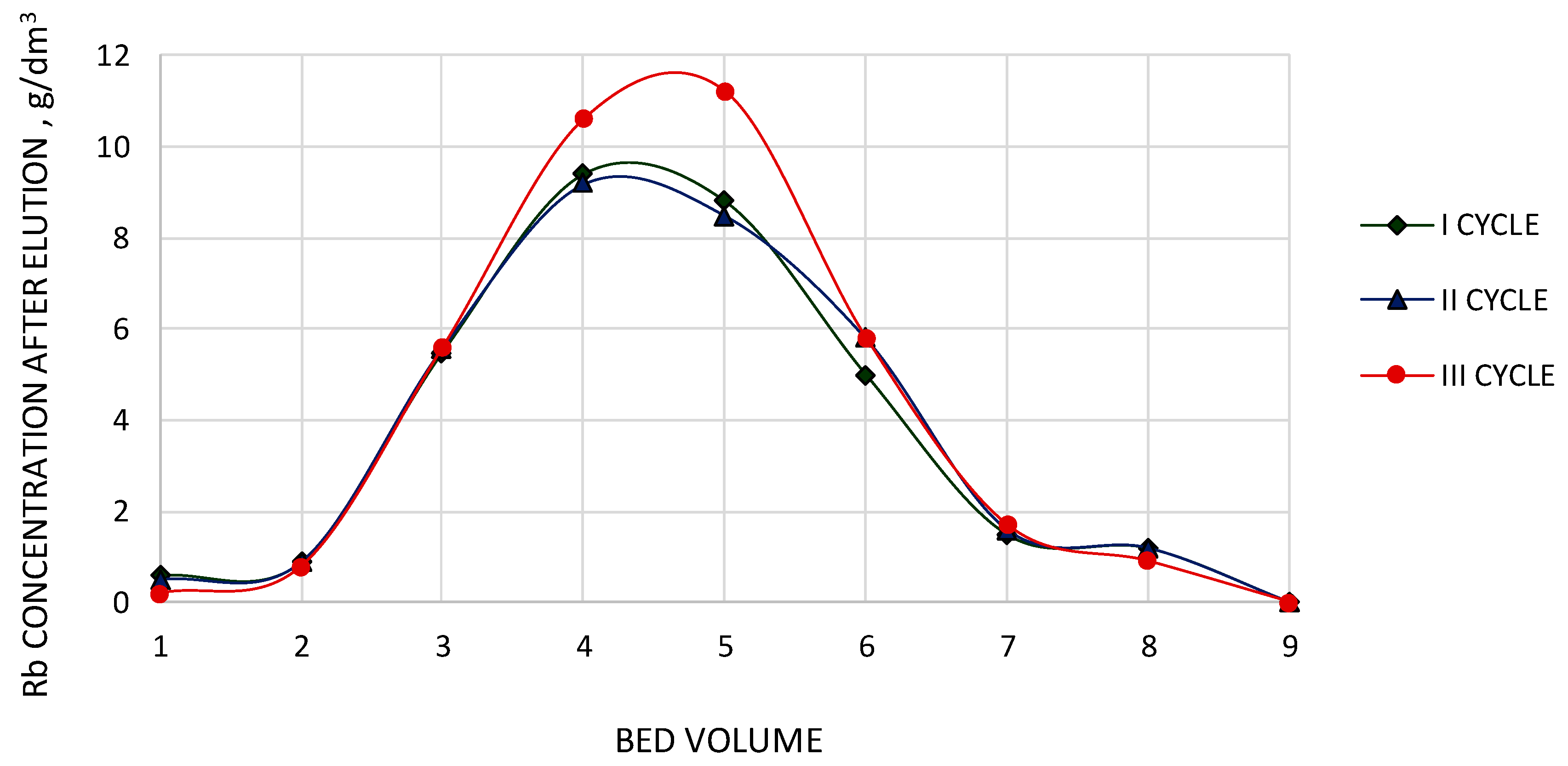
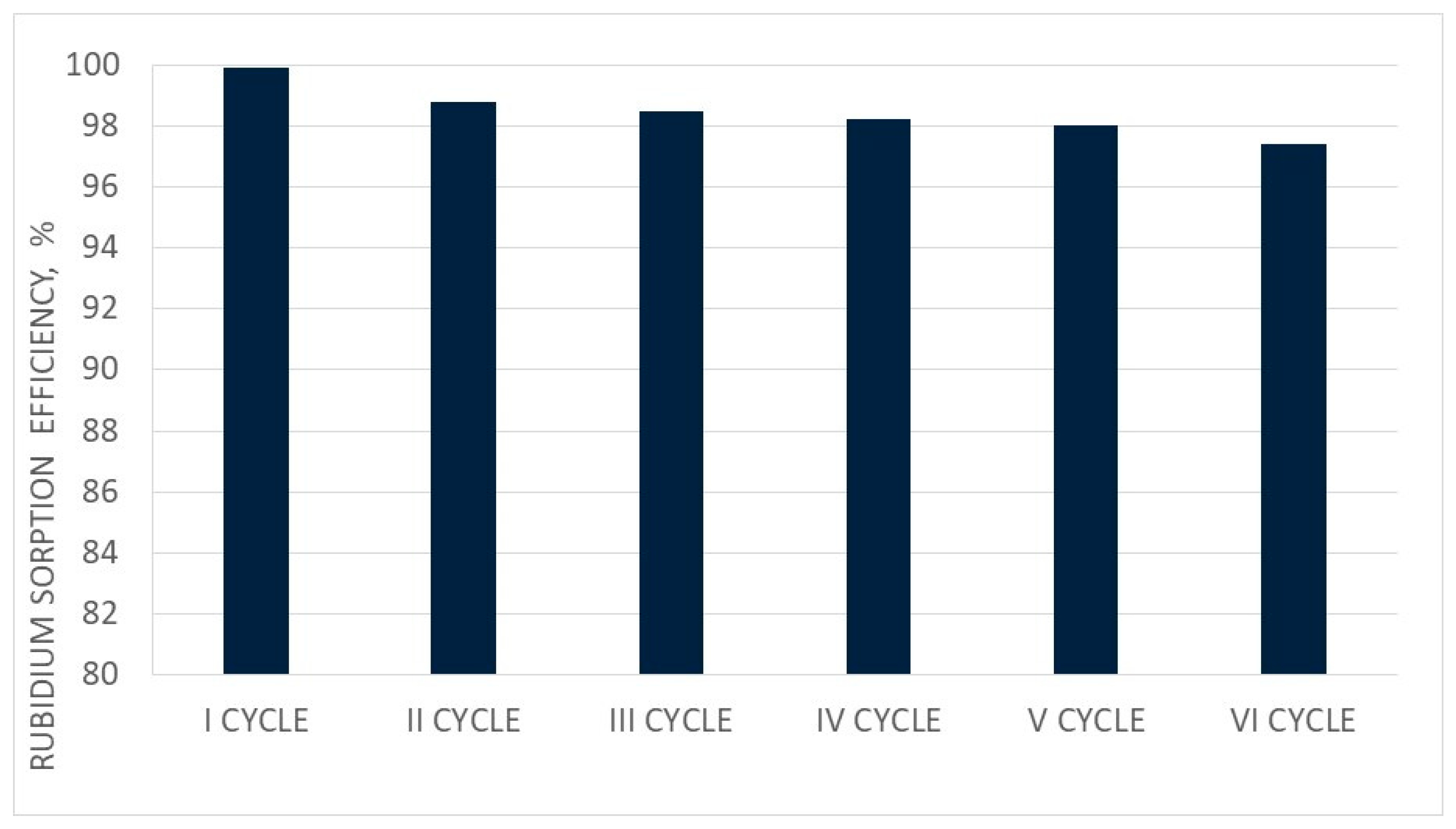
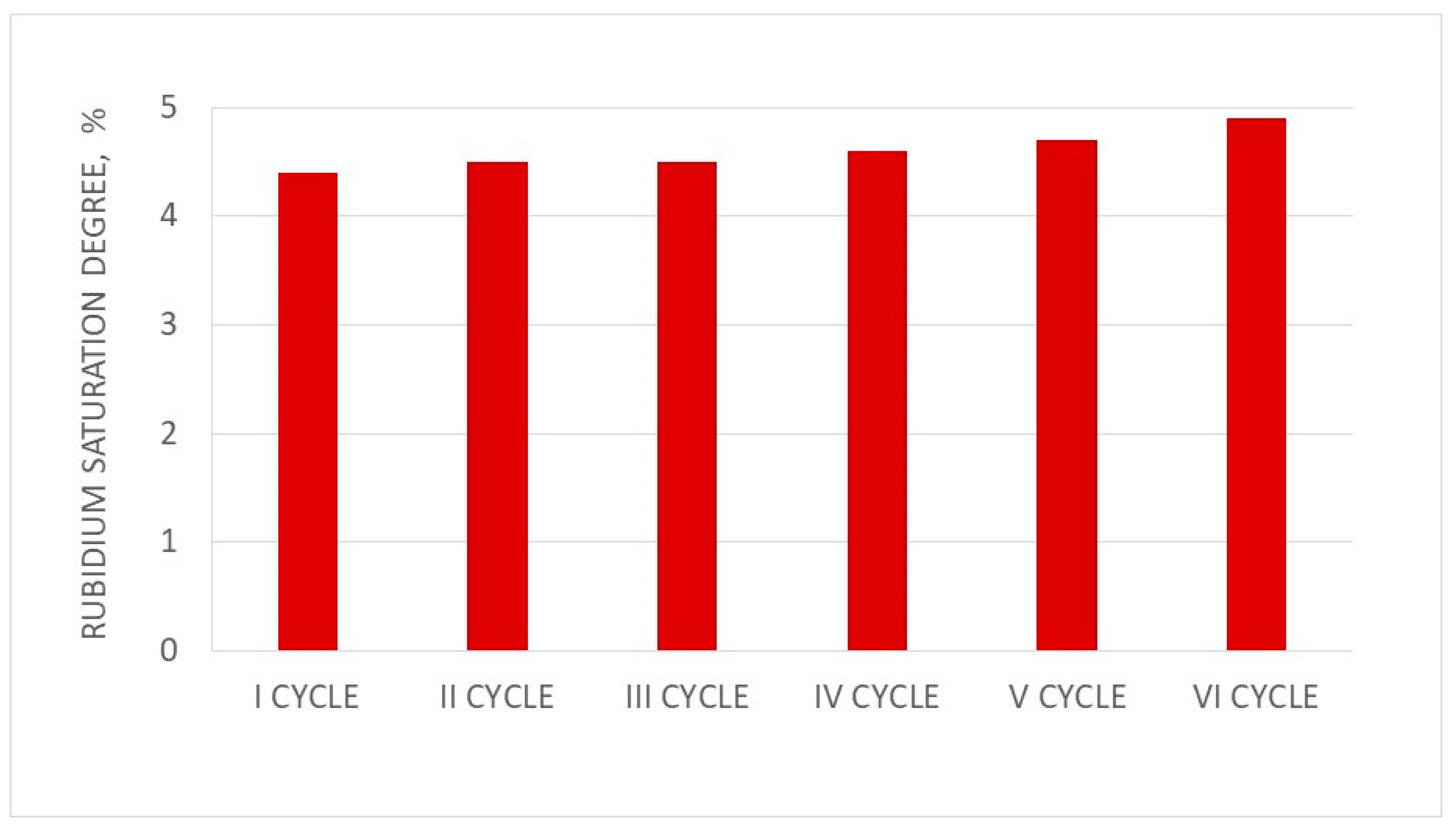
3.9. Analysis of Three Cycles of PFC100x10 under Dynamic Conditions
3.10. Crystallization and Purification Stages
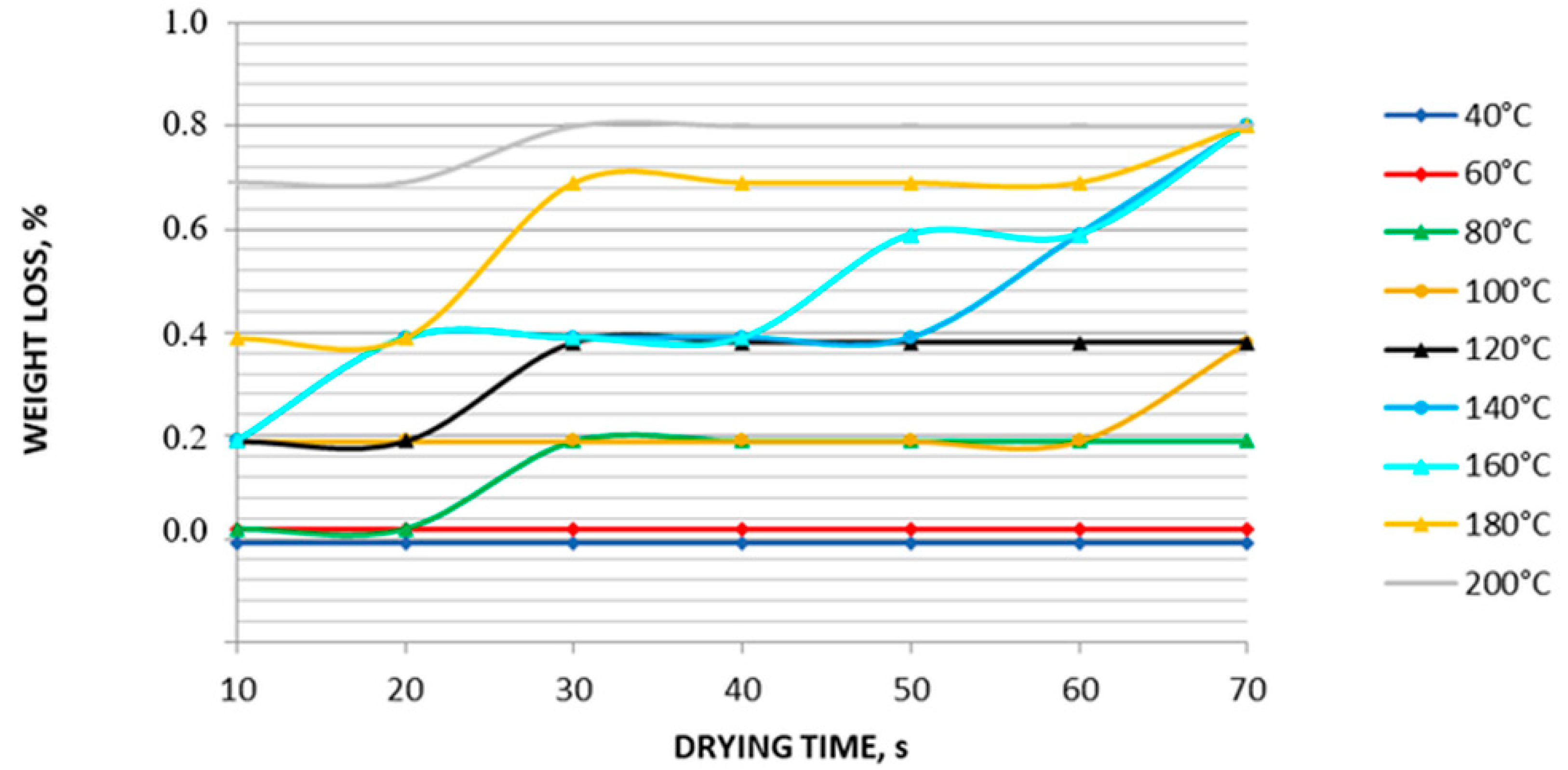
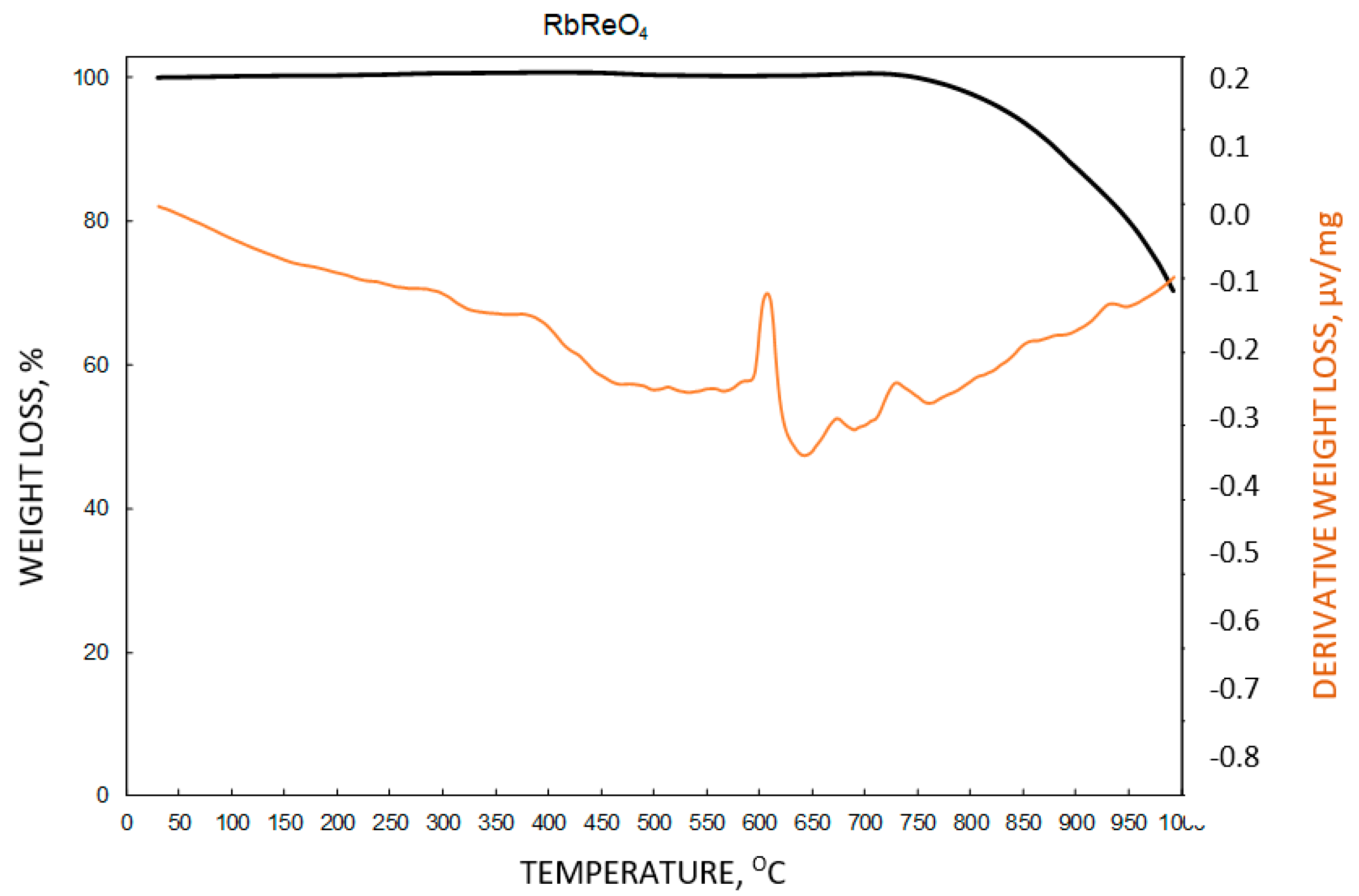
3.11. Thermal Stability
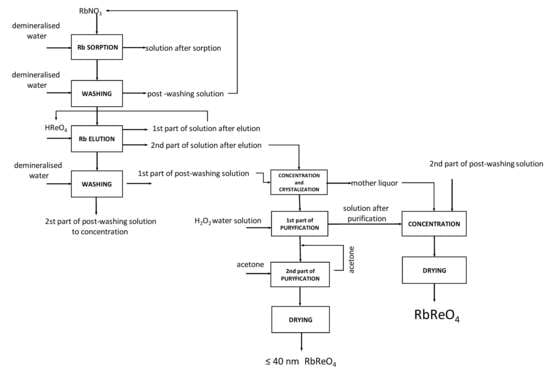
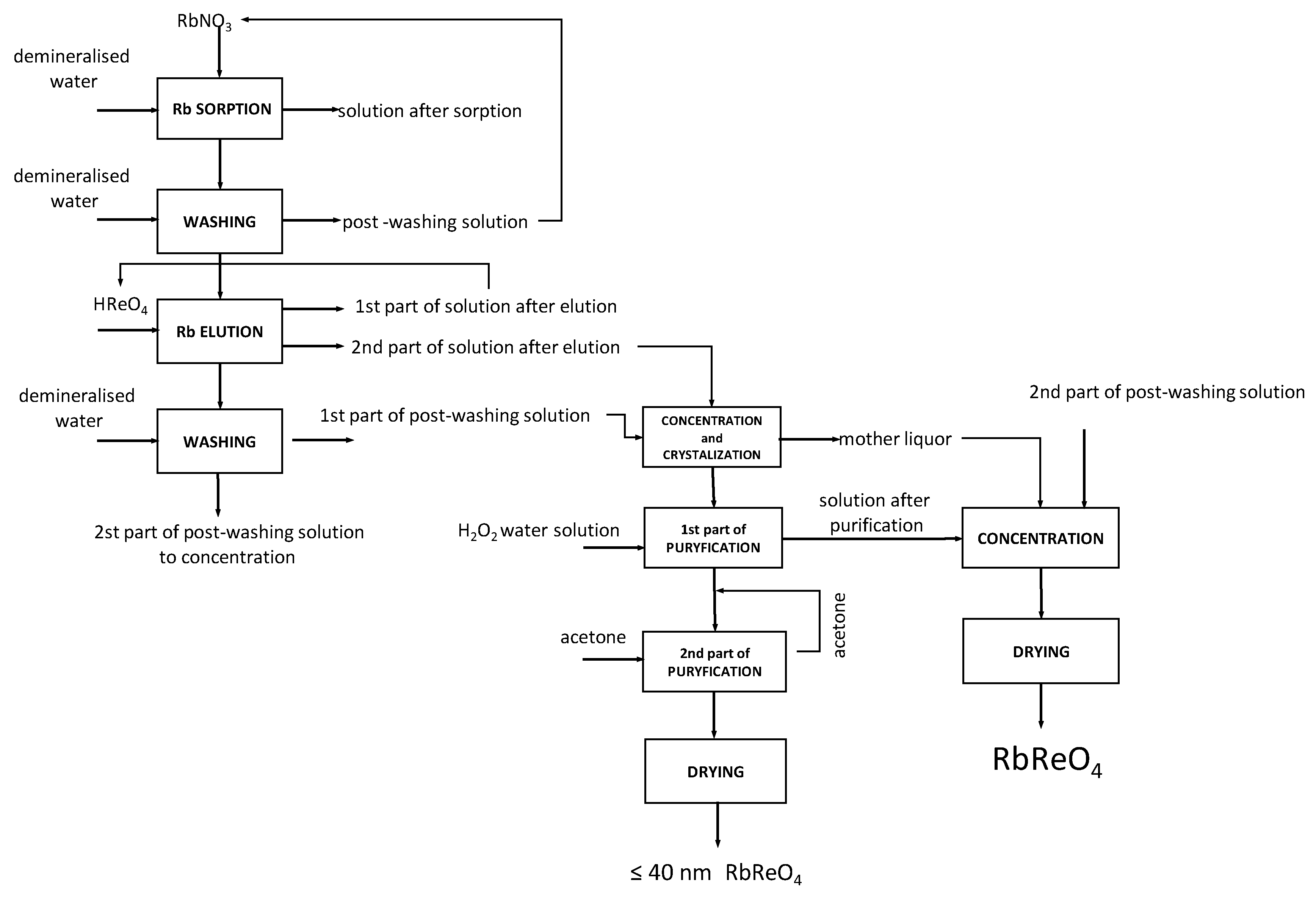
3.12. Developed Technology Scheme
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skudlarski, K. Mass spectrometric studies on the sublimation of rubidium perrhenate. J. Less Common Met. 1973, 31, 329–335. [Google Scholar] [CrossRef]
- Johnson, D.A. Thermochemistry of ammonium and rubidium perrhenates, and the effect of hydrogen bonding on the solubilities of ammonium salts. J. Chem. Soc. Dalton Trans. 1990, 3301–3304. [Google Scholar] [CrossRef]
- Solomonik, V.G.; Marenich, A.V. Isomerism and Vibrational Spectra of Alkali Metal Perrhenates: Ab Initio CISD+Q Calculations. J. Struct. Chem. 2001, 42, 914–919. [Google Scholar] [CrossRef]
- Lukas, W.; Gauneescard, M. Temperatures and enthalpies of melting alkali-metal perrhenates. J. Chem. Thermodyn. 1982, 14, 593–597. [Google Scholar] [CrossRef]
- Smith, W.T.; Long, S.H. The Salts of Perrhenic Acid. I. The Alkali Metals and Ammonium. JACS 1948, 70, 354–356. [Google Scholar] [CrossRef]
- Jaeger, F.M.; Beintema, J. The crystalstructure of cesium- thallium- and rubidium-perrhenates. Proc. Akad. Wetenschappen 1933, 36, 523–528. [Google Scholar]
- Rogner, P.; Range, K.J. Structure Refinement of Rubidium Perrhenate at 297 and 159 K. Z. Naturforsch. 1993, 48b, 233–234. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A. Hydrometallurgical methods for production of Ni(ReO4)2 and Co(ReO4)2. Erzmetall 2013, 66, 267–273. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Ciszewski, M.; Malar, Z.J.; Drzazga, M. Method of Preparation for High-Purity Nanocrystalline Anhydrous Cesium Perrhenate. Metals 2017, 7, 96. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Kopyto, D.; Majewski, T.; Drzazga, M. Production of High-Purity Anhydrous Nickel(II) Perrhenate for Tungsten-Based Sintered Heavy Alloy. Materials 2017, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska-Sejda, K.; Benke, G.; Kopyto, D.; Majewski, T.; Drzazga, M.; Ciszewski, M. Application of ion exchange for preparation of selected metal perrhenates—Precursors for superalloy production. Metals 2019, 9, 201. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K.; Chmielarz, A. Metylotrioxorhenium(VII) as catalyst of important organic reactions. Przem. Chem. 2008, 87, 1102–1105. (In Polish) [Google Scholar]
- Hermann, W.; Kratzer, R.M.; Fisher, R.W. Alkylrhenium Oxides from Perrhenates: A New, Economical Access to Organometallic Oxide Catalysts. Angew. Chem. Int. Ed. Engl. 1997, 36, 2652–2654. [Google Scholar] [CrossRef]
- Kemp, R.A. Ethylene Oxide Catalyst and Process. U.S. Patent US5663385A, 1994. [Google Scholar]
- Matusz, M. Process for Preparing Ethylene Oxide Catalysts. U.S. Patent US5739075, 2 September 1995. [Google Scholar]
- Lauritzen, A.M. Ethylene Oxide Process. U.S. Patent US4833261A, 23 May 1996. [Google Scholar]
- Boxhoorn, G. Silver-Containing Catalyst, Process for the Preparation of the Catalyst and the Catalyst prepared by the Process. U.S. Patent US4874739A, 17 October 1989. [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K. Opracowanie metod otrzymywania kwasu nadrenowego z nadrenianu amonu. Przem. Chem. 2006, 85, 847. (In Polish) [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Chmielarz, A.; Krompiec, S.; Michalik, S.; Krompiec, M. Synthesis of perrhenic acid using ion exchange method. Hydrometallurgy 2007, 89, 289–296. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Anyszkiewicz, K. Zastosowanie jonitów do sorpcji jonów amonowych z wodnych roztworów renianu(VII) amonu. Przem. Chem. 2008, 87, 289–295. (In Polish) [Google Scholar]
- Leszczyńska-Sejda, K.; Benke, G.; Krompiec, S.; Chmielarz, A.; Anyszkiewicz, K.; Gotfryd, L. Synthesis of perrhenic acid using solvent extraction method. Hydrometallurgy 2009, 95, 325–332. [Google Scholar] [CrossRef]
- Weibel, A.; Bouchet, R.; Boulc’h, F.; Kanuth, P. The Big Problem of Small Particles: A Comparison of Methods for Determination of Particle Size in Nanocrystalline Anatase Powders. Chem. Mater. 2005, 17, 2378–2385. [Google Scholar] [CrossRef]
- Leszczyńska-Sejda, K.; Benke, G.; Malarz, J.; Drzazga, M.; Ciszewski, M. Sposób Wytwarzania Bezwodnego Renianu(VII) Rubidu. Polish Patent PL228982, 16 December 2016. (In Polish). [Google Scholar]




| Resin | Structure | Functional Group | Moisture Retention % | Particle Size Range µm |
|---|---|---|---|---|
| C160 | macroporous polystyrene crosslinked with divinylbenzene | sulfonic acid | 35–40 | 300–1200 |
| SP112 | 56–60 | 670 ± 50 | ||
| CT169 | 51–57 | 425–1200 | ||
| PFC100x10 | gel polystyrene crosslinked with divinylbenzene | 44–48 | 570 ± 50 |
| Ion-Exchange Resin | Volume of a Solution after Sorption dm3 | Rb Concentration in a Solution after Sorption g/dm3 | Rb+ Sorption Efficiency % |
|---|---|---|---|
| SP112 | 0.115 | 1.4 | 67.8 ± 0.9 |
| PFC100 | 0.125 | 0.2 | 95.0 ± 0.3 |
| CT169 | 0.120 | 0.8 | 80.8 ± 0.8 |
| C160 | 0.112 | 0.5 | 88.8 ± 0.7 |
| Ion-Exchange Resin | Rb Concentration g/dm3 | Volume of a Solution after Sorption dm3 | Rb Concentration in a Solution after Sorption g/dm3 | Rb+ Sorption Efficiency % | Rb Saturation Degree % |
|---|---|---|---|---|---|
| C160 | 0.5 | 0.110 | 0.4 | 12.0 ± 0.2 | 0.02 ± 0.01 |
| 1.0 | 0.110 | 0.6 | 34.0 ± 0.2 | 0.09 ± 0.01 | |
| 2.0 | 0.121 | 0.6 | 63.7 ± 0.3 | 0.32 ± 0.02 | |
| 5.0 | 0.112 | 0.5 | 88.8 ± 0.2 | 1.11 ± 0.02 | |
| 10.0 | 0.112 | 0.9 | 89.9 ± 0.3 | 2.25 ± 0.03 | |
| 15.0 | 0.110 | 1.1 | 91.9 ± 0.3 | 3.45 ± 0.02 | |
| 20.0 | 0.112 | 1.3 | 92.7 ± 0.4 | 4.64 ± 0.03 | |
| PFC100x10 | 0.5 | 0.122 | 0.3 | 26.8 ± 0.2 | 0.03 ± 0.01 |
| 1.0 | 0.120 | 0.3 | 64.0 ± 0.2 | 0.16 ± 0.01 | |
| 2.0 | 0.120 | 0.3 | 82.0 ± 0.2 | 0.41 ± 0.02 | |
| 5.0 | 0.125 | 0.2 | 95.0 ± 0.3 | 1.19 ± 0.02 | |
| 10.0 | 0.121 | 0.8 | 96.3 ± 0.4 | 2.26 ± 0.02 | |
| 15.0 | 0.123 | 1.0 | 97.0 ± 0.4 | 3.44 ± 0.03 | |
| 20.0 | 0.121 | 1.1 | 98.0 ± 0.3 | 4.67 ± 0.02 |
| Ion-Exchange Resin | Volume of a Solution dm3 | Rb Concentration in a Solution after Elution g/dm3 | Rb+ Elution Efficiency % |
|---|---|---|---|
| C160 | 0.025 | 3.4 | 40.5 ± 0.7 |
| PFC100x10 | 0.025 | 8.2 | 93.2 ± 0.9 |
| Ion-Exchange Resin | Contact Time min | Volume of a Solution dm3 | Rb Concentration in a Solution after Elution g/dm3 | Rb+ Elution Efficiency % |
|---|---|---|---|---|
| PFC100x10 | 30 | 0.025 | 8.2 | 93.2 ± 0.8 |
| 60 | 0.024 | 8.9 | 97.1 ± 0.9 | |
| 120 | 0.023 | 9.5 | 99.3 ± 0.8 | |
| 180 | 0.024 | 9.1 | 99.3 ± 0.7 |
| Ion-Exchange Resin | Temperature °C | Volume of a Solution dm3 | Rb Concentration in a Solution after Elution g/dm3 | Rb+ Elution Efficiency % |
|---|---|---|---|---|
| PFC100x10 | 20 | 0.023 | 9.5 | 99.3 ± 0.8 |
| 40 | 0.022 | 9.3 | 93.0 ± 0.9 | |
| 60 | 0.024 | 8.6 | 93.8 ± 0.9 |
| Ion-Exchange Resin | Re:Rb Ratio | Volume of a Solution dm3 | Rb Concentration in a Solution after Elution g/dm3 | Rb+ Elution Efficiency % |
|---|---|---|---|---|
| PFC100x10 | 5:1 | 0.012 | 4.6 | 25.1 ± 0.8 |
| 10:1 | 0.023 | 9.5 | 99.3 ± 0.8 | |
| 20:1 | 0.042 | 5.2 | 99.3 ± 0.01 |
| Cycle No. | BV1 | BV2 | Rb+ Sorption Efficiency % | Rb Saturation Degree % |
|---|---|---|---|---|
| I | 6 | 13 | 75.2 | 4.5 |
| II | 7 | 13 | 72.7 | 4.7 |
| III | 9 | 14 | 75.4 | 4.9 |
| Cycle No. | BV3 | BV4 | Rb+ Elution Efficiency % |
|---|---|---|---|
| I | 4 | 6 | 72.5 |
| II | 4 | 6 | 72.5 |
| III | 4 | 6 | 79.8 |
| Test No. | c0KRb g/dm3 | c0KRe g/dm3 | Crystallite Size nm | Crystals Mass g | XRb % | XRe % | WKRbReO4 % |
|---|---|---|---|---|---|---|---|
| 1 | 1.0 | 56.0 | * | 0.5 | * | * | |
| 2 | 3.0 | 74.0 | 39 | 2.5 | 22.5 | 55.3 | 55.6 |
| 3 | 6.0 | 82.0 | 30 | 6.0 | 22.4 | 55.4 | 66.7 |
| 4 | 9.0 | 99.0 | 25 | 12.0 | 22.5 | 55.4 | 88.9 |
| Test No. | As | Bi | Ca | Cu | Fe | K | Mg | Mo | Na | Ni | Pb | Zn |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ||||||||||||
| 3 | <2 | <2 | <5 | <5 | 17 | <10 | 22 | <5 | 55 | 28 | <5 | 21 |
| 4 | 15 | 25 | 50 | 26 | 20 | |||||||
| Purification Tests | Fe | Mg | Na | Ni | Zn |
|---|---|---|---|---|---|
| ppm | |||||
| no purification | 15 | 25 | 50 | 26 | 20 |
| 5% H2O2 | 5 | 10 | 20 | 21 | 15 |
| 10% H2O2 | <3 | 10 | 10 | <10 | 12 |
| acetone | 6 | 5 | 5 | 26 | 5 |
| 10% H2O2 + acetone | <3 | <3 | <2 | <10 | <3 |
| Materials | Solution Volume dm3 | Rb Concentration g/dm3 | Rb Mass g | Re Concentration g/dm3 | Re Mass g | Process Efficiency % | Rubidium Saturation Degree % | |
|---|---|---|---|---|---|---|---|---|
| To Sorption | solution | 10.0 | 4.50 | 45.00 | - | - | - | |
| washing water | 2.5 | - | - | - | - | - | ||
| After Sorption | solution | 10.2 | 0.10 | 1.13 | 99.93 | 4.5 | ||
| washing | 2.3 | 0.08 | 0.19 | |||||
| To Elution | Ionite (1 kg) | 44.97 | - | - | - | - | ||
| eluent | 2.0 | 500.00 | - | - | - | - | ||
| washing water | 3.0 | - | - | |||||
| After Elution | elution (1st part) | 1.0 | 1.66 | 1.66 | 1.58 | 1.58 | - | - |
| elution (2nd part) | 2.0 | 17.90 | 35.80 | 468.00 | 936.00 | 94.51 | - | |
| washing (1st part) | 1.0 | 6.70 | 6.70 | 54.10 | 54.10 | |||
| washing (2nd part) | 2.0 | 0.40 | 0.80 | 4.16 | 8.32 | - | - | |
| Materials | Solution Volume dm3 | Rb Concentration g/dm3 | Rb Mass g | Re Concentration g/dm3 | Re Mass g | Process Efficiency % | Rubidium Saturation Degree % | |
|---|---|---|---|---|---|---|---|---|
| To Sorption | solution | 11.2 | 4.52 | 50.62 | - | - | - | |
| washing water | 2.5 | - | - | - | - | - | ||
| After Sorption | solution | 11.3 | 0.05 | 0.070 | 97.39 | 5.7 | ||
| washing | 2.4 | 0.01 | 0.017 | |||||
| To Elution | ionite (1 kg) | 49.30 | - | - | - | - | ||
| eluent | 2.0 | 500.00 | - | - | - | - | ||
| washing water | 3.0 | - | - | - | - | - | - | |
| After Elution | Elution (1st part) | 1.0 | 3.20 | 3.20 | 1.67 | 1.67 | ||
| Elution (2nd part) | 2.0 | 14.0 | 28.0 | 468.00 | 936.00 | 83.16 | - | |
| Washing (1st part) | 1.0 | 13.0 | 12.00 | 53.30 | 53.30 | |||
| Washing (2nd part) | 2.0 | 6.3 | 12.6 | 4.50 | 9.00 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leszczyńska-Sejda, K.; Benke, G.; Ciszewski, M.; Drzazga, M. Technology to Produce High-Purity Anhydrous Rubidium Perrhenate on an Industrial Scale. Materials 2019, 12, 1130. https://doi.org/10.3390/ma12071130
Leszczyńska-Sejda K, Benke G, Ciszewski M, Drzazga M. Technology to Produce High-Purity Anhydrous Rubidium Perrhenate on an Industrial Scale. Materials. 2019; 12(7):1130. https://doi.org/10.3390/ma12071130
Chicago/Turabian StyleLeszczyńska-Sejda, Katarzyna, Grzegorz Benke, Mateusz Ciszewski, and Michał Drzazga. 2019. "Technology to Produce High-Purity Anhydrous Rubidium Perrhenate on an Industrial Scale" Materials 12, no. 7: 1130. https://doi.org/10.3390/ma12071130
APA StyleLeszczyńska-Sejda, K., Benke, G., Ciszewski, M., & Drzazga, M. (2019). Technology to Produce High-Purity Anhydrous Rubidium Perrhenate on an Industrial Scale. Materials, 12(7), 1130. https://doi.org/10.3390/ma12071130