Application of Reverse Micelle Sol–Gel Synthesis for Bulk Doping and Heteroatoms Surface Enrichment in Mo-Doped TiO2 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
Physico-Chemical Characterization of the Prepared Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.T.; Lee, A.T.; Hoffmann, M.R. Chemical mechanism of inorganic oxidants in the TiO2/UV process: Increased rates of degradation of chlorinated hydrocarbons. Environ. Sci. Technol. 1995, 29, 2567–2573. [Google Scholar] [CrossRef]
- Choi, W.; Hoffmann, M.R. Novel Photocatalytic Mechanisms for CHCl3, CHBr3, and CCl3CO2− Degradation and the Fate of Photogenerated Trihalomethyl Radicals on TiO2. Environ. Sci. Technol. 1997, 31, 89–95. [Google Scholar] [CrossRef]
- Prairie, M.R.; Evans, L.R.; Stange, B.M.; Martinez, S.L. An investigation of titanium dioxide photocatalysis for the treatment of water contaminated with metals and organic chemicals. Environ. Sci. Technol. 1993, 27, 1776–1782. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Understanding Polymorphic Phase Transformation Behavior during Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Wang, C.C.; Zakaria, R.; Ying, J.Y. Role of particle size in nanocrystalline TiO2-based photocatalysts. J. Phys. Chem. Biol. 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J.F. Thermodynamic analysis of phase stability of nanocrystalline titania. J. Mater. Chem. 1998, 8, 2073–2076. [Google Scholar] [CrossRef]
- Oskam, G.; Nellore, A.; Penn, R.L.; Searson, P.C. The growth kinetics of TiO2 nanoparticles from titanium (IV) alkoxide at high water/titanium ratio. J. Phys. Chem. B 2003, 107, 1734–1738. [Google Scholar] [CrossRef]
- Wu, M.; Lin, G.; Chen, D.; Wang, G.; He, D.; Feng, S.; Xu, R. Sol-hydrothermal synthesis and hydrothermally structural evolution of nanocrystal titanium dioxide. Chem. Mater. 2002, 14, 1974–1980. [Google Scholar] [CrossRef]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and Photoelectrochemical Investigation of Single-Crystal Anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Taga, Y.; Mannstadt, W. Electronic and optical properties of anatase. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 7459–7465. [Google Scholar] [CrossRef]
- Ozawa, K.; Emori, M.; Yamamoto, S.; Yukawa, R.; Yamamoto, S.; Hobara, R.; Fujikawa, K.; Sakama, H.; Matsuda, I. Electron–Hole Recombination Time at TiO2 Single-Crystal Surfaces: Influence of Surface Band Bending. J. Phys. Chem. Lett. 2014, 5, 1953–1957. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts-Applications and Attributes; IntechOpen: London, UK, 2018. [Google Scholar]
- Clarizia, L.; Vitiello, G.; Pallotti, D.K.; Silvestri, B.; Nadagouda, M.; Lettieri, S.; Luciani, G.; Andreozzi, R.; Maddalena, P.; Marotta, R. Effect of surface properties of copper-modified commercial titanium dioxide photocatalysts on hydrogen production through photoreforming of alcohols. Int. J. Hydrogen Energy 2017, 42, 28349–28362. [Google Scholar] [CrossRef]
- Freyria, F.S.; Compagnoni, M.; Ditaranto, N.; Rossetti, I.; Piumetti, M.; Ramis, G.; Bonelli, B. Pure and Fe-doped mesoporous titania catalyse the oxidation of acid orange 7 by H2O2 under different illumination conditions: Fe doping improves photocatalytic activity under simulated solar light. Catalysts 2017, 7, 213. [Google Scholar] [CrossRef]
- Piumetti, M.; Freyria, F.S.; Armandi, M.; Geobaldo, F.; Garrone, E.; Bonelli, B. Fe- and V-doped mesoporous titania prepared by direct synthesis: Characterization and role in the oxidation of AO7 by H2O2 in the dark. Catal. Today 2014, 227, 71–79. [Google Scholar] [CrossRef]
- Zeng, L.; Lu, Z.; Li, M.; Yang, J.; Song, W.; Zeng, D.; Xie, C. A modular calcination method to prepare modified N-doped TiO2 nanoparticle with high photocatalytic activity. Appl. Catal. B Environ. 2016, 183, 308–316. [Google Scholar] [CrossRef]
- Hossain, M.A.; Elias, M.; Sarker, D.R.; Diba, Z.R.; Mithun, J.M.; Azad, M.A.K.; Siddiquey, I.A.; Rahman, M.M.; Uddin, J.; Uddin, M.N. Synthesis of Fe- or Ag-doped TiO2–MWCNT nanocomposite thin films and their visible-light-induced catalysis of dye degradation and antibacterial activity. Res. Chem. Intermed. 2018, 44, 2667–2683. [Google Scholar] [CrossRef]
- Guayaquil-Sosa, J.F.; Serrano-Rosales, B.; Valadés-Pelayo, P.J.; de Lasa, H. Photocatalytic hydrogen production using mesoporous TiO2 doped with Pt. Appl. Catal. B Environ. 2017, 211, 337–348. [Google Scholar] [CrossRef]
- Lavorato, C.; Argurio, P.; Mastropietro, T.F.; Pirri, G.; Poerio, T.; Molinari, R. Pd/TiO2 doped faujasite photocatalysts for acetophenone transfer hydrogenation in a photocatalytic membrane reactor. J. Catal. 2017, 353, 152–161. [Google Scholar] [CrossRef]
- Jin, C.; Dai, Y.; Wei, W.; Ma, X.; Li, M.; Huang, B. Effects of single metal atom (Pt, Pd, Rh and Ru) adsorption on the photocatalytic properties of anatase TiO2. Appl. Surf. Sci. 2017, 426, 639–646. [Google Scholar] [CrossRef]
- Zou, Z.; Zhou, Z.; Wang, H.; Yang, Z. Effect of Au clustering on ferromagnetism in Au doped TiO2 films: theory and experiments investigation. J. Phys. Chem. Solids 2017, 100, 71–77. [Google Scholar] [CrossRef]
- Gai, Y.; Li, J.; Li, S.; Xia, J.; Wei, S. Design of Narrow-Gap TiO2: A Passivated Codoping Approach for Enhanced Photoelectrochemical Activity. Phys. Rev. Lett. 2009, 36402, 23–26. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J. Phys. Chem. Solids 2002, 63, 1909–1920. [Google Scholar] [CrossRef]
- Bonelli, B.; Esposito, S.; Freyria, F.S. Mesoporous Titania: Synthesis, properties and comparison with non-porous titania. In Titanium Dioxide; Intech: London, UK, 2017; ISBN 978-953-51-5493-8. [Google Scholar]
- Chandra, P.; Doke, D.S.; Umbarkar, S.B.; Biradar, A.V. One-pot synthesis of ultrasmall MoO3 nanoparticles supported on SiO2, TiO2, and ZrO2 nanospheres: An efficient epoxidation catalyst. J. Mater. Chem. A 2014, 2, 19060–19066. [Google Scholar] [CrossRef]
- Ghosh, S. Comparative studies on brij reverse micelles prepared in benzene/surfactant/ethylammonium nitrate systems: Effect of head group size and polarity of the hydrocarbon chain. J. Colloid Interface Sci. 2011, 360, 672–680. [Google Scholar] [CrossRef]
- Gadhi, T.A.; Hernández, S.; Castellino, M.; Chiodoni, A.; Husak, T.; Barrera, G.; Allia, P.; Russo, N.; Tagliaferro, A. Single BiFeO3 and mixed BiFeO3/Fe2O3/Bi2Fe4O9 ferromagnetic photocatalysts for solar light driven water oxidation and dye pollutants degradation. J. Ind. Eng. Chem. 2018, 63, 437–448. [Google Scholar] [CrossRef]
- Esposito, S.; Turco, M.; Bagnasco, G.; Cammarano, C.; Pernice, P. New insight into the preparation of copper/zirconia catalysts by sol–gel method. Appl. Catal. A Gen. 2011, 403, 128–135. [Google Scholar] [CrossRef]
- Esposito, S. “Traditional” Sol-Gel Chemistry as a Powerful Tool for the Preparation of Supported Metal and Metal Oxide Catalysts. Materials 2019, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Zhang, S.; Lu, R. Facile synthesis of ultrasmall monodisperse “raisin–bun”-type MoO3/SiO2 nanocomposites with enhanced catalytic properties. Nanoscale 2013, 5, 4823. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Ishigaki, T. Fe-doped TiO2 nanopowders by oxidative pyrolysis of organometallic precursors in induction thermal plasma: Synthesis and structural characterization. Thin Solid Films 2006, 506–507, 278–282. [Google Scholar] [CrossRef]
- Batzill, M.; Morales, E.H.; Diebold, U. Influence of nitrogen doping on the defect formation and surface properties of TiO2 rutile and anatase. Phys. Rev. Lett. 2006, 96, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ciambelli, P.; Sannino, D.; Palma, V.; Vaiano, V.; Bickley, R.I. Reaction mechanism of cyclohexane selective photo-oxidation to benzene on molybdena/titania catalysts. Appl. Catal. A Gen. 2008, 349, 140–147. [Google Scholar] [CrossRef]
- Del Arco, M.; Carrazhn, S.R.G.; Rives, V.; Garcla-Ramos, J.V. A Laser Raman Spectroscopy Study of Surface Species Existing in MoO3/A12O3 Catalysts. Spectrosc. Lett. 1992, 25, 73–82. [Google Scholar] [CrossRef]
- Stampfl, S.R.; Chen, Y.; Dumesic, J.A.; Niu, C.; Hill, C.G. Interactions of molybdenum oxide with various oxide supports: Calcination of mechanical mixtures. J. Catal. 1987, 105, 445–454. [Google Scholar] [CrossRef]
- Nasi, R.; Gadhi, T.A.; Freyria, F.S.; Ditaranto, N.; Esposito, S.; Hernandez, S.; Armandi, M.; Bonelli, B. Surface chemical characterization of Mo doped TiO2 nanoparticles for photocatalytic dye degradation. In Proceedings of the Incontro Spettroscopia Analitica ISA 2018, Cagliari, Italy, 5–8 June 2018; Volume 1, pp. 5–6. [Google Scholar]
- Gomathi Devi, L.; Narasimha Murthy, B. Characterization of Mo Doped TiO2 and its Enhanced Photo Catalytic Activity Under Visible Light. Catal. Lett. 2008, 125, 320–330. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of Surface Area, Primary Particle Size, and Crystal Phase on Titanium Dioxide Nanoparticle Dispersion Properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar] [CrossRef]
- Holmberg, J.P.; Ahlberg, E.; Bergenholtz, J.; Hassellöv, M.; Abbas, Z. Surface charge and interfacial potential of titanium dioxide nanoparticles: Experimental and theoretical investigations. J. Colloid Interface Sci. 2013, 407, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Pirinejad, L.; Maleki, A.; Shahmoradi, B.; Daraei, H.; Yang, J.-K.; Lee, S.-M. Synthesis and application of Fe-N-Cr-TiO2 nanocatalyst for photocatalytic degradation of Acid Black 1 under LED light irradiation. J. Mol. Liq. 2019, 279, 232–240. [Google Scholar] [CrossRef]
- Allard, M.M.; Merlos, S.N.; Springer, B.N.; Cooper, J.; Zhang, G.; Boskovic, D.S.; Kwon, S.R.; Nick, K.E.; Perry, C.C. Role of TiO2 Anatase Surface Morphology on Organophosphorus Interfacial Chemistry. J. Phys. Chem. C 2018, 122, 29237–29248. [Google Scholar] [CrossRef]
- Al-Hetlani, E.; Amin, M.O.; Madkour, M. Detachable photocatalysts of anatase TiO2 nanoparticles: Annulling surface charge for immediate photocatalyst separation. Appl. Surf. Sci. 2017, 411, 355–362. [Google Scholar] [CrossRef]
- He, H.; Cheng, Y.; Yang, C.; Zeng, G.; Zhu, C.; Yan, Z. Influences of anion concentration and valence on dispersion and aggregation of titanium dioxide nanoparticles in aqueous solutions. J. Environ. Sci. 2017, 54, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 7104. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.L.; Wu, G.S.; Liao, B.Q. Zeta potential of shape-controlled TiO2 nanoparticles with surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2009, 348, 270–275. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di, K.; Dumontel, B.; Garino, N. Applied Catalysis B: Environmental Sonophotocatalytic degradation mechanisms of Rhodamine B dye via radicals generation by micro- and nano-particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, M.; Pasternak, S.; Paz, Y.; Rochkind, M.; Pasternak, S.; Paz, Y. Using Dyes for Evaluating Photocatalytic Properties: A Critical Review. Molecules 2014, 20, 88–110. [Google Scholar] [CrossRef]
- Gadhi, T.A.; Hernández-Gordillo, A.; Bizarro, M.; Jagdale, P.; Tagliaferro, A.; Rodil, S.E. Efficient α/β-Bi2O3 composite for the sequential photodegradation of two-dyes mixture. Ceram. Int. 2016, 42, 13065–13073. [Google Scholar] [CrossRef]
- Boppella, R.; Basak, P.; Manorama, S.V. Viable method for the synthesis of biphasic TiO2 nanocrystals with tunable phase composition and enabled visible-light photocatalytic performance. ACS Appl. Mater. Interfaces 2012, 4, 1239–1246. [Google Scholar] [CrossRef]


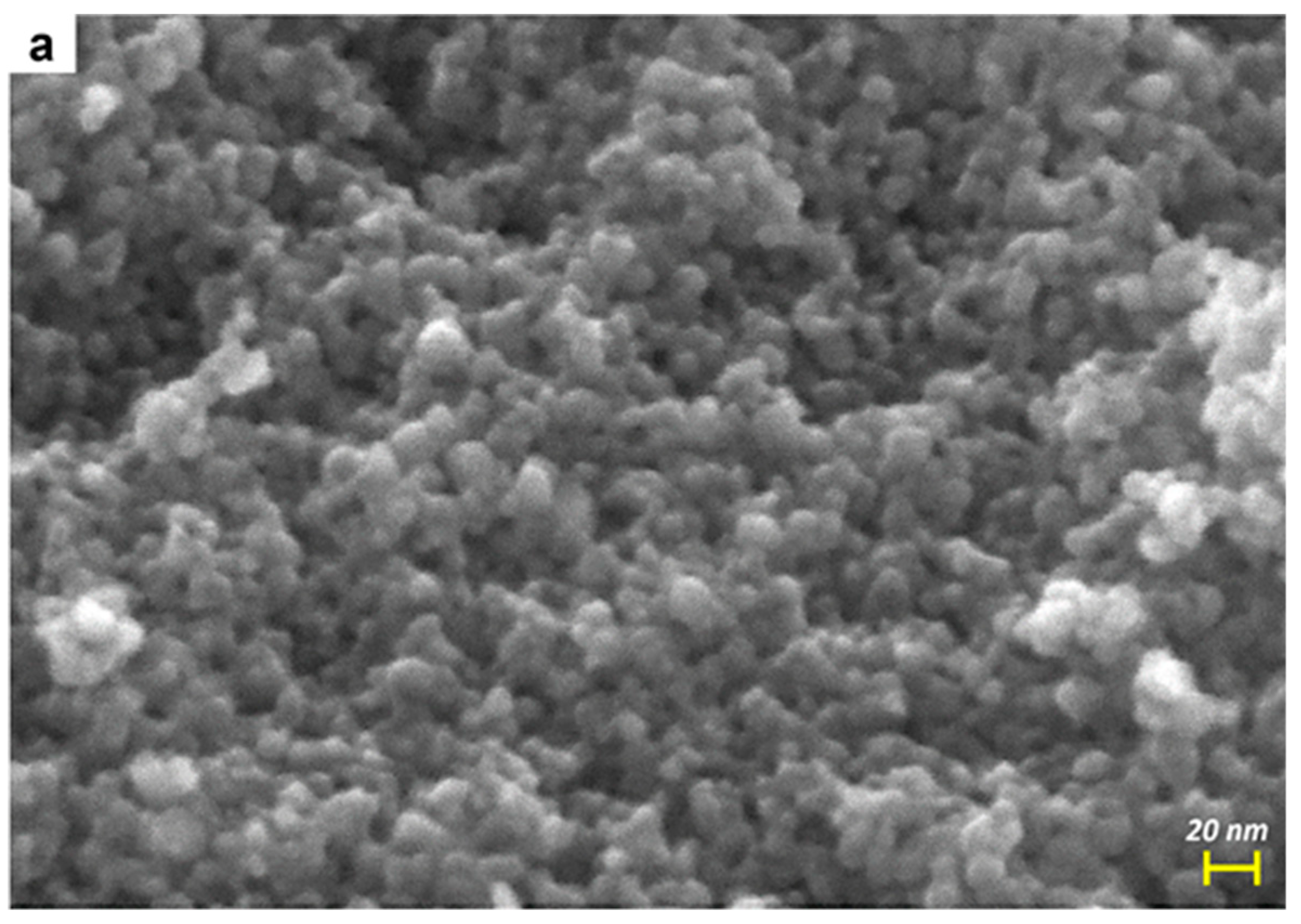


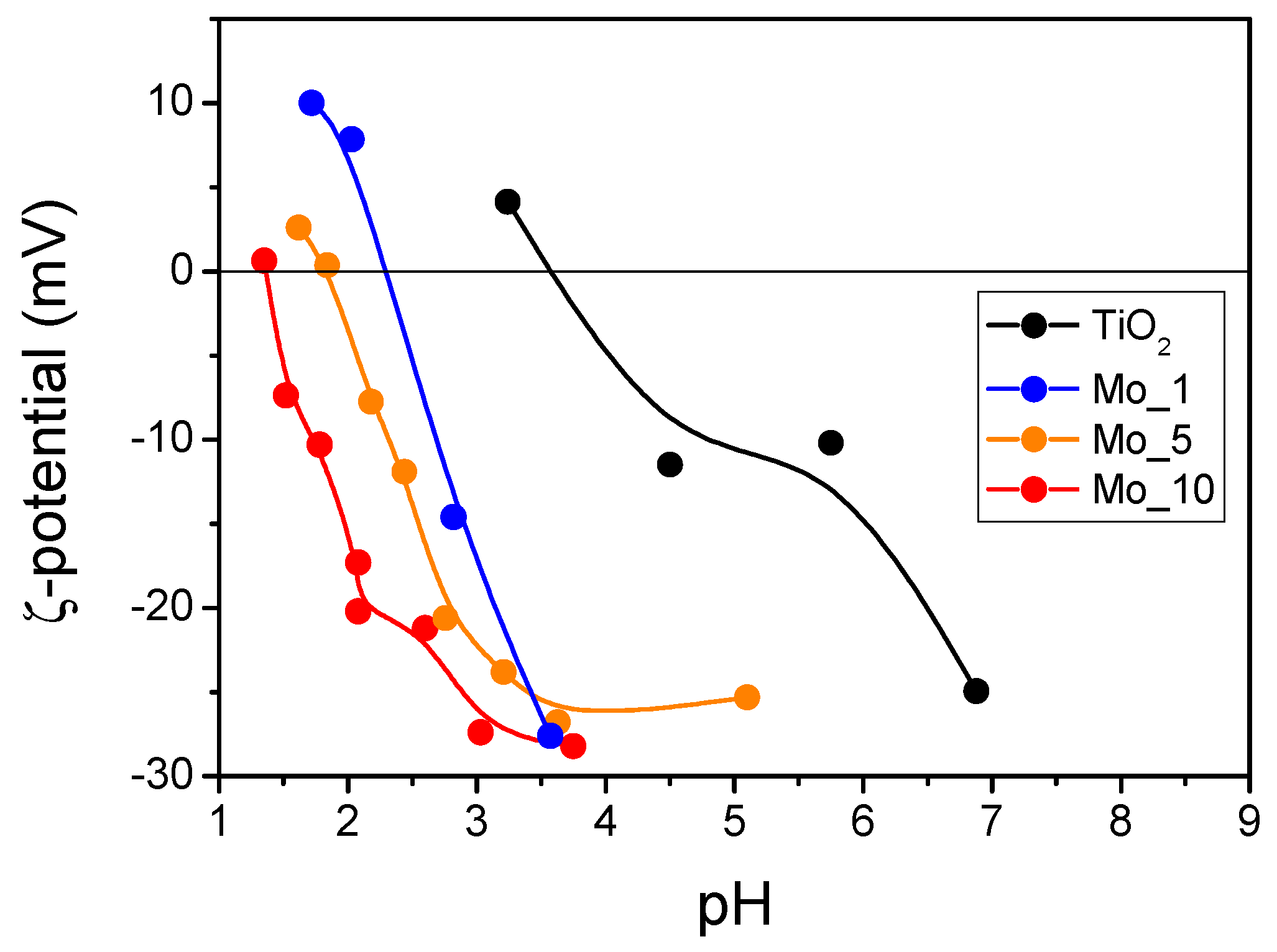
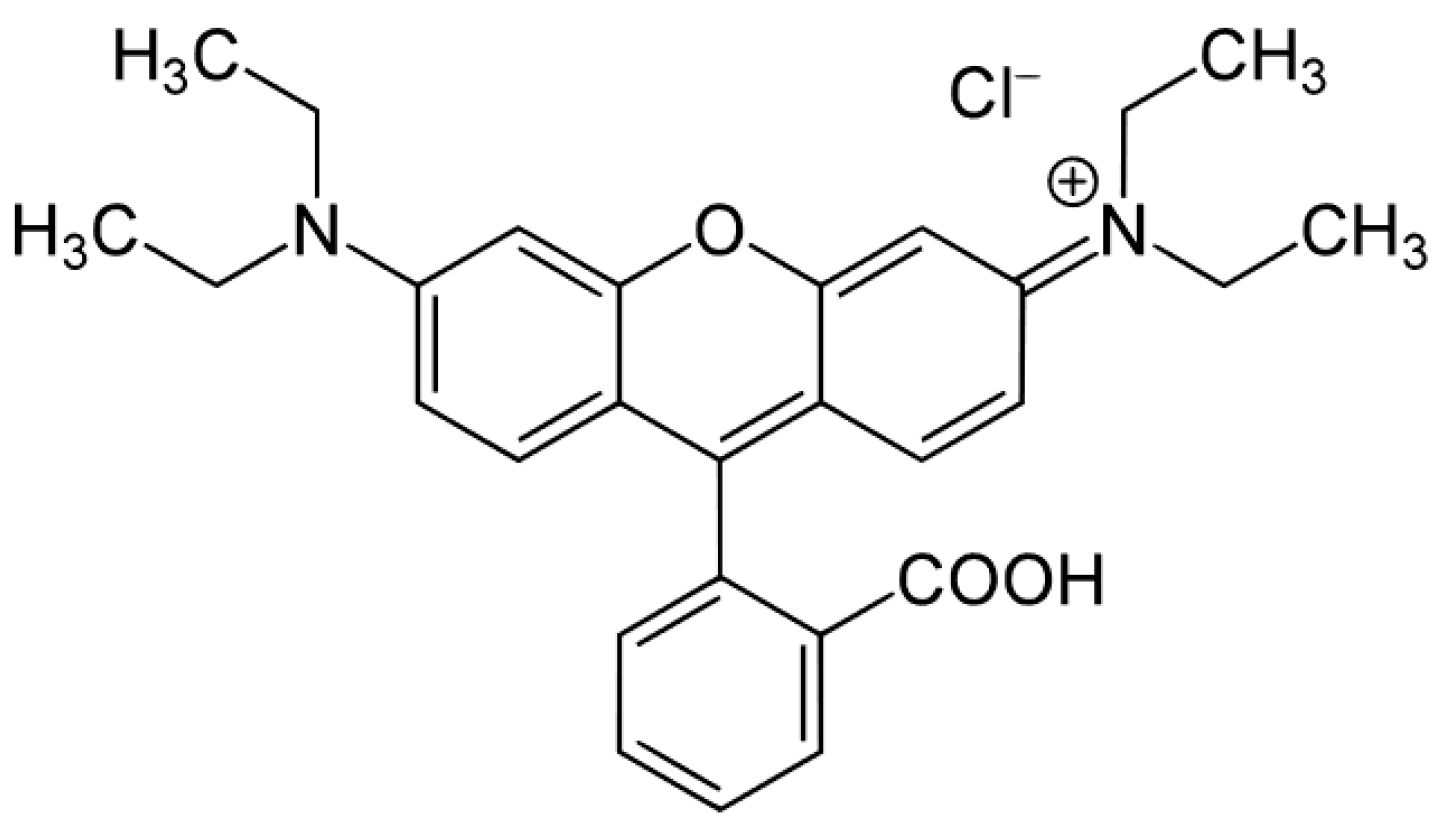

| Sample | BET SSA (m2 g−1) | Total Pore Volume (cm3 g−1) | Average Particle Size (±s.d. nm) | Average Crystallite Size (±s.d. nm) * | EDX Determined Mo/Ti (Nominal Mo/Ti) Atomic Ratios | XPS Determined Surface Mo/Ti Atomic Ratio [32] |
|---|---|---|---|---|---|---|
| TiO2 | 71 | 0.091 | 12 (3) | 10.3 (0.5) | - | - |
| Mo_1 | 76 | 0.112 | 21 (5) | 12.5 (0.9) | 0.0070 (0.0084) | 0.042 |
| Mo_5 | 74 | 0.141 | 22 (5) | 12.5 (0.4) | 0.05 (0.044) | 0.150 |
| Mo_10 | 96 | 0.137 | 18 (4) | 9.9 (0.5) | 0.090 (0.092) | 0.194 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasi, R.; Esposito, S.; Freyria, F.S.; Armandi, M.; Gadhi, T.A.; Hernandez, S.; Rivolo, P.; Ditaranto, N.; Bonelli, B. Application of Reverse Micelle Sol–Gel Synthesis for Bulk Doping and Heteroatoms Surface Enrichment in Mo-Doped TiO2 Nanoparticles. Materials 2019, 12, 937. https://doi.org/10.3390/ma12060937
Nasi R, Esposito S, Freyria FS, Armandi M, Gadhi TA, Hernandez S, Rivolo P, Ditaranto N, Bonelli B. Application of Reverse Micelle Sol–Gel Synthesis for Bulk Doping and Heteroatoms Surface Enrichment in Mo-Doped TiO2 Nanoparticles. Materials. 2019; 12(6):937. https://doi.org/10.3390/ma12060937
Chicago/Turabian StyleNasi, Roberto, Serena Esposito, Francesca S. Freyria, Marco Armandi, Tanveer A. Gadhi, Simelys Hernandez, Paola Rivolo, Nicoletta Ditaranto, and Barbara Bonelli. 2019. "Application of Reverse Micelle Sol–Gel Synthesis for Bulk Doping and Heteroatoms Surface Enrichment in Mo-Doped TiO2 Nanoparticles" Materials 12, no. 6: 937. https://doi.org/10.3390/ma12060937
APA StyleNasi, R., Esposito, S., Freyria, F. S., Armandi, M., Gadhi, T. A., Hernandez, S., Rivolo, P., Ditaranto, N., & Bonelli, B. (2019). Application of Reverse Micelle Sol–Gel Synthesis for Bulk Doping and Heteroatoms Surface Enrichment in Mo-Doped TiO2 Nanoparticles. Materials, 12(6), 937. https://doi.org/10.3390/ma12060937












