Improved DC Dielectric Performance of cPP-g-MAH/iPP/SEBS Composite with Chemical Graft Modification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterization and Testing Scheme
2.3. Theoretical Calculation Methodology
3. Results and Discussion
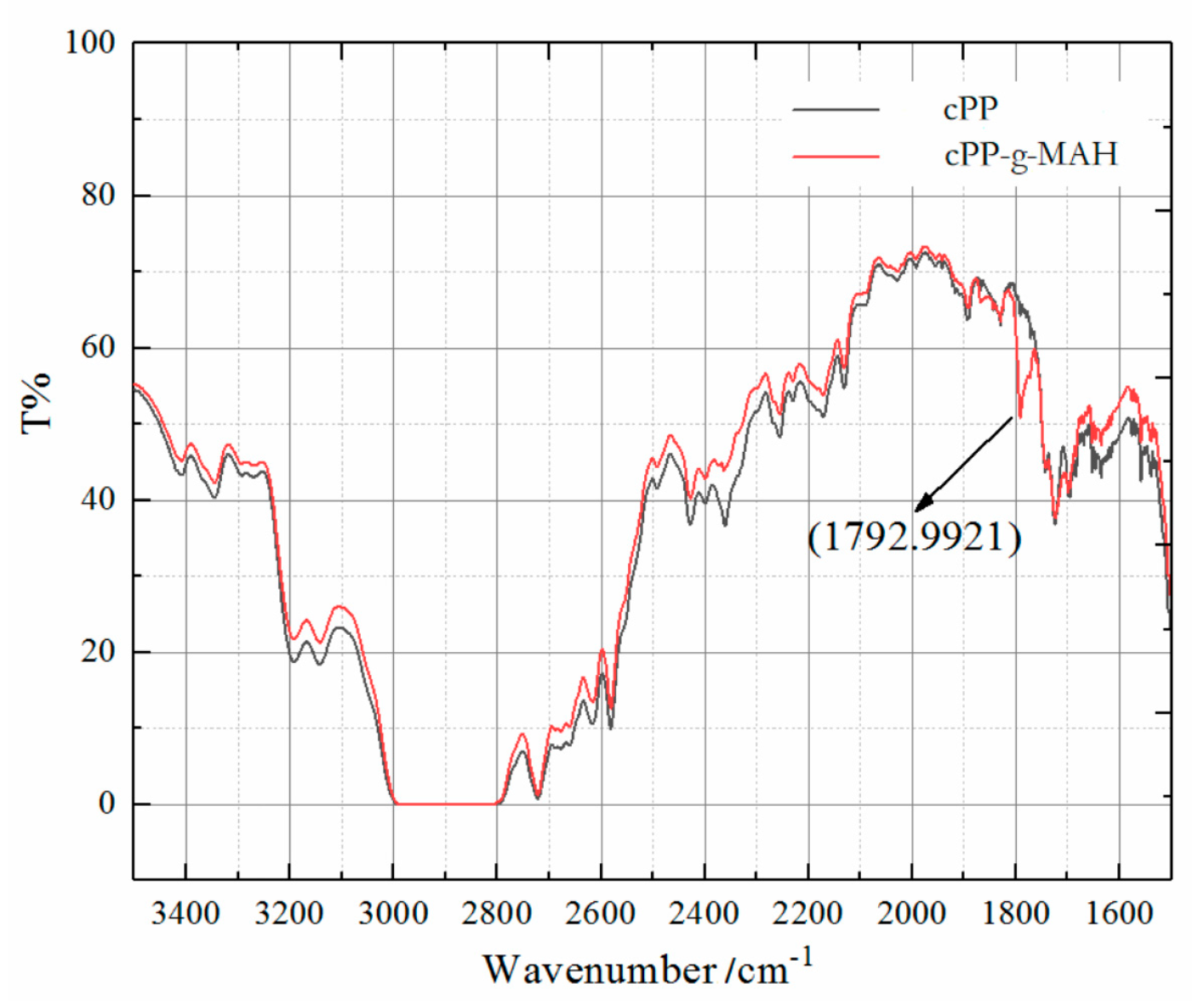
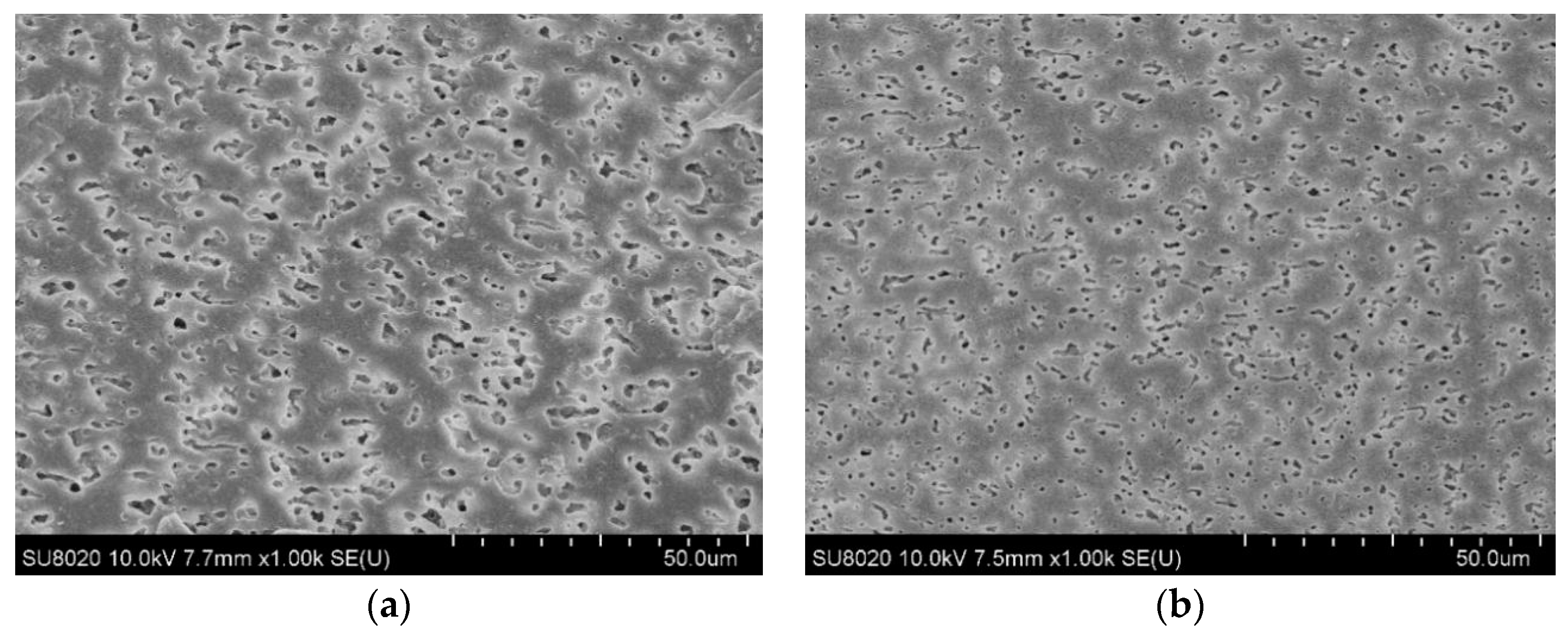
3.1. Structure Characterization
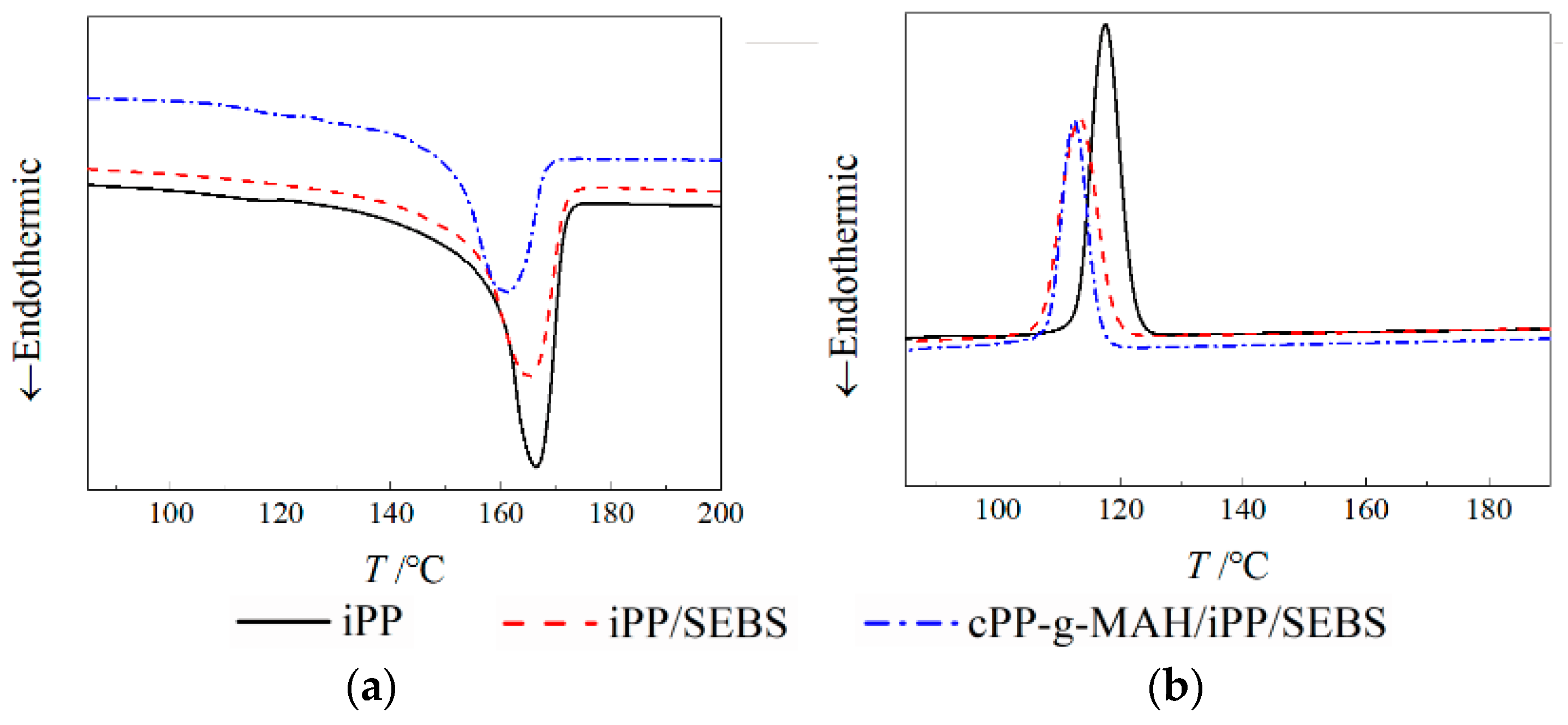
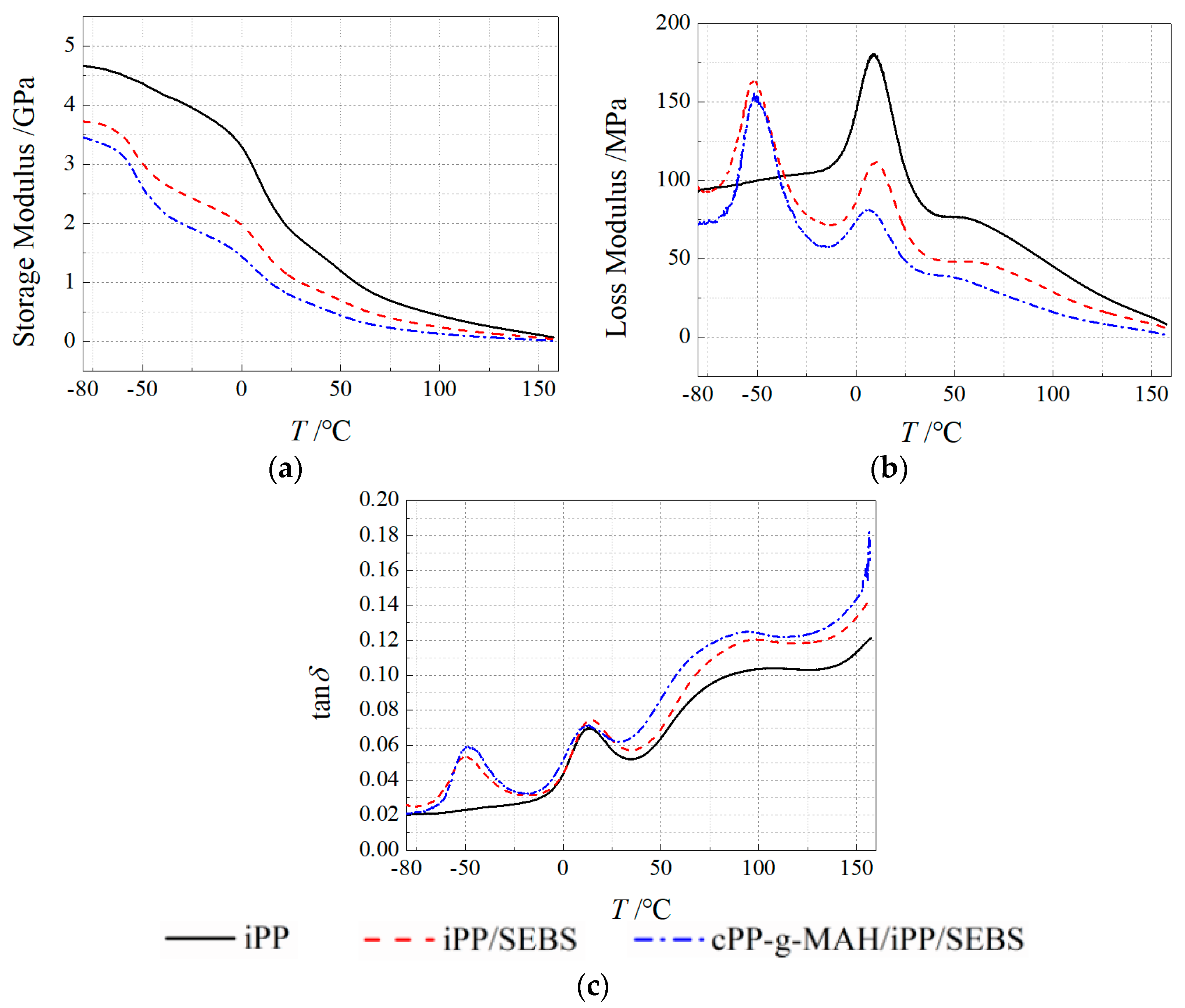
3.2. Thermal–Mechanical Performance
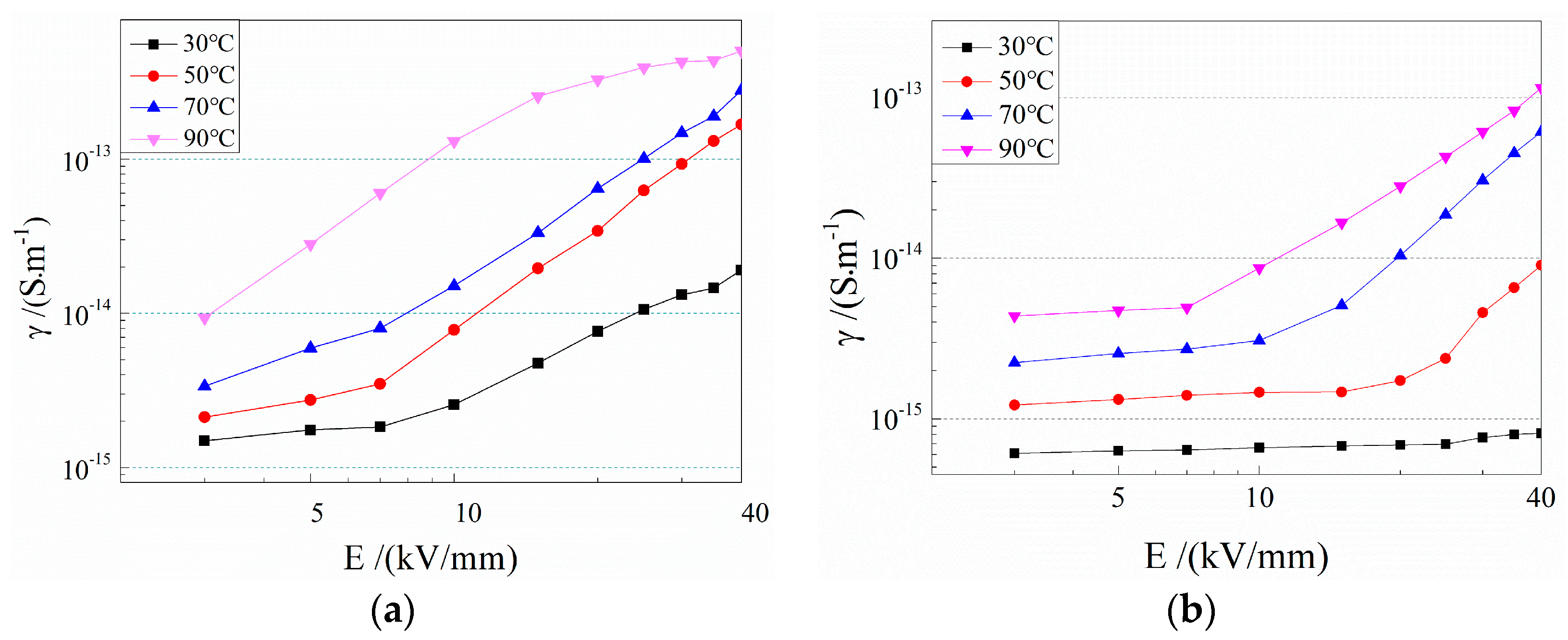
3.3. Electrical Conductivity
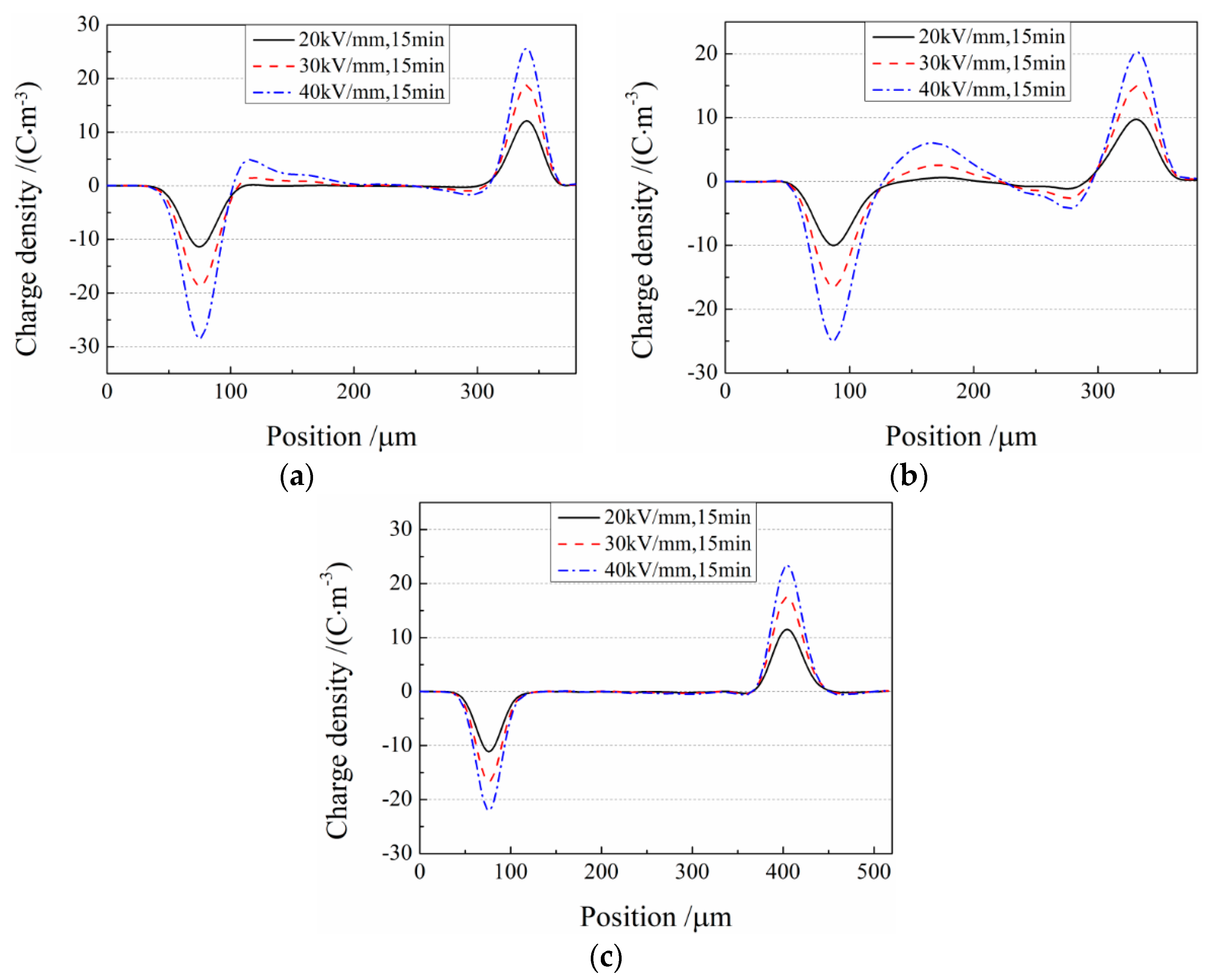
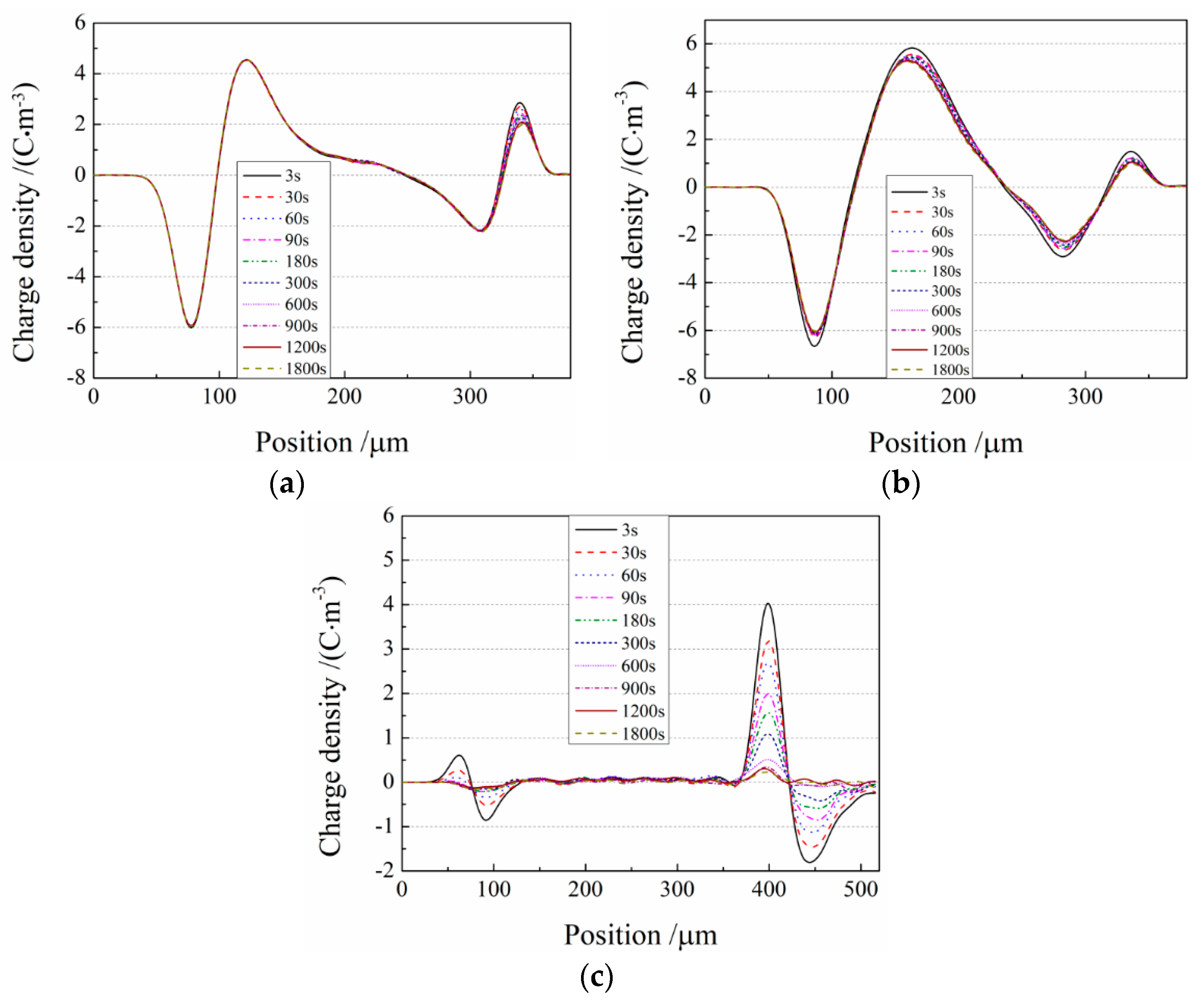
3.4. Space Charge Characteristics
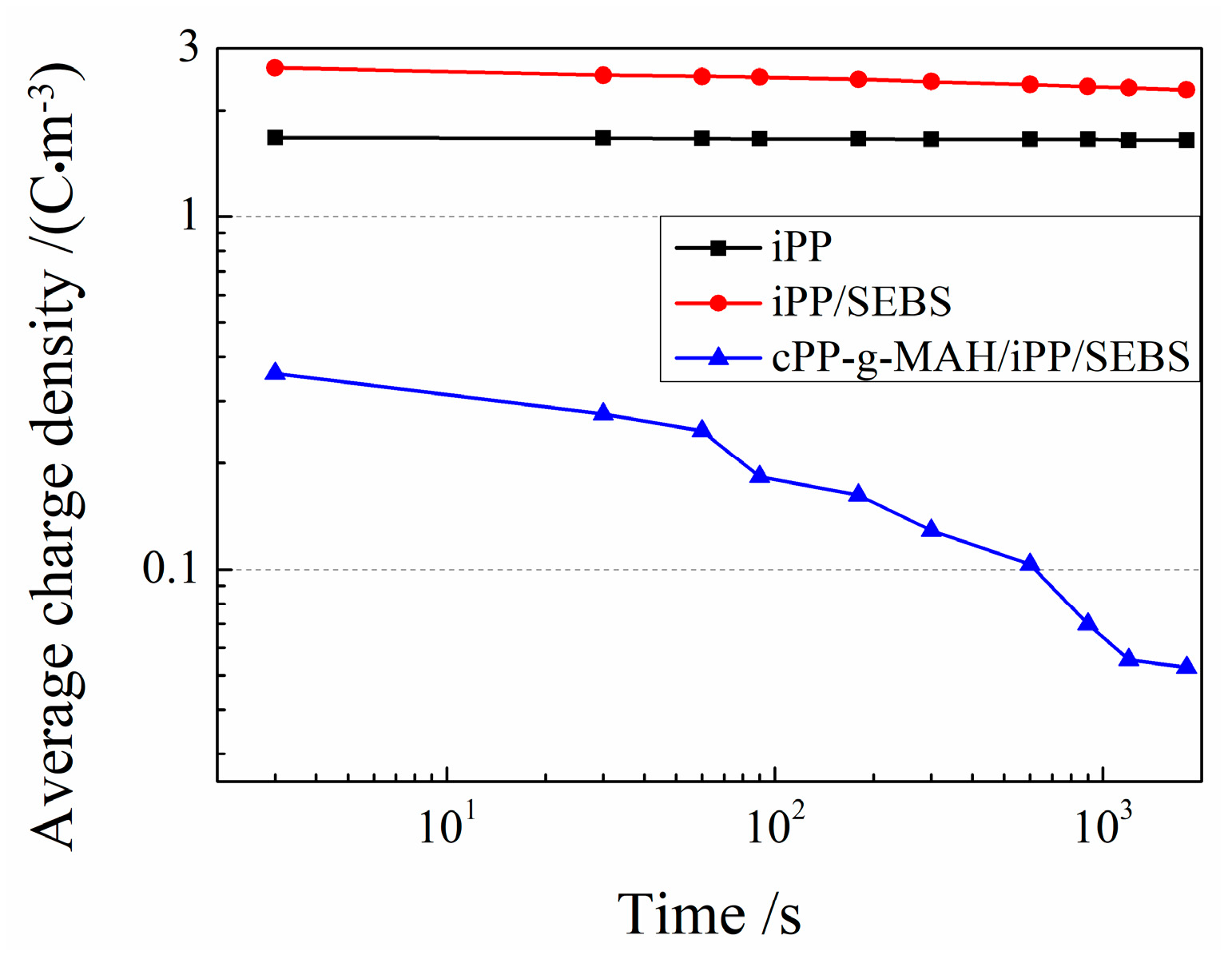
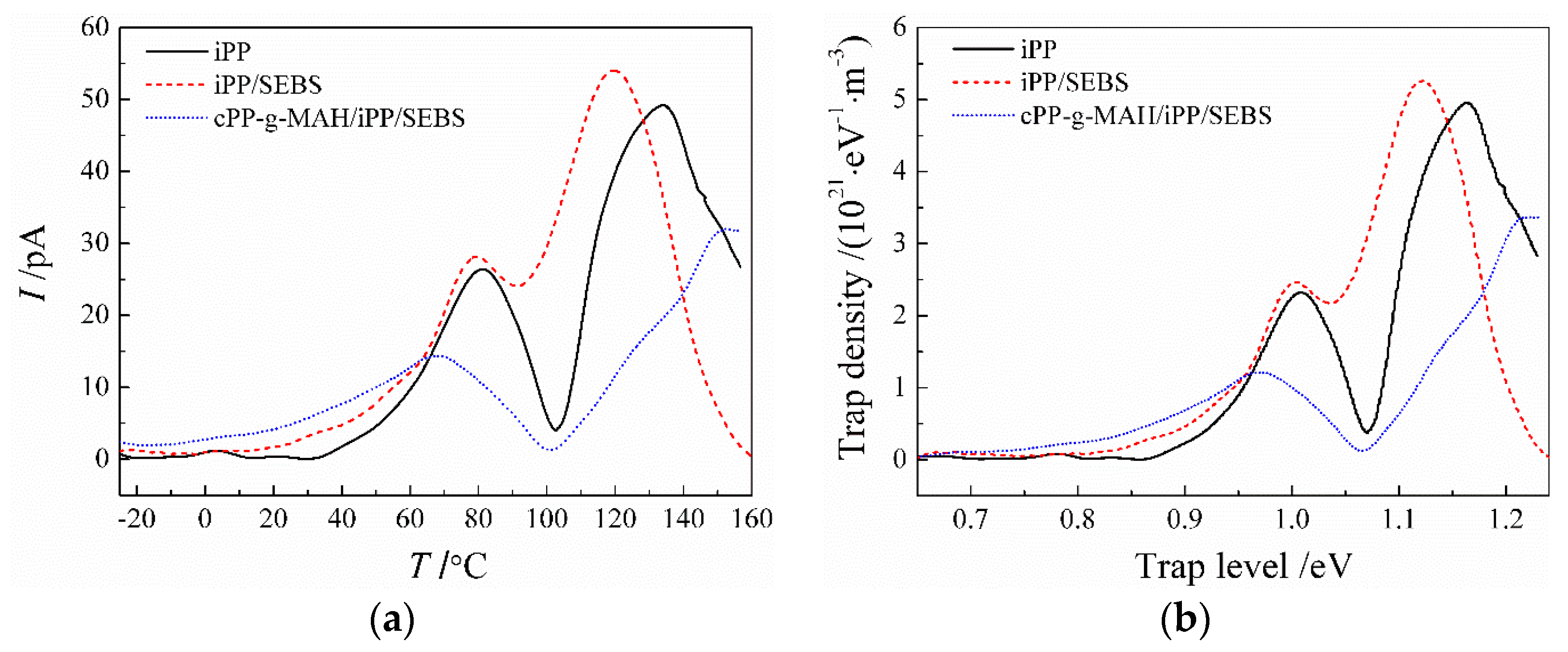
3.5. Charge Trap Characteristics
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ou, M.; Xue, Y.; Zhang, X. Iterative DC Optimal power flow considering transmission network loss. Electr. Power Compon. Syst. 2016, 44, 955965. [Google Scholar] [CrossRef]
- Feng, D.; Chen, Z. System Control of Power Electronics Interfaced Distribution Generation Units. In Proceedings of the IEEE International Power Electronics & Motion Control Conference, Shanghai, China, 14–16 August 2009. [Google Scholar]
- Zhou, Y.; He, J.; Hu, J.; Huang, X.; Jiang, P. Evaluation of polypropylene/polyolefin elastomer blends for potential recyclable HVDC cable insulation applications. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 673–681. [Google Scholar] [CrossRef]
- Huang, X.; Fan, Y.; Zhang, J.; Jiang, P. Polypropylene based thermoplastic polymers for potential recyclable HVDC cable insulation applications. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1446–1456. [Google Scholar] [CrossRef]
- Diao, J.; Huang, X.; Jia, Q.; Liu, F.; Jiang, P. Thermoplastic isotactic polypropylene/ethylene-octene polyolefin co-polymer nanocomposite for recyclable HVDC cable insulation. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1416–1429. [Google Scholar] [CrossRef]
- Yuan, X.; Chung, T.C.M. Cross-linking effect on dielectric properties of polypropylene thin films and applications in electric energy storage. Appl. Phys. Lett. 2011, 98, 062901. [Google Scholar] [CrossRef]
- Dang, B.; Hu, J.; Zhou, Y.; He, J. Remarkably improved electrical insulating performances of lightweight poly-propylene nanocomposites with fullerene. J. Phys. D 2017, 50, 455303. [Google Scholar] [CrossRef]
- Hampton, R.N. Feature article-some of the considerations for materials operating under high-voltage, direct-current stresses. IEEE Electr. Insul. Mag. 2008, 24, 5–13. [Google Scholar] [CrossRef]
- Khalil, M.S. International research and development trends and problems of HVDC cables with polymeric insulation. IEEE Electr. Insul. Mag. 1997, 13, 35–47. [Google Scholar] [CrossRef]
- Hanley, T.L.; Burford, R.P.; Fleming, R.J.; Baber, K.W. A general review of polymeric insulation for use in HVDC cables. IEEE Electr. Insul. Mag. 2003, 19, 13–24. [Google Scholar] [CrossRef]
- Ghosh, S.; Bhowmick, A.K.; Roychowdhury, N.; Holden, G. Influence of block molecular weight on the proper-ties of styrene-ethylenebutylene-styrene block copolymers. J. Appl. Polym. Sci. 2000, 77, 1621–1628. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Wilkinson, A.; Liauw, C.M.; Mourelatou, D.; Barrio, J.A.; Maria, A.M. Degradation and stabilisation of styrene-ethylene-butadiene-styrene (SEBS) block copolymer. Polym. Degrad. Stab. 2000, 71, 113–122. [Google Scholar] [CrossRef]
- Allen, N.S.; Edge, M.; Mourelatou, D.; Wilkinson, A.; Liauw, C.M.; Parellada, M.D.; Barrio, J.A.; Quiterio, R.S. Influence of ozone on styrene–ethylene–butylene–styrene (SEBS) copolymer. Polym. Degrad. Stab. 2003, 79, 297–307. [Google Scholar] [CrossRef]
- Tjong, S.C.; Shi, A.X.; Li, R.K.Y.; Yiu, W.M. Preparation and performance characteristics of short-glass-fiber/maleated styrene–ethylene–butylene–styrene/polypropylene hybrid composites. J. Appl. Polym. Sci. 2010, 86, 1303–1311. [Google Scholar] [CrossRef]
- Ohlsson, B.; Törnell, B. Melt miscibility in blends of polypropylene, polystyrene-block-poly(ethylene-statbutylene)-block-polystyrene, and processing oil from melting point depression. Polym. Eng. Sci. 1996, 36, 1547–1556. [Google Scholar] [CrossRef]
- Montanari, G.C. Bringing an insulation to failure: The role of space charge. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 339–364. [Google Scholar] [CrossRef]
- Ling, Z.; Zhou, Y.; Cui, X.; Sha, Y.; Le, T.; Ye, Q.; Tian, J. Effect of nanoparticle surface modification on breakdown and space charge behavior of XLPE/SiO2 nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1554–1564. [Google Scholar]
- Cavallini, A.; Montanari, G.C.; Tozzi, M.; Chen, X. Diagnostic of HVDC systems using partial discharges. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 275–284. [Google Scholar] [CrossRef]
- Milliere, L.; Maskasheva, K.; Laurent, C.; Despax, B.; Boudou, L.; Teyssedre, G. Silver nanoparticles as a key feature of a plasma polymer composite layer in mitigation of charge injection into polyethylene under dc stress. J. Phys. D Appl. Phys. 2016, 49, 015304. [Google Scholar] [CrossRef]
- Hosier, I.L.; Reaud, S.; Vaughan, A.S.; Swinler, S.G. Morphology, thermal, mechanical and electrical properties of propylene-based materials for cable applications. In Proceedings of the Conference Record of the IEEE International Symposium on Electrical Insulation, Vancouver, BC, Canada, 9–12 June 2008. [Google Scholar]
- Hosier, I.L.; Cozzarini, L.; Vaughan, A.S.; Swinler, S.G. Propylene based systems for high voltage cable insulation applications. J. Phys. Conf. Ser. 2009, 183, 012015. [Google Scholar] [CrossRef]
- Dang, B.; He, J.; Hu, J.; Zhou, Y. Tailored sPP/silica nanocomposite for ecofriendly insulation of extruded HVDC cable. J. Nanomater. 2015, 2015, 686248. [Google Scholar] [CrossRef]
- Takada, T.; Hayase, Y.; Tanaka, Y.; Okamoto, T. Space charge trapping in electrical potential well caused by permanent and induced dipoles for LDPE/MgO nanocomposite. IEEE Trans. Dielectr. Electr. Insul. 2007, 15, 152–160. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of a multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Han, B.; Wang, X.; Sun, Z.; Yang, J.; Lei, Q. Space charge suppression induced by deep traps in polyethylene/zeolite nanocomposite. Appl. Phys. Lett. 2013, 102, 012902. [Google Scholar] [CrossRef]
- Huang, X.; Jiang, P.; Yi, Y. Nanoparticle surface modification induced space charge suppression in linear lowdensity polyethylene. Appl. Phys. Lett. 2009, 95, 242905. [Google Scholar] [CrossRef]
- Xu, W.; Liang, G.; Wang, W.; Tang, S.; He, P.; Pan, W.P. Poly(propylene)-poly(propylene)-grafted maleic anhydride-organic montmorillonite (PP/PP-g-MAH/Org-MMT) nanocomposites. II. Nonisothermal crystallization kinetics. J. Appl. Polym. Sci. 2003, 88, 3093–3099. [Google Scholar] [CrossRef]
- Ao, Y.; Tang, K.; Xu, N.; Yang, H.; Zhang, H. Compatibilization of PP/SEBS-MAH blends by grafting glycidyl methacrylate onto polypropylene. Polym. Bull. 2007, 59, 279–288. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Dang, B.; He, J. Mechanism of highly improved electrical properties in polypropylene by chemical modification of grafting maleic anhydride. J. Phys. D Appl. Phys. 2016, 49, 415301. [Google Scholar] [CrossRef]
- Zha, J.; Wu, Y.; Wang, S.; Wu, D.; Yan, D.; Dang, Z. Improvement of space charge suppression of polypropylene for potential application in HVDC cables. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2337–2343. [Google Scholar] [CrossRef]
- Gupta, A.K.; Purwar, S.N. Crystallization of PP in PP/SEBS blends and its correlation with tensile properties. J. Appl. Polym. Sci. 2010, 29, 1595–1609. [Google Scholar] [CrossRef]
- Ieda, M. Electrical conduction and carrier traps in polymeric materials. IEEE Trans. Electr. Insul. 1984, EI-19, 162–178. [Google Scholar] [CrossRef]
- Tian, F.; Lei, Q.; Wang, X.; Wang, Y. Effect of deep trapping states on space charge suppression in polyethylene/ZnO nanocomposite. Appl. Phys. Lett. 2011, 99, 142903. [Google Scholar] [CrossRef]
- Wang, S.J.; Zha, J.W.; Wu, Y.H.; Ren, L.; Dang, Z.M.; Wu, J. Preparation, microstructure and properties of polyethylene/alumina nanocomposites for HVDC insulation. IEEE Trans. Dielectr. Electr. Insul. 2016, 22, 3350–3356. [Google Scholar] [CrossRef]
- Lim, F.N.; Fleming, R.J.; Naybour, R.D. Space charge accumulation in power cable XLPE insulation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 273–281. [Google Scholar] [CrossRef]
- Tian, F.; Bu, W.; Shi, L.; Yang, C.; Wang, Y.; Lei, Q. Theory of modified thermally stimulated current and direct determination of trap level distribution. J. Electrost. 2011, 69, 7–10. [Google Scholar] [CrossRef]

| Samples | cPP-g-MAH | iPP | SEBS |
|---|---|---|---|
| iPP/SEBS | 0 | 75 | 25 |
| cPP-g-MAH/iPP/SEBS | 37.5 | 37.5 | 25 |
| Electronic Hamiltonian | Scheme | Condition and Parameter |
|---|---|---|
| Exchange–correlation energy | Meta-generalized-gradient approximation | Multi-configurational dual-range local exchange (M11-L) |
| Integration accuracy | – | 2000 grid points /atom |
| Self-consistent field | Tolerance | 1 × 10−6 eV/atom |
| Multipolar expansion | Octupole | |
| Charge density mixing | Charge = 0.3, Direct inversion iterative subspace (DIIS) = 5 | |
| Core treatment | All-electron | – |
| Numerical basis set | Double numerical polarized | Basis file 4.4 |
| Orbital cutoff | Global | 5.0 Å |
| Sample | iPP | iPP/SEBS | cPP-g-MAH/iPP/SEBS |
|---|---|---|---|
| Tc (°C) | 117.5 | 113.7 | 111.9 |
| Tm (°C) | 166.5 | 165.4 | 162.2 |
| XC (%) | 39.91 | 35.65 | 28.23 |
| Samples | iPP | iPP/SEBS | cPP-g-MAH/iPP/SEBS |
|---|---|---|---|
| 155 °C | 5 | 9 | 10 |
| 165 °C | 7 | 17 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yang, J.; Zhao, H.; Sun, W.; Gao, M.; Zhao, X.; Hu, M.; Xie, S. Improved DC Dielectric Performance of cPP-g-MAH/iPP/SEBS Composite with Chemical Graft Modification. Materials 2019, 12, 1094. https://doi.org/10.3390/ma12071094
Zhou Y, Yang J, Zhao H, Sun W, Gao M, Zhao X, Hu M, Xie S. Improved DC Dielectric Performance of cPP-g-MAH/iPP/SEBS Composite with Chemical Graft Modification. Materials. 2019; 12(7):1094. https://doi.org/10.3390/ma12071094
Chicago/Turabian StyleZhou, Yu, Jiaming Yang, Hong Zhao, Weifeng Sun, Mingze Gao, Xindong Zhao, Ming Hu, and Shuhong Xie. 2019. "Improved DC Dielectric Performance of cPP-g-MAH/iPP/SEBS Composite with Chemical Graft Modification" Materials 12, no. 7: 1094. https://doi.org/10.3390/ma12071094
APA StyleZhou, Y., Yang, J., Zhao, H., Sun, W., Gao, M., Zhao, X., Hu, M., & Xie, S. (2019). Improved DC Dielectric Performance of cPP-g-MAH/iPP/SEBS Composite with Chemical Graft Modification. Materials, 12(7), 1094. https://doi.org/10.3390/ma12071094






