Experimental Study on Corrosion and Mechanical Behavior of Main Cable Wires Considering the Effect of Strain
Abstract
:1. Introduction
2. Accelerated Corrosion Test of Cable Wires with Constant Strain
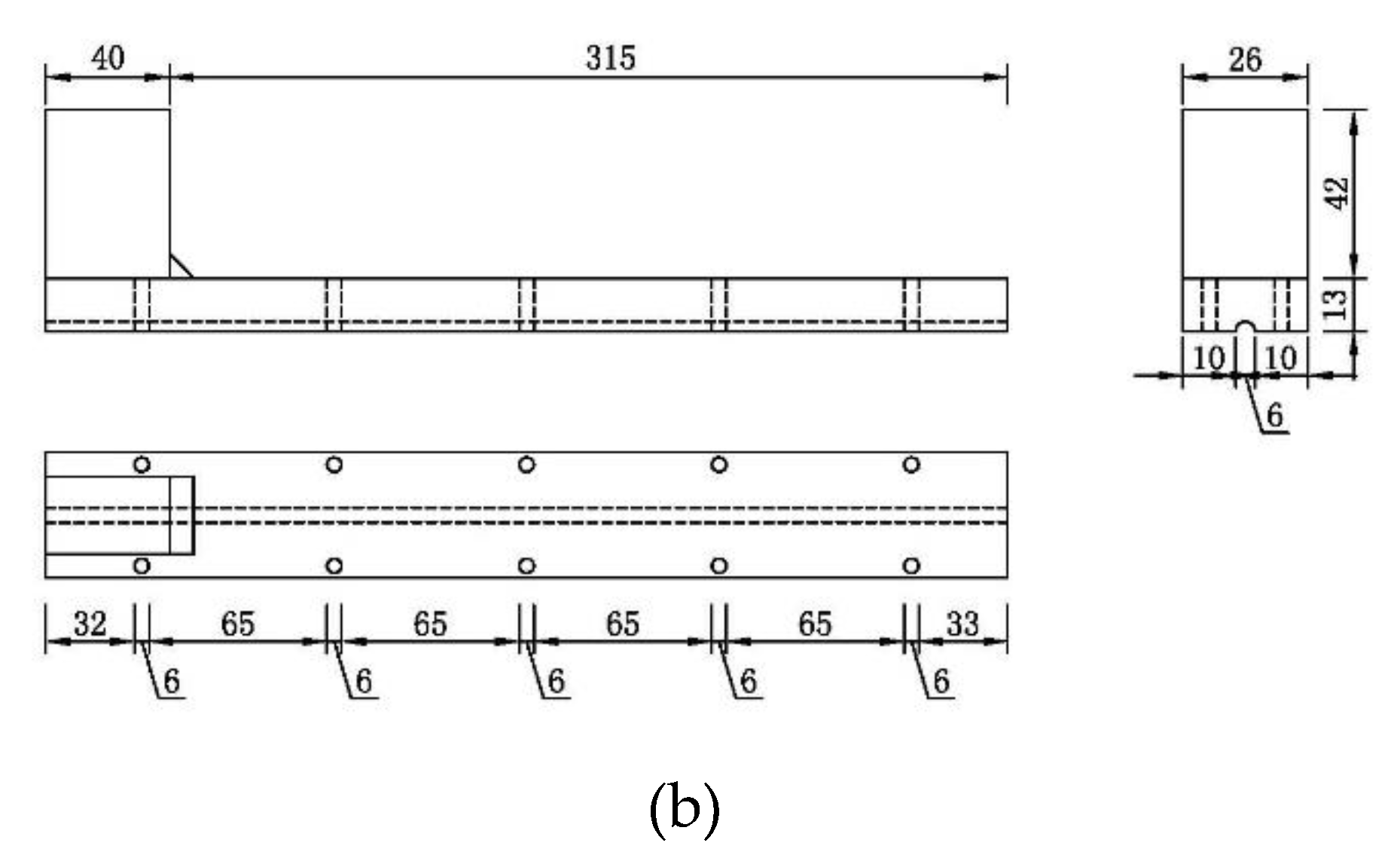
2.1. Material and Specimens
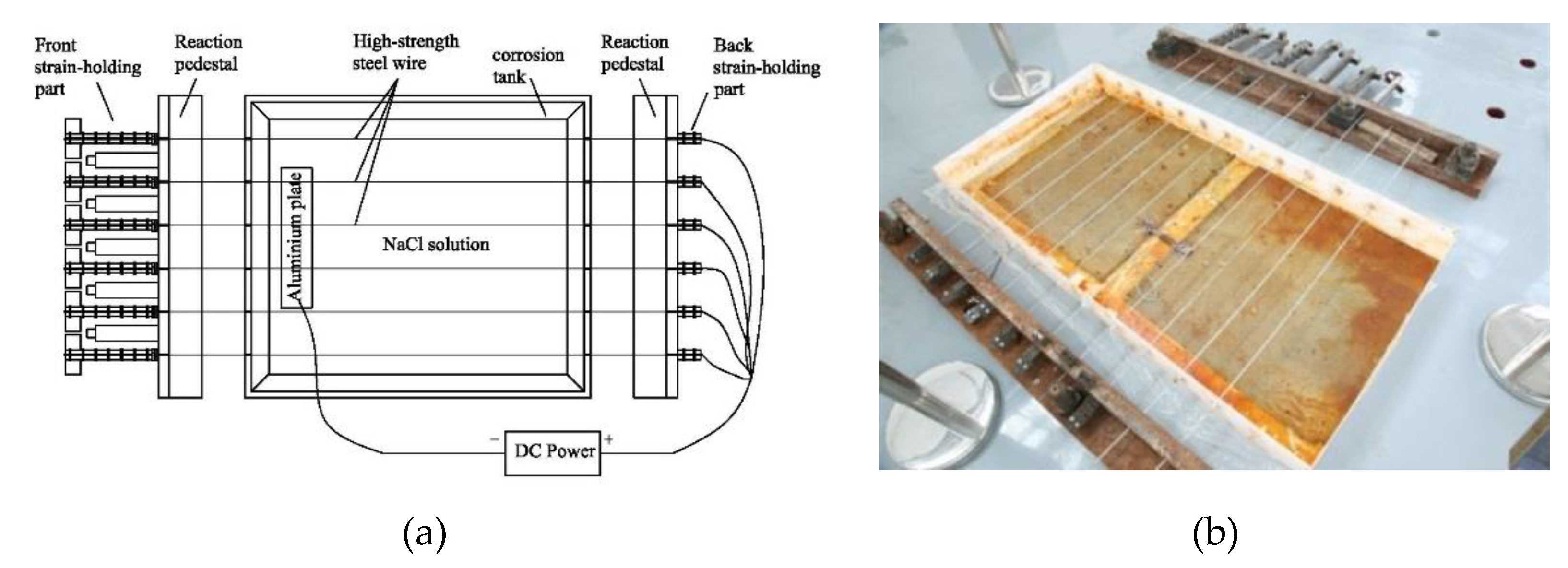
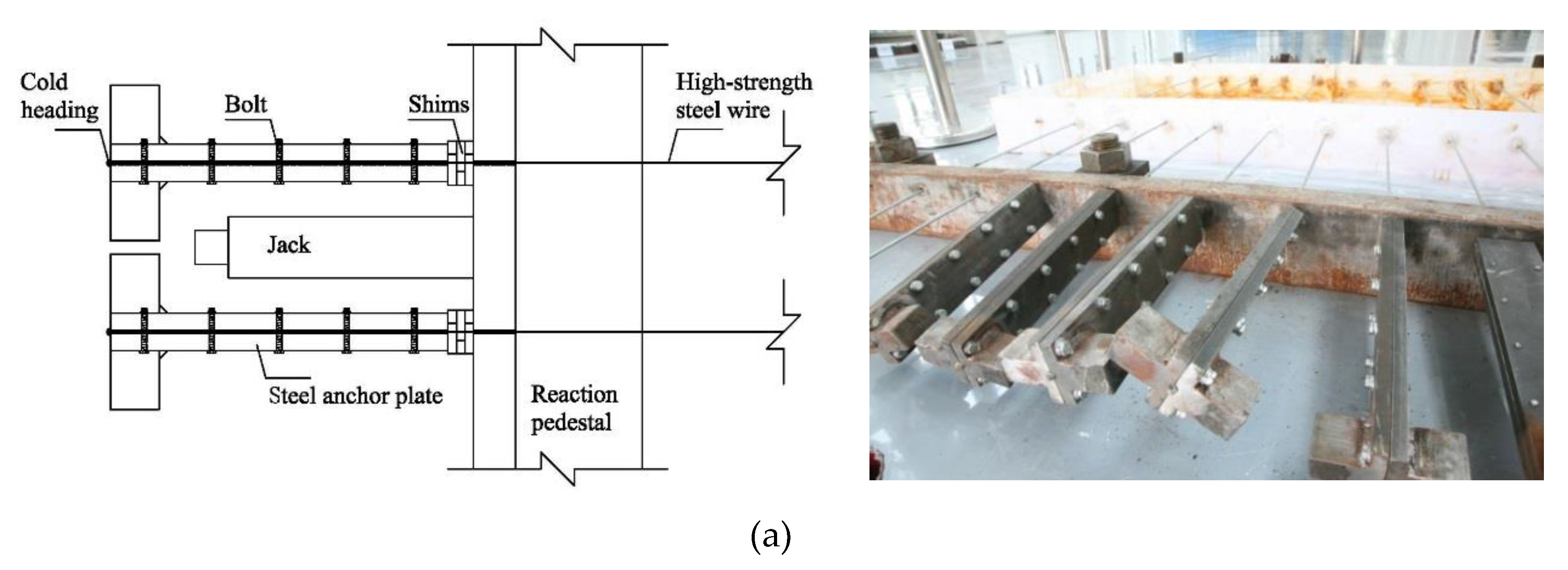
2.2. Precise Strain-Holding Device
2.3. Electrochemical Accelerated Corrosion Test and Static Tensile Test
3. Analysis of Experimental Results
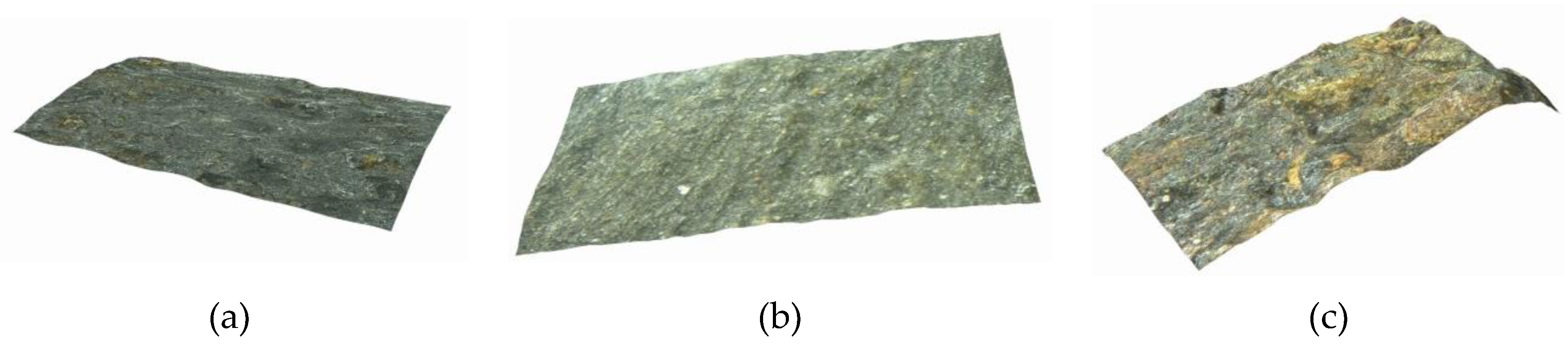
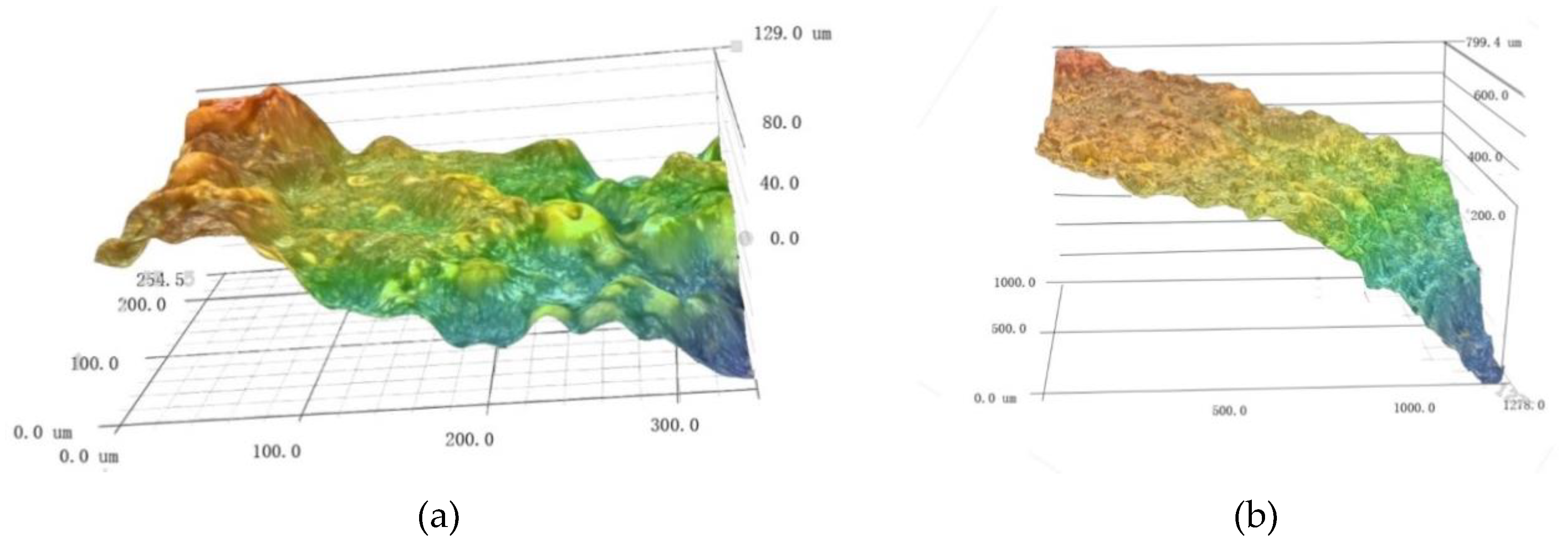
3.1. Morphology of Corroded Steel Wires
3.2. Mass Loss Ratio and Sectional Diameter of Corroded Steel Wire
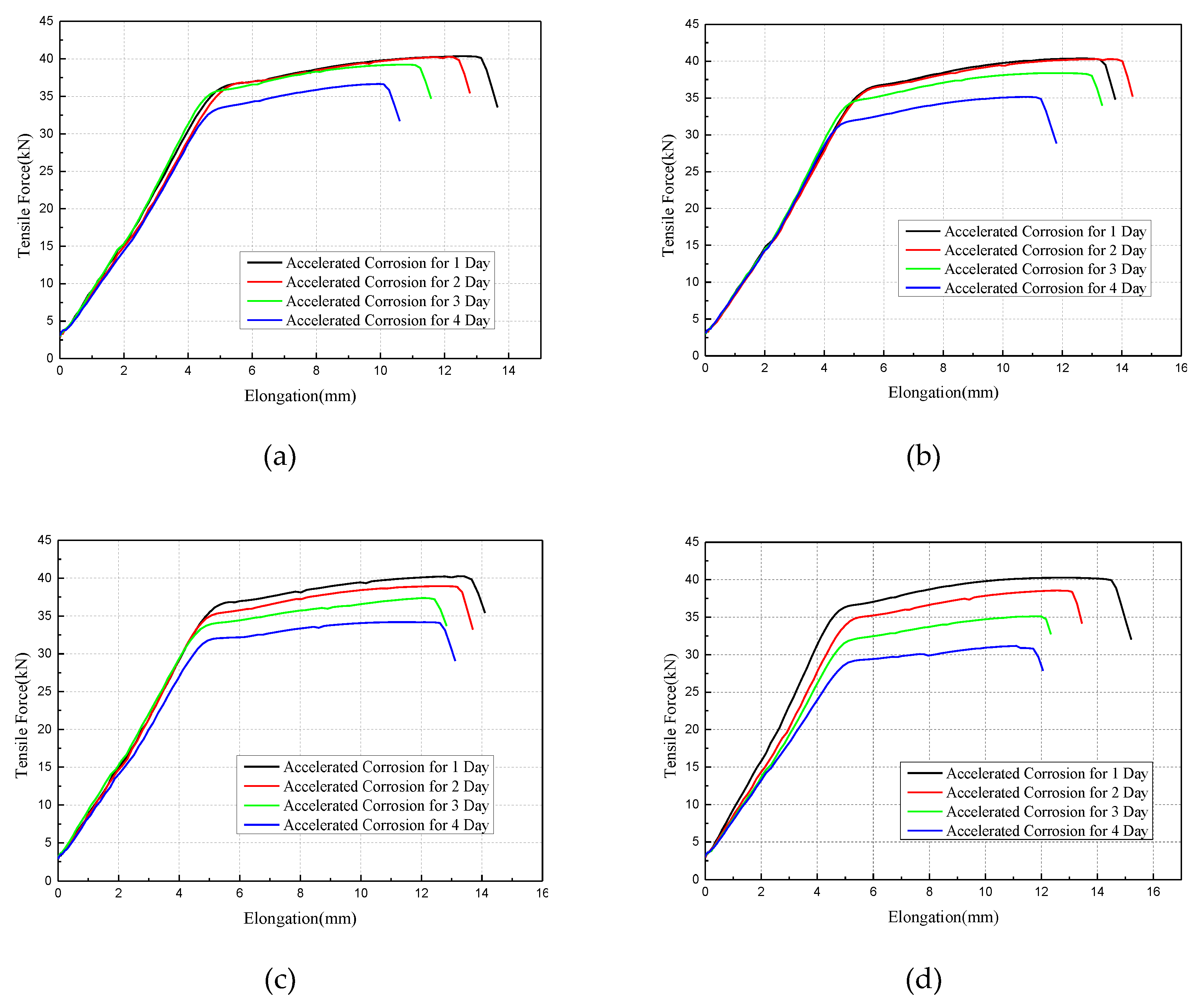
3.3. Mechanical Properties of Corroded Wires
4. Correlation Analysis between Corrosion Degree and Mechanical Properties of Cable Wires under Constant Strains
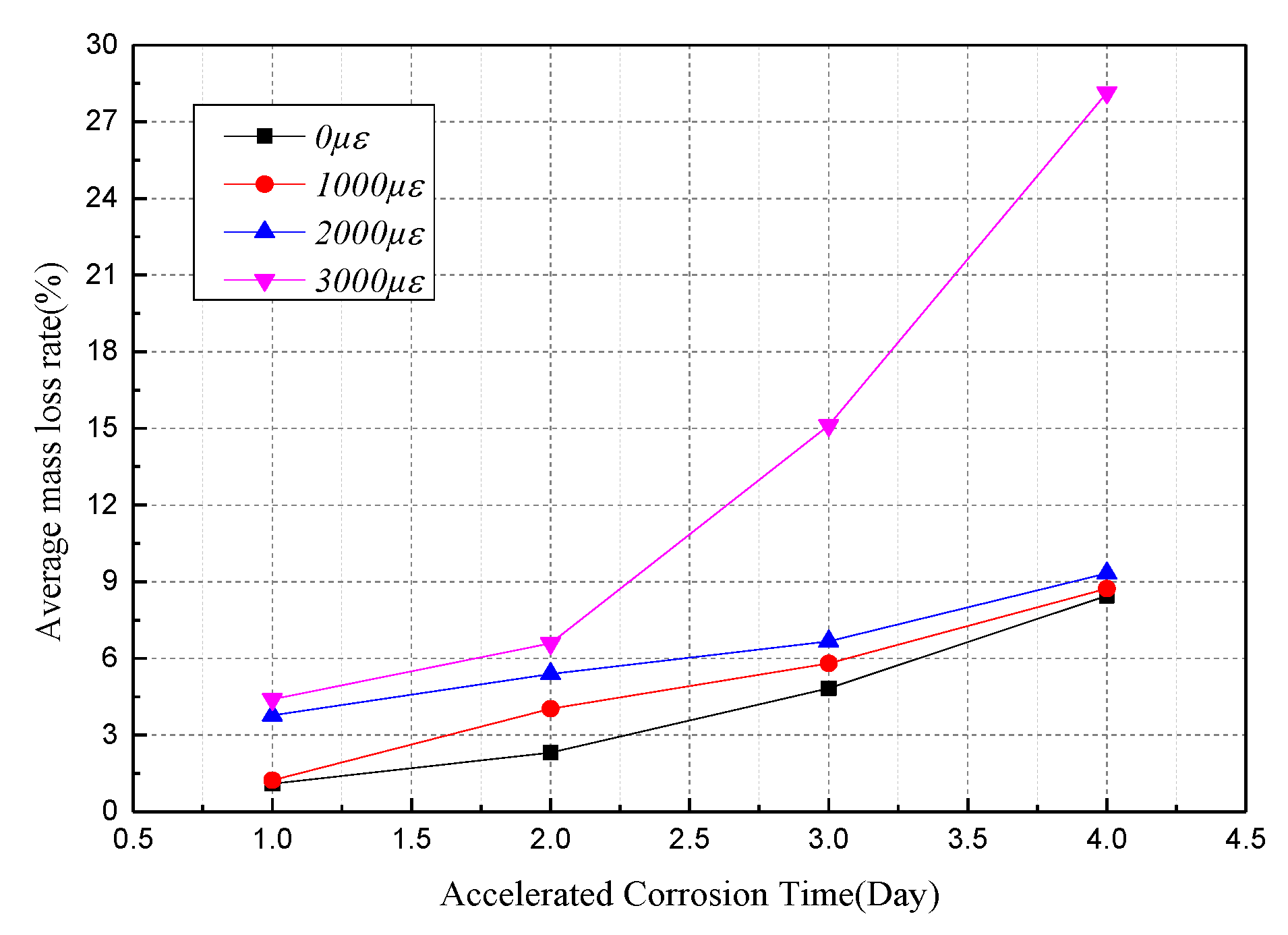
4.1. Tensile Behavior with the Average Mass Loss Rate
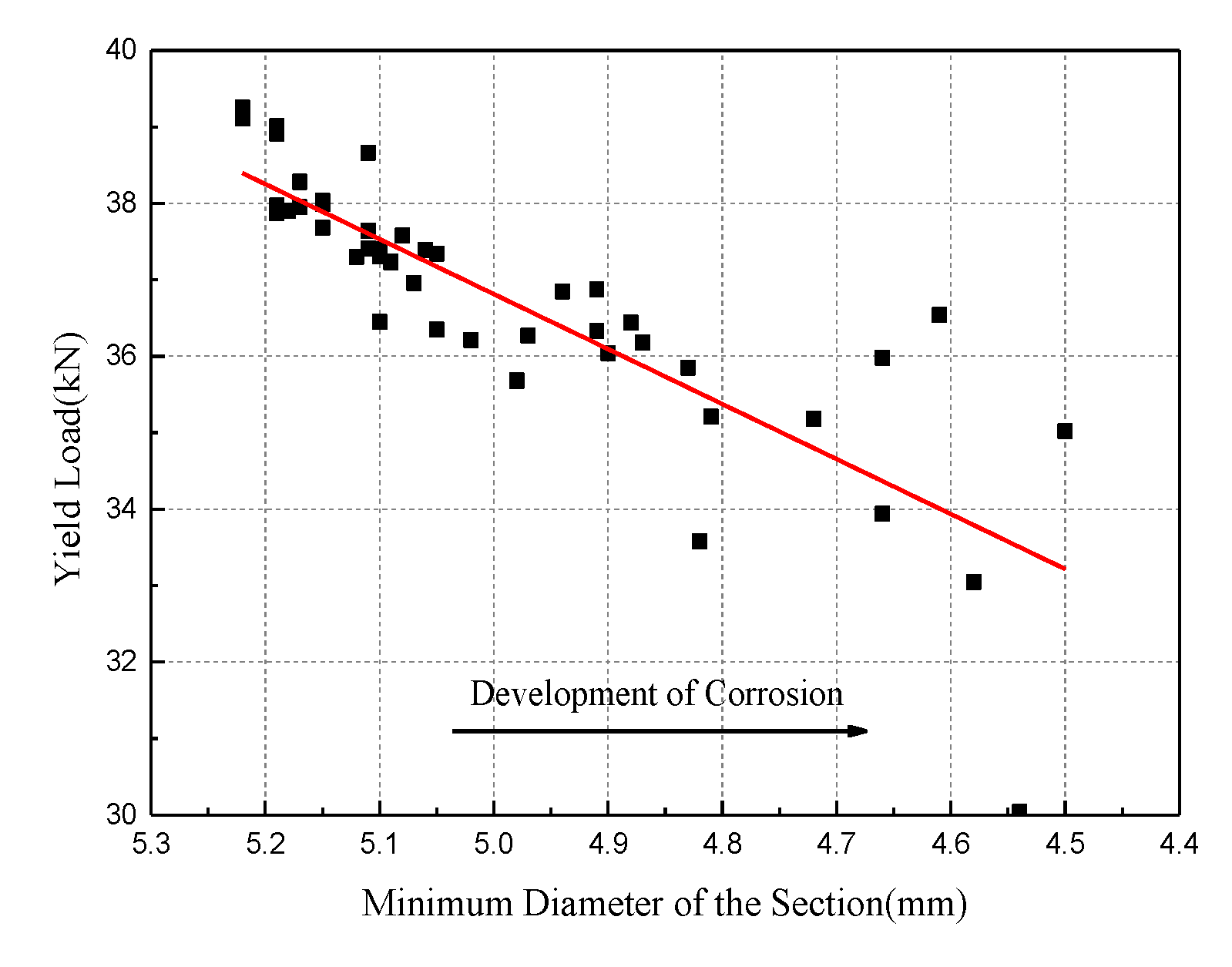
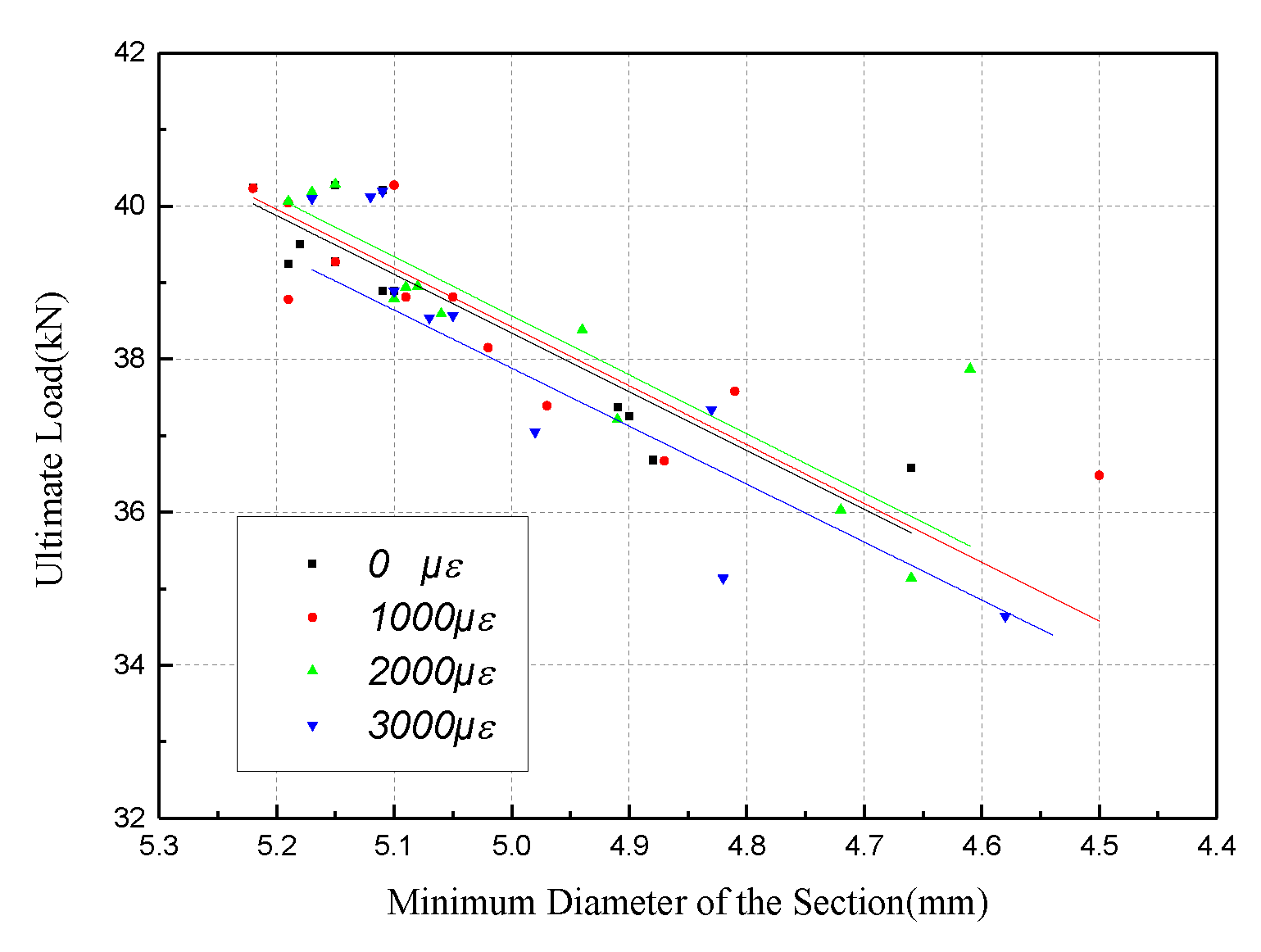
4.2. Tensile Behavior with the Minimum Diameter of the Section
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, G. Corrosion and Protection under Natural Environment; Chemical Industry Press: Beijing, China, 1997. [Google Scholar]
- Eriksson, P.; Carlsson, B.; Wallinder, I.O. Design of accelerated corrosion tests for electronic components in automotive applications. IEEE Trans. Compon. Packag. Technol. 2001, 24, 99–107. [Google Scholar] [CrossRef]
- Zeng, Y.; Gu, X.; Zhang, W.; Huang, Q. Accelerated corrosion technique for reinforcement steel bars in concrete. J. Struct. Eng. 2009, 25, 101–105. [Google Scholar]
- Xia, J. Mechanical Behavior of Corroded Reinforced Concrete Structure. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2010. [Google Scholar]
- Chen, X. Experimental analysis of a non-destructive corrosion monitoring system for main cables of suspension bridges. J. China Foreign Highw. 2016, 36, 72–76. [Google Scholar]
- Suzumura, K.; Nakamura, S. Environmental factors affecting corrosion of galvanized steel wires. J. Mater. Civ. Eng. 2004, 16, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Dong, C.; Xiao, K.; Du, C.; Zhou, H.; Lin, C. Initial Behavior and Mechanism of Metal Atmospheric Corrosion; Science Press: Beijing, China, 2009. [Google Scholar]
- Miao, C.; Sun, C.; Chen, L. Research on equivalent conversion method of environmental corrosion for cable components of large span bridges. J. Water Resour. Archit. Eng. 2010, 8, 5–7. [Google Scholar]
- Li, X.; Liu, Q.; Kong, L.; Zhou, J. Corrosion behavior of galvanized steel under stress in simulated acid rain solution. Corros. Sci. Prot. Technol. 2008, 20, 44–46. [Google Scholar]
- Betti, R.; West, A.; Vermaas, G.; Cao, Y. Corrosion and embrittlement in high-strength wires of suspension bridge cables. J. Bridge Eng. 2005, 10, 151–162. [Google Scholar] [CrossRef]
- Barton, S.C.; Vermaas, G.W.; Duby, P.F.; Alan, C.V.; Betti, R. Accelerated corrosion and embrittlement of high-strength bridge wire. J. Mater. Civ. Eng. 2000, 12, 33–38. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzumura, K.; Taruit, T. Mechanical properties and remaining strength of corroded bridge wires. Struct. Eng. Int. 2004, 14, 50–54. [Google Scholar] [CrossRef]
- Nakamura, S.; Suzumura, K. Hydrogen embrittlement and corrosion fatigue of corroded bridge wires. J. Constr. Steel Res. 2009, 65, 269–277. [Google Scholar] [CrossRef]
- Li, X.-M.; Zhou, J.; Liu, Q.; Kong, L.-F.; Zhou, J. Effect of tension on corrosion behavior of galvanized steel wire for cable—Stayed bridge. Electrochemistry 2007, 13, 297–304. [Google Scholar]
- Yang, W.J.; Yang, P.; Li, X.M.; Feng, W.L. Influence of tensile stress on corrosion behavior of high-strength galvanized steel bridge wires in simulated acid rain. Mater. Corros. 2012, 63, 401–408. [Google Scholar] [CrossRef]
- Wu, S.; Chen, H.; Ramandi, H.L.; Hagan, P.C.; Crosky, A.; Saydam, S. Effects of environmental factors on stress corrosion cracing of cold-drawn high-carbon steel wires. Corros. Sci. 2018, 132, 234–243. [Google Scholar] [CrossRef]
- Melchers, R.E. Statistical characterization of pitting corrosion part 1: Data analysis. Corrosion 2005, 61, 655–664. [Google Scholar] [CrossRef]
- Paik, J.K.; Lee, J.M.; Ko, M.J. Ultimate compressive strength of plate elements with pit corrosion wastage. J. Eng. Marit. Environ. 2003, 217, 185–200. [Google Scholar] [CrossRef]
- Mao, J.; Xu, F.; Jin, W.; Gao, Q.; Xu, Y.; Xu, C. An optical fiber sensor method for simultaneously monitoring corrosion and structural strain induced by loading. J. Test. Eval. 2018, 46, 1443–1451. [Google Scholar] [CrossRef]
- Li, X.; Li, D. Wavelet analysis and evaluation of ultrasonic guided wave signal on corrosion damage for reinforced concrete. Prestress Tech. 2014, 2, 20–23. [Google Scholar]
- Zhang, W.; Li, S.; Gu, X.; Zhu, C. Experiment on axial tensile fatigue of naturally corroded steel bar. China J. Highw. Transp. 2009, 22, 53–58. [Google Scholar]
- Ramandi, H.L.; Chen, H.; Crosky, A.; Saydam, S. Interactions of stress corrosion cracks in cold drawn pearlitic steel wires: An X-ray micro–computed tomography study. Corros. Sci. 2018, 145, 170–179. [Google Scholar] [CrossRef]
- Vehovar, L.; Kuhar, V.; Vehovar, A. Hydrogen-assisted stress-corrosion of prestressing wires in a motorway viaduct. In Engineering Failure Analysis; Elsevier: Amsterdam, The Netherlands, 1998; Volume 5, pp. 21–27. [Google Scholar]
- Liu, W.; Li, W.; Ma, Q. Corrosion test of high strength steel wire strand in marine environment. HVM Prestress. Technol. 2001, 2, 34–38. [Google Scholar]
- Li, F.; Yuan, Y.; Du, J.; Ma, H. Deterioration of tensile behavior of steel strands corroded by chloride. J. Southeast Univ. 2009, 39, 340–344. [Google Scholar]
- Zheng, Y.; Ou, Y.; An, L. Study on the test of mechanical properties of corroded steel bar embedded in concrete. Mod. Transp. Technol. 2005, 2, 33–36. [Google Scholar]
- Zhang, H.; Zhang, L.; Hu, S.; Zhang, J.; Hu, D. The effect of chloride salt corrosion on deterioration of loaded steel strands. Sci. Tech. Eng. 2018, 18, 232–236. [Google Scholar]
- Wu, X.; Li, L.; Li, H.; Li, B.; Ling, Z. Effect of strain level on corrosion of stainless steel bar. Construc. Build. Mater. 2018, 163, 189–199. [Google Scholar] [CrossRef]
- Zhang, W.; Song, X.; Gu, X.; Li, S. Tensile and fatigue behavior of corroded rebars. Construc. Build. Mater. 2012, 34, 409–417. [Google Scholar] [CrossRef]









| Specimens Items | Corrosion Time/Day | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Strain level | 0 με | 0-1-1(/2/3) 1 | 0-2-1(/2/3) | 0-3-1(/2/3) | 0-4-1(/2/3) |
| 1000 με | 1000-1-1(/2/3) | 1000-2-1(/2/3) | 1000-3-1(/2/3) | 1000-4-1(/2/3) | |
| 2000 με | 2000-1-1(/2/3) | 2000-2-1(/2/3) | 2000-3-1(/2/3) | 2000-4-1(/2/3) | |
| 3000 με | 3000-1-1(/2/3) | 3000-2-1(/2/3) | 3000-3-1(/2/3) | 3000-4-1(/2/3) | |
| Strain Level | 1 Day | 2 Day | 3 Day | 4 Day |
|---|---|---|---|---|
| 0 με | 1.10% | 2.31% | 4.83% | 8.45% |
| 1000 με | 1.23% | 4.03% | 5.81% | 8.73% |
| 2000 με | 3.77% | 5.39% | 6.67% | 9.33% |
| 3000 με | 4.40% | 6.59% | 15.12% | 28.14% |
| Mechanical Property | Strain Level | 1 Day | 2 Day | 3 Day | 4 Day |
|---|---|---|---|---|---|
| Yield Load (kN) | 0 με | 38.65 | 37.63 | 37.11 | 36.43 |
| 1000 με | 38.47 | 37.63 | 36.28 | 35.47 | |
| 2000 με | 38.32 | 37.09 | 36.86 | 35.22 | |
| 3000 με | 37.74 | 37.23 | 35.04 | 31.55 | |
| Ultimate Load (kN) | 0 με | 40.24 | 39.22 | 38.46 | 36.88 |
| 1000 με | 40.18 | 38.95 | 38.12 | 36.91 | |
| 2000 με | 40.17 | 38.89 | 38.06 | 36.35 | |
| 3000 με | 40.14 | 38.67 | 36.51 | 32.77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Chen, Y.; Zheng, X.; Ma, R.; Tian, H. Experimental Study on Corrosion and Mechanical Behavior of Main Cable Wires Considering the Effect of Strain. Materials 2019, 12, 753. https://doi.org/10.3390/ma12050753
Xu F, Chen Y, Zheng X, Ma R, Tian H. Experimental Study on Corrosion and Mechanical Behavior of Main Cable Wires Considering the Effect of Strain. Materials. 2019; 12(5):753. https://doi.org/10.3390/ma12050753
Chicago/Turabian StyleXu, Fangyuan, Yuanli Chen, Xianglong Zheng, Rujin Ma, and Hao Tian. 2019. "Experimental Study on Corrosion and Mechanical Behavior of Main Cable Wires Considering the Effect of Strain" Materials 12, no. 5: 753. https://doi.org/10.3390/ma12050753
APA StyleXu, F., Chen, Y., Zheng, X., Ma, R., & Tian, H. (2019). Experimental Study on Corrosion and Mechanical Behavior of Main Cable Wires Considering the Effect of Strain. Materials, 12(5), 753. https://doi.org/10.3390/ma12050753






