1. Introduction
In the past decade, high-entropy alloys (HEAs), containing at least four principle elements in equimolar or near-equimolar ratios, have received more and more attention due to the remarkable comprehensive performance. Unlike conventional alloys materials based on one or two principal constituent elements, HEAs tend to form a simple multi-component solid solution structure, such as face-centered cubic (FCC) structure, body-centered cubic (BCC) structure, and hexagonal close-packed (HCP) structure [
1,
2,
3,
4,
5].
Among these, CoCrFeNi system HEAs, which possess the typical FCC solid solution structure, exhibit a remarkable combination of high ductility, cryogenic fracture toughness, corrosion resistance, and thermal stability. Moreover, such FCC-typed HEAs have been proven to achieve high strength while maintaining sufficient ductility by means of precipitation strengthening mechanisms. For instance, He et al. developed high-performance HEAs with nano-sized coherent precipitates, L1
2-Ni
3(Ti, Al), in CoCrFeNi matrix by adding a small amount of Ti and Al elements [
6]. Liu et al. confirmed that the precipitation of ordered Nb-enriched Laves phase can effectively enhance the strength of CoCrFeNiNb
x HEAs [
7]. Thus, CoCrFeNi system HEAs are considered a potential high-performance structural material.
However, compared to conventional Ni-based superalloys and steel, high density (~8 g/cm
3) and cost severely limit its engineer application. This is caused by the expensive metal elements used, such as Co and Ni, for bulk CoCrFeNi system HEAs. At present, a recognized and effective solution is to develop high performance HEAs coatings on substrates of traditional materials, which could give full play to its performance advantages, and an increasing number of work on HEAs coating have been reported [
8,
9,
10,
11,
12].
Traditional HEAs coating preparation methods are mainly focused on laser cladding [
8,
9], laser metal deposition [
10], mechanical alloying, and hot pressing sintering [
11,
12]. Different from these technologies, the plasma spray process has more advantages: (1) the ingredient of coating is easy to control precisely; (2) the temperature rise of the substrate is small and does not affect the shape and performance of the substrate. Therefore, plasma spraying technology has great application potential for preparing HEA coatings with excellent performance.
In this work, (CoCrFeNi)95Nb5 HEA were selected as the composition of coating because of its high strength and corrosion resistance in bulk state, while the corresponding properties of coating remain unknown. Therefore, (CoCrFeNi)95Nb5 HEA coating on the Q235 steel substrate was fabricated by using the plasma spray technique, and the microstructure evolution during preparation process, microhardness, and corrosion behavior in 3.5 wt.% NaCl solution were investigated. Furthermore, the phase formation and the corrosion behavior of the coatings were specifically analyzed.
2. Experimental Procedures
The Q235 steel was used as the substrate, and its chemical composition was Fe (base), C (≤0.22%), Si (≤0.35%), Mn (≤1.4%), P (≤0.045%), and S (≤0.05%). First, surface impurities were removed by acetone in an ultrasonic cleaner. Metal powder of Co, Cr, Fe, Ni, and Nb with purity more than 99.9% was used to prepare (CoCrFeNi)95Nb5 HEA powder by atomization method. Then, the (CoCrFeNi)95Nb5 HEA powder was used as raw material to prepare HEA coating by vacuum plasma spraying system (ZP-2000, DWAUTO, Xi’an, China). Plasma spraying process parameters: arc power was 30 kW, current was 500 A, main gas Ar flow was 45 L/min, and powder delivery gas flow was 4 L/min; the spraying distance was 100 mm.
The crystal structures of the HEA powder and coating were identified by X-ray diffractometer (XRD, Rigaku D/MAX-RB, Rigaku Corporation, Tokyo, Japan) using Cu-Kα radiation scanning from 20° to 100° at a scanning rate of 10°/min. The microstructure and chemical composition of the HEA powder and coating were observed by scanning electron microscope (SEM, ZEISS SUPRA 55, ZEISS, Oberkochen, Germany) coupled with energy dispersive spectrometry (EDS). The microhardness measurements of HEA coatings at different depths were tested by digital micro Vickers (401MVD, WOLPERT Co., Norwood, MA, USA) under a load of 500 g for 15 s. For each depth, 10 repetitions were performed. After removing the maximum and minimum values, the average value was calculated.
The electrochemical polarization curve of the (CoCrFeNi)95Nb5 HEA coating was investigated by the electrochemical workstation (Versa STAT MC, PARSTAT 4000, AMETEK Co., Princeton, NJ, USA) in the 3.5 wt.% NaCl solution open to air at room temperature of 298 K. A three-electrode system was used for the electrochemical test, in which the working electrode was the HEA coating sample. The platinum plate was used as an auxiliary electrode, and the reference electrode was a saturated calomel electrode (SCE) with E = 0.244 VSHE. The potential scanning range was −0.5 to 0.5 V, and the scan rate was 1 mV/s. After the electrochemical test, the sample was washed with deionized water in an ultrasonic cleaner and dried. Subsequently, the morphology and chemical composition of the sample was observed by SEM coupled with EDS.
An X-ray photoelectron spectrometer (XPS, ESCALAB 250Xi, Thermo Fisher Scientific, Waltham, MA, USA) was employed to study the oxide phases formed on the HEA coating surface, and the XPS spectra were analyzed by Thermo Avantage 5.9 software.
3. Results
3.1. Microstructure of (CoCrFeNi)95Nb5 HEA Powder
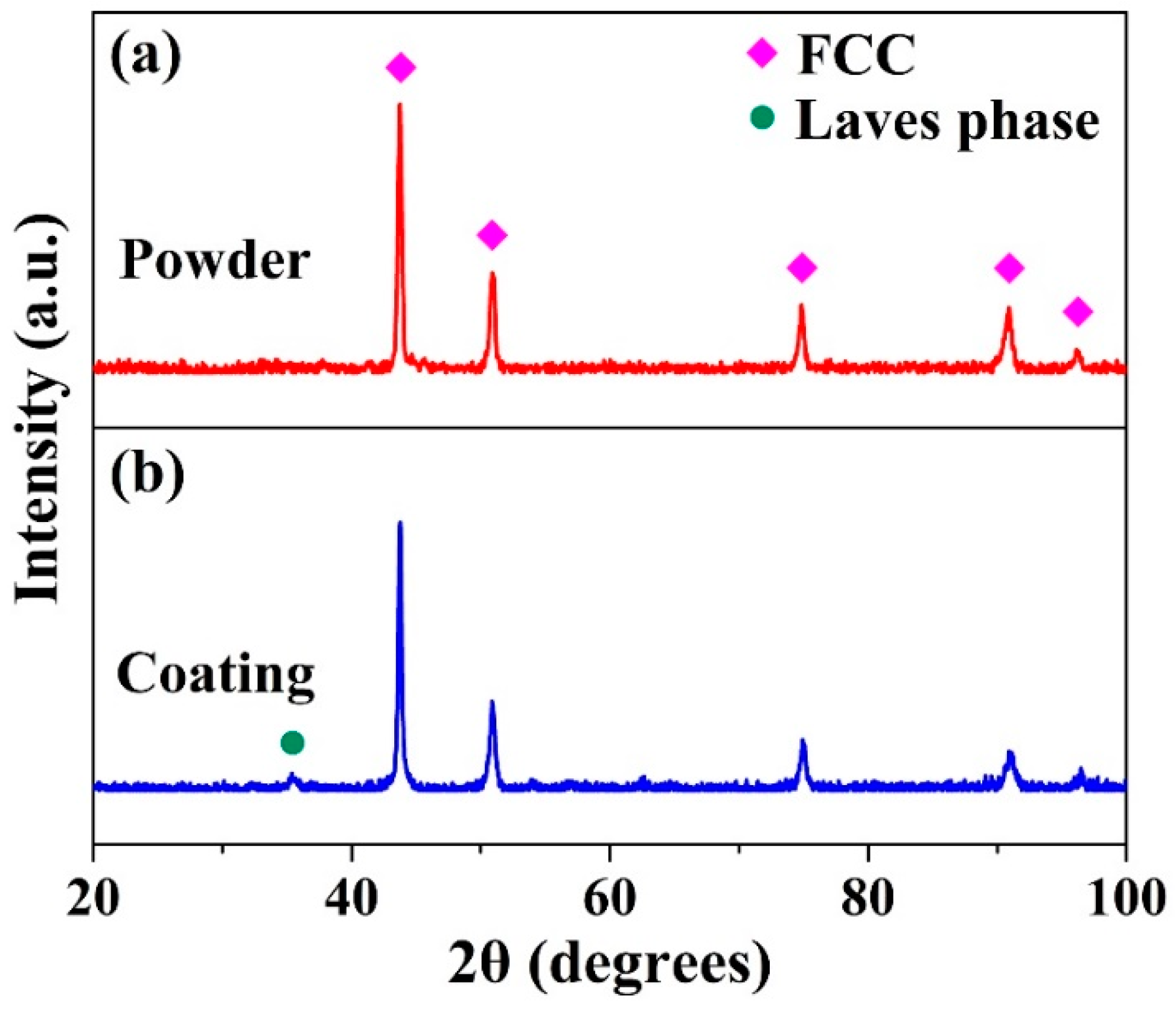
The XRD pattern of the (CoCrFeNi)
95Nb
5 HEA powder is shown in
Figure 1a. As can be seen, only diffraction peak of simple FCC phase is found on the pattern, indicating no precipitation of second phase. This result is significantly different from the as-cast CoCrFeNiNb
x HEAs with similar composition, where the ordered Laves phase with hexagonal close-packed (HCP) structure could form in FCC matrix with additions of a small amount of Nb. Obviously, during the preparation process, the second phase is inhibited and the supersaturated solid solution structure in high temperature state is retained to room temperature during atomization process.
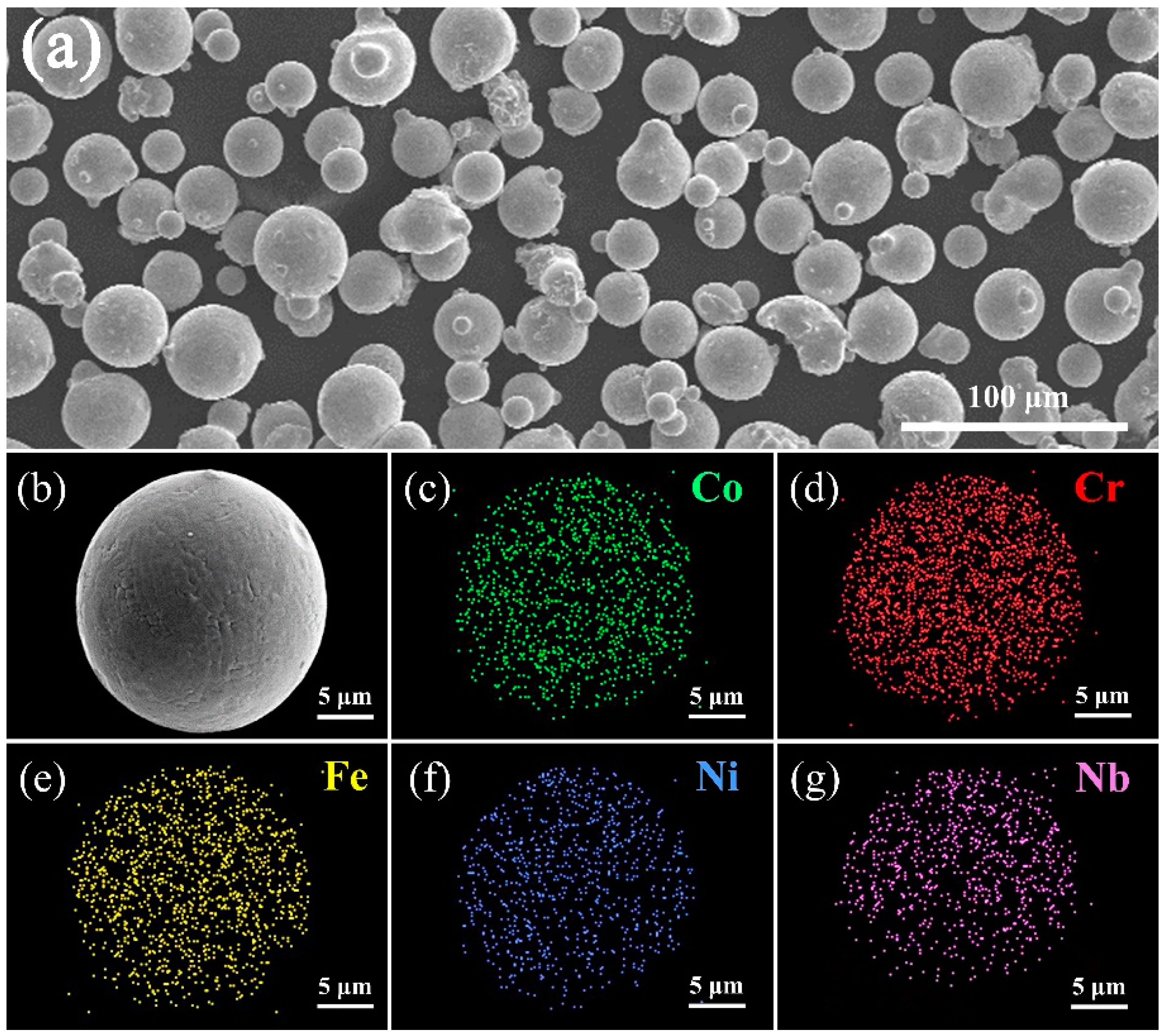
The SEM image of (CoCrFeNi)
95Nb
5 HEA powder is shown in
Figure 2a. It can be seen that the powder is nearly spherical, and the diameter of the particles is from 10 μm to 30 μm. As seen from
Figure 2b, the surface of the spherical powder is not smooth, distributing inhomogeneously as a grid. The EDS results (
Table 1) and mappings (
Figure 2c–g) of HEA powder indicate that composite of powder is very close to the designed nominal composition and the distribution of each element in the single powder is highly uniform.
3.2. Microstructure of (CoCrFeNi)95Nb5 HEA Coating
Using the above HEA powder, the HEA coating was successfully prepared by plasma spraying.
Figure 1b shows the XRD pattern of the HEA coating. Compared to the XRD pattern of the HEA powder, there was one minor diffraction peak determined as the Laves phase on the pattern at about 36°, which is consistent with the phase constituent of as-cast CoCrFeNiNb
x HEAs reported by Jiang et al. [
13], and the peak intensity of the Laves phase was significantly lower than that of the FCC phase, indicating the FCC phase is the main phase of the alloy.
Figure 3a exhibits the low magnification morphology of longitudinal section, illustrating that the thickness of the coating on the Q235 steel substrate was approximately 500 μm and that the surface of the coating was uneven.
Figure 3b is an enlarged view of the longitudinal section of the bonding surface between the HEA coating and the substrate. It can be seen that there is a clear interface between the coating and the substrate; however, the combination between the two parts was tight and only a small amount of pores was observed. The longitudinal section of the coating shows a distinct elongated dendritic and interdendritic structure parallel to the surface of the substrate, which exhibits the traces of expansion and subsequent solidification of the melt HEAs droplets on the substrate during plasma spraying.
Figure 3c presents the microstructure of cross section of the (CoCrFeNi)
95Nb
5 HEA coating. It was found that the dendrites with white contrast (marked as DR) and interdendrites with gray contrast (marked as ID) show an approximately equiaxed morphology, and black areas are pores. The chemical composition of the dendritic phase and the interdendritic phase was analyzed by EDS, and the results are shown in
Table 1. It has been demonstrated that Cr and Nb elements are enriched in the interdendritic phase, while Co, Fe and Ni are mainly concentrated in the dendritic region. Similar composition segregation phenomena have also appeared in the study of Liu et al. [
7]. It is noteworthy that a lot of rounded precipitates (marked as P) are found in the gray phases, as shown in the inset of
Figure 3d and the corresponding elemental composition was analyzed by EDS. From
Table 1, the precipitated phase is rich in Co, Fe, and Nb.
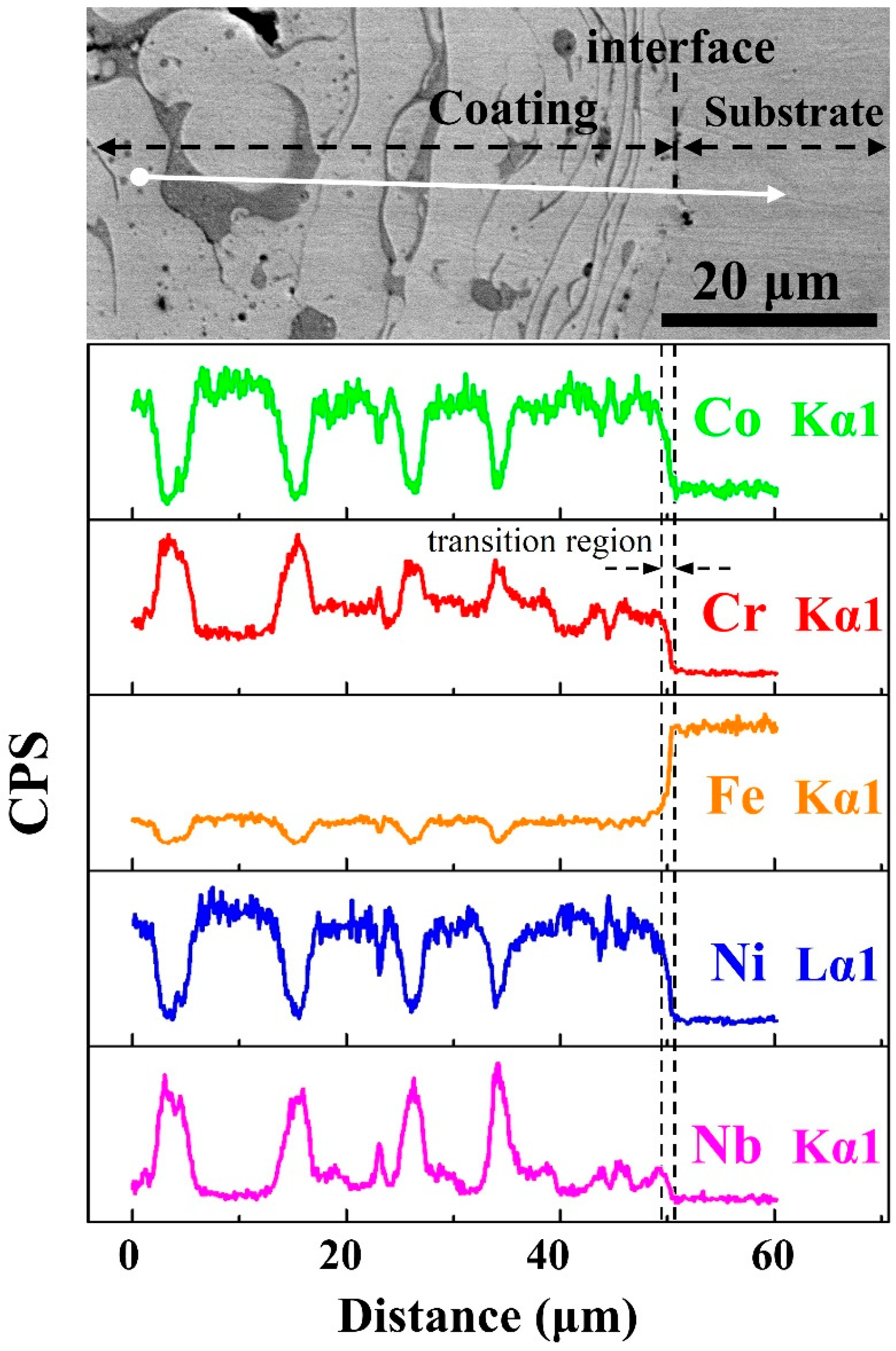
In order to analyze the elemental partitioning behavior across the coating-substrate interface, the EDS line scan performs on the white line on
Figure 4a, and the distributions of different alloying elements are shown in
Figure 4b–f. Elemental composition does not sharply change at the interface; however, there is a distinct diffusion transition zone with a width of about 2 μm between coating and substrate, indicating that the HEA coating and Q235 steel substrate are combined by metallurgical bonding, and the heat-affected zone is narrow during plasma spraying process.
3.3. Microhardness of the (CoCrFeNi)95Nb5 HEA Coating
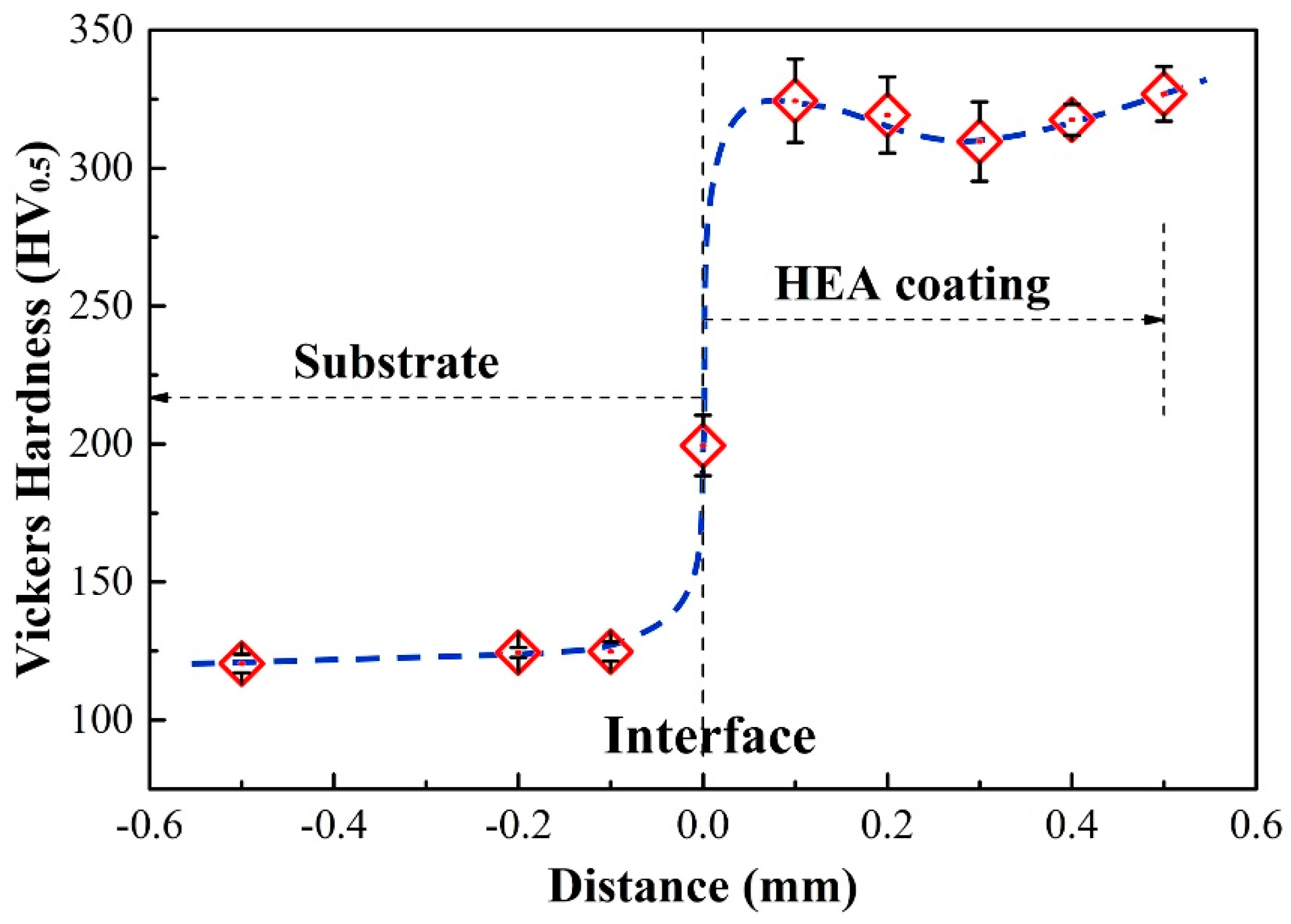
In order to investigate the mechanical behavior of HEAs coating, the microhardness varying from the substrate to the surface of the (CoCrFeNi)
95Nb
5 HEA coating is tested as shown in
Figure 5. The results show that the microhardness of the HEA coating, 310~325 HV0.5, is significantly higher than that of the Q235 steel substrate, ~122 HV0.5, and the hardness of the interface is between such two parts. The change trend of microhardness corresponds to that of microstructure and composition, as shown in
Figure 3 and
Figure 4. It means that HEAs coating with high microhardness, which is attributed to the solution strengthening caused by the high size mismatch between Nb with Co, Cr, Fe, and Ni, and the precipitation strengthening caused by the precipitation of the ordered Laves phase, can effectively improve the comprehensive mechanical property of the substrate.
3.4. Corrosion Performance of (CoCrFeNi)95Nb5 HEA Coating
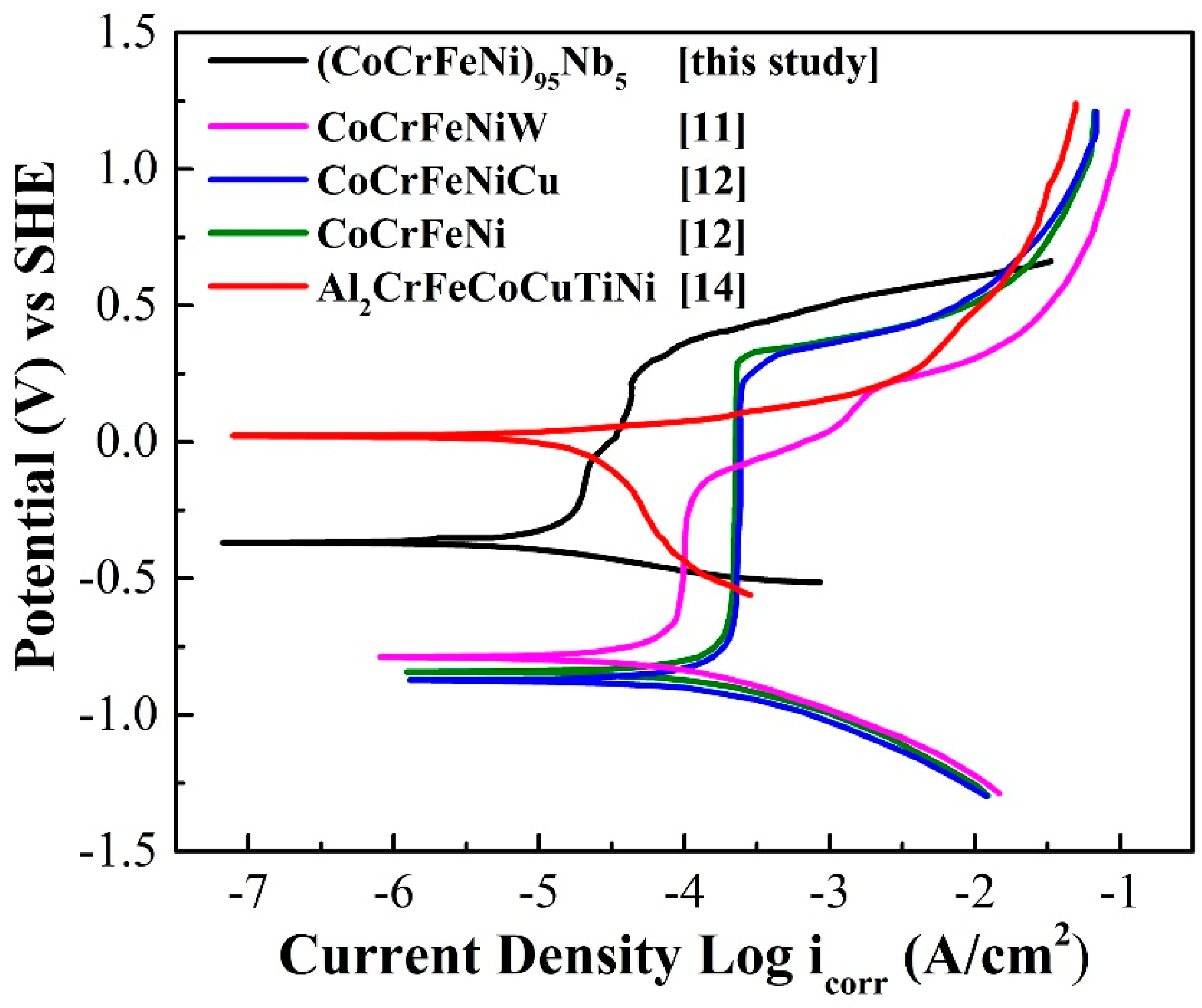
The polarization curve of (CoCrFeNi)
95Nb
5 HEA coating in 3.5 wt.% NaCl solution is shown in
Figure 6. For comparison,
Figure 6 also exhibits the polarization curves of other HEA coatings prepared by other processes in 3.5 wt.% NaCl solution, and the corrosion dynamics parameters of all HEA coatings obtained by linear fit are given in
Table 2. As can be seen, the (CoCrFeNi)
95Nb
5 HEA and Al
2CrFeCoCuTiNi HEA coatings have higher corrosion potential than other coatings, and the (CoCrFeNi)
95Nb
5 HEA coating also possesses the lowest corrosion current density, indicating that the (CoCrFeNi)
95Nb
5 HEA coating has excellent comprehensive corrosion resistance in 3.5 wt.% NaCl solution. Furthermore, although the pitting potential of (CoCrFeNi)
95Nb
5 HEA coating is similar to that of other CoCrFeNi-based coating, the lower passivation current density of (CoCrFeNi)
95Nb
5 HEA coating means the better pitting resistance.
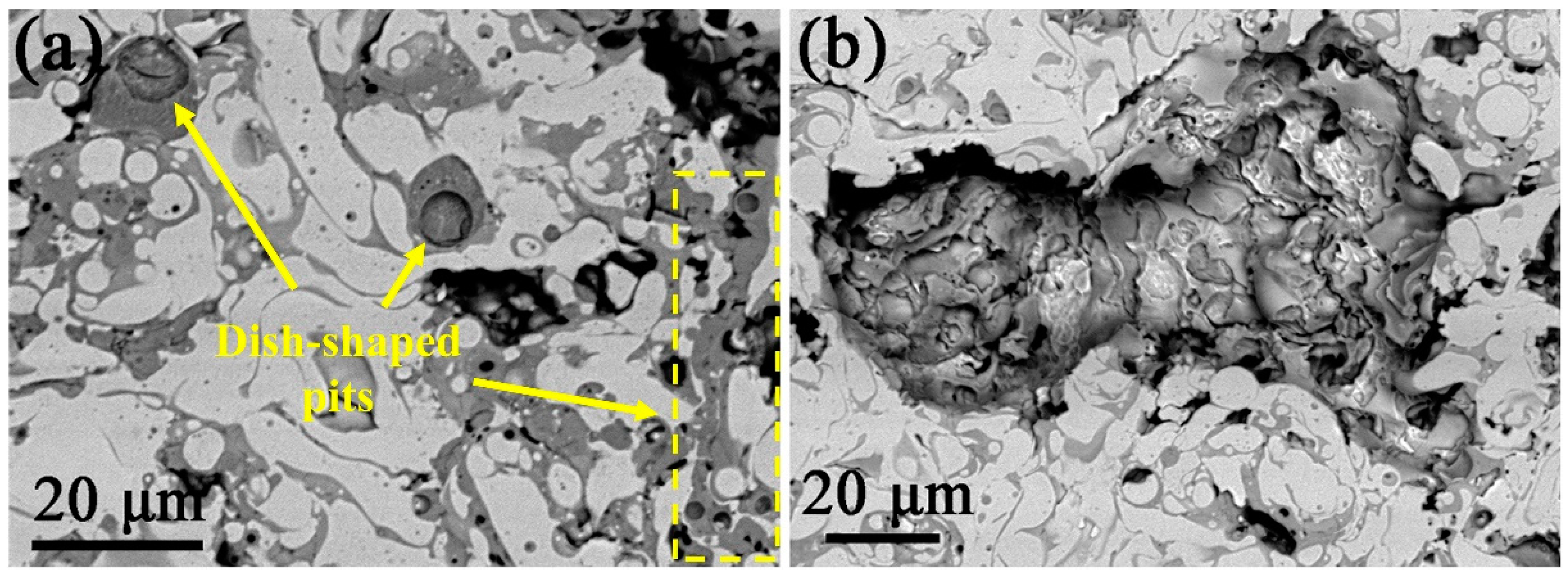
Figure 7 shows the SEM images of the surface of the (CoCrFeNi)
95Nb
5 HEA coating after electrochemical experiment in 3.5 wt.% NaCl solution. Obviously, a large number of tiny dish-shaped pitting pits with diameter of 1~5 μm are observed on the surface of the coating (
Figure 7a), and such pitting pits are only distributed in gray phase region.
Figure 7b presents a large corrosion pit where many secondary pitting holes are formed on the inner wall of the pit. Furthermore, those pitting holes are mainly displayed in the interdendritic region; however, the dendritic phase is undamaged, indicating that selective corrosion occurs on the surface of the HEA coating. According to the foregoing EDS results (listed in
Table 1), it can be inferred that in the 3.5 wt.% NaCl solution, the interdendritic phase rich in Nb and Cr is more easily corroded, while the dendritic phase rich in Co, Ni, and Fe is less susceptible to corrosion.
4. Discussion
4.1. Phase Formation
According to the phase analysis results obtained above, it was found that, although having the same chemical composition, the phase composition of the sample changed significantly at different preparation stages. That is, the ordered Laves phase is suppressed in (CoCrFeNi)95Nb5 HEA powder, and only a single FCC solid solution phase is formed. However, in the coating, the thermodynamically stable ordered Laves phase could precipitate out of the FCC solid solution matrix during plasma spraying process. This indicates that in addition to thermodynamic factors, kinetic factors play a very important role in the process of phase formation.
The results of research on as-cast CoCrFeNiNb
x alloys have proven that Nb tends to form ordered phases with other elements because of negative mixing enthalpy, and with increment of Nb, the proportion of Laves phase increases in the alloy [
17,
18]. However, the effect of mixing enthalpy on phase formation during coating preparation is not significant. The mixing enthalpy between the elements of the high-entropy alloy is shown in
Table 3. The mixed enthalpy of Nb and Cr is larger than the other three elements, indicating that Nb has a relatively weak binding force with Cr; however, the actual situation is that Nb and Cr are enriched in the interdendritic phase. This phenomenon may be due to the difference in melting point of the element and the characteristics of the plasma spraying process. The melting points of Cr and Nb are 2133 K and 2750 K, respectively, significantly higher than Co (1770 K), Fe (1811 K) and Ni (1728 K). Due to the rapid solidification of plasma spraying, the Cr and Nb-rich phases with high melting point have solidified before they converted into stable phases, which leads to the enrichment of Cr and Nb elements in the interdendritic phase. In addition, a small amount of the Co- and Fe-saturated precipitates formed in the interdendritic region as shown in the new phase in
Figure 3d, which are rich in Co, Fe and Nb, and have better thermodynamic stability than interdendritic phase. This indicates that the interdendritic phase tends to shift to a more stable morphology.
4.2. Corrosion Resistance
Electrochemical experiments have demonstrated that the (CoCrFeNi)
95Nb
5 HEA coating exhibits excellent corrosion resistance, and that the passivation film of the coating may play an important role. To prove this guess, chemical compositions of the passive film formed on the surface of the (CoCrFeNi)
95Nb
5 HEA coating after air oxidation were investigated by XPS.
Figure 8 shows the XPS spectra of the passivation film on the surface of HEA coating in air. The full survey spectrum (
Figure 8a) exhibits that the passivation film contains seven elements of Co, Cr, Fe, Ni, Nb, O, and C [
19]. The peaks of Ni 2p correspond to Ni and NiO (
Figure 8b). The Co 2p peaks are determined as Co and CoO (
Figure 8c). The peaks of Fe 2p are identified as Fe and Fe
2O
3 (
Figure 7d). The Cr 2p peaks correspond to Cr
2O
3 (
Figure 8e). Lastly, Nb
2O
5 (
Figure 8f) is observed in the peaks of Nb 3d.
Early research reported that stainless steel, Fe-Cr alloy, and CrFe
15Ni
15 alloy have excellent corrosion resistance due to the dense Cr
2O
3 passivation film formed on the surface [
20,
21,
22]. Similarly, it was speculated that the Cr
2O
3 passivation film has the same effect in the corrosion resistance of HEAs. AlCoCrFeNi [
23], Al
xCrFe
1.5MnNi
0.5 [
24], and Al
0.5CoCrCuFeNiB
x [
25] verified the foregoing speculation. Therefore, for the (CoCrFeNi)
95Nb
5 HEA coating passivation film of this study, it can be obtained from the results of XPS that the Cr
2O
3 in the passivation film provides good corrosion resistance for the coating, and the addition of trace amount of Nb results in forming a stable metal oxide of Nb
2O
5 in the passivation film of the coating, which also plays a positive role in the corrosion resistance [
26,
27].
Nevertheless, although the passivation film can improve the corrosion resistance, the (CoCrFeNi)
95Nb
5 HEA coating will inevitably be subjected to some local corrosion as with other corrosion-resistant alloys. Owing to the more negative the potential of the metal standard electrode, the easier it is to lose electrons and dissolve. Moreover, for the alloy of this paper, the standard potential of Nb (−1.1 V) and Cr (−0.74 V) is significantly lower than that of Fe (−0.44 V), Ni (−0.25 V) and Co (−0.28 V) [
28,
29]. Therefore, during the electrochemical experiment, once the local passivation film is destroyed, the interdendritic phase rich in Nb and Cr is preferentially corroded as an anode, and the dendritic phase rich in Co, Ni, and Fe is protected as a cathode, which results in selective corrosion on the surface of the (CoCrFeNi)
95Nb
5 HEA coating. Furthermore, as the pitting behavior continues, the adjacent interdendritic regions are completely corroded, causing catastrophic corrosion (
Figure 7b). Similarly, this phenomenon of selective corrosion was observed in CrMnFeCoNi HEA [
30] and AlCoCrFeNi [
31].
5. Conclusions
(1) The (CoCrFeNi)95Nb5 HEA coating was prepared by plasma spraying on substrate of the Q235 steel, consisting of a simple FCC solid solution with Laves phase, and the coating bonded well to the substrate.
(2) Elemental segregation occurred between the dendrites and the interdendrites of the coating, and the elements Cr and Nb with high melting point were enriched between the interdendrites. This phenomenon may be due to the difference in melting point of the element and the characteristics of the plasma spraying process.
(3) The coating has a microhardness of 321 HV0.5, which can effectively improve the surface hardness of the Q235 steel part.
(4) The (CoCrFeNi)95Nb5 HEA coating exhibited excellent corrosion resistance in the electrochemical corrosion test of a 3.5 wt.% NaCl solution comparing to previously studied coatings prepared by other processes.
(5) In the 3.5 wt.% NaCl solution, the selective corrosion occurred on the surface of (CoCrFeNi)95Nb5 HEA coating, while the interdendritic phase rich in Nb and Cr was preferentially corroded as an anode, and the dendritic phase rich in Co, Ni, and Fe was protected as a cathode.
Author Contributions
Conceptualization, W.W.; methodology, W.Q. and X.Y.; software, L.X. and W.Q.; formal analysis, W.W., X.Y. and W.Q.; resources, W.W. and L.X.; writing—original draft preparation, W.Q.; writing—review and editing, W.Q., J.L. and Y.Z.
Funding
This work was supported by the National Natural Science Foundation of China (51702332 and 21703007), Key Laboratory of Cryogenics, TIPC, CAS (CRYOQN201705).
Acknowledgments
The authors would like to thank the project team assistance with funding. Thanks to the tutor’s patience and thorough guidance, as well as the help of Zhao Li and Jieqian Wang in the experimental stage, and Shuying Chen’s guidance in English grammar.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tong, C.J.; Chen, M.R.; Yeh, J.W.; Lin, S.J.; Chen, S.K.; Shun, T.T.; Chang, S.Y. Mechanical performance of the AlxCoCrCuFeNi high-entropy alloy system with multiprincipal elements. Metall. Mater. Trans. A 2005, 36, 1263–1271. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Wang, Y.; Chen, G. Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl. Phys. Lett. 2007, 90, 181904. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P. Alloy design and properties optimization of high-entropy alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Liu, N.; Chen, C.; Chang, I.; Zhou, P.; Wang, X. Compositional dependence of phase selection in CoCrCu0.1FeMoNi-based high-entropy alloys. Materials 2018, 11, 1290. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Su, K.; Zhang, J.; Liang, X.; Peng, H.; Li, X. Enhanced strength of a mechanical alloyed NbMoTaWVTi refractory high entropy alloy. Materials 2018, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, W.; Wang, H.; Wu, Y.; Liu, X.; Nieh, T.; Lu, Z. Effects of Al addition on structural evolution and tensile properties of the FeCoNiCrMn high-entropy alloy system. Acta Mater. 2014, 62, 105–113. [Google Scholar] [CrossRef]
- Liu, W.; He, J.; Huang, H.; Wang, H.; Lu, Z.; Liu, C. Effects of Nb additions on the microstructure and mechanical property of CoCrFeNi high-entropy alloys. Intermetallics 2015, 60, 1–8. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, Q. MoFeCrTiWAlNb refractory high-entropy alloy coating fabricated by rectangular-spot laser cladding. Intermetallics 2018, 102, 78–87. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.L.; Zhang, C.H. Phase evolution characteristics of FeCoCrAlCuVxNi high entropy alloy coatings by laser high-entropy alloying. Mater. Lett. 2015, 141, 7–9. [Google Scholar] [CrossRef]
- Chen, X.; Yan, L.; Karnati, S.; Zhang, Y.; Liou, F. Fabrication and characterization of AlxCoFeNiCu1−x high entropy alloys by laser metal deposition. Coatings 2017, 7, 47. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Sun, J.; Li, X.; Li, P.; Du, J.; Qiao, P.; Wang, Y. CoCrFeNi(W1−xMox) high-entropy alloy coatings with excellent mechanical properties and corrosion resistance prepared by mechanical alloying and hot pressing sintering. Mater. Des. 2017, 117, 193–202. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Ge, W.; Zhang, Z.; Wang, Y. High-entropy alloy coatings with excellent mechanical, corrosion resistance and magnetic properties prepared by mechanical alloying and hot pressing sintering. Surf. Interfaces 2017, 9, 36–43. [Google Scholar] [CrossRef]
- Jiang, H.; Jiang, L.; Qiao, D.; Lu, Y.; Wang, T.; Cao, Z.; Li, T. Effect of Niobium on microstructure and properties of the CoCrFeNbxNi high entropy alloys. J. Mater. Sci. Technol. 2017, 33, 712–717. [Google Scholar] [CrossRef]
- Qiu, X.W.; Liu, C.G. Microstructure and properties of Al2CrFeCoCuTiNix high-entropy alloys prepared by laser cladding. J. Alloys Compd. 2013, 553, 216–220. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of bulk metallic glasses by atomic size difference, heat of mixing and period of constituent elements and its application to characterization of the main alloying element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Huo, W.; Zhou, H.; Fang, F.; Xie, Z.; Jiang, J. Microstructure and mechanical properties of CoCrFeNiZrx eutectic high-entropy alloys. Mater. Des. 2017, 134, 226–233. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sool, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Olefjord, I.; Brox, B.; Froment, M. Passivity of Metals and Semiconductors; Elsevier Science Publishers: Amsterdam, The Netherlands, 1983; p. 561. [Google Scholar]
- Olefjord, I.; Brox, B. Quantitative ESCA analysis of the passive state of an Fe-Cr alloy and an Fe-Cr-Mo alloy. In Passivity of Metals and Semiconductors; Elsevier: Amsterdam, The Netherlands, 1983; pp. 561–570. [Google Scholar]
- Zhang, B.; Wang, J.; Wu, B.; Guo, X.; Wang, Y.; Chen, D.; Zhang, Y.; Du, K.; Oguzie, E.; Ma, X. Unmasking chloride attack on the passive film of metals. Nat. Commun. 2018, 9, 2559. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.H.; Yue, T.M.; Guo, Z.N.; Lin, X. Microstructure and corrosion properties of AlCoCrFeNi high entropy alloy coatings deposited on AISI 1045 steel by the electrospark process. Metall. Mater. Trans. A 2013, 44, 1767–1778. [Google Scholar] [CrossRef]
- Lee, C.; Chen, Y.; Hsu, C.; Yeh, J.; Shih, H. Enhancing pitting corrosion resistance of AlxCrFe1.5MnNi0.5 high-entropy alloys by anodic treatment in sulfuric acid. Thin Solid Films 2008, 517, 1301–1305. [Google Scholar] [CrossRef]
- Lee, C.; Chen, Y.; Hsu, C.; Yeh, J.; Shih, H. The effect of boron on the corrosion resistance of the high entropy alloys Al0.5CoCrCuFeNiBx. J. Electrochem. Soc. 2007, 154, C424–C430. [Google Scholar] [CrossRef]
- Souza, C.A.C.; de Oliveira, M.F.; May, J.E.; Mariano, N.A.; Kuri, S.E.; Kiminami, C.S. Corrosion resistance of amorphous and nanocrystalline Fe–M–B (M = Zr, Nb) alloys. J. Non-Cryst. Solids 2000, 273, 282–288. [Google Scholar] [CrossRef]
- Kiminami, C.S.; Souza, C.A.C.; Bonavina, L.F.; de Andrade Lima, L.R.P.; Suriñach, S.; Baró, M.D.; Bolfarini, C.; Botta, W.J. Partial crystallization and corrosion resistance of amorphous Fe-Cr-M-B (M=Mo, Nb) alloys. J. Non-Cryst. Solids 2010, 356, 2651–2657. [Google Scholar] [CrossRef]
- Paunović, M.; Schlesinger, M. Fundamentals of Electrochemical Deposition; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Bard, A.J.; Faulkner, L.R.; Leddy, J.; Zoski, C.G. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Kao, Y.F.; Lee, T.D.; Chen, S.K.; Chang, Y.S. Electrochemical passive properties of AlxCoCrFeNi (x = 0, 0.25, 0.50, 1.00) alloys in sulfuric acids. Corros. Sci. 2010, 52, 1026–1034. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).