Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity
Abstract
1. Introduction
2. Materials and Methods
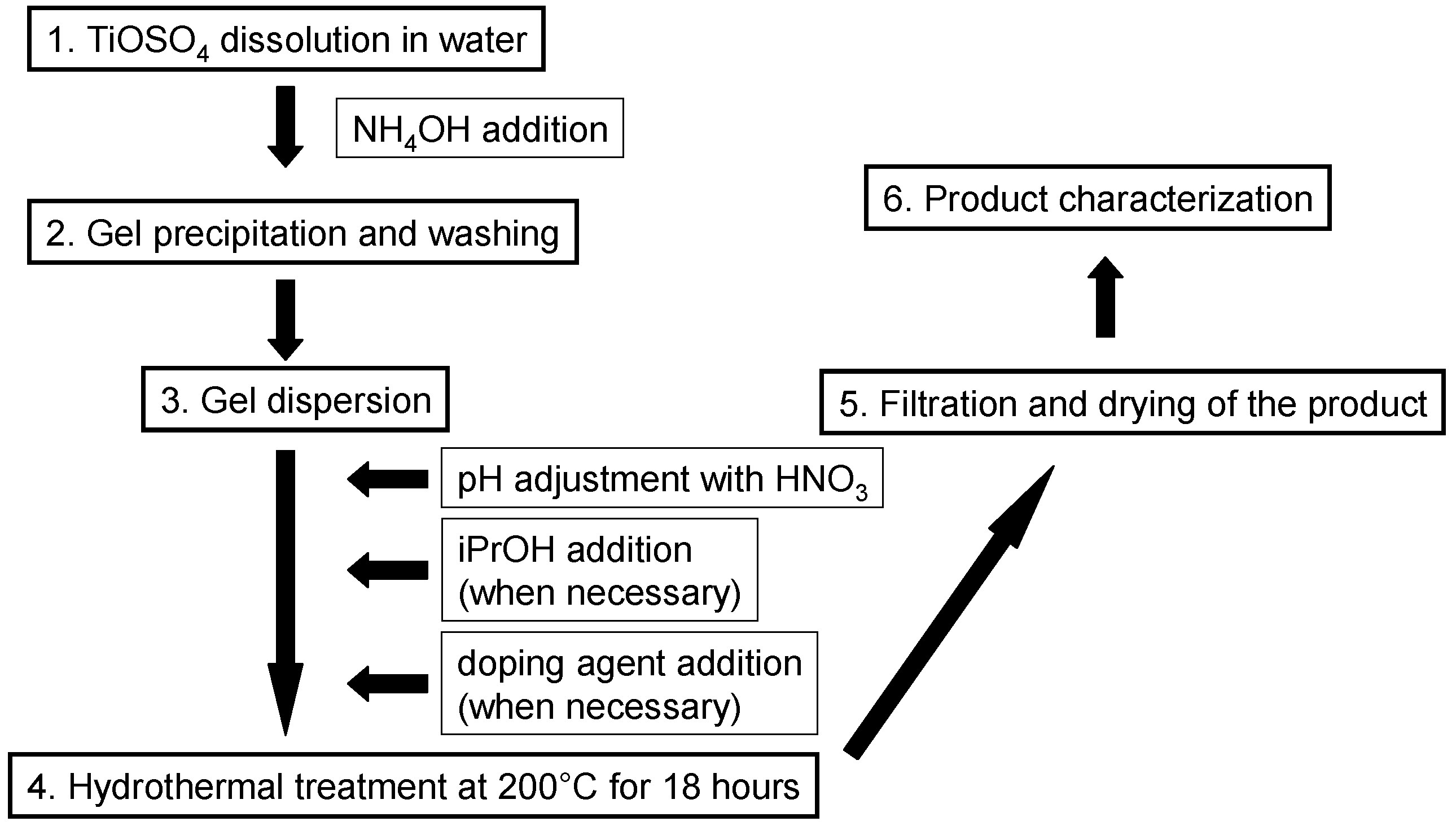
2.1. Sample Preparation
2.2. Sample Analysis
2.3. Photocatalytic Activity Measurement
2.3.1. Photocatalytic Activity of IPA Degradation
2.3.2. NOx Removal Measurement
2.3.3. Liquid-Solid Photocatalytic Removal of Methylene Blue (MB)
3. Results and Discussion
3.1. X-ray Powder Diffraction
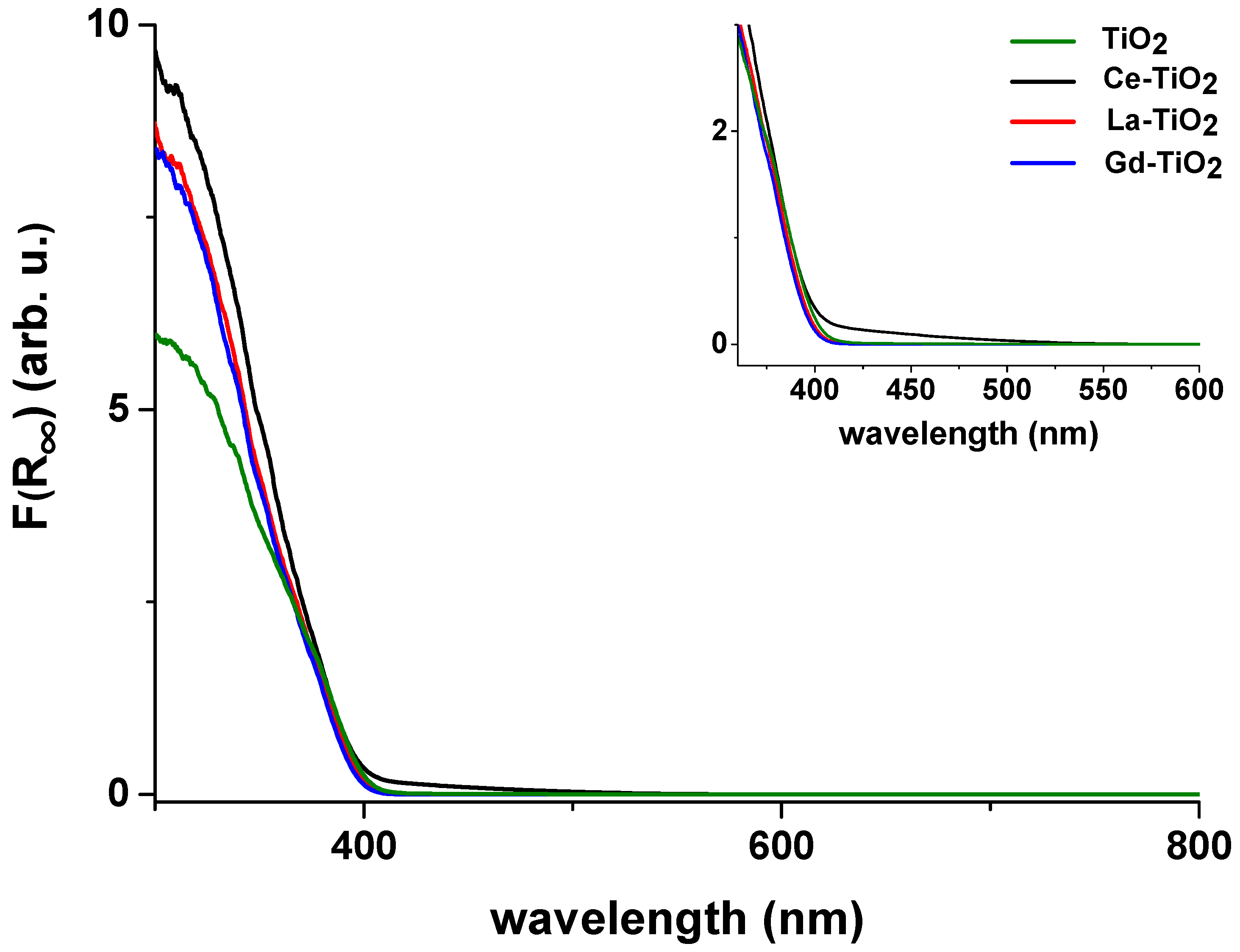
3.2. UV-Vis Spectroscopy
3.3. FE-SEM
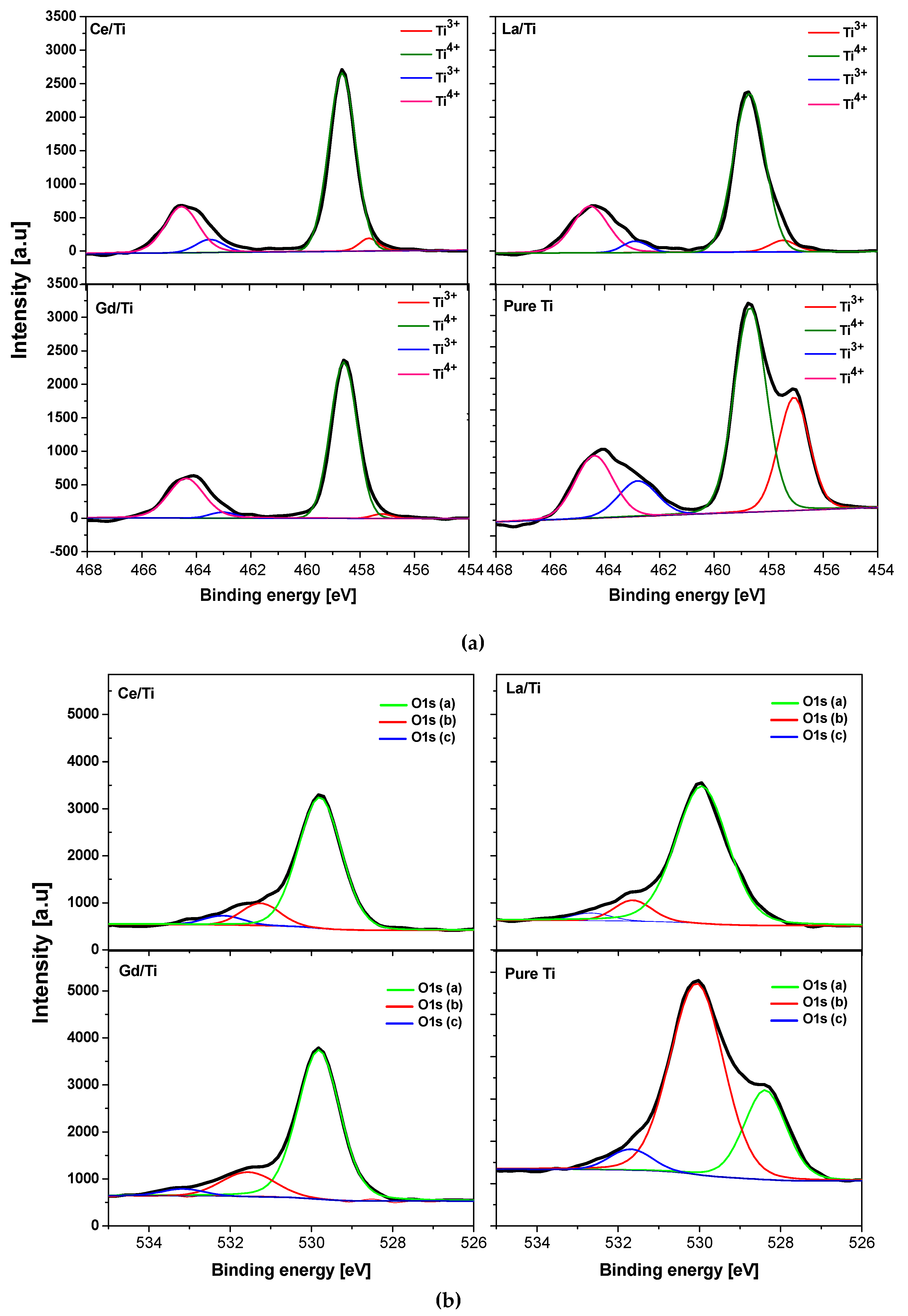
3.4. XPS Analysis
3.5. Photocatalytic Activity
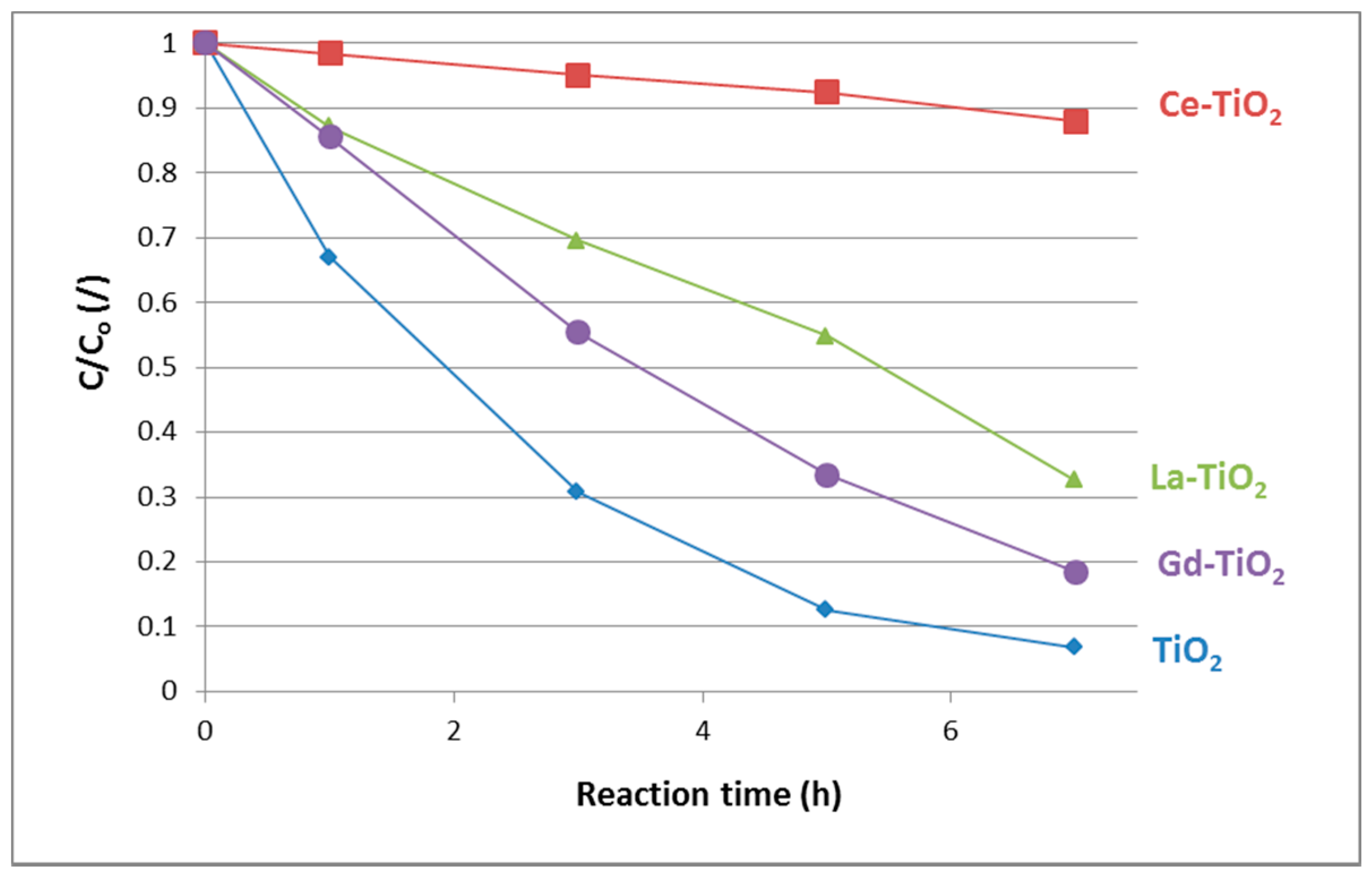
3.5.1. Liquid-Solid
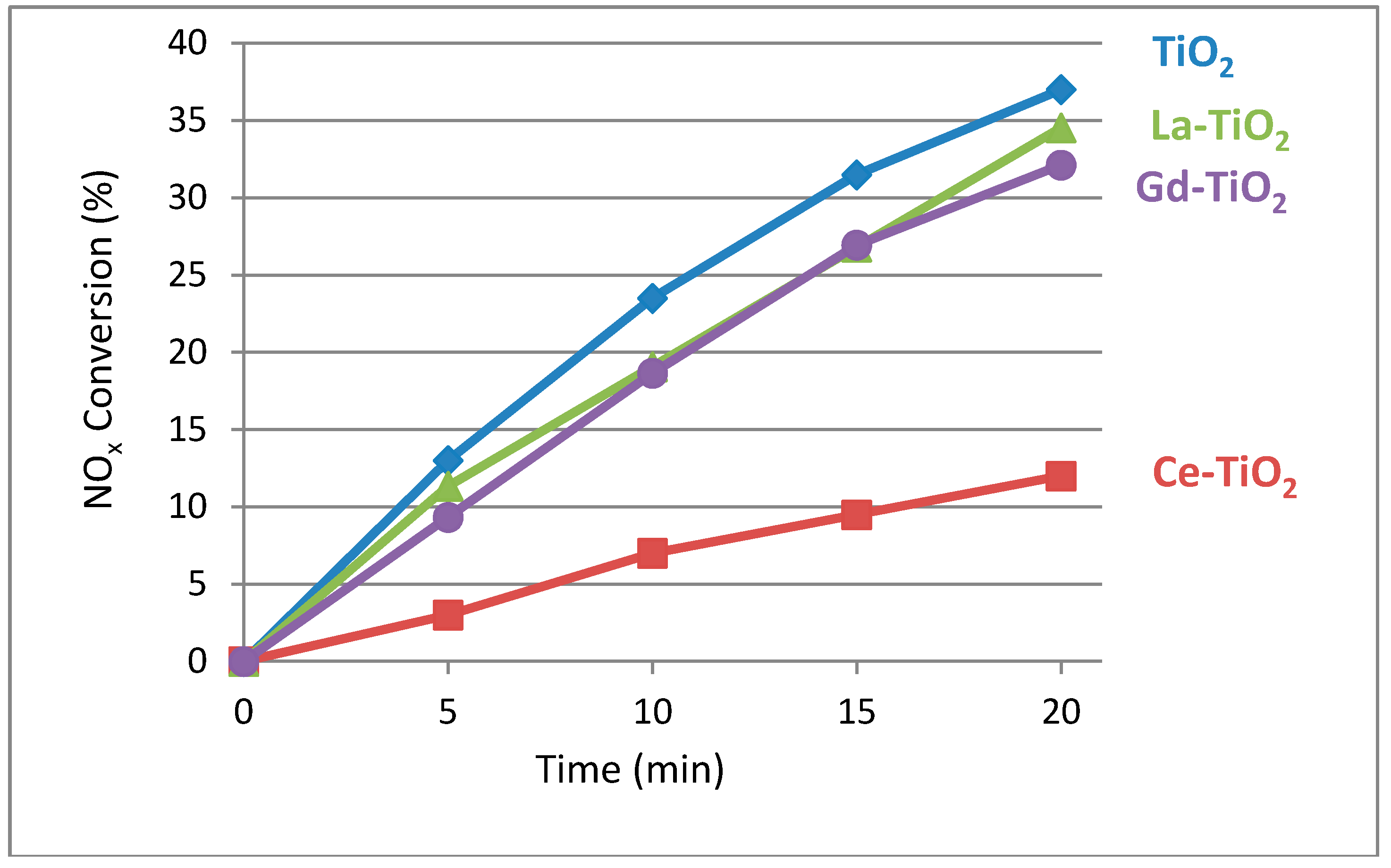
3.5.2. Gas-Solid NOx Removal
3.5.3. Gas-Solid VOC Removal
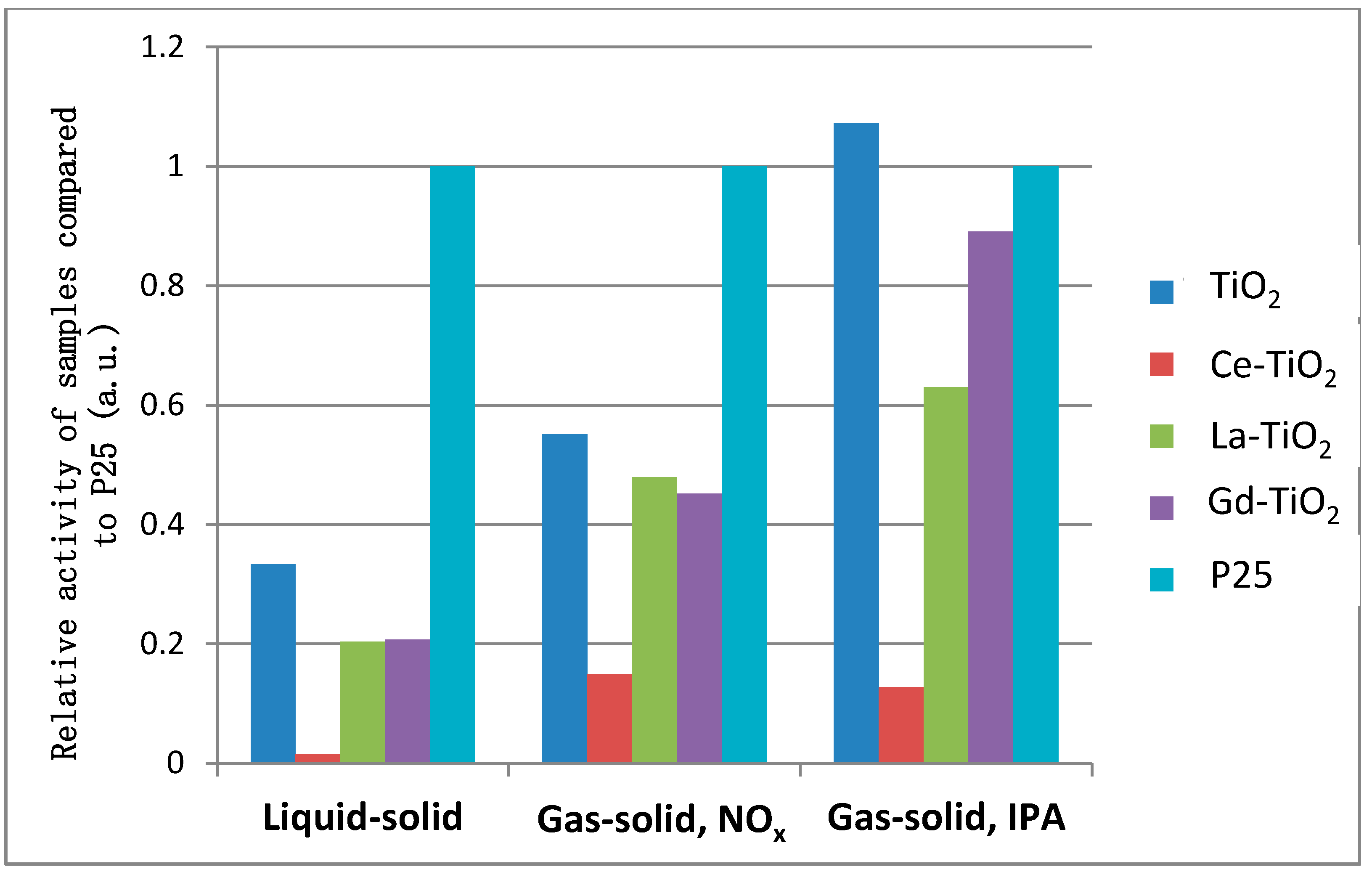
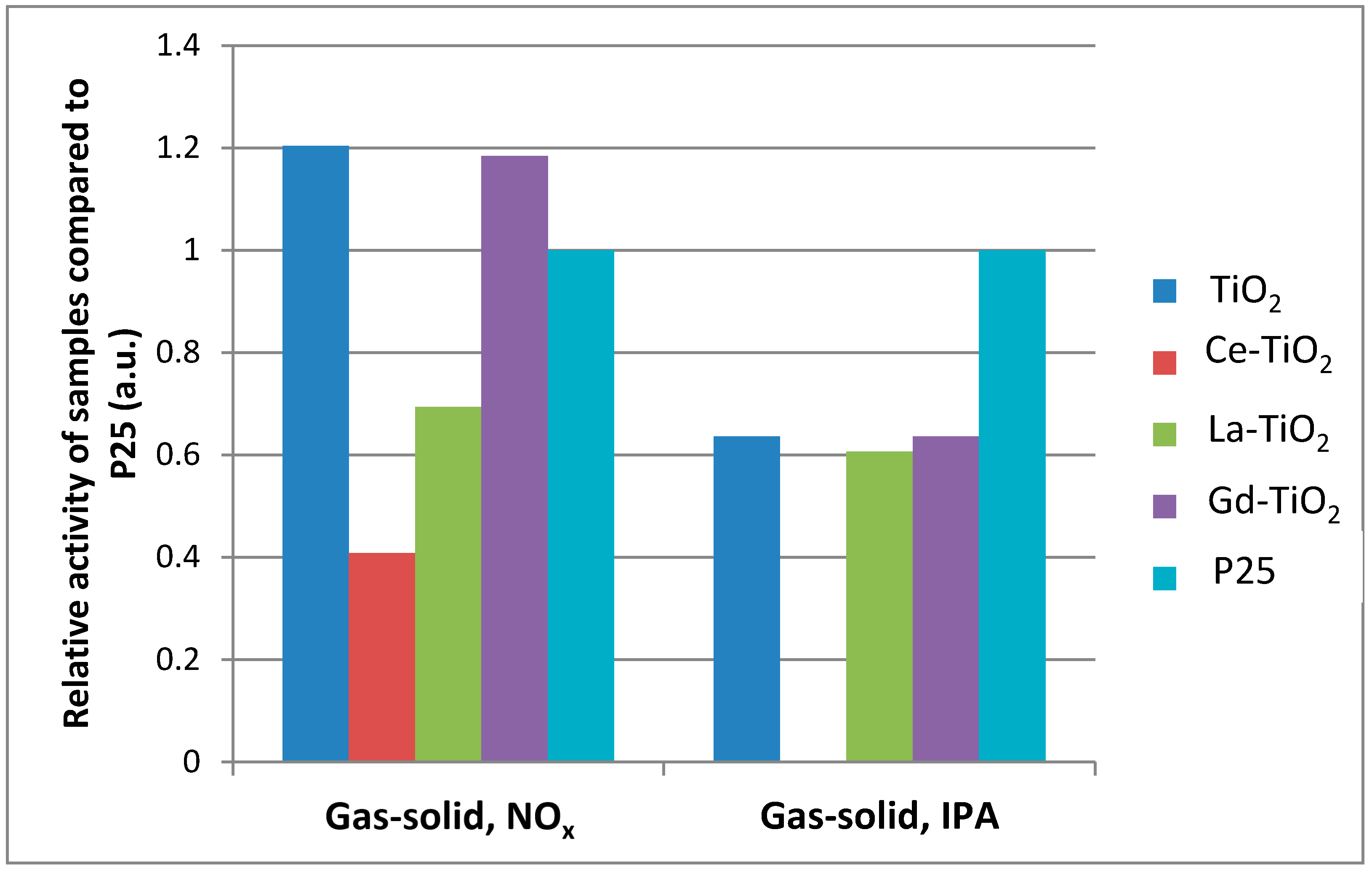
3.5.4. Comparison between Photocatalytic Activities of Samples in Different Environments
Activity under UV Irradiation or Simulated Solar Irradiation (UV + Vis)
Activity under Visible Light Irradiation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, D.M.; Pullar, R.C.; Gualtieri, A.F.; Seabra, M.P.; Labrincha, J.A. Phase composition, crystal structure and microstructure of silver and tungsten doped TiO2 nanopowders with tuneable photochromic behaviour. Acta Mater. 2013, 61, 5571–5585. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic water treatment by titanium dioxide: Recent updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.G.; Leva, P.; Kotzias, D. Application of photocatalytic technology for NOx removal. Appl. Phys. A-Mater. Sci. Process. 2007, 89, 81–84. [Google Scholar] [CrossRef]
- Mo, J.H.; Zhang, Y.P.; Xu, Q.J.; Lamson, J.J.; Zhao, R.Y. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Bingham, S.; Daoud, W.A. Recent advances in making nano-sized TiO2 visible-light active through rare-earth metal doping. J. Mater. Chem. 2011, 21, 2041–2050. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.M. Titanium dioxide crystals with tailored facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Mao, S.S. Titanium dioxide nanomaterials: synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N. Is the Band Gap of Pristine TiO2 Narrowed by Anion- and Cation-Doping of Titanium Dioxide in Second-Generation Photocatalysts? J. Phys. Chem. B 2006, 110, 24287–24293. [Google Scholar] [CrossRef] [PubMed]
- Anpo, M. Utilization of TiO2 photocatalysts in green chemistry. Pure Appl. Chem. 2000, 72, 1265–1270. [Google Scholar] [CrossRef]
- Wang, F.; Ma, Z.Z.; Ban, P.P.; Xu, X.H. C, N and S codoped rutile TiO2 nanorods for enhanced visible-light photocatalytic activity. Mater. Lett. 2017, 195, 143–146. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Umebayashi, T.; Yamaki, T.; Itoh, H.; Asai, K. Analysis of electronic structures of 3d transition metal-doped TiO2 based on band calculations. J. Phys. Chem. Solids 2002, 63, 1909–1920. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Gualtieri, A.F.; Seabra, M.P.; Labrincha, J.A. Sol-gel synthesis, characterisation and photocatalytic activity of pure, W-, Ag-and W/Ag co-doped TiO2 nanopowders. Chem. Eng. J. 2013, 214, 364–375. [Google Scholar] [CrossRef]
- Colon, G.; Maicu, M.; Hidalgo, M.C.; Navio, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B-Environ. 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Li, H.; Zhao, G.L.; Chen, Z.J.; Han, G.R.; Song, B. Low temperature synthesis of visible light-driven vanadium doped titania photocatalyst. J. Colloid Interface Sci. 2010, 344, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ding, J.J.; Bao, J.; Gao, C.; Qi, Z.M.; Yang, X.Y.; He, B.; Li, C.X. Photocatalytic degradation of gaseous toluene on Fe-TiO2 under visible light irradiation: a study on the structure, activity and deactivation mechanism. Appl. Surf. Sci. 2012, 258, 5031–5037. [Google Scholar] [CrossRef]
- Lu, X.N.; Ma, Y.F.; Tian, B.Z.; Zhang, J.L. Preparation and characterization of Fe-TiO2 films with high visible photoactivity by autoclaved-sol method at low temperature. Solid State Sci. 2011, 13, 625–629. [Google Scholar] [CrossRef]
- Umar, K.; Haque, M.M.; Muneer, M.; Harada, T.; Matsumura, M. Mo, Mn and La doped TiO2: Synthesis, characterization and photocatalytic activity for the decolourization of three different chromophoric dyes. J. Alloys Compd. 2013, 578, 431–438. [Google Scholar] [CrossRef]
- Ranjitha, A.; Muthukumarasamy, N.; Thambidurai, M.; Velauthapillai, D.; Balasundaraprabhu, R.; Agilan, S. Fabrication of Ni-doped TiO2 thin film photoelectrode for solar cells. Sol. Energy 2014, 106, 159–165. [Google Scholar] [CrossRef]
- Di Valentin, C.; Finazzi, E.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Paganini, M.C.; Giamello, E. N-doped TiO2: Theory and experiment. Chem. Phys. 2007, 339, 44–56. [Google Scholar] [CrossRef]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol–gel TiO2. J. Hazard. Mater. 2012, 211, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Ren, W.J.; Ai, Z.H.; Jia, F.L.; Zhang, L.Z.; Fan, X.X.; Zou, Z.G. Low temperature preparation and visible light photocatalytic activity of mesoporous carbon-doped crystalline TiO2. Appl. Catal. B-Environ. 2007, 69, 138–144. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xue, X.X.; Yang, H. Preparation and characterization of carbon or/and boron-doped titania nano-materials with antibacterial activity. Ceram. Int. 2014, 40, 12533–12537. [Google Scholar] [CrossRef]
- Stengl, V.; Bakardjieva, S.; Murafa, N. Preparation and photocatalytic activity of rare earth doped TiO2 nanoparticles. Mater. Chem. Phys. 2009, 114, 217–226. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Skapin, A.S.; Seabra, M.P.; Labrincha, J.A. Visible light activated photocatalytic behaviour of rare earth modified commercial TiO2. Mater. Res. Bull. 2014, 50, 183–190. [Google Scholar] [CrossRef]
- Habibi, M.H.; Vosooghian, H. Photocatalytic degradation of some organic sulfides as environmental pollutants using titanium dioxide suspension. J. Photochem. Photobiol. A-Chem. 2005, 174, 45–52. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, W. Substrate-Specific Photocatalytic Activities of TiO2 and Multiactivity Test for Water Treatment Application. Environ. Sci. Technol. 2007, 42, 294–300. [Google Scholar] [CrossRef]
- Wang, C.Y.; Pagel, R.; Dohrmann, J.K.; Bahnemann, D.W. Antenna mechanism and deaggregation concept: novel mechanistic principles for photocatalysis. Comptes Rendus Chim. 2006, 9, 761–773. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Dillert, R.; Feldhoff, A.; Bahnemann, D.W. Direct Synthesis of Photocatalytically Active Rutile TiO2 Nanorods Partly Decorated with Anatase Nanoparticles. J. Phys. Chem. C 2010, 114, 4909–4915. [Google Scholar] [CrossRef]
- Rozman, N.; Skrlep, L.; Gaberscek, M.; Skapin, A.S. Tuning the Photocatalytic Activity of Nanocrystalline Titania by Phase Composition Control and Nitrogen Doping, Using Different Sources of Nitrogen. Acta Chim. Slov. 2014, 61, 506–516. [Google Scholar] [PubMed]
- Meagher, E.P.; Lager, G.A. Polyhedral thermal expansion in the TiO2 polymorphs; refinement of the crystal structures of rutile and brookite at high temperature. Can. Mineral. 1979, 17, 77–85. [Google Scholar]
- Howard, C.J.; Sabine, T.M.; Dickson, F. Structural and thermal parameters for rutile and anatase. Acta Crystallogr. Sect. B-Struct. Sci. 1991, 47, 462–468. [Google Scholar] [CrossRef]
- Marolt, T.; Skapin, A.S.; Bernard, J.; Zivec, P.; Gaberscek, M. Photocatalytic activity of anatase-containing facade coatings. Surf. Coat. Technol. 2011, 206, 1355–1361. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Piccirillo, C.; Pullar, R.C.; Gualtieri, A.F.; Seabra, M.P.; Castro, P.M.L.; Labrincha, J.A. Silver-modified nano-titania as an antibacterial agent and photocatalyst. J. Phys. Chem. C 2014, 118, 4751–4766. [Google Scholar] [CrossRef]
- Munuera, G.; Moreno, F.; Prieto, J.A. Temperature Programmed Desorption of Water Adsorbed on Anatase Surfaces. Zeitschrift Fur Physikalische Chemie-Frankfurt 1972, 78, 113. [Google Scholar] [CrossRef]
- Bickley, R.I.; Munuera, G.; Stone, F.S. Photoadsorption and photocatalysis at rutile surfaces: II. Photocatalytic oxidation of isopropanol. J. Catal. 1973, 31, 398–407. [Google Scholar] [CrossRef]
- Larson, S.A.; Widegren, J.A.; Falconer, J.L. Transient studies of 2-propanol photocatalytic oxidation on titania. J. Catal. 1995, 157, 611–625. [Google Scholar] [CrossRef]
- Lucas, S.S.; Ferreira, V.M.; de Aguiar, J.L.B. Incorporation of titanium dioxide nanoparticles in mortars—Influence of microstructure in the hardened state properties and photocatalytic activity. Cement Concr. Res. 2013, 43, 112–120. [Google Scholar] [CrossRef]
- Skapin, A.S.; Skrlep, L.; Suvorov, D.; Zunic, V.; Skapin, S.D. Photocatalytic activity of hierarchically structured, thermally stable, anatase particles. RSC Adv. 2015, 5, 26769–26776. [Google Scholar] [CrossRef]
- Burns, A.; Hayes, G.; Li, W.; Hirvonen, J.; Demaree, J.D.; Shah, S.I. Neodymium ion dopant effects on the phase transformation in sol–gel derived titania nanostructures. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2004, 111, 150–155. [Google Scholar] [CrossRef]
- Xu, Y.H.; Chen, H.R.; Zeng, Z.X.; Lei, B. Investigation on mechanism of photocatalytic activity enhancement of nanometer cerium-doped titania. Appl. Surf. Sci. 2006, 252, 8565–8570. [Google Scholar] [CrossRef]
- Hawthorne, F.C.A.; Brown, G.E.A.; Mineralogical Society of America. Spectroscopic Methods in Mineralogy and Geology; Mineralogical Society of America: Chantilly, VA, USA, 1988. [Google Scholar]
- Galindo-Hernandez, F.; Gomez, R. Degradation of the herbicide 2,4-dichlorophenoxyacetic acid over TiO2-CeO2 sol-gel photocatalysts: effect of the annealing temperature on the photoactivity. J. Photochem. Photobiol. A-Chem. 2011, 217, 383–388. [Google Scholar] [CrossRef]
- Nguyen-Phan, T.D.; Song, M.B.; Kim, E.J.; Shin, E.W. The role of rare earth metals in lanthanide-incorporated mesoporous titania. Microporous Mesoporous Mater. 2009, 119, 290–298. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, Z.Y.; Wang, X.Y.; Yu, T.; Guan, J.; Yu, Z.T.; Li, Z.S.; Zou, Z.G. Increasing the Oxygen Vacancy Density on the TiO2 Surface by La-Doping for Dye-Sensitized Solar Cells. J. Phys. Chem. C 2010, 114, 18396–18400. [Google Scholar] [CrossRef]
- Li, J.H.; Yang, X.; Yu, X.D.; Xu, L.L.; Kang, W.L.; Yan, W.H.; Gao, H.F.; Liu, Z.H.; Guo, Y.H. Rare earth oxide-doped titania nanocomposites with enhanced photocatalytic activity towards the degradation of partially hydrolysis polyacrylamide. Appl. Surf. Sci. 2009, 255, 3731–3738. [Google Scholar] [CrossRef]
- Lin, J.; Jimmy, C.Y. An investigation on photocatalytic activities of mixed TiO2-rare earth oxides for the oxidation of acetone in air. J. Photochem. Photobiol. A-Chem. 1998, 116, 63–67. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A. Fully quantitative X-ray characterisation of Evonik Aeroxide TiO2 P25®. Mater. Lett. 2014, 122, 345–347. [Google Scholar] [CrossRef]
- Hurum, D.C.; Agrios, A.G.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Explaining the Enhanced Photocatalytic Activity of Degussa P25 Mixed-Phase TiO2 Using EPR. J. Phys. Chem. B 2003, 107, 4545–4549. [Google Scholar] [CrossRef]
- Zalas, M. Gadolinium-modified titanium oxide materials for photoenergy applications: A review. J. Rare Earths 2014, 32, 487–495. [Google Scholar] [CrossRef]
- Bao, R.Y.; Yu, Y.M.; Chen, H.Y.; Wang, W.Z.; Xia, J.X.; Li, H. Effects of Rare Earth Elements and Nitrogen Co-Doped on the Photocatalytic Performance of TiO2. Cryst. Res. Technol. 2018, 53, 8. [Google Scholar] [CrossRef]
- Lee, H.; Park, I.S.; Bang, H.J.; Park, Y.K.; Kim, H.; Ha, H.H.; Kim, B.J.; Jung, S.C. Fabrication of Gd-La codoped TiO2 composite via a liquid phase plasma method and its application as visible-light photocatalysts. Appl. Surf. Sci. 2019, 471, 893–899. [Google Scholar] [CrossRef]
- Karmaoui, M.; Tobaldi, D.M.; Skapin, A.S.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A.; Amaral, V.S. Non-aqueous sol-gel synthesis through a low-temperature solvothermal process of anatase showing visible-light photocatalytic activity. RSC Adv. 2014, 4, 46762–46770. [Google Scholar] [CrossRef]

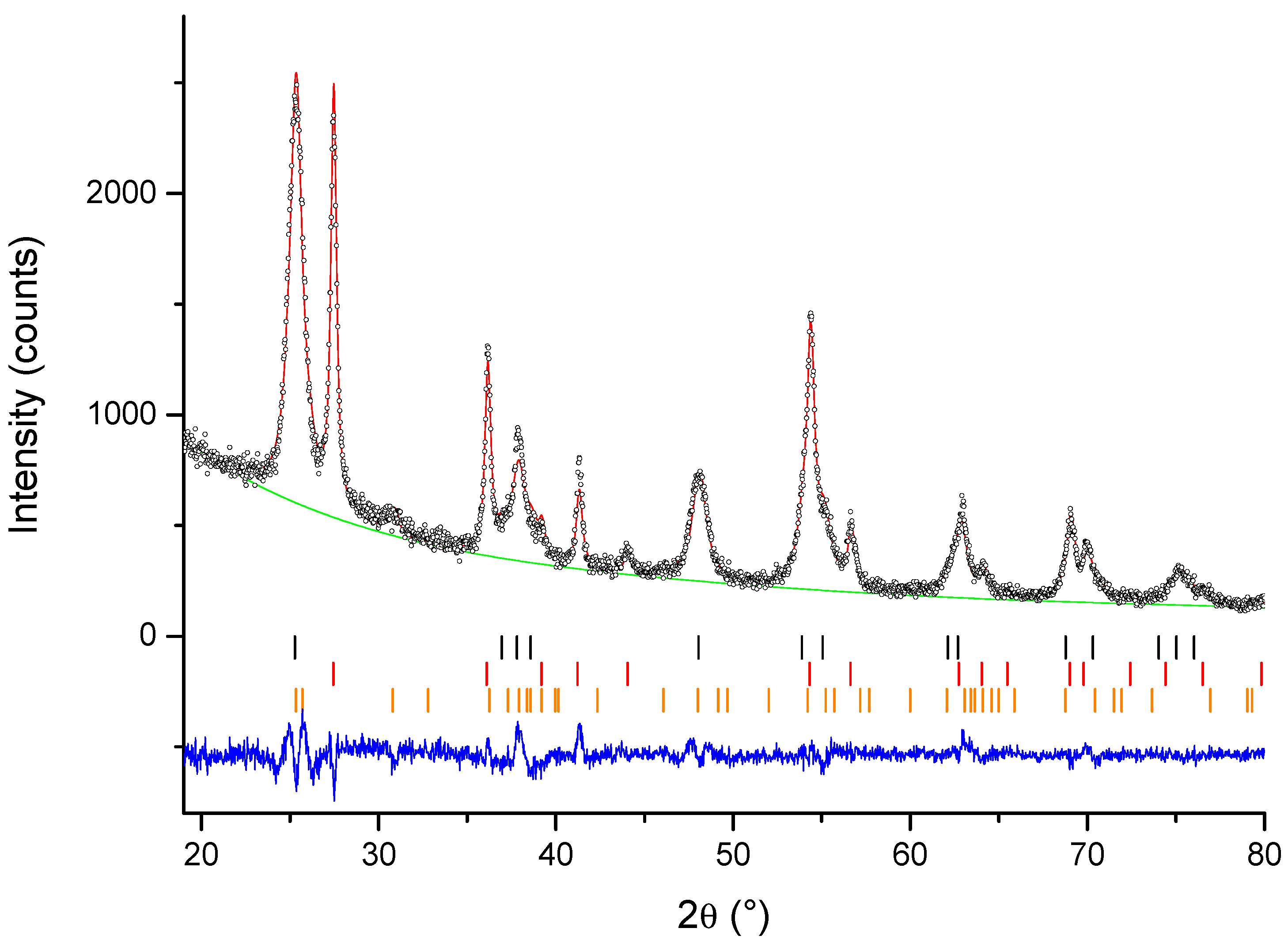

| Sample No. of Variables | Agreement Factors | Phase Composition | |||||
|---|---|---|---|---|---|---|---|
| Rf2 (%) | Rwp (%) | χ2 | Anatase (wt.%) | Rutile (wt.%) | Brookite (wt.%) | ||
| TiO2 | 12 | 5.68 | 8.59 | 2.117 | / | 100 | / |
| Ce-TiO2 | 22 | 5.83 | 5.95 | 1.999 | 56.1 ± 0.3 | 35.6 ± 0.2 | 8.3 ± 0.7 |
| La-TiO2 | 15 | 5.49 | 7.27 | 2.389 | 26.0 ± 0.4 | 74.0 ± 0.1 | / |
| Gd-TiO2 | 19 | 4.66 | 7.29 | 2.427 | 25.4 ± 0.5 | 74.6 ± 0.1 | / |
| Sample | 1st Derivative Maximum | |
|---|---|---|
| Wavelength (nm) | Eg (eV) | |
| TiO2 | 401 | 3.09 |
| Ce-TiO2 | 395 | 3.14 |
| La-TiO2 | 400 | 3.10 |
| Gd-TiO2 | 399 | 3.11 |
| Sample | k′app (h−1) |
|---|---|
| TiO2 | 0.390 |
| Ce-TiO2 | 0.018 |
| La-TiO2 | 0.238 |
| Gd-TiO2 | 0.242 |
| P25 | 1.17 |
| Sample | Photocatalytic Activity | |
|---|---|---|
| k′app Solar (min−1) | k′app VIS (min−1) | |
| TiO2 | 0.0244 | 0.0042 |
| Ce-TiO2 | 0.0066 | 0.0000 |
| La-TiO2 | 0.0212 | 0.0040 |
| Gd-TiO2 | 0.0200 | 0.0042 |
| P25 | 0.04427 | 0.0066 |
| Sample | Photocatalytic Activity | |
|---|---|---|
| Vis (ppm/h) | UV + Vis (ppm/h) | |
| TiO2 | 59 | 885 |
| Ce-TiO2 | 20 | 105 |
| La-TiO2 | 34 | 520 |
| Gd-TiO2 | 58 | 735 |
| P25 | 49 | 825 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozman, N.; Tobaldi, D.M.; Cvelbar, U.; Puliyalil, H.; Labrincha, J.A.; Legat, A.; Sever Škapin, A. Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity. Materials 2019, 12, 713. https://doi.org/10.3390/ma12050713
Rozman N, Tobaldi DM, Cvelbar U, Puliyalil H, Labrincha JA, Legat A, Sever Škapin A. Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity. Materials. 2019; 12(5):713. https://doi.org/10.3390/ma12050713
Chicago/Turabian StyleRozman, Nejc, David M. Tobaldi, Uroš Cvelbar, Harinarayanan Puliyalil, João A. Labrincha, Andraž Legat, and Andrijana Sever Škapin. 2019. "Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity" Materials 12, no. 5: 713. https://doi.org/10.3390/ma12050713
APA StyleRozman, N., Tobaldi, D. M., Cvelbar, U., Puliyalil, H., Labrincha, J. A., Legat, A., & Sever Škapin, A. (2019). Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity. Materials, 12(5), 713. https://doi.org/10.3390/ma12050713







