Release of Cationic Drugs from Charcoal
Abstract
1. Introduction
2. Experimental
2.1. Materials, Oxidation Procedures and Preparation of Compounds
2.2. Characterization Methods
3. Results and Discussion
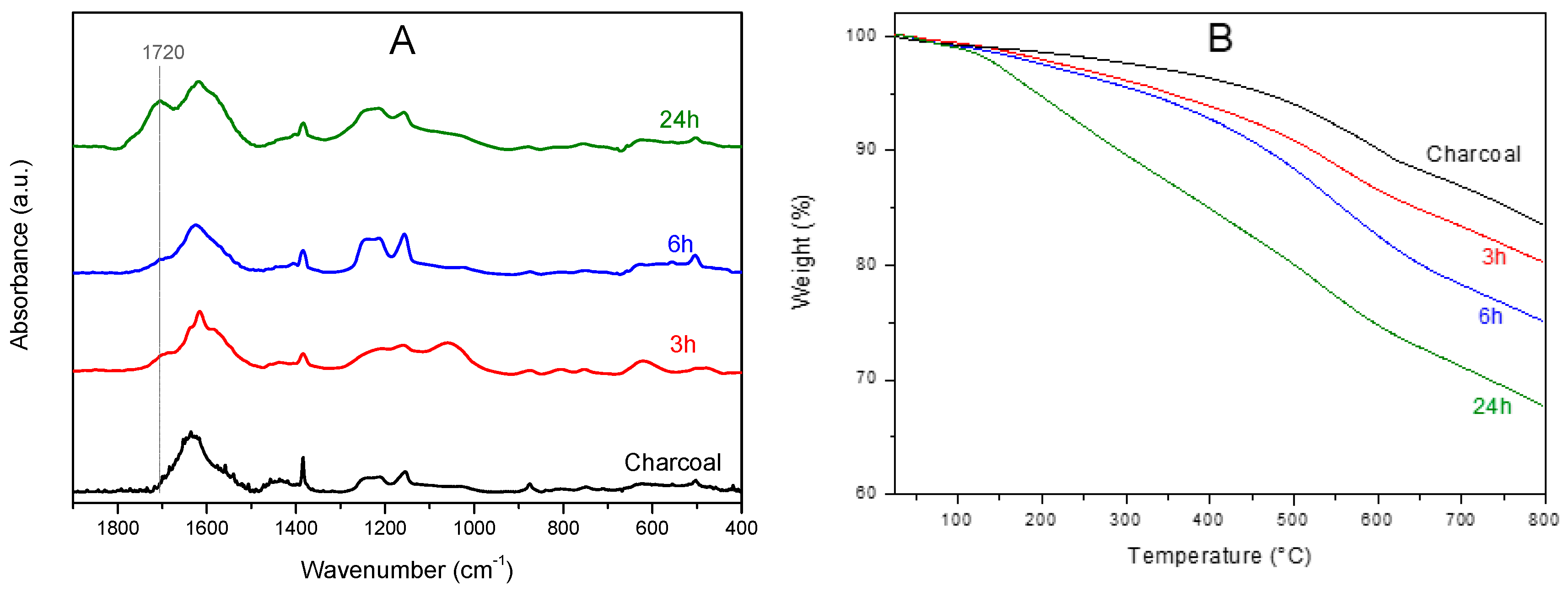
3.1. Oxidation of Charcoal by Hydrogen Peroxide
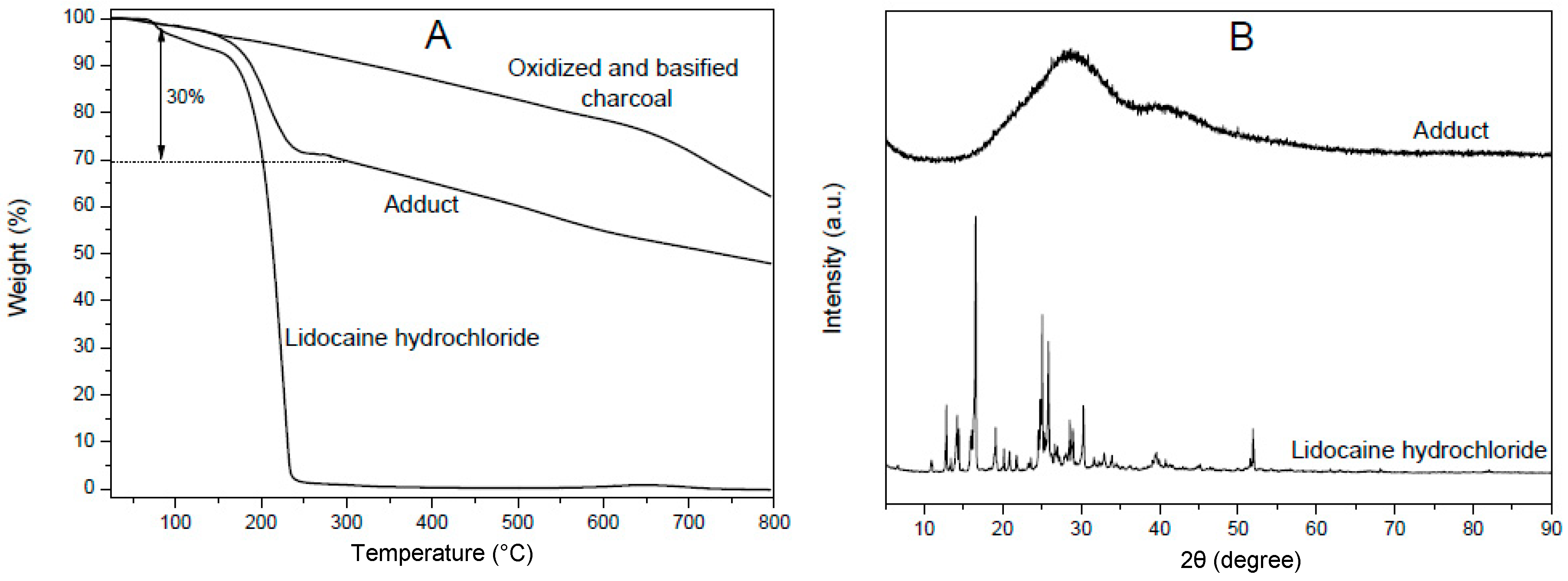
3.2. Formation of Adducts between Oxidized Charcoal and Organic Cations
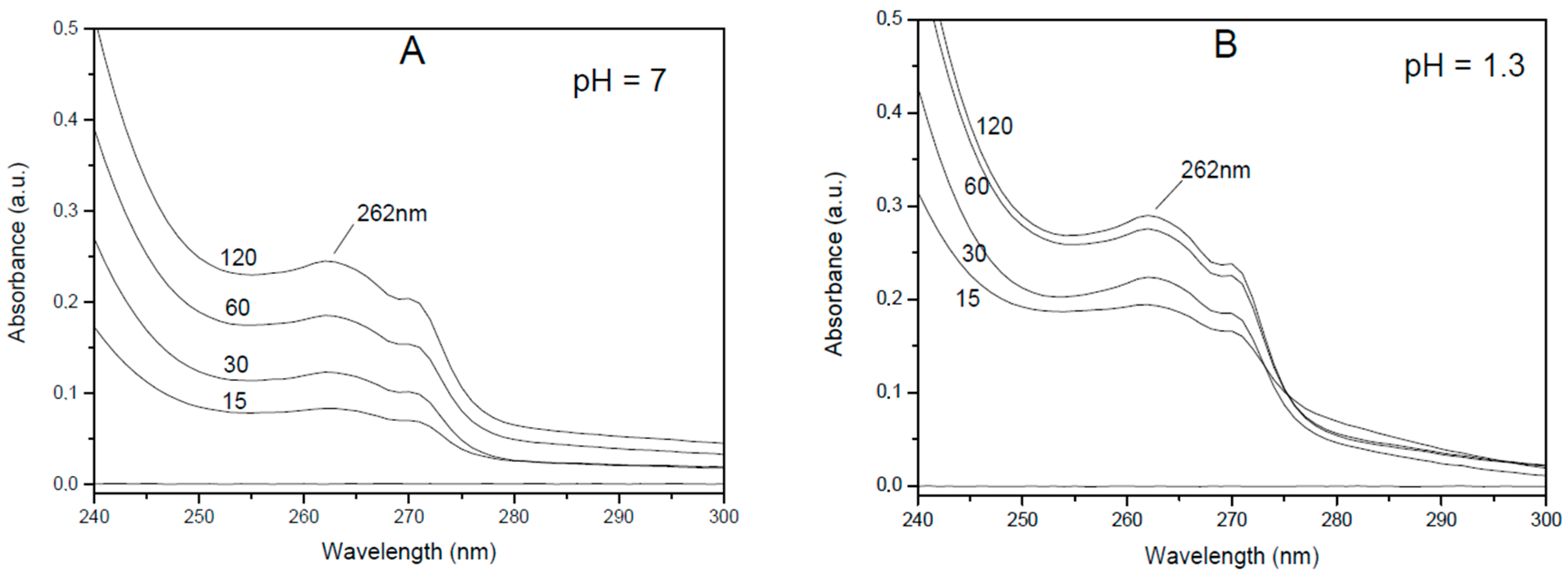
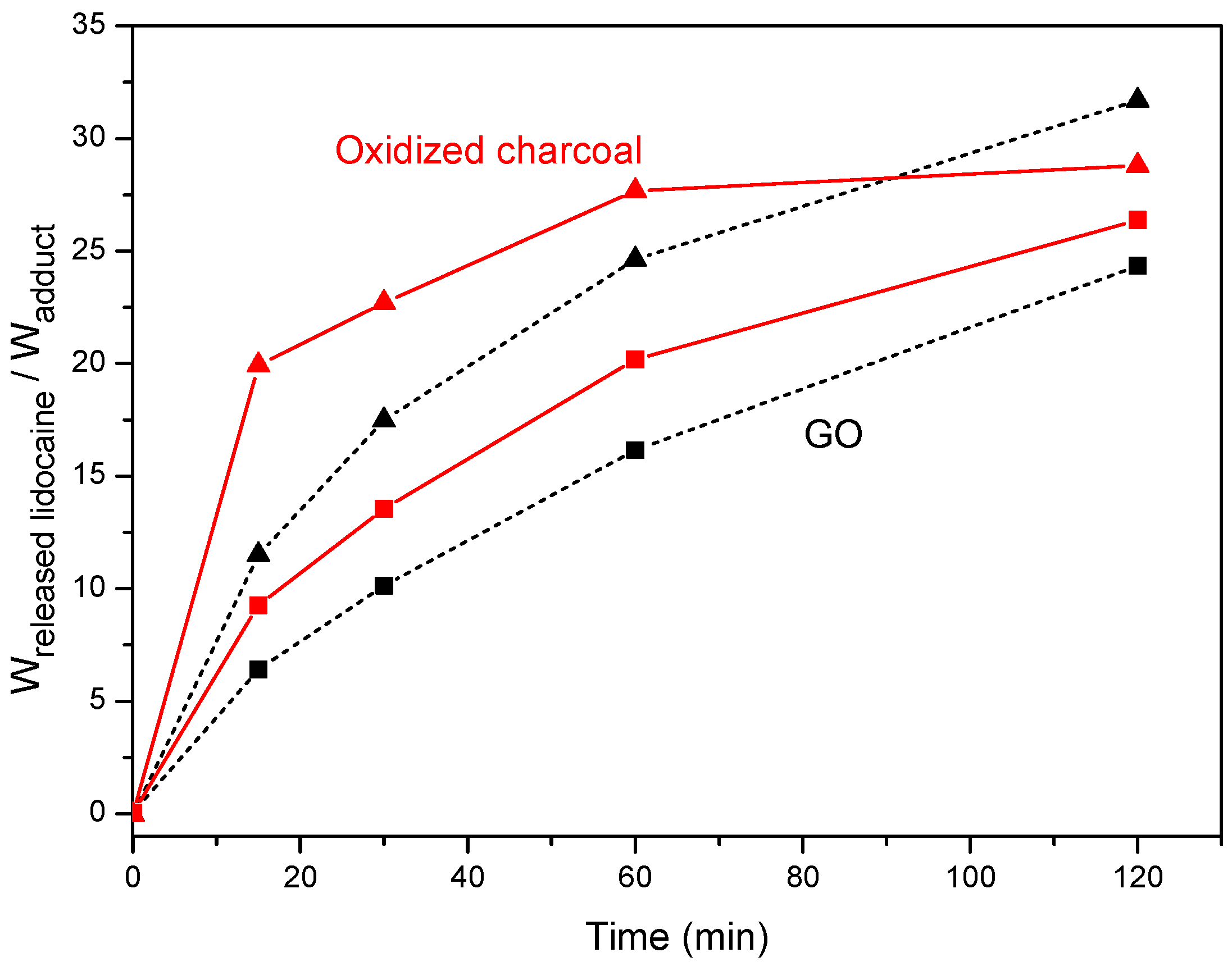
3.3. Lidocaine Release from Adducts with Oxidized Charcoal and Graphite Oxide
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Severina, I.I.; Muntyan, M.S.; Lewis, K.; Skulachev, V.P. Transfer of cationic antibacterial agents berberine, palmatine, and benzalkonium through bimolecular planar phospholipid film and staphylococcus aureus membrane. IUBMB Life 2001, 52, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.B.; Li, C.; Taotafa, U.; Ding, B.; Guan, Q. Antibacterial Properties of cationic steroid antibiotics. FEMS Microbiol. Lett. 2002, 217, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Strom, M.B.; Haug, B.E.; Skar, M.L.; Stensen, W.; Stiberg, T.; Svendsen, J.S. The Pharmacophore of short cationic antibacterial peptides. J. Med. Chem. 2003, 46, 1567–1570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Yu, J.C.; Yip, H.Y.; Li, Q.; Kwong, K.W.; Xu, W.; Wong, P.K. Ambient light reduction strategy to synthesize silver nanoparticles and silver-coated TiO2 with enhanced photocatalytic and bactericidal activities. Langmuir 2003, 19, 10372–10380. [Google Scholar] [CrossRef]
- Ohashi, F.; Ueda, S.; Taguri, T.; Kawachi, S.; Abe, H. Antimicrobial activity and thermostability of silver 6-benzylaminopurine montmorillonite. Appl. Clay Sci. 2009, 46, 296–299. [Google Scholar] [CrossRef]
- Findlay, B.; Zhanel, G.G.; Schweizer, F. Cationic amphiphiles, a new generation of antimicrobials inspired by the natural antimicrobial peptide scaffold. Antimicrob. Agents Chemother. 2010, 54, 4049–4058. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable cationic antibacterial amphiphiles: Synthesis, mechanism of action, and cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef] [PubMed]
- van der Heyden, M.A.; Stary-Weinzinger, A.; Sanchez-Chapula, J.A. Inhibition of cardiac inward rectifier currents by cationic amphiphilic drugs. Curr. Mol. Med. 2013, 13, 1284–1298. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Yasir, M.; Berry, T.; Cranfield, G.C.; Nizalapur, S.; Yee, E.; Kimyon, O.; Taunk, A.; Ho, K.K.K.; Cornell, B.; et al. Design and synthesis of short amphiphilic cationic peptidomimetics based on biphenyl backbone as antibacterial agents. Eur. J. Med. Chem. 2018, 143, 1702–1722. [Google Scholar] [CrossRef] [PubMed]
- Walvekar, P.; Gannimani, R.; Rambharose, S.; Mocktar, C.; Govender, T. Fatty acid conjugated pyridinium cationic amphiphiles as antibacterial agents and self-assembling nano carriers. Chem. Phys. Lipids 2018, 214, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dailey, J.W. Pharmaceutical Industry; Encyclopædia Britannica, Inc.: Chicago, IL, USA, 2018. [Google Scholar]
- Tan, J.P.K.; Goh, C.H.; Tam, K.C. Comparative drug release studies of two cationic drugs from pH-responsive nanogels. Eur. J. Pharm. Sci. 2007, 32, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Herrera, P.; Burghardt, R.C.; Phillips, T.D. Adsorption of salmonella enteritidis by cetylpyridinium-exchanged montmorillonite clays. Vet. Microbiol. 2000, 74, 259–272. [Google Scholar] [CrossRef]
- Cai, X.; Tan, S.Z.; Liao, M.-H.; Wu, T.; Liu, R.F.; Yu, B. Thermal stability and long-acting antibacterial activity of phosphonium montmorillonites. J. Cent. South. Univ. Technol. 2010, 17, 485–491. [Google Scholar] [CrossRef]
- Galimberti, M.; Giudice, S.; Cipolletti, V.; Guerra, G. Control of organoclay structure in hydrocarbon polymers. Polym. Adv. Technol. 2010, 21, 679–684. [Google Scholar] [CrossRef]
- Cipolletti, V.; Galimberti, M.; Mauro, M.; Guerra, G. Organoclays with hexagonal rotator order for the paraffinic chains of the compensating cation. Implications on the structure of clay polymer nanocomposites. Appl. Clay Sci. 2014, 87, 179–188. [Google Scholar] [CrossRef]
- Ramorino, G.; Bignotti, F.; Pandini, S.; Ricco, T. Mechanical reinforcement in naturalrubber/organoclay nanocomposites. Compos. Sci. Technol. 2009, 69, 1206–1211. [Google Scholar] [CrossRef]
- Rhim, J.W.; Hong, S.I.; Ha, C.S. Tensile, water vapor barrier and antimicrobial properties of PLA/nanoclay composite films. LWT-Food Sci. Technol. 2009, 42, 612–617. [Google Scholar] [CrossRef]
- Cai, X.; Tan, S.; Lin, M.; Xie, A.; Mai, W.; Zhang, X.; Lin, Z.; Wu, T.; Liu, Y. Synergistic antibacterial brilliant blue/reduced graphene oxide/quaternary phosphonium salt composite with excellent water solubility and specific targeting capability. Langmuir 2011, 27, 7828–7835. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.-G.; Cai, X.; Lin, M.-S.; Wu, T.; Zhang, X.-J.; Lin, Z.-D.; Tan, S. Long-acting antibacterial activity of quaternary phosphonium salts functionalized few-layered graphite. Mater. Sci. Eng. B 2011, 176, 1222–1226. [Google Scholar] [CrossRef]
- Mauro, M.; Maggio, M.; Cipolletti, V.; Galimberti, M.; Longo, P.; Guerra, G. Graphite oxide intercalation compounds with rotator hexagonal order in the intercalated layers. Carbon 2013, 61, 395–403. [Google Scholar] [CrossRef]
- Mauro, M.; Maggio, M.; Antonelli, A.; Acocella, M.R.; Guerra, G. Intercalation and exfoliation compounds of graphite oxide with quaternary phosphonium ions. Chem. Mater. 2015, 27, 1590–1596. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, X.; Yan, Y.; Chen, D.; Huang, L.; Zhang, J.; Ke, Y.; Tan, S. The utilization of a three-dimensional reduced graphene oxide and montmorillonite composite aerogel as a multifunctional agent for wastewater treatment. RSC Adv. 2018, 8, 4239–4248. [Google Scholar] [CrossRef]
- Maggio, M.; Acocella, M.R.; Guerra, G. Intercalation compounds of oxidized carbon black. RSC Adv. 2016, 6, 105565–105572. [Google Scholar] [CrossRef]
- Park, I.S.; Park, M.O.; Hur, J.W.; Kim, D.S.; Chang, Y.J.; Kim, Y.J.; Park, J.Y.; Johnson, S.C. Anesthetic effects of lidocaine-hydrochloride on water parameters in simulated transport experiment of juvenile winter flounder, Pleuronectes americanus. Aquaculture 2009, 294, 76–79. [Google Scholar] [CrossRef]
- Meng, Q.-T.; Xia, Z.-Y.; Liu, J.; Bayliss, D.A.; Chen, X. Local anesthetic inhibits hyperpolarization-activated cationic currents. Mol. Pharm. 2011, 79, 866–873. [Google Scholar] [CrossRef]
- Chen-Chow, P.-C.; Frank, S.G. In vitro release of lidocaine from pluronic F-127 gels. Int. J. Pharm. 1981, 8, 89–99. [Google Scholar] [CrossRef]
- Chen, P.-C.; Kohane, D.S.; Park, Y.J.; Bartlett, R.H.; Langer, R.; Yang, V.C. Injectable microparticle-gel system for prolonged and localized lidocaine release.II.In vivo anesthetic effects. J. Biomed. Mater. Res. A 2004, 70, 459–466. [Google Scholar] [CrossRef]
- Ricci, E.J.; Lunardi, L.O.; Nanclares, D.M.A.; Marchetti, J.M. Sustained release of lidocaine from Poloxamer 407 gels. Int. J. Pharm. 2005, 288, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-Z.; Sheu, M.-T.; Chen, C.-H.; Yang, Y.-R.; Ho, H.-O. Release characteristics of lidocaine from local implant of polyanionic and polycationic hydrogels. J. Control. Release 2007, 118, 333–339. [Google Scholar] [CrossRef]
- Sawant, P.D.; Luu, D.; Ye, R.; Buchta, R. Drug release from hydroethanolic gels. Effect of drug’s lipophilicity (log P), polymer-drug interactions and solvent lipophilicity. Int. J. Pharm. 2010, 396, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mueller-Goymann, C.C.; Frank, S.G. Interaction of lidocaine and lidocaine hydrochloride with the liquid crystal structure of topical preparations. Int. J. Pharm. 1986, 29, 147–159. [Google Scholar] [CrossRef]
- Perale, G.; Casalini, T.; Barri, V.; Mueller, M.; Maccagnan, S.; Masi, M.J. Lidocaine release from polycaprolactone threads. J. Appl. Polym. Sci. 2010, 117, 3610–3614. [Google Scholar] [CrossRef]
- Vittore, A.; Acocella, M.R.; Guerra, G. Edge oxidation of graphite by hydrogen peroxide. Langmuir 2019, 35, 2244–2250. [Google Scholar] [CrossRef] [PubMed]
- Hummers, S.W.R.; Offeman, E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Mauro, M.; Cipolletti, V.; Galimberti, M.; Longo, P.; Guerra, G. Chemically reduced graphite oxide with improved shape anisotropy. J. Phys. Chem. C 2012, 116, 24809–24813. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Hamidi, S.; Moradi, Y.; Barzegar, S.; Ahmadi, M.; Naebe, M. A renewable bio-based epoxy resin with improved mechanical performance that can compete with DGEBA. RSC Adv. 2017, 7, 8694–8701. [Google Scholar] [CrossRef]
- Canavate, J.; Colom, X.; Pages, P.; Carrasco, F. Study of the curing process of an epoxy resin by FTIR spectroscopy. Polym. Plast. Technol. Eng. 2000, 39, 937–943. [Google Scholar] [CrossRef]

| Charcoal | %N | %C | %H | %O | O/C | %H2O |
|---|---|---|---|---|---|---|
| Untreated | 0.3 | 74.8 | 4.2 | 20.7 | 0.29 | 4.0 |
| H2O2 oxidized, 3 h | 1.0 | 68.8 | 1.9 | 28.2 | 0.41 | 5.6 |
| H2O2 oxidized, 6 h | 1.0 | 67.4 | 1.8 | 29.8 | 0.44 | 6.1 |
| H2O2 oxidized, 24 h | 0.9 | 57.2 | 1.5 | 40.4 | 0.70 | 7.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Ruocco, C.; Acocella, M.R.; Guerra, G. Release of Cationic Drugs from Charcoal. Materials 2019, 12, 683. https://doi.org/10.3390/ma12040683
Di Ruocco C, Acocella MR, Guerra G. Release of Cationic Drugs from Charcoal. Materials. 2019; 12(4):683. https://doi.org/10.3390/ma12040683
Chicago/Turabian StyleDi Ruocco, Chiara, Maria Rosaria Acocella, and Gaetano Guerra. 2019. "Release of Cationic Drugs from Charcoal" Materials 12, no. 4: 683. https://doi.org/10.3390/ma12040683
APA StyleDi Ruocco, C., Acocella, M. R., & Guerra, G. (2019). Release of Cationic Drugs from Charcoal. Materials, 12(4), 683. https://doi.org/10.3390/ma12040683




