In Situ Controlled Surface Microstructure of 3D Printed Ti Alloy to Promote Its Osteointegration
Abstract
1. Introduction
2. Materials and Methods
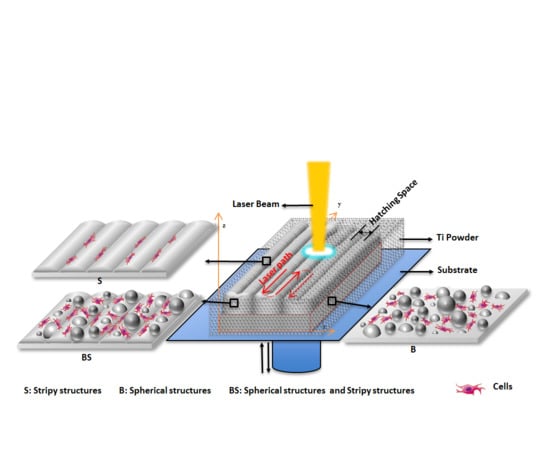
2.1. 3D Printing of Ti Alloy Implants
2.2. Hydrothermal Treatment
2.3. Characterization of Prepared Ti Alloy Implants
2.4. Protein Adsorption
2.5. Cell Culture
2.6. Cell Viability
2.7. Cell Differentiation
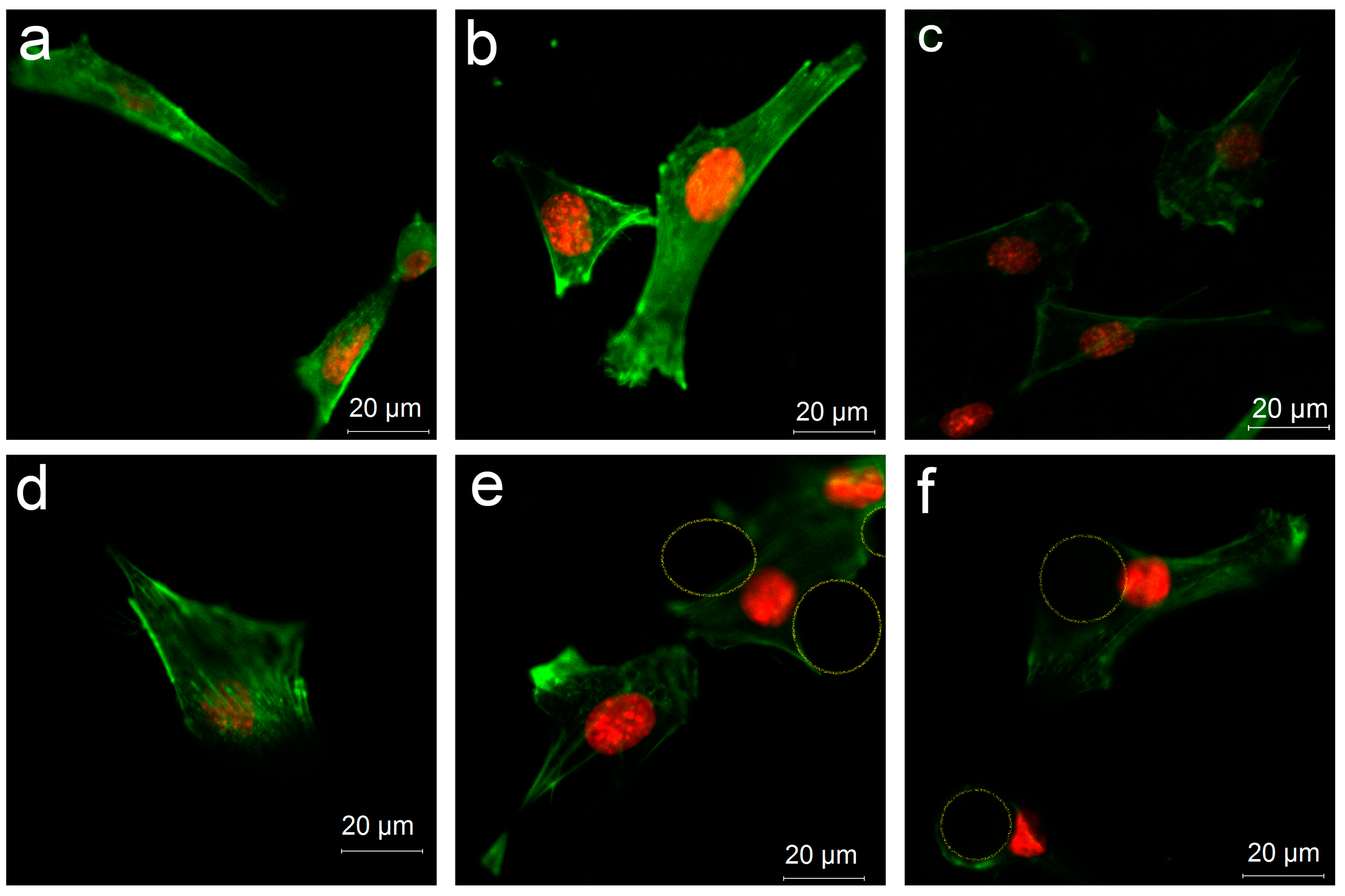
2.8. Cellular Cytoskeleton Morphology
2.9. Statistical Analysis
3. Results
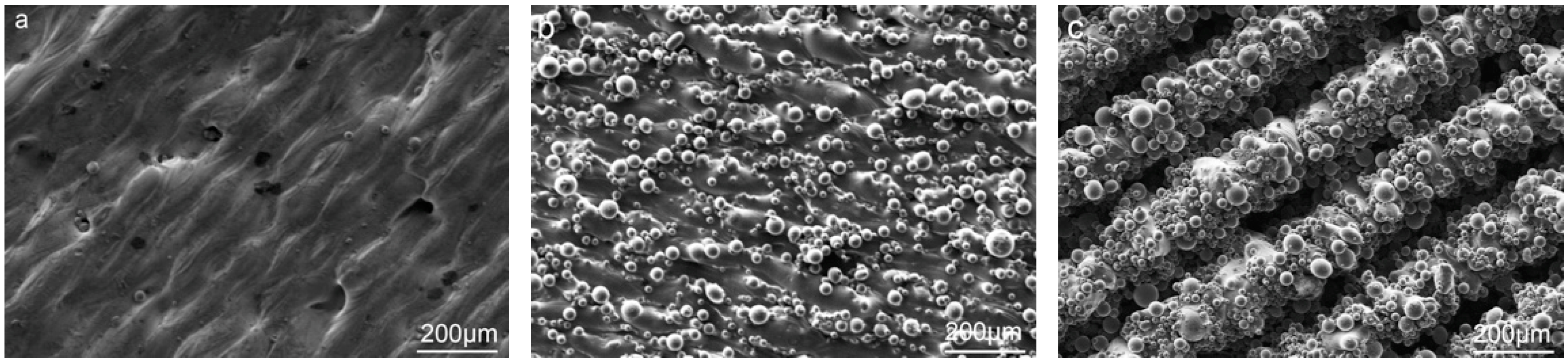
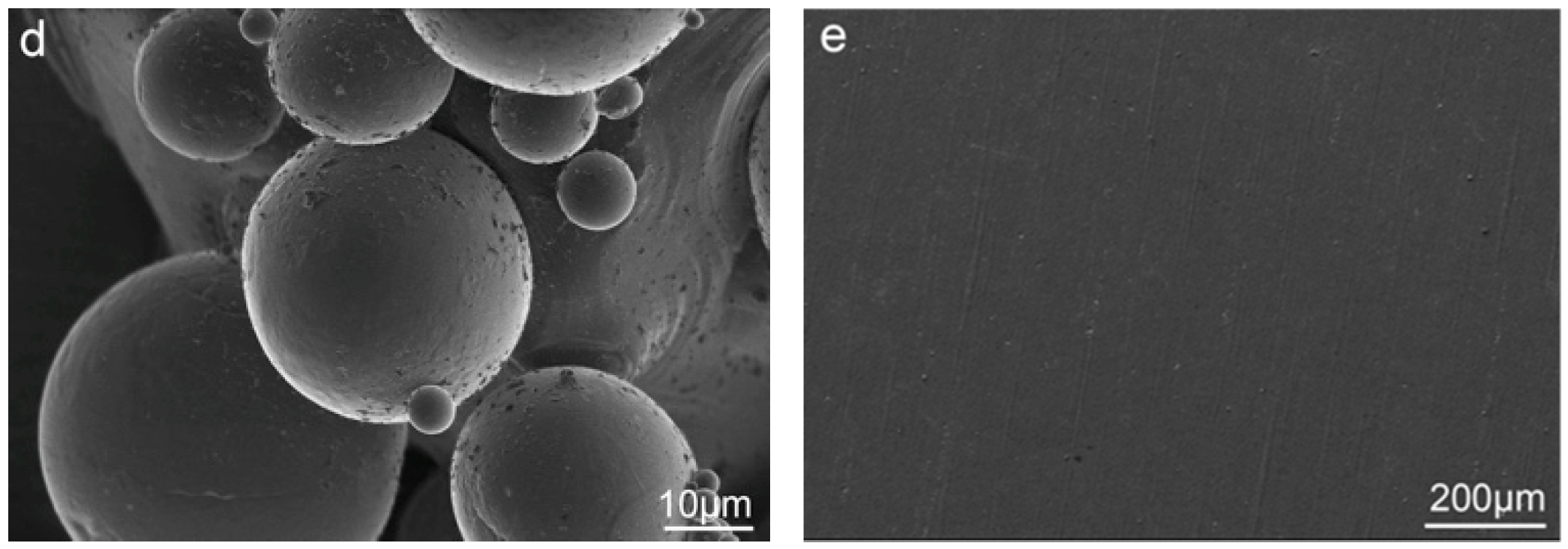
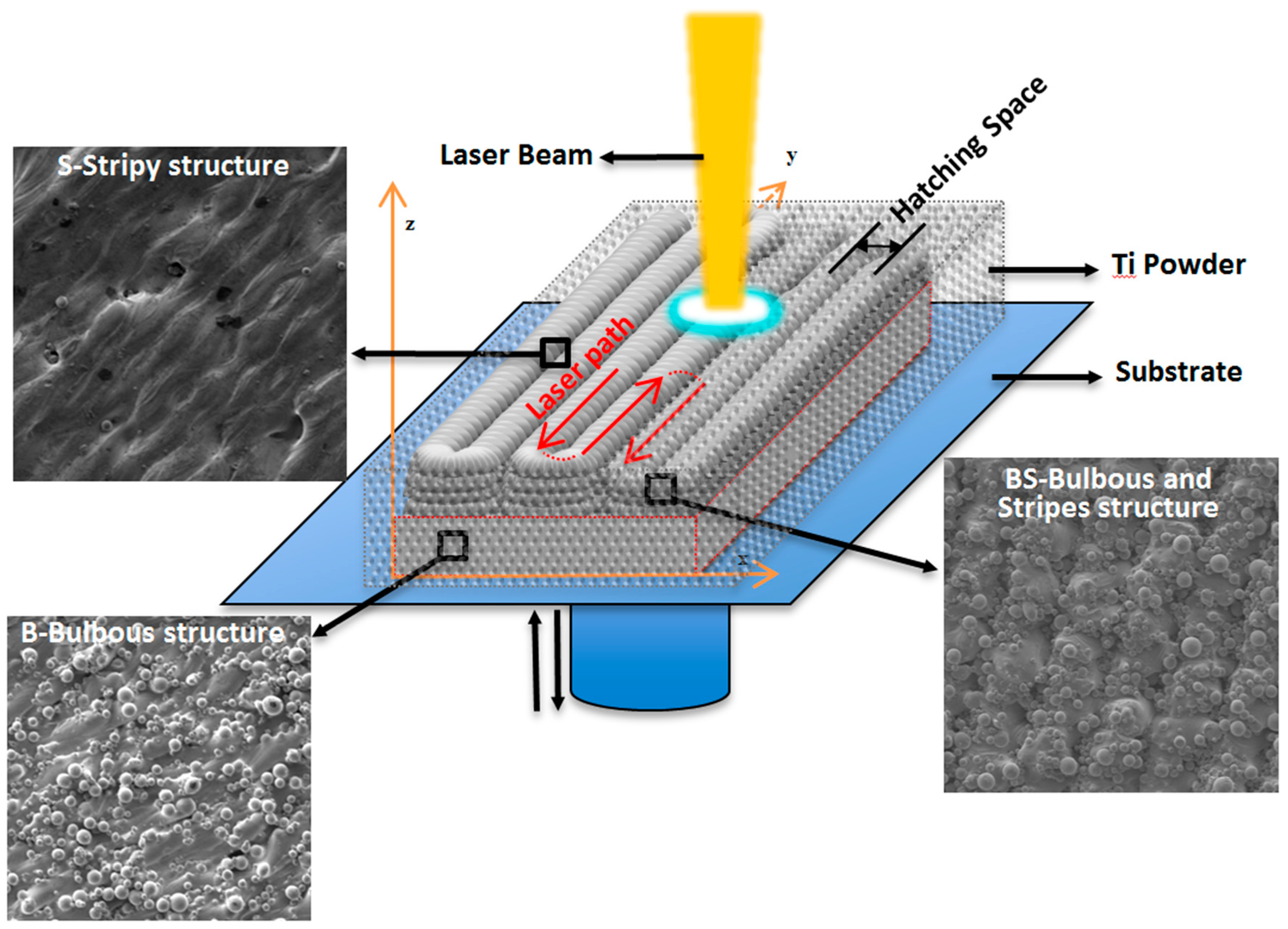
3.1. The In Situ Tailored Surface Structures of 3D-Printed Ti Alloy Implants
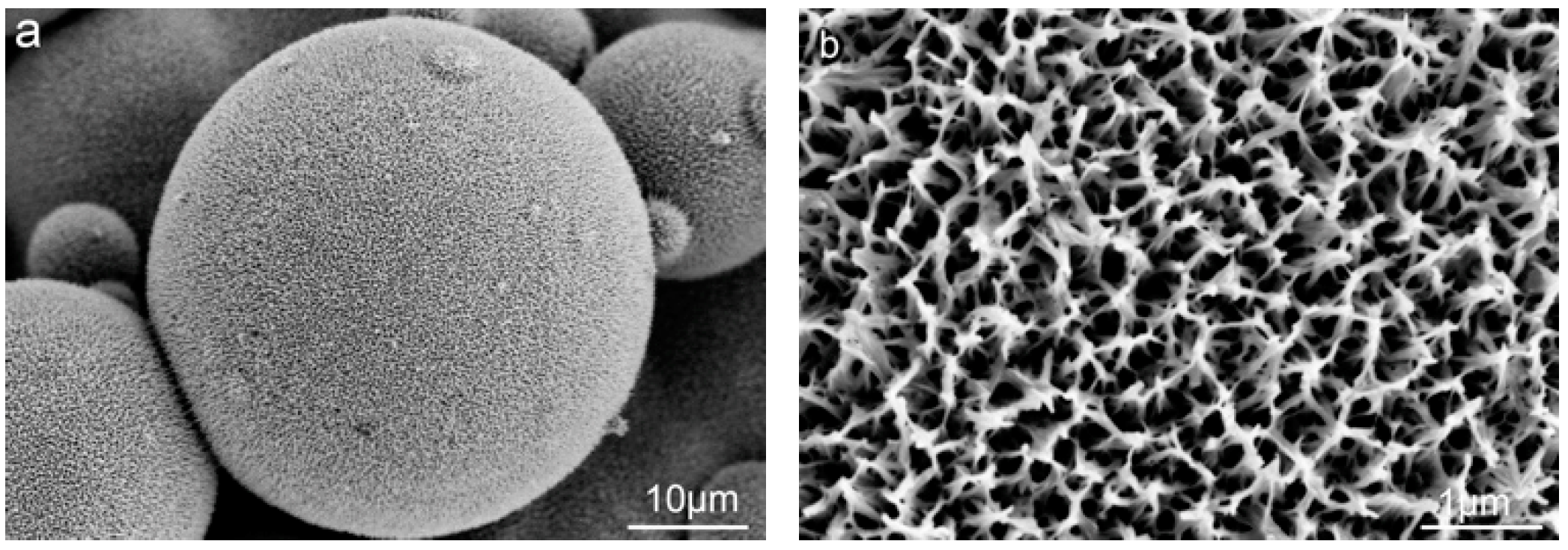
3.2. The Post Hydrothermal Treatment of the Tailored Surface Structures
3.3. Surface Composition
3.4. In-Vitro Evaluation
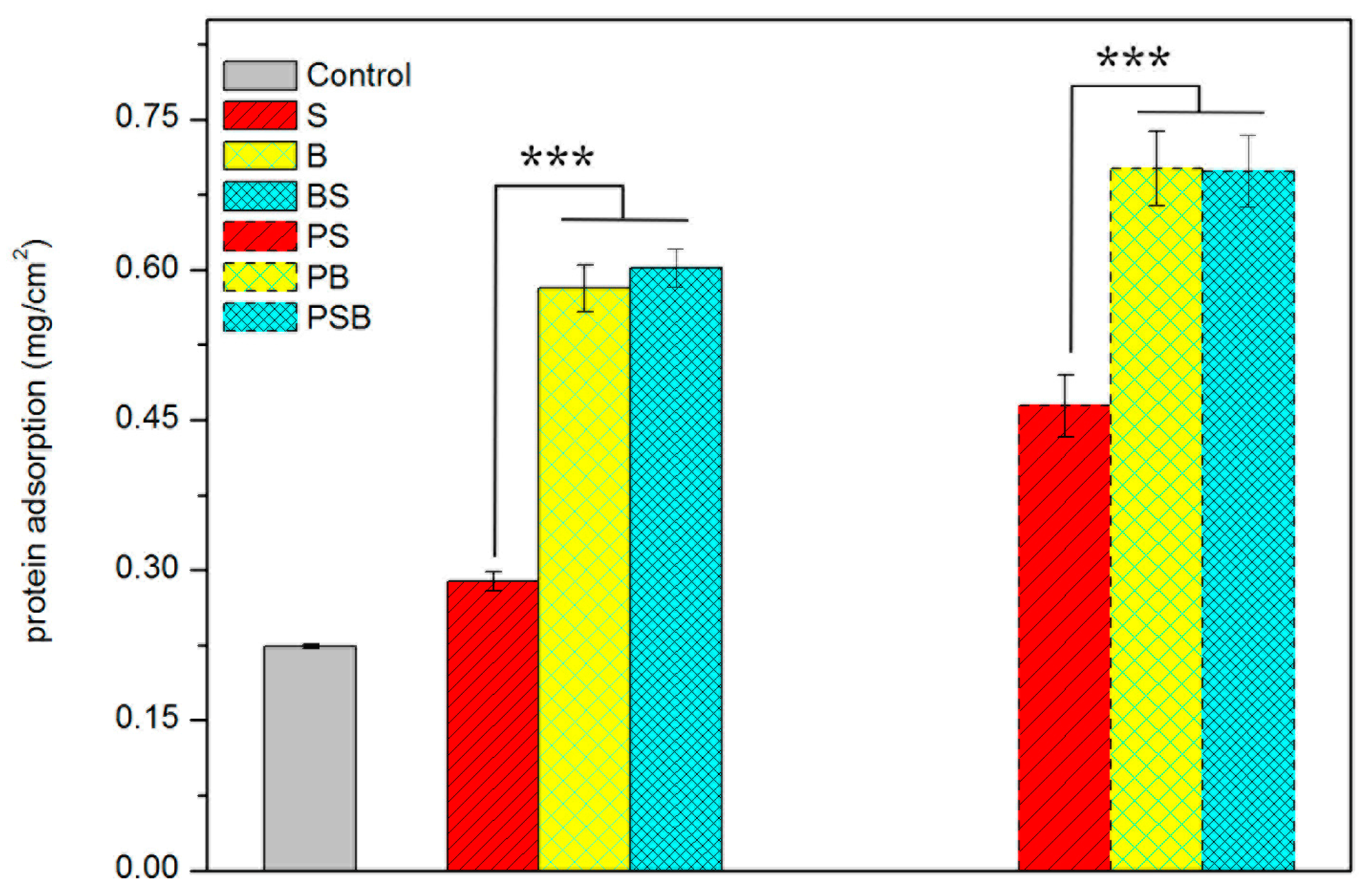
3.4.1. Protein Adsorption
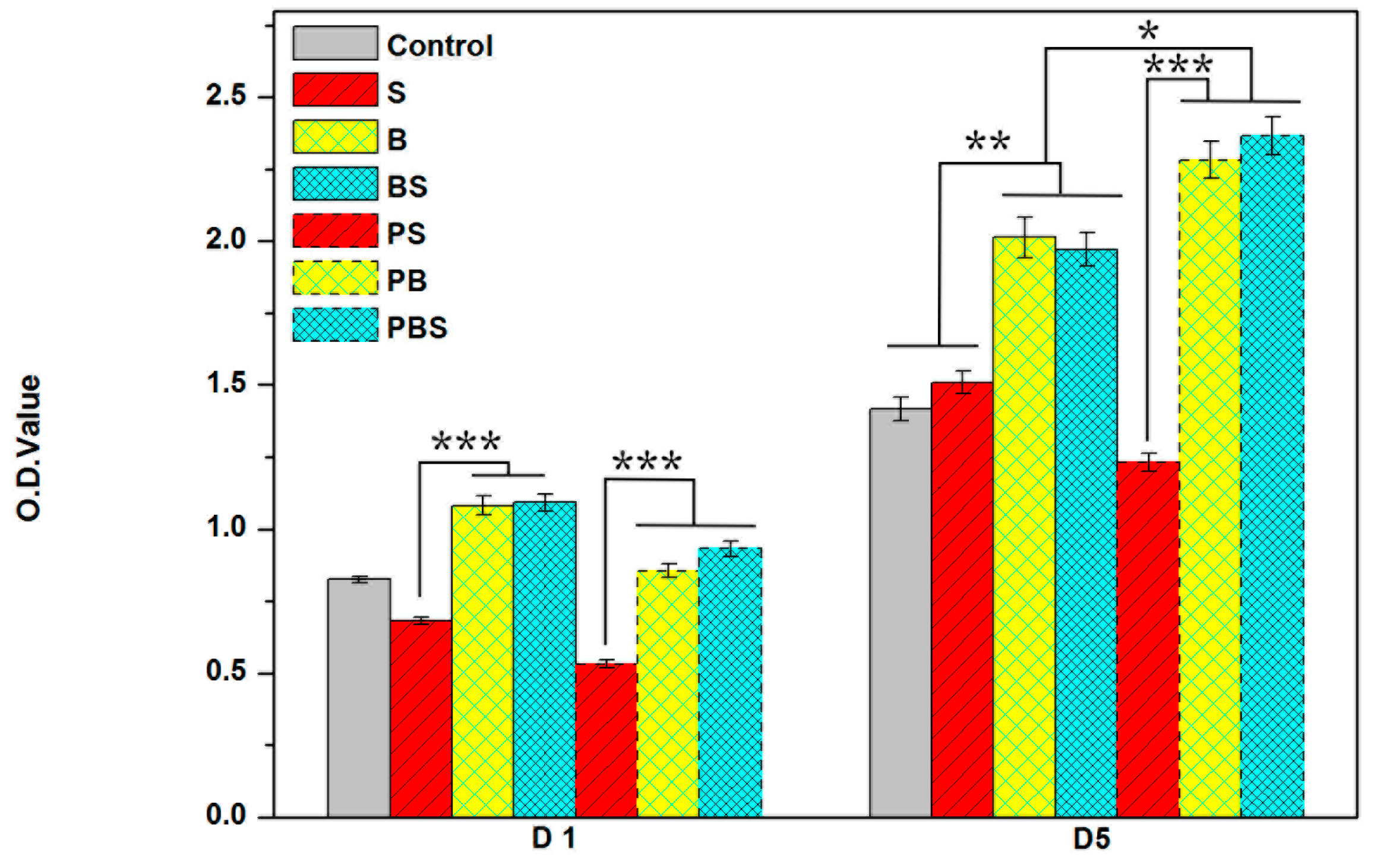
3.4.2. Cell Viability
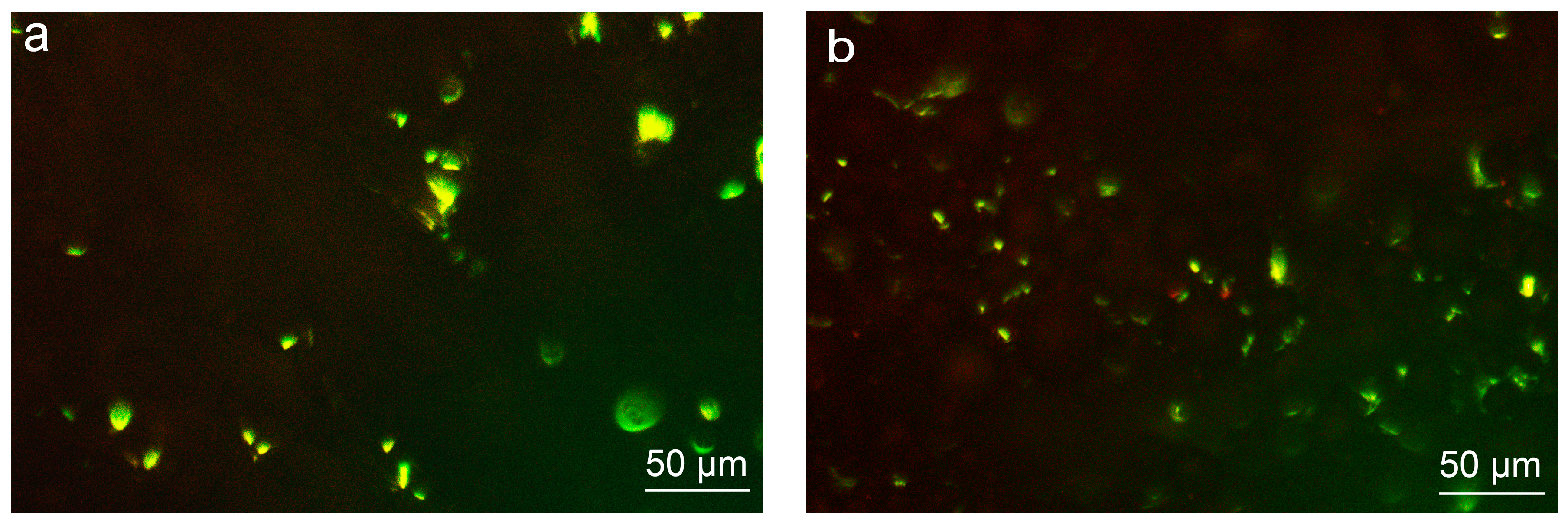
3.4.3. Cell Distribution on Various Surfaces
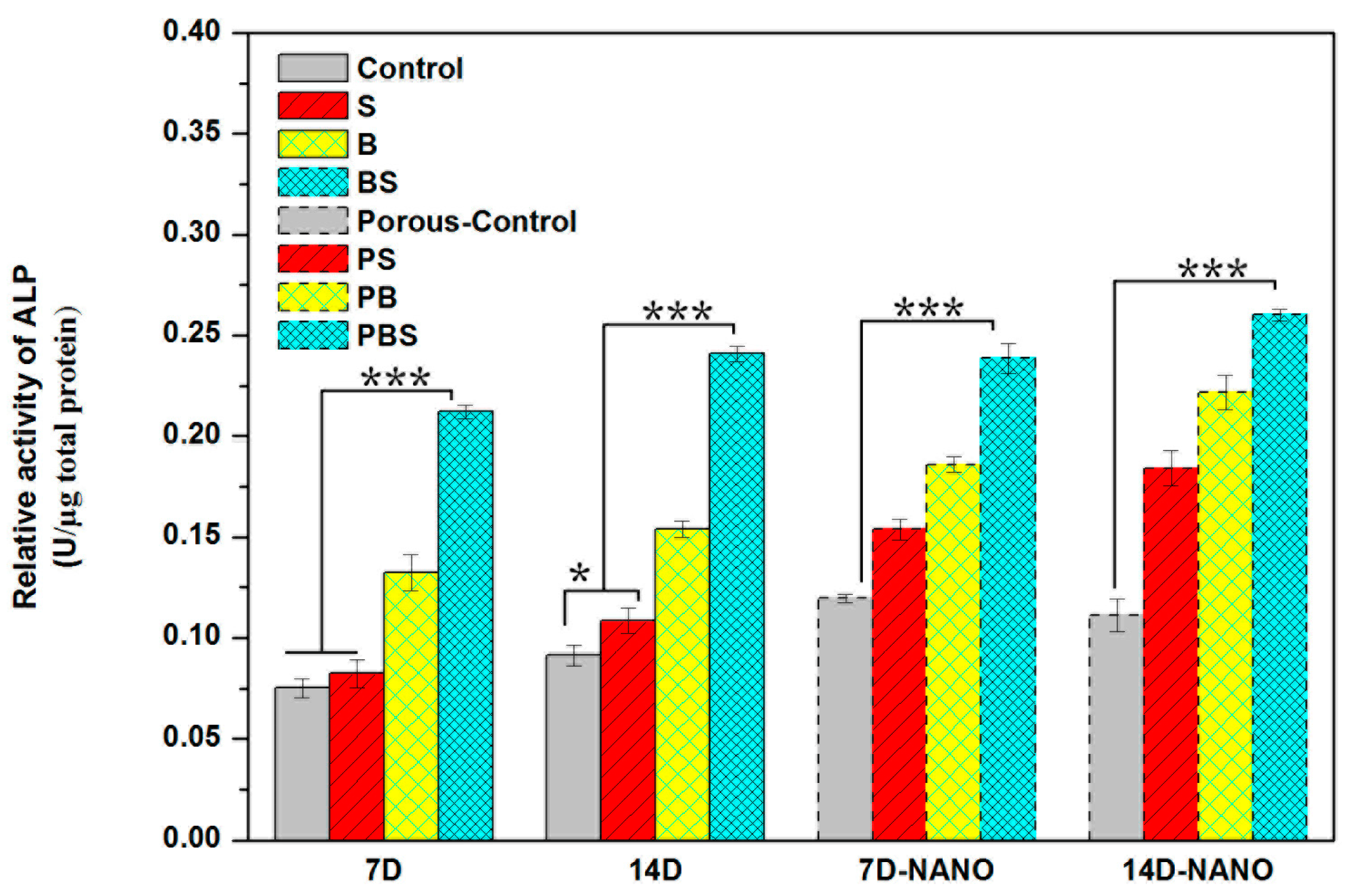
3.4.4. Cell Differentiation
4. Discussion
4.1. In Situ, Controlled Surface Structure
4.1.1. In Situ, Controlled Surface Microstructure
4.1.2. Construction of Nanostructure
4.2. Analysis of Cellular Responses on the Tailored Surface Structures of the Implants
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Song, Z.-L.; Feng, C.-K.; Chiu, F.-Y.; Liu, C.-L. The clinical significance of rapid prototyping technique in complex spinal deformity surgery—Case sharing and literature review. Formos. J. Musculoskelet. Disord. 2013, 4, 88–93. [Google Scholar] [CrossRef]
- Chua, C.K.; Leong, K.F. 3D Printing and Additive Manufacturing: Principles and Applications (with Companion Media Pack) of Rapid Prototyping, 4th ed.; World Scientific Publishing Company: Singapore, 2014. [Google Scholar]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.M.; Daurka, J.; Akhtar, K. Use of 3D printing in orthopaedic surgery. Br. Med. J. 2014, 348, g2963. [Google Scholar] [CrossRef]
- Eltorai, A.E.; Nguyen, E.; Daniels, A.H. Three-dimensional printing in orthopedic surgery. Orthopedics 2015, 38, 684–687. [Google Scholar] [PubMed]
- Giannopoulos, A.A.; Mitsouras, D.; Yoo, S.-J.; Liu, P.P.; Chatzizisis, Y.S.; Rybicki, F.J. Applications of 3D printing in cardiovascular diseases. Nat. Rev. Cardiol. 2016, 13, 701–718. [Google Scholar] [CrossRef] [PubMed]
- Meier, L.; Meineri, M.; Hiansen, J.Q.; Horlick, E. Structural and congenital heart disease interventions: The role of three-dimensional printing. Neth. Heart J. 2017, 25, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, B.; Xiong, G.; Dunham, S.; Min, J.K. Current progress in 3D printing for cardiovascular tissue engineering. Biomed. Mater. 2015, 10, 034002. [Google Scholar] [CrossRef] [PubMed]
- Mulford, J.S.; Babazadeh, S.; Mackay, N. Three-dimensional printing in orthopaedic surgery: Review of current and future applications. ANZ J. Surg. 2016, 86, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Özcan, M. Additive Manufacturing Technologies Used for 3D Metal Printing in Dentistry. Curr. Oral Health Rep. 2017, 4, 201–208. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.; Wang, B.; Wang, C.; Wang, Z.; Yang, F.; Gao, C.; Liu, H.; Qin, Y.; Wang, J. Heat treatment effect on the mechanical properties, roughness and bone ingrowth capacity of 3D printing porous titanium alloy. RSC Adv. 2018, 8, 12471–12483. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Brooks, W.K.; Jones, E.; Sutcliffe, C.J. Selective Laser Melting: A regular unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Heat treatment of Ti6Al4V produced by Selective Laser Melting: Microstructure and mechanical properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef]
- Joddar, B.; Ito, Y. Biological modifications of materials surfaces with proteins for regenerative medicine. J. Mater. Chem. 2011, 21, 13737–13755. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New fronties for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 19, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.; Batzer, R.; Kieswetter, K.; Liu, Y.; Cochran, D.; Szmuckler-Moncler, S.; Dean, D.; Schwartz, Z. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1α, 25-(OH) 2D3. J. Biomed. Mater. Res. 1998, 39, 77–85. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.; Ladas, S.; Sotiropoulou, D.; Amedee, J.; Missirlis, Y. Effect of surface roughness of the titanium alloy Ti–6Al–4V on human bone marrow cell response and on protein adsorption. Biomaterials 2001, 22, 1241–1251. [Google Scholar] [CrossRef]
- Rosa, A.L.; Beloti, M.M. Effect of cpTi surface roughness on human bone marrow cell attachment, proliferation, and differentiation. Braz. Dent. J. 2003, 14, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Starý, V.; Douděrová, M.; Bačáková, L. Influence of surface roughness of carbon materials on human osteoblast-like cell growth. J. Biomed. Mater. Res. Part A 2014, 102, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yan, C.; Ye, K.; He, Y.; Li, Z.; Ding, J. Effect of RGD nanospacing on differentiation of stem cells. Biomaterials 2013, 34, 2865–2874. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-P.; Stehle, T.; Diefenbach, B.; Zhang, R.; Dunker, R.; Scott, D.L.; Joachimiak, A.; Goodman, S.L.; Arnaout, M.A. Crystal structure of the extracellular segment of integrin αVβ3. Science 2001, 294, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of biomaterial–cell interactions by adsorbed proteins: A review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-W.; Liu, D.-Z.; Wang, S.-Y.; Wang, S.-S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Huang, X.; Shan, L.; Cheng, K.; Weng, W. Cytocompatibility of Titanium Microsphere-Based Surfaces. ACS Biomater. Sci. Eng. 2017, 3, 3254–3260. [Google Scholar] [CrossRef]
- Qin, J.; Yang, D.; Maher, S.; Lima-Marques, L.; Zhou, Y.; Chen, Y.; Atkins, G.J.; Losic, D. Micro-and nano-structured 3D printed titanium implants with a hydroxyapatite coating for improved osseointegration. J. Mater. Chem. B 2018, 6, 3136–3144. [Google Scholar] [CrossRef]
- Biemond, J.; Hannink, G.; Verdonschot, N.; Buma, P. Bone ingrowth potential of electron beam and selective laser melting produced trabecular-like implant surfaces with and without a biomimetic coating. J. Mater. Sci. Mater. Med. 2013, 24, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Mines, R.; Fox, P. Characterization of selectively laser melted Ti-6Al-4 V micro-lattice struts. Procedia Eng. 2011, 10, 536–541. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, D.; Yoon, T.R.; Kim, H.K.; Jo, H.H.; Park, J.S.; Lee, J.H.; Kim, W.D.; Kwon, I.K.; Park, S.A. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomater. 2016, 40, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Pattanayak, D.K.; Fukuda, A.; Matsushita, T.; Takemoto, M.; Fujibayashi, S.; Sasaki, K.; Nishida, N.; Nakamura, T.; Kokubo, T. Bioactive Ti metal analogous to human cancellous bone: Fabrication by selective laser melting and chemical treatments. Acta Biomater. 2011, 7, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, Y.; Lu, S.; Chen, T.; Zhao, Y.; Chen, H.; Tang, Z. Fabrication and characterization of selective laser melting printed Ti–6Al–4V alloys subjected to heat treatment for customized implants design. Prog. Nat. Sci. Mater. Int. 2016, 26, 671–677. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Humbeeck, J.; Kruth, J. A study of the microstructural evolution during selective laser melting of Ti-6Al-4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Mumtaz, M.; Hopkinson, N. Top surface and side roughness of inconel 625 parts processed using selective laser melting. Rapid Prototyp. J. 2009, 15, 96–103. [Google Scholar] [CrossRef]
- Andrukhov, O.; Huber, R.; Shi, B.; Berner, S.; Rausch-Fan, X.; Moritz, A.; Spencer, N.D.; Schedle, A. Proliferation, behavior, and differentiation of osteoblasts on surfaces of different microroughness. Dent. Mater. 2016, 32, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Gagg, G.; Ghassemieh, E.; Wiria, F.E. Effects of sintering temperature on morphology and mechanical characteristics of 3D printed porous titanium used as dental implant. Mater. Sci. Eng. C 2013, 33, 3858–3864. [Google Scholar] [CrossRef] [PubMed]
- Pakkanen, J.; Calignano, F.; Trevisan, F.; Lorusso, M.; Ambrosio, E.P.; Manfredi, D.; Fino, P. Study of internal channel surface roughnesses manufactured by selective laser nelting in aluminum and titanium allys. Metall. Mater. Trans. A 2016, 47, 3837–3844. [Google Scholar] [CrossRef]
- Wild, M.D.; Schumacher, R.; Mayer, K.; Schkommodau, E.; Thoma, D.; Bredell, M.; Gujer, A.K.; Gratz, K.W.; Weber, F.E. Bone regeneration by the osteoconductivity of porous titanium implants manufactured by selective laser melting: A histological and micro computed tomography study in rabbit. Tissue Eng. A 2013, 19, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lawn, B.R.; Rekow, E.D.; Thompson, V.P. Effect of sandblasting on the long-term performance of dental ceramics. J. Biomed. Mater. Res. B Appl. Biomater 2004, 71B, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; McLachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-S.; Liu, J.-F.; Wu, C.-P.; Huang, H.-H. Nanoporous surface topography enhances bone cell differentiation on Ti–6Al–7Nb alloy in bone implant applications. J. Alloys Compd. 2015, 643, S124–S132. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Osathanon, T.; Bespinyowong, K.; Arksornnukit, M.; Takahashi, H.; Pavasant, P. Human osteoblast-like cell spreading and proliferation on Ti-6Al-7Nb surfaces of varying roughness. J. Oral Sci. 2011, 53, 23–30. [Google Scholar] [CrossRef] [PubMed]
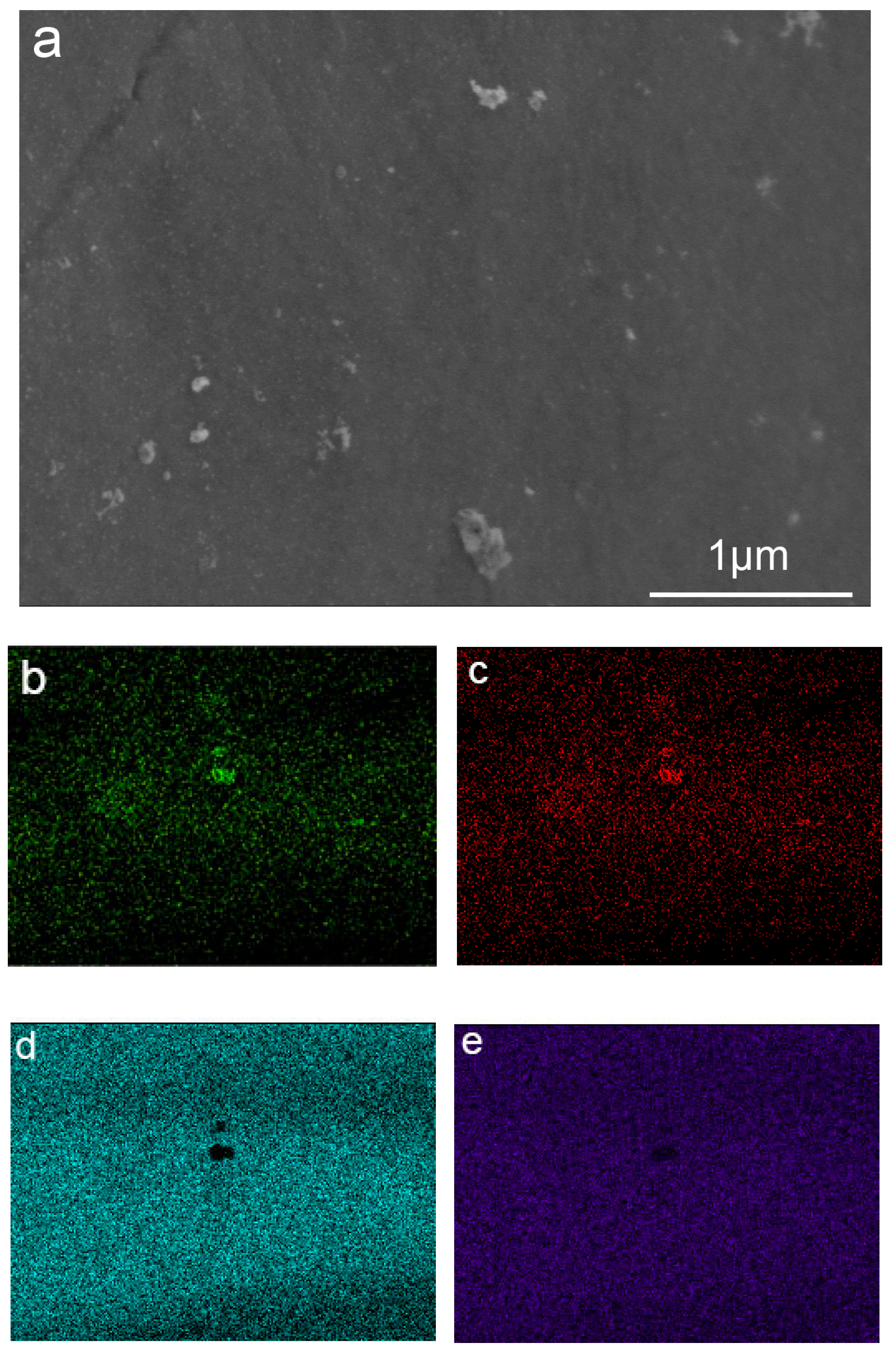

| Area | Ti K | Al K | V K | O K |
|---|---|---|---|---|
| Substrate | 73.98 | 5.51 | 3.28 | 14.86 |
| Microsphere | 72.44 | 5.46 | 3.16 | 15.87 |
| Graininess | 35.22 | 16.00 | 2.01 | 46.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, L.; Kadhum, A.A.H.; Al-Furjan, M.S.H.; Weng, W.; Gong, Y.; Cheng, K.; Zhou, M.; Dong, L.; Chen, G.; Takriff, M.S.; et al. In Situ Controlled Surface Microstructure of 3D Printed Ti Alloy to Promote Its Osteointegration. Materials 2019, 12, 815. https://doi.org/10.3390/ma12050815
Shan L, Kadhum AAH, Al-Furjan MSH, Weng W, Gong Y, Cheng K, Zhou M, Dong L, Chen G, Takriff MS, et al. In Situ Controlled Surface Microstructure of 3D Printed Ti Alloy to Promote Its Osteointegration. Materials. 2019; 12(5):815. https://doi.org/10.3390/ma12050815
Chicago/Turabian StyleShan, Lijun, Abdul Amir H. Kadhum, M.S.H. Al-Furjan, Wenjian Weng, Youping Gong, Kui Cheng, Maoying Zhou, Lingqing Dong, Guojin Chen, Mohd S. Takriff, and et al. 2019. "In Situ Controlled Surface Microstructure of 3D Printed Ti Alloy to Promote Its Osteointegration" Materials 12, no. 5: 815. https://doi.org/10.3390/ma12050815
APA StyleShan, L., Kadhum, A. A. H., Al-Furjan, M. S. H., Weng, W., Gong, Y., Cheng, K., Zhou, M., Dong, L., Chen, G., Takriff, M. S., & Sulong, A. B. (2019). In Situ Controlled Surface Microstructure of 3D Printed Ti Alloy to Promote Its Osteointegration. Materials, 12(5), 815. https://doi.org/10.3390/ma12050815