Thermal, Mechanical, and Microstructural Study of PBO Fiber during Carbonization
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Thermal Analysis of PBO Fiber
3.2. Mechanical Performance of PBO-Based Carbon Fiber
3.3. Evolution of Elemental Composition of PBO Fiber during the Carbonization Process
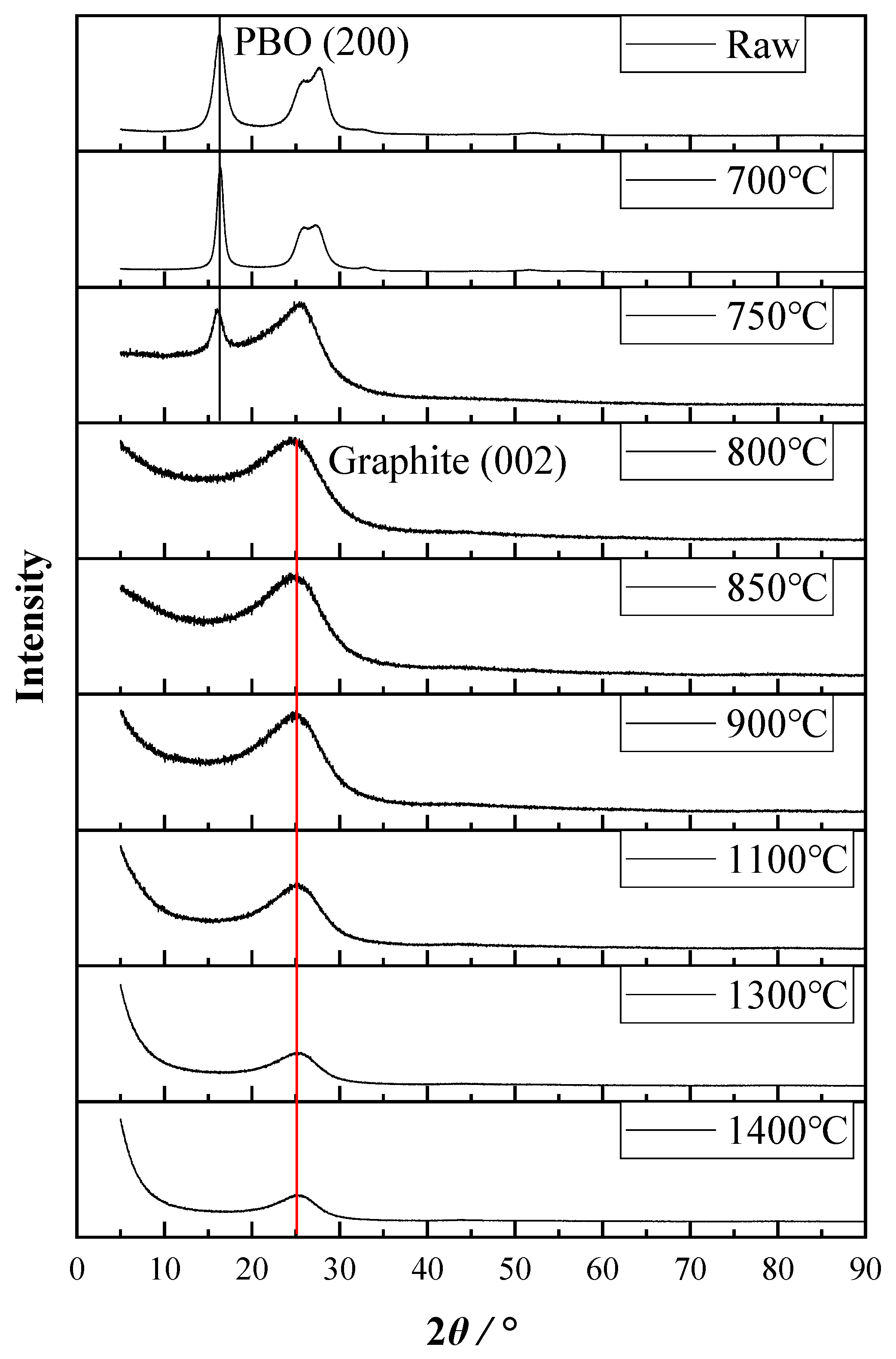
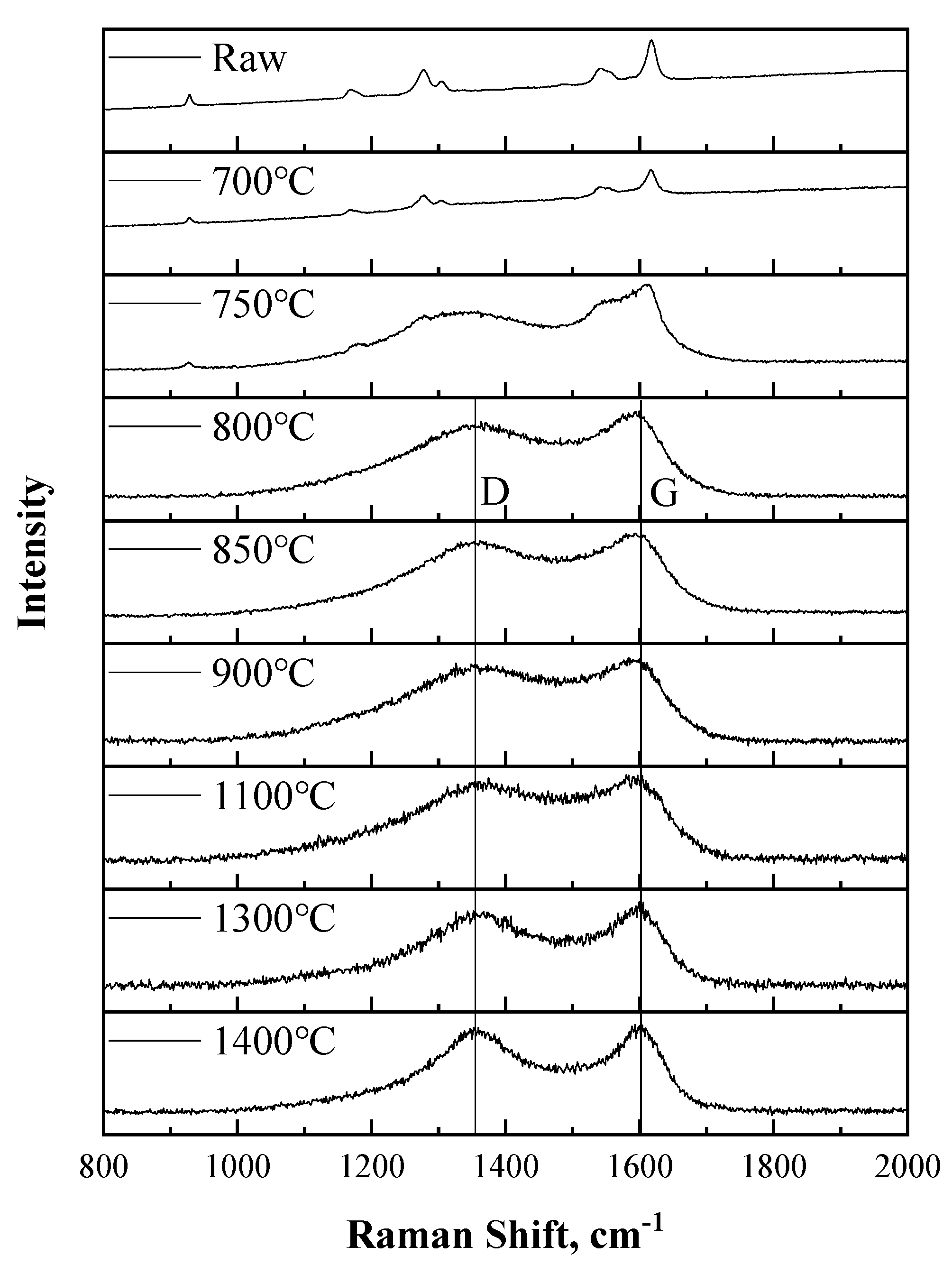
3.4. Change of Microstructure of PBO Fiber during Carbonization Process
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PBO | Poly(p-phenylene benzobisoxazole) |
| HTT | Heat treatment temperature |
| TGA | Thermalgravimetric analysis |
| TGA-MS | Thermalgravimetric analysis coupled with mass spectroscopy |
| PAN | Polyacrylonitrile |
| PAN-CF | Polyacrylonitrile-based carbon fiber |
| OEA | Organic elemental analysis |
| SEM | Scanning electron microscopy |
| XRD | X-ray diffraction |
| FWHM | Full width at half maximum |
References
- Naito, K.; Tanaka, Y.; Yang, J.-M.; Kagawa, Y. Tensile properties of ultrahigh strength PAN-based, ultrahigh modulus pitch-based and high ductility pitch-based carbon fibers. Carbon 2008, 46, 189–195. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, C. Graphene fiber: A new trend in carbon fibers. Mater. Today 2015, 18, 480–492. [Google Scholar] [CrossRef]
- Yao, S.S.; Jin, F.L.; Rhee, K.Y.; Hui, D.; Park, S.J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. Pt. B-Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Hamim, S.U.; Akbari, M.K.; Fakhrhoseini, S.M.; Khayyam, H.; Pakseresht, A.H.; Ghasali, E.; Zabet, M.; Munir, K.S.; Jia, S.; Davim, J.P.; Naebe, M. Carbon fiber reinforced metal matrix composites: Fabrication processes and properties. Compos. Pt. A-Appl. Sci. Manuf. 2017, 92, 70–96. [Google Scholar] [CrossRef]
- Soutis, C. Carbon fiber reinforced plastics in aircraft construction. Mater. Sci. Eng. A 2005, 412, 171–176. [Google Scholar] [CrossRef]
- Che, D.; Saxena, I.; Han, P.; Guo, P.; Ehmann, K.F. Machining of carbon fiber reinforced plastics/polymers: A literature review. J. Manuf. Sci. Eng. 2014, 136, 034001. [Google Scholar] [CrossRef]
- Chand, S. Review carbon fibers for composites. J. Mater. Sci. 2000, 35, 1303–1313. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Ge, Y.; Ran, L.; Peng, K.; Yi, M. Surface structures of PAN-based carbon fibers and their influences on the interface formation and mechanical properties of carbon-carbon composites. Compos. Pt. A-Appl. Sci. Manuf. 2016, 90, 480–488. [Google Scholar] [CrossRef]
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon fibers: Precursors, manufacturing, and properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrolysis. 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Wazir, A.H.; Kakakhel, L. Preparation and characterization of pitch-based carbon fibers. New Carbon Mater. 2009, 24, 83–88. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.-H. Preparation of pitch based carbon fibers using Hyper-coal as a raw material. Carbon 2016, 106, 28–36. [Google Scholar] [CrossRef]
- Kim, B.-J.; Kotegawa, T.; Eom, Y.; An, J.; Hong, I.-P.; Kato, O.; Nakabayashi, K.; Miyawaki, J.; Kim, B.C.; Mochida, I. Enhancing the tensile strength of isotropic pitch-based carbon fibers by improving the stabilization and carbonization properties of precursor pitch. Carbon 2016, 99, 649–657. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, D.-L.; Li, Y.; Li, X.-G. Preparation and hydrogen storage of activated rayon-based carbon fibers with high specific surface area. J. Phys. Chem. Solids. 2010, 71, 444–447. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Lu, W. Effect on Tensile Strength of Wood-based Carbon Fiber Impregnated by Boron. Bioresource 2016, 11, 5075–5082. [Google Scholar] [CrossRef]
- Rahaman, M.S.A.; Ismail, A.F.; Mustafa, A. A review of heat treatment on polyacrylonitrile fiber. Polym. Degrad. Stab. 2007, 92, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.A.; Jang, D.; Tejima, S.; Cruz-Silva, R.; Joh, H.I.; Kim, H.C.; Lee, S.; Endo, M. Strengthened PAN-based carbon fibers obtained by slow heating rate carbonization. Nature 2016, 6, 22988. [Google Scholar] [CrossRef]
- Yu, M.-J.; Bai, Y.-J.; Wang, C.-G.; Xu, Y.; Guo, P.-Z. A new method for the evaluation of stabilization index of polyacrylonitrile fibers. Mater. Lett. 2007, 61, 2292–2294. [Google Scholar] [CrossRef]
- Hiremath, N.; Mays, J.; Bhat, G. Recent developments in carbon fibers and carbon nanotube-based fibers: A review. Poly. Rev. 2017, 57, 339–368. [Google Scholar] [CrossRef]
- Gelayol, G.; Minoo, N.; Khashayar, B.; Abbas, M.; Reza, J.; Hamid, K. Production of low cost carbon-fiber through energy optimization of stabilization process. Materials 2018, 11, 385–397. [Google Scholar]
- Fratini, A.V.; Lenhert, P.G.; Resch, T.J.; Adams, W.W. Molecular packing and crystalline order in polybenzobisoxazole and polybenzobisthiazole fibers. MRS Online Proc. Libr. Arch. 1988, 134, 431–445. [Google Scholar] [CrossRef]
- So, Y.-H. Rigid-rod polymers with enhanced lateral interactions. Prog. Polym. Sci. 2000, 25, 137–157. [Google Scholar] [CrossRef]
- Zhang, C.H.; Huang, Y.D.; Yuan, W.J.; Zhang, J.N. UV aging resistance properties of PBO fiber coated with nano-ZnO hybrid sizing. J. Appl. Polym. Sci. 2011, 120, 2468–2476. [Google Scholar] [CrossRef]
- Krause, S.; Haddock, T.; Vezie, D.; Lenhert, P.; Hwang, W.-F.; Price, G.; Helminiak, T.; O’brien, J.; Adams, W. Morphology and properties of rigid-rod poly (p-phenylene benzobisoxazole) (PBO) and stiff-chain poly (2, 5 (6)-benzoxazole) (ABPBO) fibres. Polymer 1988, 29, 1354–1364. [Google Scholar] [CrossRef]
- Kitagawa, T.; Ishitobi, M.; Yabuki, K. An analysis of deformation process on poly-p-phenylenebenzobisoxazole fiber and a structural study of the new high-modulus type PBO HM+ fiber. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1605–1611. [Google Scholar] [CrossRef]
- Kumar, S.; Dang, T.D.; Arnold, F.E.; Bhattacharyya, A.R.; Min, B.G.; Zhang, X.; Vaia, R.A.; Park, C.; Adams, W.W.; Hauge, R.H. Synthesis, structure, and properties of PBO/SWNT Composites. Macromolecules 2002, 35, 9039–9043. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, D.-S.; Kim, S.-R. Improvement of interfacial adhesion and nondestructive damage evaluation for plasma-treated PBO and Kevlar fibers/epoxy composites using micromechanical techniques and surface wettability. J. Colloid Interface Sci. 2003, 264, 431–445. [Google Scholar] [CrossRef]
- Wang, J.; Liang, G.; Zhao, W.; Zhang, Z. Enzymatic surface modification of PBO fibers. Surf. Coat. Technol. 2007, 201, 4800–4804. [Google Scholar] [CrossRef]
- Vázquez-Santos, M.B.; Geissler, E.; László, K.; Rouzaud, J.-N.; Martínez-Alonso, A.; Tascón, J.M. Comparative XRD, Raman, and TEM study on graphitization of PBO-derived carbon fibers. J. Phys. Chem. C 2011, 116, 257–268. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Z.-H.; Ma, W.-J.; Ma, Q.-S. Influence of heat treatment on physical–chemical properties of PAN-based carbon fiber. Ceram. Int. 2006, 32, 291–295. [Google Scholar] [CrossRef]
- Tamargo-MartíNez, K.; Villar-Rodil, S.; Paredes, J.; Montes-Moran, M.; MartíNez-Alonso, A.; Tascon, J. Thermal decomposition of poly (p-phenylene benzobisoxazole) fibres: Monitoring the chemical and nanostructural changes by Raman spectroscopy and scanning probe microscopy. Polym. Degrad. Stab. 2004, 86, 263–268. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.-P.; Froigneux, E.; Moreau, M.; Rouzaud, J.-N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
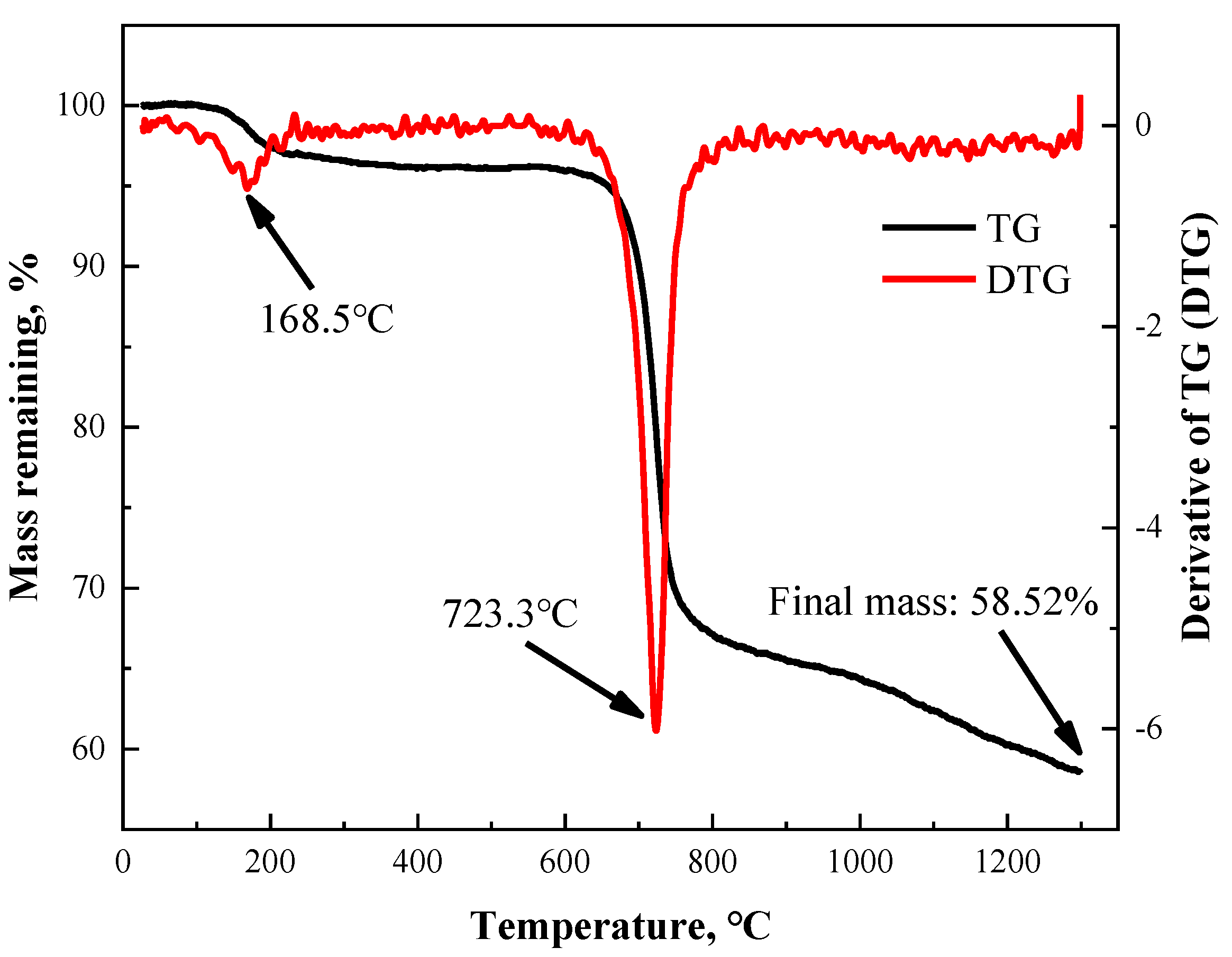

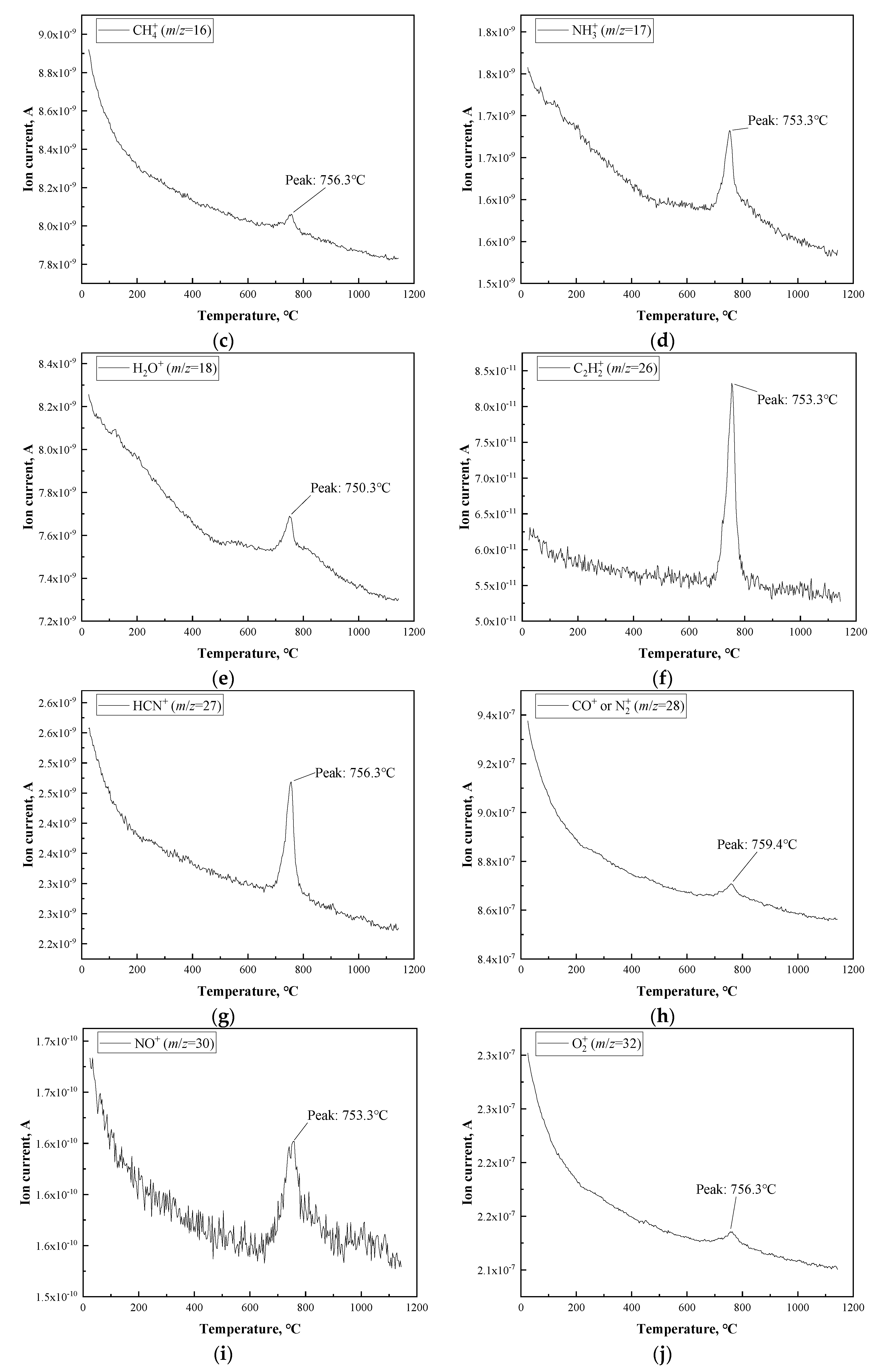
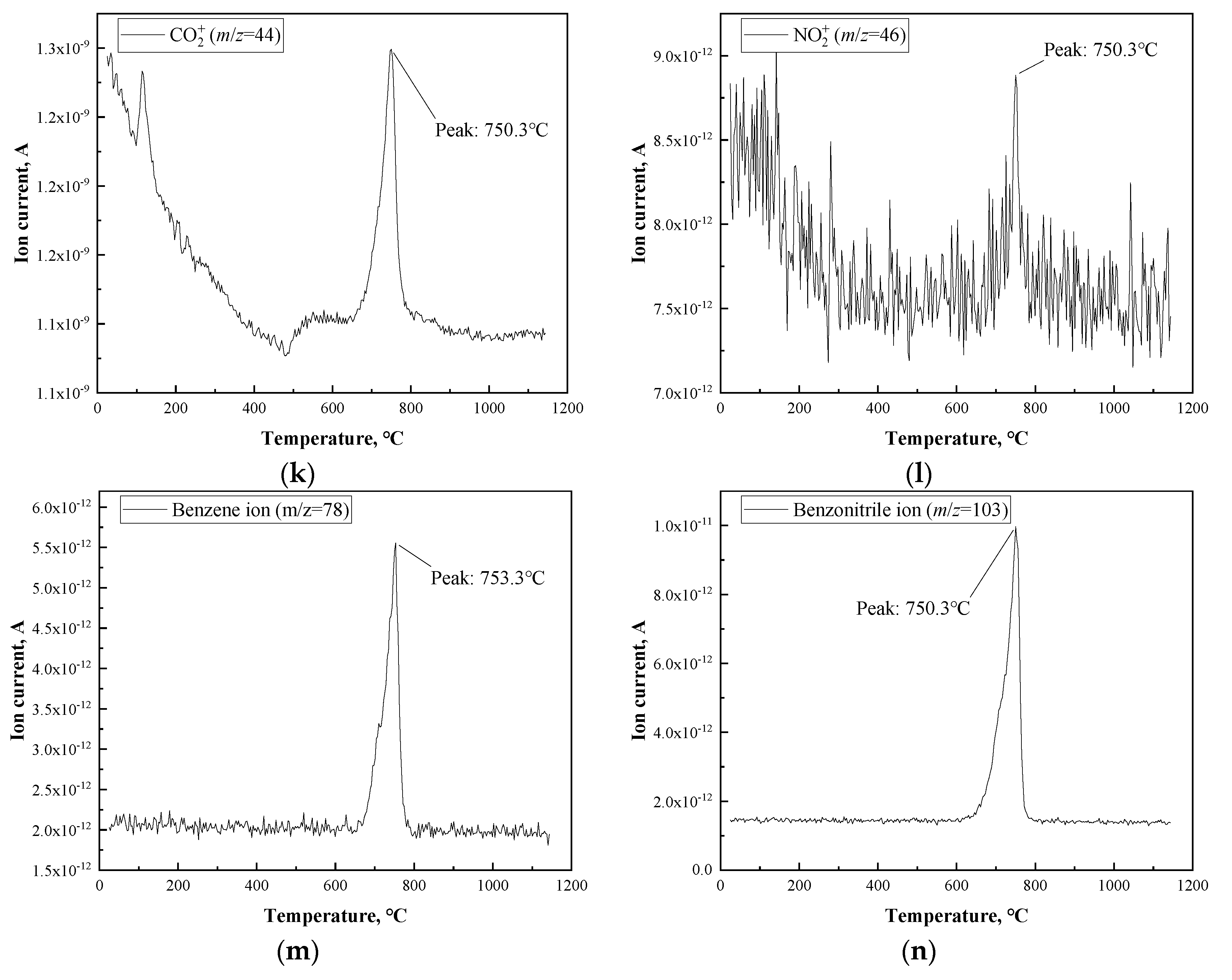
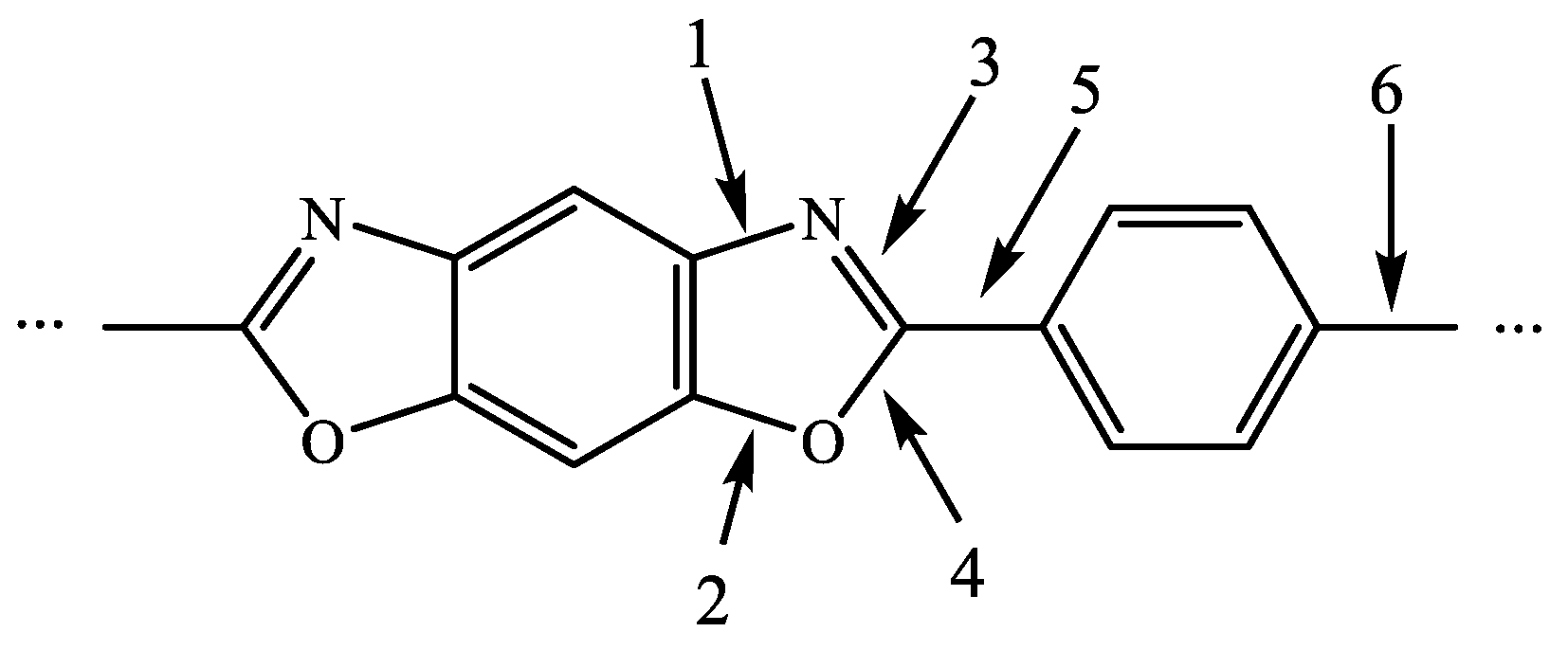
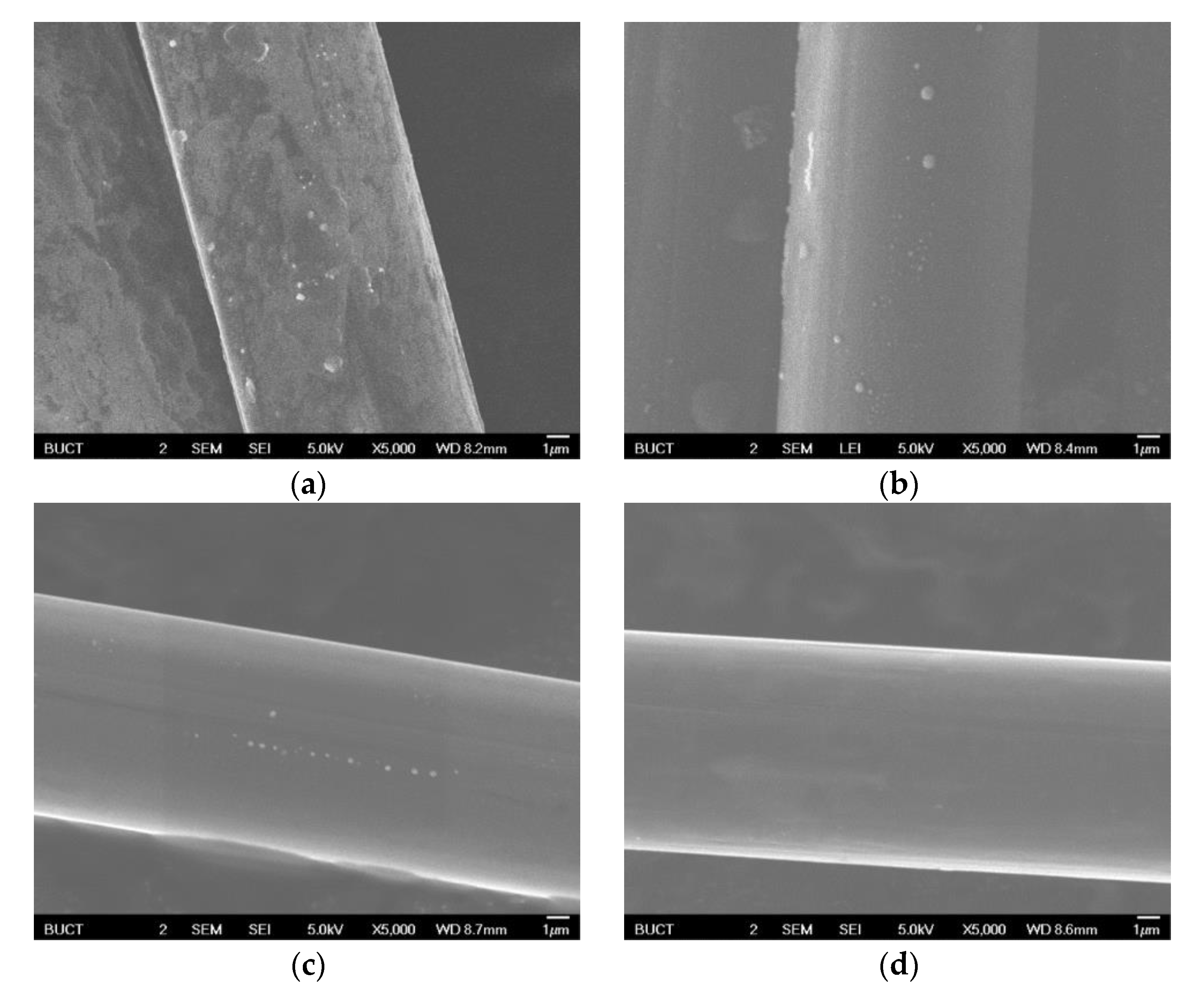
| HTT (°C) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Strain at Failure (%) |
|---|---|---|---|
| 700 | 484 ± 41 | 116 ± 8 | 0.8 |
| 750 | 350 ± 38 | 53 ± 5 | 1.3 |
| 800 | 429 ± 35 | 65 ± 6 | 1.3 |
| 850 | 438 ± 33 | 75 ± 6 | 1.1 |
| 900 | 470 ± 41 | 92 ± 8 | 1.1 |
| 1100 | 516 ± 42 | 130 ± 8 | 0.9 |
| 1300 | 521 ± 40 | 131 ± 11 | 0.8 |
| 1400 | 523 ± 36 | 131 ± 10 | 0.7 |
| HTT (°C) | Element Content (wt.%) | C/H | C/N | C/O | |||
|---|---|---|---|---|---|---|---|
| C | H | N | O | ||||
| untreated | 69.25 | 3.069 | 11.52 | 17.95 | 22.56 | 6.011 | 3.858 |
| 700 | 70.80 | 2.124 | 11.58 | 15.75 | 33.33 | 6.113 | 4.496 |
| 750 | 73.83 | 2.697 | 9.849 | 13.64 | 27.37 | 7.496 | 5.412 |
| 800 | 76.02 | 2.335 | 8.396 | 13.48 | 32.56 | 9.054 | 5.639 |
| 850 | 76.73 | 2.039 | 8.153 | 13.72 | 37.63 | 9.411 | 5.592 |
| 900 | 77.58 | 1.852 | 7.988 | 12.23 | 41.89 | 9.712 | 6.343 |
| 1100 | 83.39 | 1.163 | 6.554 | 8.548 | 71.70 | 12.72 | 9.755 |
| 1300 | 93.20 | 0.522 | 3.228 | 3.258 | 178.5 | 28.87 | 28.61 |
| 1400 | 95.76 | 0.418 | 2.381 | 2.090 | 229.1 | 40.21 | 45.82 |
| HTT (°C) | Diameter (μm) | Relative Reduction (%) |
|---|---|---|
| untreated | 13.97 | - |
| 700 | 13.03 | 6.729 |
| 750 | 12.92 | 7.516 |
| 800 | 12.28 | 12.10 |
| 850 | 11.87 | 15.03 |
| 900 | 11.67 | 16.46 |
| 1100 | 11.05 | 20.90 |
| 1300 | 10.98 | 21.40 |
| 1400 | 10.95 | 21.62 |
| HTT (°C) | 2θ (°) | d (nm) | FWHM (°) | L (nm) | |
|---|---|---|---|---|---|
| PBO (200) | Graphite (002) | ||||
| untreated | 16.197 | - | 0.547 | 1.509 | 5.263 |
| 700 | 16.303 | - | 0.544 | 0.890 | 8.924 |
| 750 | 15.856 | - | 0.559 | 1.381 | 5.748 |
| 800 | - | 25.080 | 0.355 | 7.117 | 1.132 |
| 850 | - | 25.374 | 0.351 | 6.937 | 1.162 |
| 900 | - | 25.533 | 0.349 | 6.748 | 1.195 |
| 1100 | - | 25.690 | 0.347 | 6.170 | 1.307 |
| 1300 | - | 25.540 | 0.349 | 5.398 | 1.493 |
| 1400 | - | 25.602 | 0.348 | 5.195 | 1.552 |
| HTT (°C) | D Peak Position (cm−1) | G Peak Position (cm−1) | R’s Value |
|---|---|---|---|
| 800 | 1373 | 1592 | 3.423 |
| 850 | 1373 | 1593 | 3.297 |
| 900 | 1379 | 1592 | 3.164 |
| 1100 | 1380 | 1595 | 3.009 |
| 1300 | 1372 | 1597 | 2.737 |
| 1400 | 1370 | 1596 | 2.467 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Zhang, X.; Tian, Y. Thermal, Mechanical, and Microstructural Study of PBO Fiber during Carbonization. Materials 2019, 12, 608. https://doi.org/10.3390/ma12040608
Hao W, Zhang X, Tian Y. Thermal, Mechanical, and Microstructural Study of PBO Fiber during Carbonization. Materials. 2019; 12(4):608. https://doi.org/10.3390/ma12040608
Chicago/Turabian StyleHao, Weizhe, Xuejun Zhang, and Yanhong Tian. 2019. "Thermal, Mechanical, and Microstructural Study of PBO Fiber during Carbonization" Materials 12, no. 4: 608. https://doi.org/10.3390/ma12040608
APA StyleHao, W., Zhang, X., & Tian, Y. (2019). Thermal, Mechanical, and Microstructural Study of PBO Fiber during Carbonization. Materials, 12(4), 608. https://doi.org/10.3390/ma12040608




