The Supramolecular Organogel Formed by Self-Assembly of Ursolic Acid Appended with Aromatic Rings
Abstract
1. Introduction
2. Materials and Methods
2.1. General Procedure
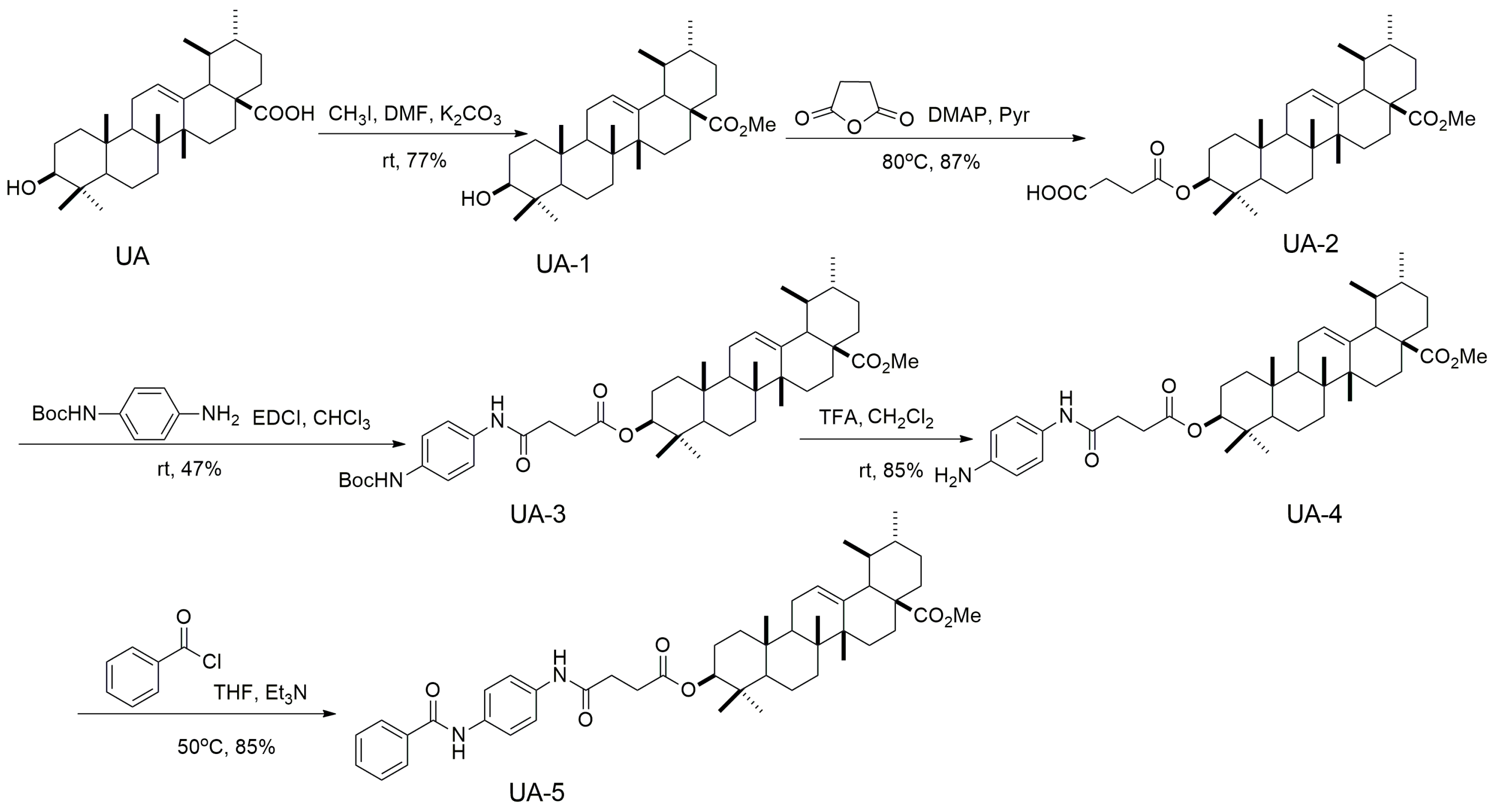
2.2. Synthesis
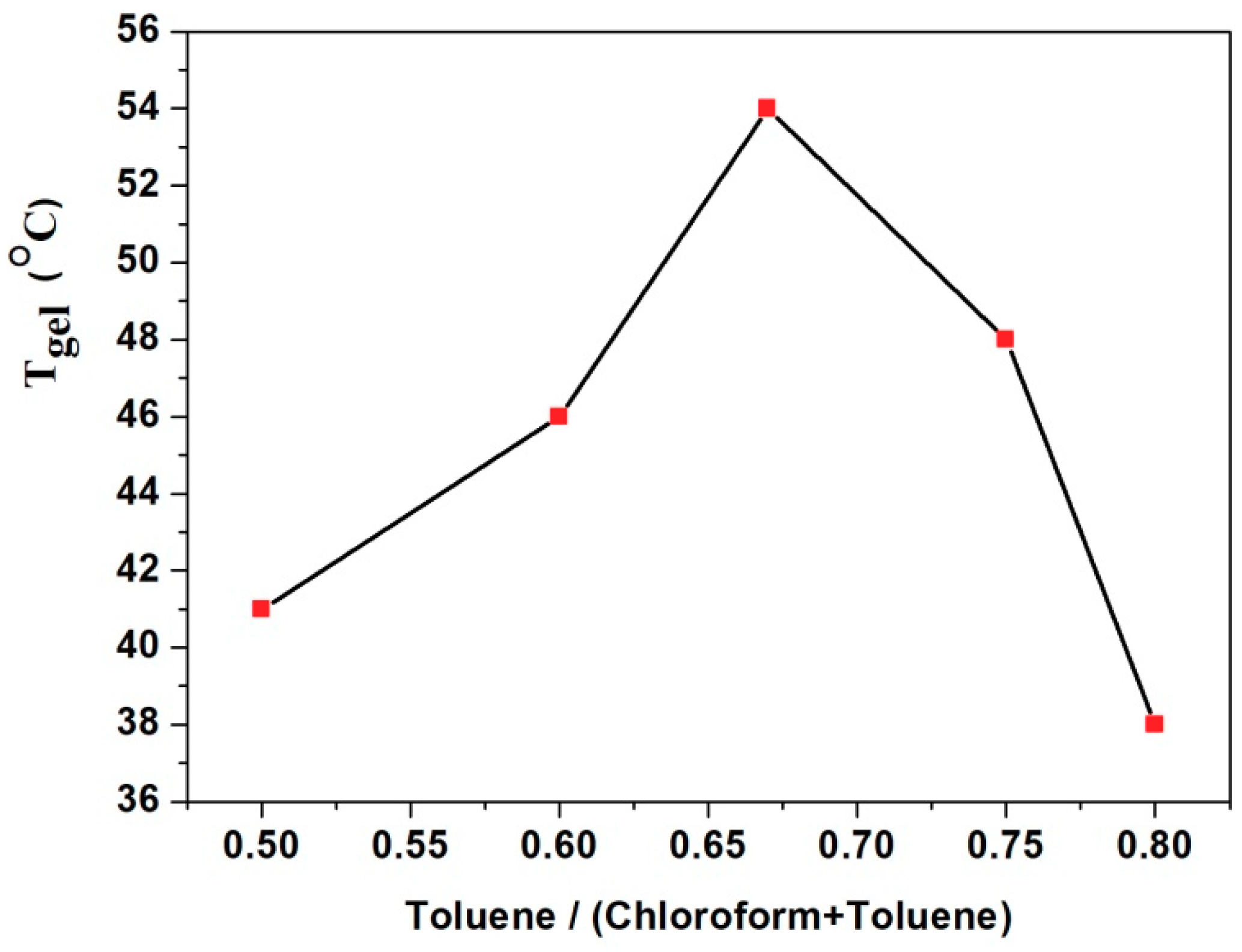
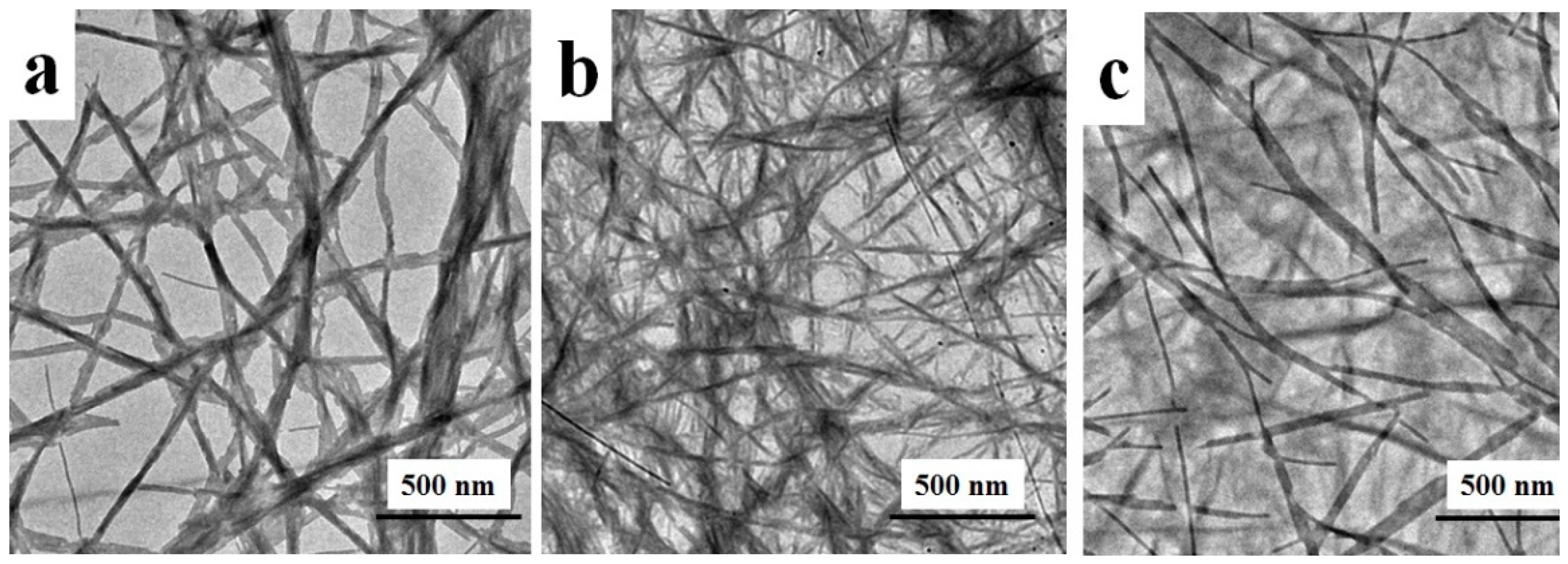
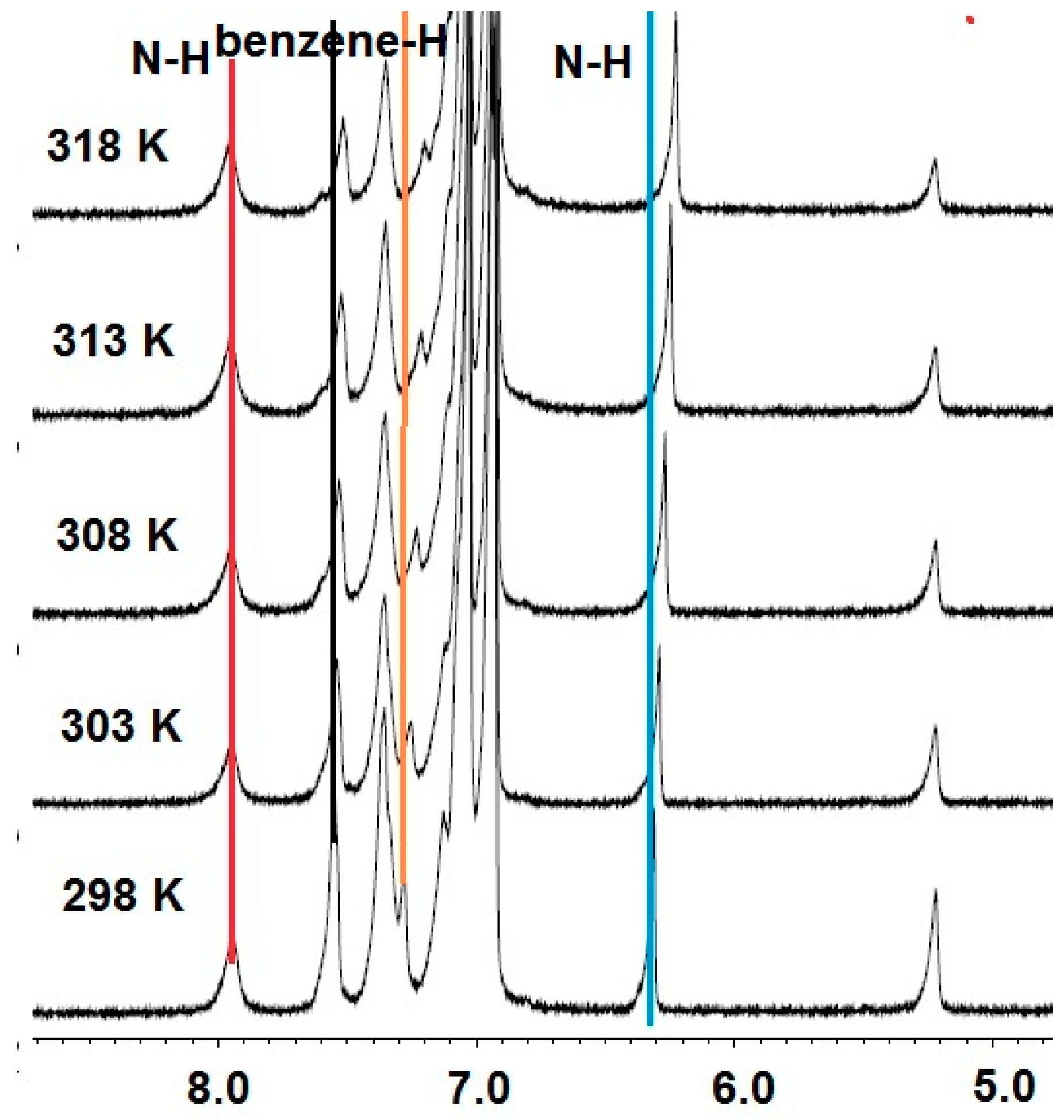
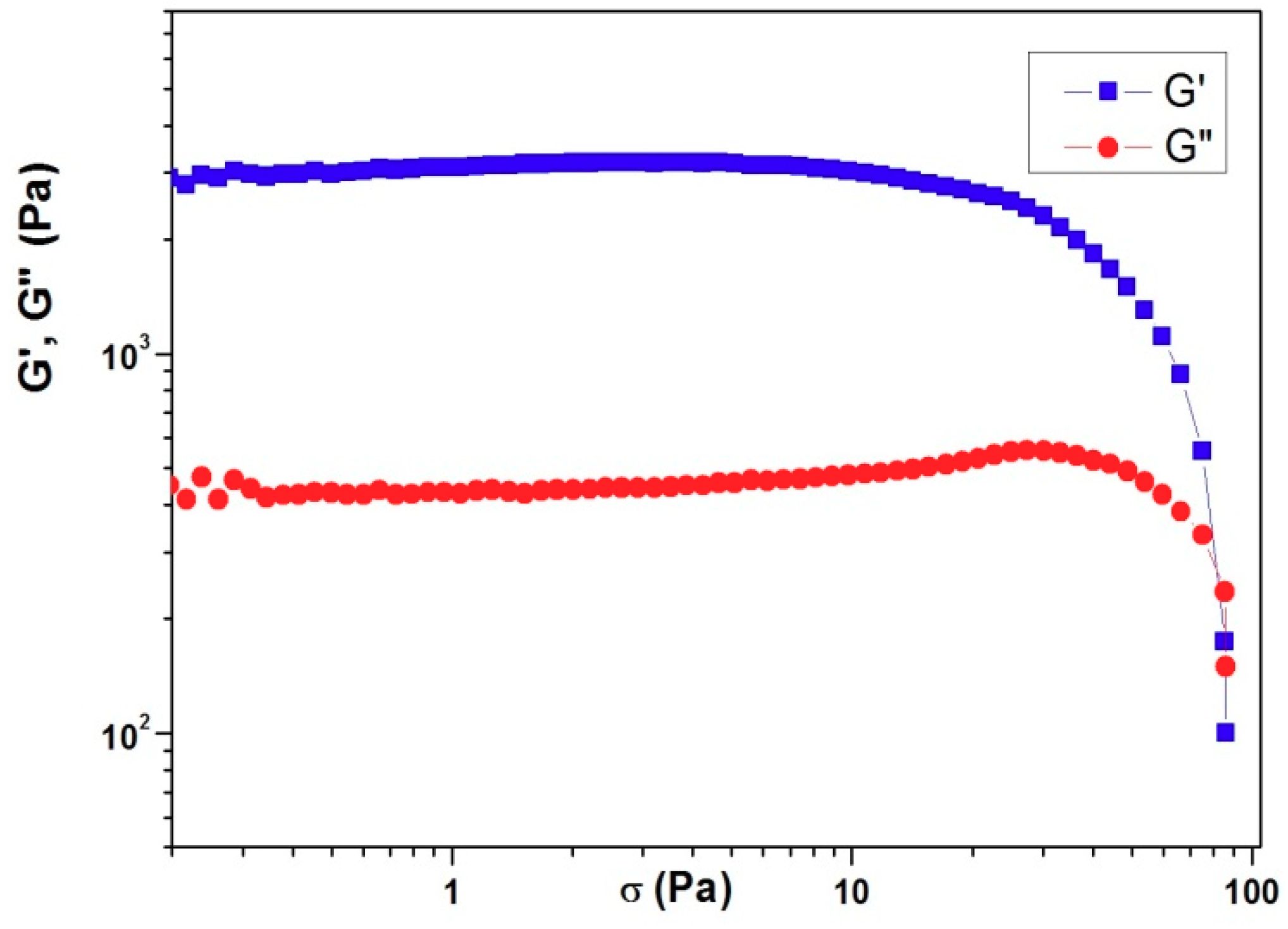
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steed, J.W. Supramolecular gel chemistry: Developments over the last decade. Chem. Commun. 2011, 47, 1379–1383. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.; Zhu, H. Recent Advance between Supramolecular Gels and Catalysis. Chem. Asian. J. 2018, 13, 712–729. [Google Scholar]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Jin, Q.X.; Lv, K.; Zhang, L.; Liu, M.H. Water tuned the helical nanostructures and supramolecular chirality in organogels. Chem. Commun. 2014, 50, 3702–3705. [Google Scholar] [CrossRef] [PubMed]
- Suthiwangcharoen, N.; Li, T.; Wu, L.; Reno, H.B.; Thompson, P.; Wang, Q. Facile co-assembly process to generate core-shell nanoparticles with functional protein corona. Biomacromolecules 2014, 15, 948–956. [Google Scholar] [CrossRef]
- Gao, Y.X.; Hu, J.; Ju, Y. Supramolecular Self-Assembly Based on Natural Small Molecules. Acta.Chim. Sinica. 2016, 74, 312–329. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Xu, B. Supramolecular hydrogels made of basic biological building blocks. Chem. Asian J. 2014, 9, 1446–1472. [Google Scholar] [CrossRef] [PubMed]
- Nelli, S.R.; Chakravarthy, R.D.; Mohiuddin, M.; Lin, H.C. The role of amino acids on supramolecular co-assembly of naphthalenediimide–pyrene based hydrogelators. RSC Adv. 2018, 8, 14753–14759. [Google Scholar] [CrossRef]
- Lu, J.; Ju, Y. Organogels Based on Natural Products. Chin. J. Org. Chem. 2013, 33, 469–482. [Google Scholar] [CrossRef]
- Bag, B.G.; Majumdar, R.; Laguerre, M. Natural triterpenoids as renewable nanos. Struct. Chem. 2012, 23, 393–398. [Google Scholar] [CrossRef]
- Bag, B.G.; Dinda, S.K.; Dey, P.P.; Mallia, V.A.; Weiss, R.G. Self-assembly of esters of arjunolic acid into fibrous networks and the properties of their organogels. Langmuir 2009, 25, 8663–8671. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Majumdar, R. Vesicular self-assembly of a natural triterpenoid arjunolic acid in aqueous medium: Study of entrapment properties and in situ generation of gel–gold nanoparticle hybrid material. RSC Adv. 2014, 4, 53327–53334. [Google Scholar] [CrossRef]
- Bag, B.G.; Majumdar, R. Self-assembly of a renewable nano-sized triterpenoid 18β-glycyrrhetinic acid. RSC Adv. 2012, 2, 8623–8626. [Google Scholar] [CrossRef]
- Gao, Y.; Hao, J.; Wu, J.; Hu, J.; Ju, Y. Cooperative supramolecular helical assembly of a pyridinium-tailored methyl glycyrrhetate. Soft Matter 2016, 12, 8979–8982. [Google Scholar] [CrossRef]
- Lu, J.R.; Hu, J.; Song, Y.; Ju, Y. A new dual-responsive organogel based on uracil-appended glycyrrhetinic acid. Org. Lett. 2011, 13, 3372–3375. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.R.; Gao, Y.X.; Wu, J.D.; Ju, Y. Organogels of triterpenoid–tripeptide conjugates: Encapsulation of dye molecules and basicity increase associated with aggregation. RSC Adv. 2013, 3, 23548–23552. [Google Scholar] [CrossRef]
- Lu, J.R.; Wu, J.D.; Ju, Y. Tuning the aggregation mode to induce different chiralities in organogels of mono- and bistriterpenoid derivatives and the preparation of gold nanoparticles for use as a template. New J. Chem. 2014, 38, 6050–6056. [Google Scholar] [CrossRef]
- Saha, A.; Adamcik, J.; Bolisetty, S.; Handschin, S.; Mezzenga, R. Fibrillar Networks of Glycyrrhizic Acid for Hybrid Nanomaterials withCatalytic Features. Angew. Chem. Int. Ed. 2015, 54, 5408–5412. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Dash, S.S. First self-assembly study of betulinic acid, a renewable nano-sized, 6-6-6-6-5 pentacyclic monohydroxy triterpenic acid. Nanoscale 2011, 3, 4564–4566. [Google Scholar] [CrossRef] [PubMed]
- Bag, B.G.; Dash, S.S. Hierarchical Self-Assembly of a Renewable Nanosized Pentacyclic Dihydroxy-triterpenoid Betulin Yielding Flower-Like Architectures. Langmuir 2015, 31, 13664–13672. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hao, J.; Wu, J.; Zhang, X.; Hu, J.; Ju, Y. Solvent-Directed Assembly of a Pyridinium-Tailored Methyl Oleanolate Amphiphile: Stepwise Growth of Microrods and Nanofibers. Langmuir 2016, 32, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.R.; Hu, J.; Liu, C.L.; Gao, H.X.; Ju, Y. Water-induced gel formation of an oleanlic acid–adenine conjugate and the effects of uracil derivative on the gel stability. Soft Matter 2012, 8, 9576–9580. [Google Scholar] [CrossRef]
- Lu, J.R.; Ju, Y. Supramolecular Gels Based on Natural Product-Triterpenoids. Process. Chem. 2016, 28, 260–268. [Google Scholar]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol. Nutr. Food. Res. 2010, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Dar, B.A.; Lone, A.M.; Shah, W.A.; Qurishi, M.A. Synthesis and screening of ursolic acid-benzylidine derivatives as potential anti-cancer agents. Eur. J. Med. Chem. 2016, 111, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, S.H.; Ma, B.L.; Wang, W.W.; Yu, B.Y.; Zhang, J. New derivatives of ursolic acid through the biotransformation by Bacillus megaterium CGMCC 1.1741 as inhibitors on nitric oxide production. Bioorg. Med. Chem. Lett. 2017, 27, 2575–2578. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.R.; Wu, X.N.; Chen, H.P.; Liang, Y.H. First Organogelation Study of Ursolic Acid, a Natural Ursane Triterpenoid. Chem. Lett. 2016, 45, 860–862. [Google Scholar]
- Ma, M.; Kuang, Y.; Gao, Y.; Zhang, Y.; Gao, P.; Xu, B. Aromatic-aromatic interactions induce the self-assembly of penta peptidic derivatives in water to form nanofibers and supramolecular hydrogels. J. Am. Chem. Soc. 2010, 132, 2719–2728. [Google Scholar] [CrossRef]
- Shin, S.; Lim, S.; Kim, Y.; Kim, T.; Choi, T.L.; Lee, M. Supramolecular Switching between Flat Sheets and Helical Tubules Triggered by Coordination Interaction. J. Am. Chem. Soc. 2013, 135, 2156–2159. [Google Scholar] [CrossRef]
- Velazquez, D.G.; Diaz, D.D.; Ravelo, A.G.; Marrero-Tellado, J.J. Hunter’s Oligoamide: A Functional C2-Symmetric Molecule with Unusual Topology for Selective Organic Gel Formation. Eur. J. Org. Chem. 2007, 2007, 1841–1845. [Google Scholar] [CrossRef]
- Niu, L.; Song, J.; Li, J.; Tai, N.; Lu, M.; Fan, K. Solvent effects on the gelation performance of melamine and 2-ethylhexylphosphoric acid mono-2-ethylhexyl ester in water–organic mixtures. Soft Matter 2013, 9, 7780–7786. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Wang, B.; Hao, J.; Hu, J.; Ju, Y. Natural Triterpenoid- and Oligo(Ethylene Glycol)-Pendant-Containing Block and Random Copolymers: Aggregation and pH-Controlled Release. Chem. Asian. J. 2018, 13, 2723–2729. [Google Scholar] [CrossRef] [PubMed]
- Kar, H.D.; Gehrig, W.; Laquai, F.; Ghosh, S. J-aggregation, its impact on excited state dynamics and unique solvent effects on macroscopic assembly of a core-substituted naphthalenediimide. Nanoscale 2015, 7, 6729–6736. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hao, J.; Yan, Q.; Du, F.; Ju, Y.; Hu, J. A Natural Triterpenoid-Tailored Phosphate: In Situ Reduction of Heavy Metals Spontaneously to Generate Electrochemical Hybrid Gels. Acs Appl. Mater. Interfaces 2018, 10, 17352–17358. [Google Scholar] [CrossRef] [PubMed]
- Dyakonova, M.A.; Stavrouli, N.; Popescu, M.T.; Kyriakos, K.; Grillo, I.; Philipp, M.; Jaksch, S.; Tsitsilianis, C.; Papadakis, C.M. Physical Hydrogels via Charge Driven Self-Organization of a TriblockPolyampholyte—Rheological and Structural Investigations. Macromolecules 2014, 47, 7561–7572. [Google Scholar] [CrossRef]
- Kirilov, P.; Palomo, M.C. Colloidal dispersions of gelled nanoparticles (GLN): Concept and potential applications. Gels 2017, 3, 33–46. [Google Scholar]


| Entry | Solvent | State a | MGC b (g/100 cm3) |
|---|---|---|---|
| 1 | chlorobenzene | G | 1.8±0.1 |
| 2 | bromobenzene | G | 2.6±0.1 |
| 3 | o-dichlorobenzene | G | 1.0±0.1 |
| 4 | chloroform/toluene(1/2) | G | 0.8±0.1 |
| 5 | chloroform/p-xylene(1/2) | G | 1.0±0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Hu, J.; Liang, Y.; Cui, W. The Supramolecular Organogel Formed by Self-Assembly of Ursolic Acid Appended with Aromatic Rings. Materials 2019, 12, 614. https://doi.org/10.3390/ma12040614
Lu J, Hu J, Liang Y, Cui W. The Supramolecular Organogel Formed by Self-Assembly of Ursolic Acid Appended with Aromatic Rings. Materials. 2019; 12(4):614. https://doi.org/10.3390/ma12040614
Chicago/Turabian StyleLu, Jinrong, Jinshan Hu, Yinghua Liang, and Wenquan Cui. 2019. "The Supramolecular Organogel Formed by Self-Assembly of Ursolic Acid Appended with Aromatic Rings" Materials 12, no. 4: 614. https://doi.org/10.3390/ma12040614
APA StyleLu, J., Hu, J., Liang, Y., & Cui, W. (2019). The Supramolecular Organogel Formed by Self-Assembly of Ursolic Acid Appended with Aromatic Rings. Materials, 12(4), 614. https://doi.org/10.3390/ma12040614




