Improvement of Transparencies and Mechanical Properties of Poly(cyclohexylene dimethylene cyclohexanedicarboxylate) Parts Using a Compounding Nucleating Agent to Control Crystallization
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Sample Preparations
2.3. Optical Performance Tests
2.4. Tensile Tests
2.5. Thermal Analyses
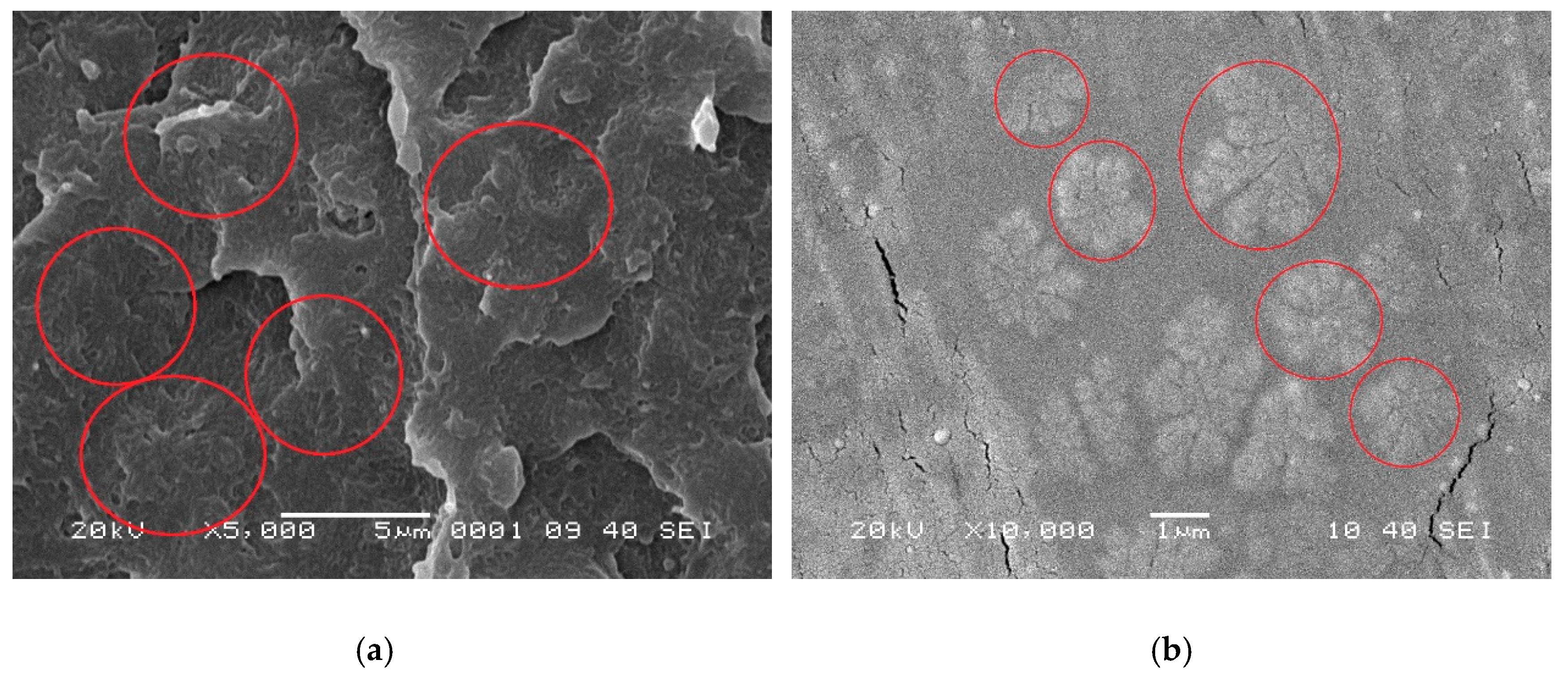
2.6. Morphological Observations
3. Results and Discussion
3.1. Comparison among PCCE/MDBSs
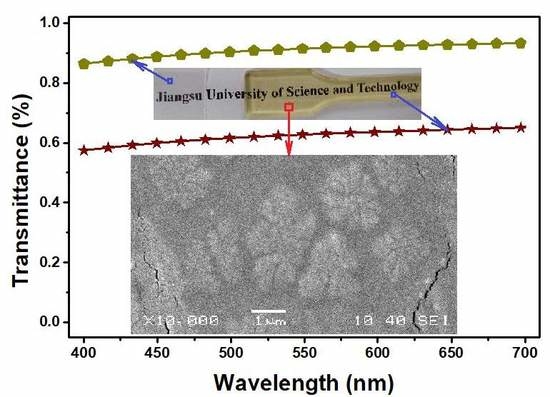
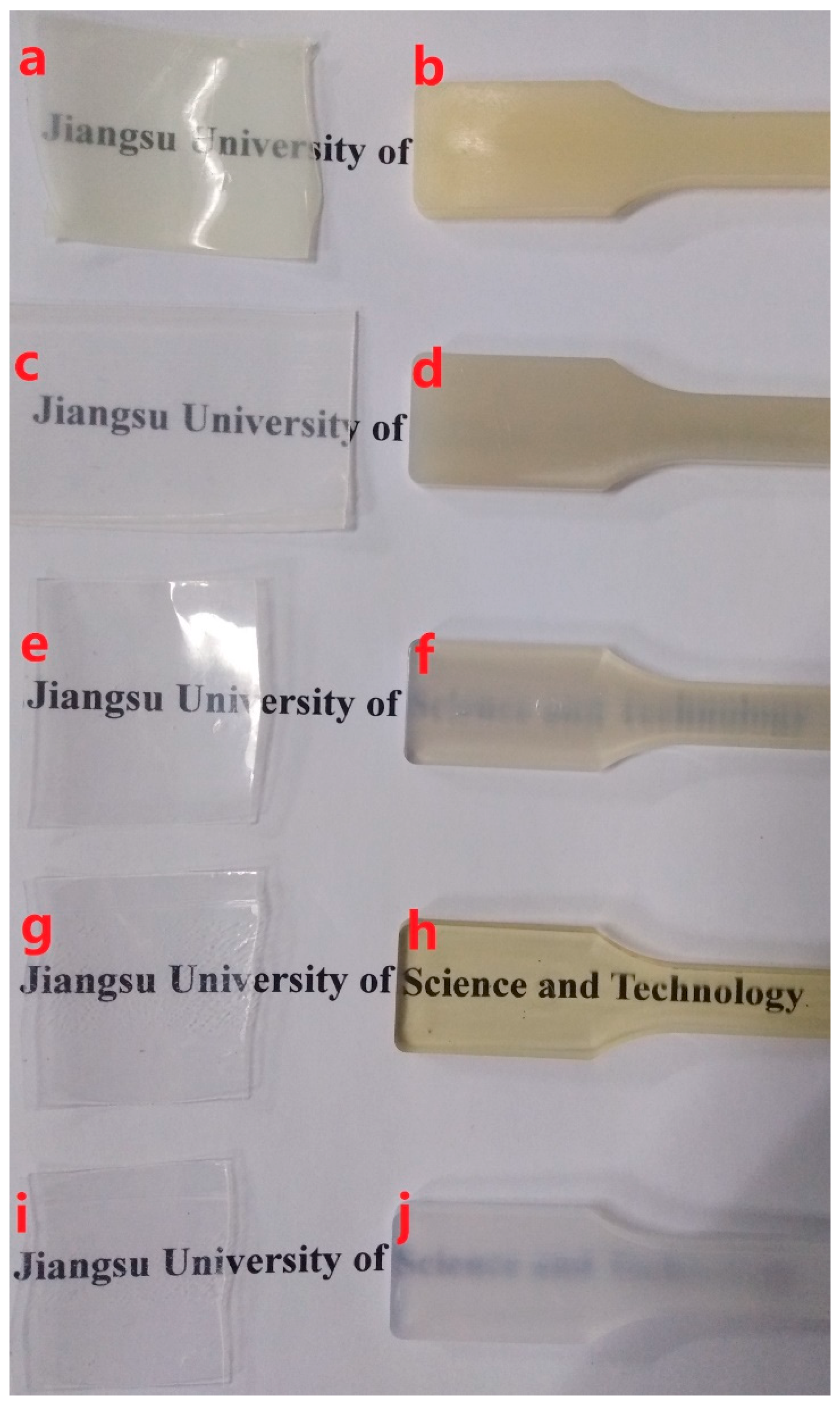
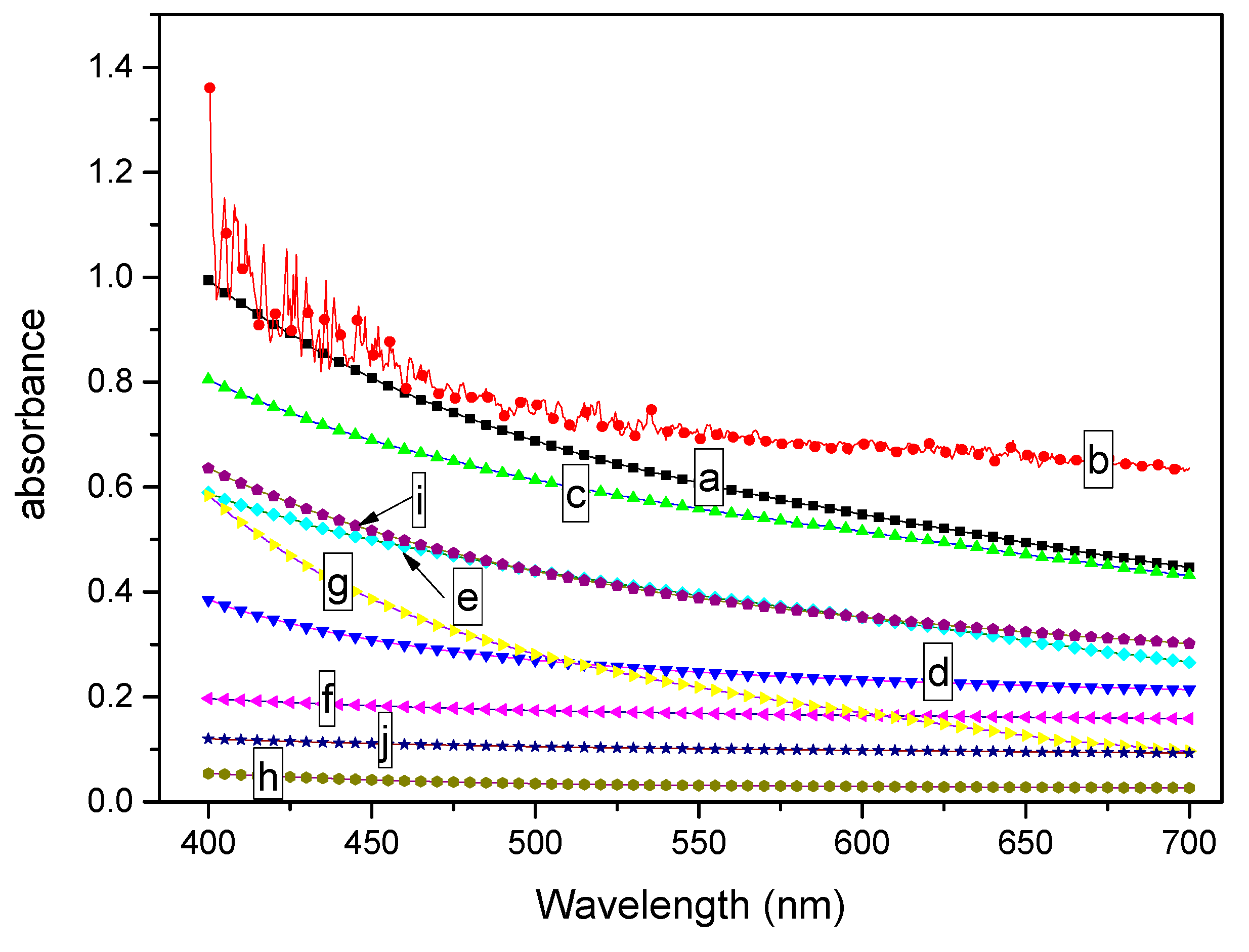
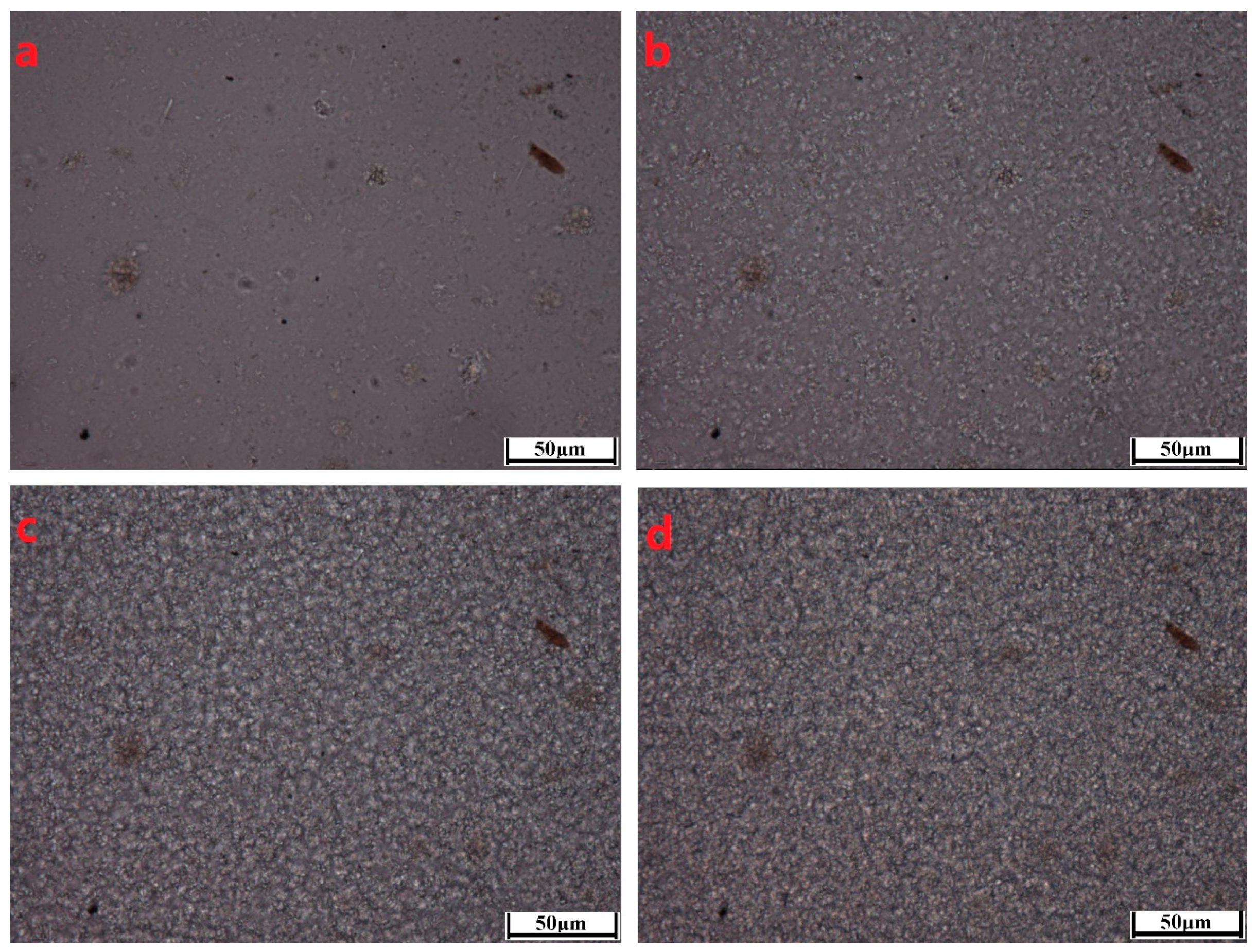
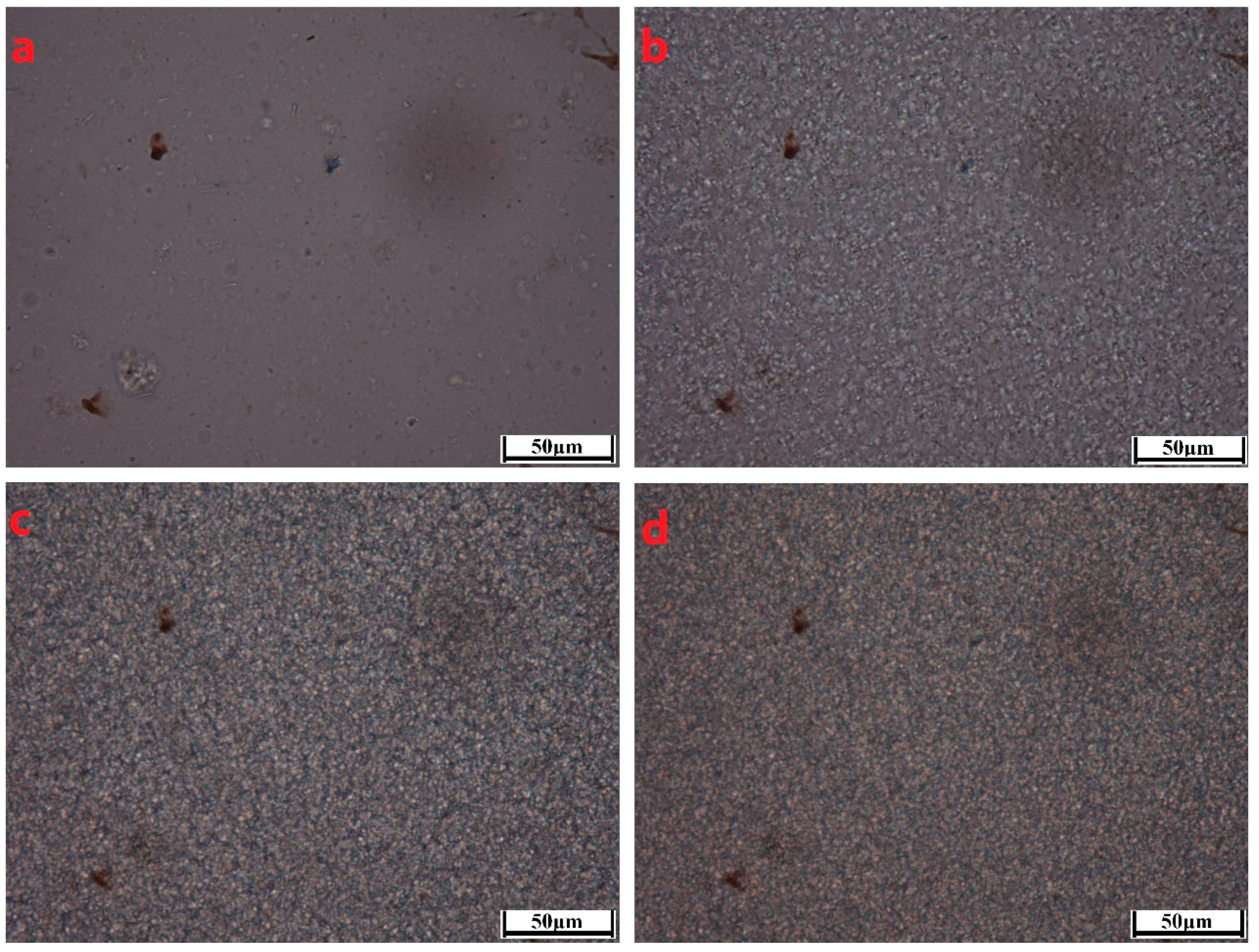
3.1.1. Transparencies
3.1.2. Mechanical Properties
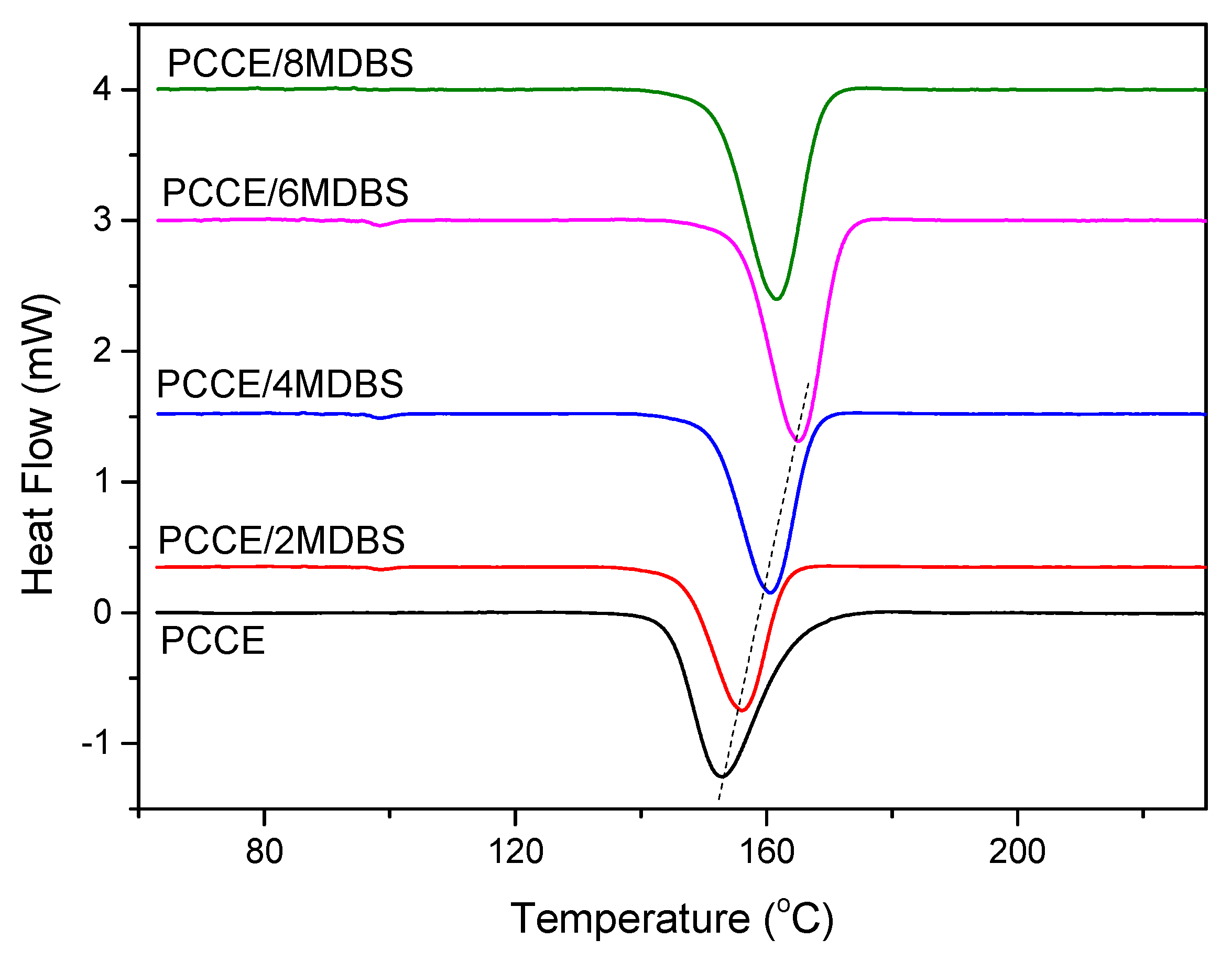
3.1.3. Crystallization Behaviors
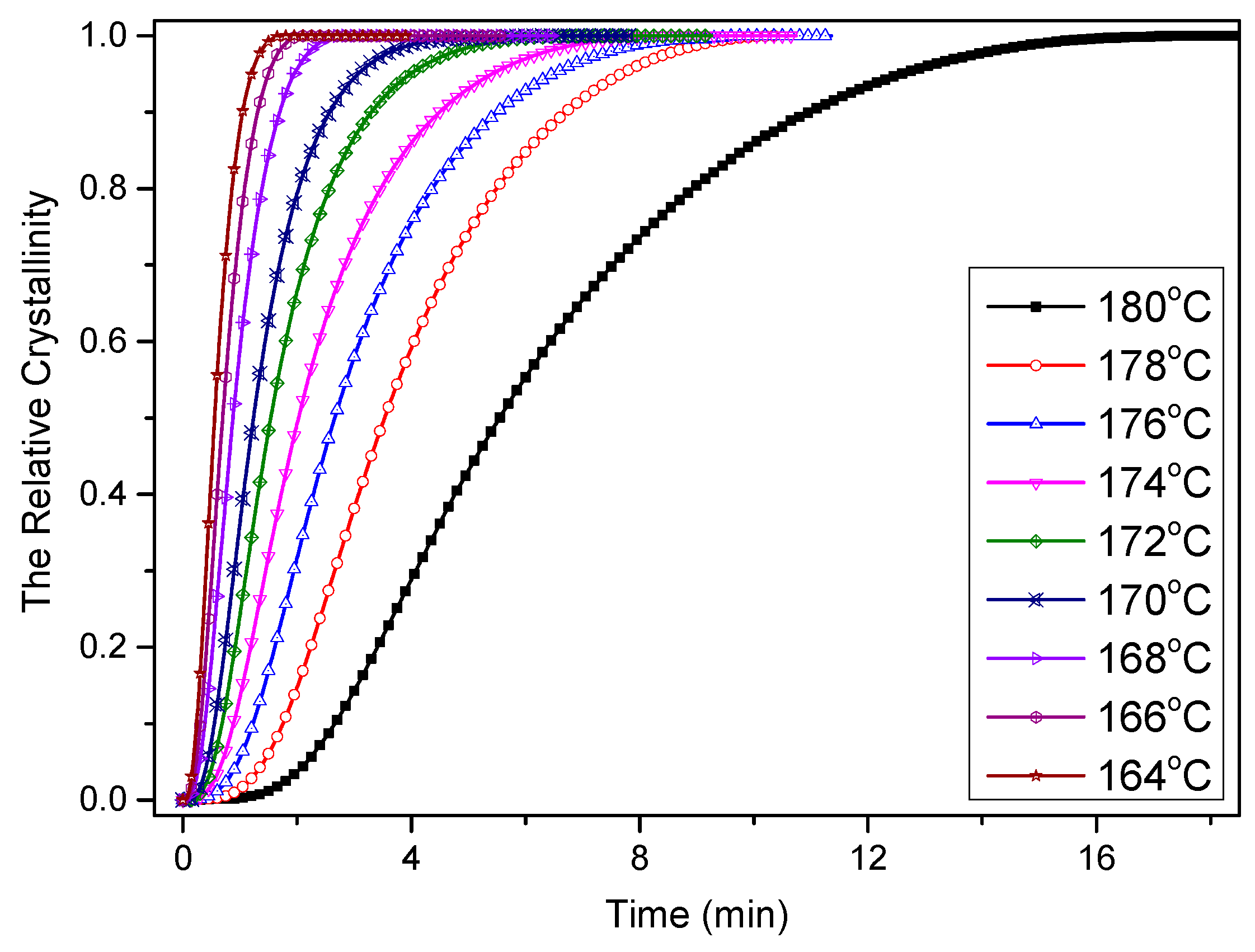
3.2. Crystallization Kinetics of PCCE/6MDBS
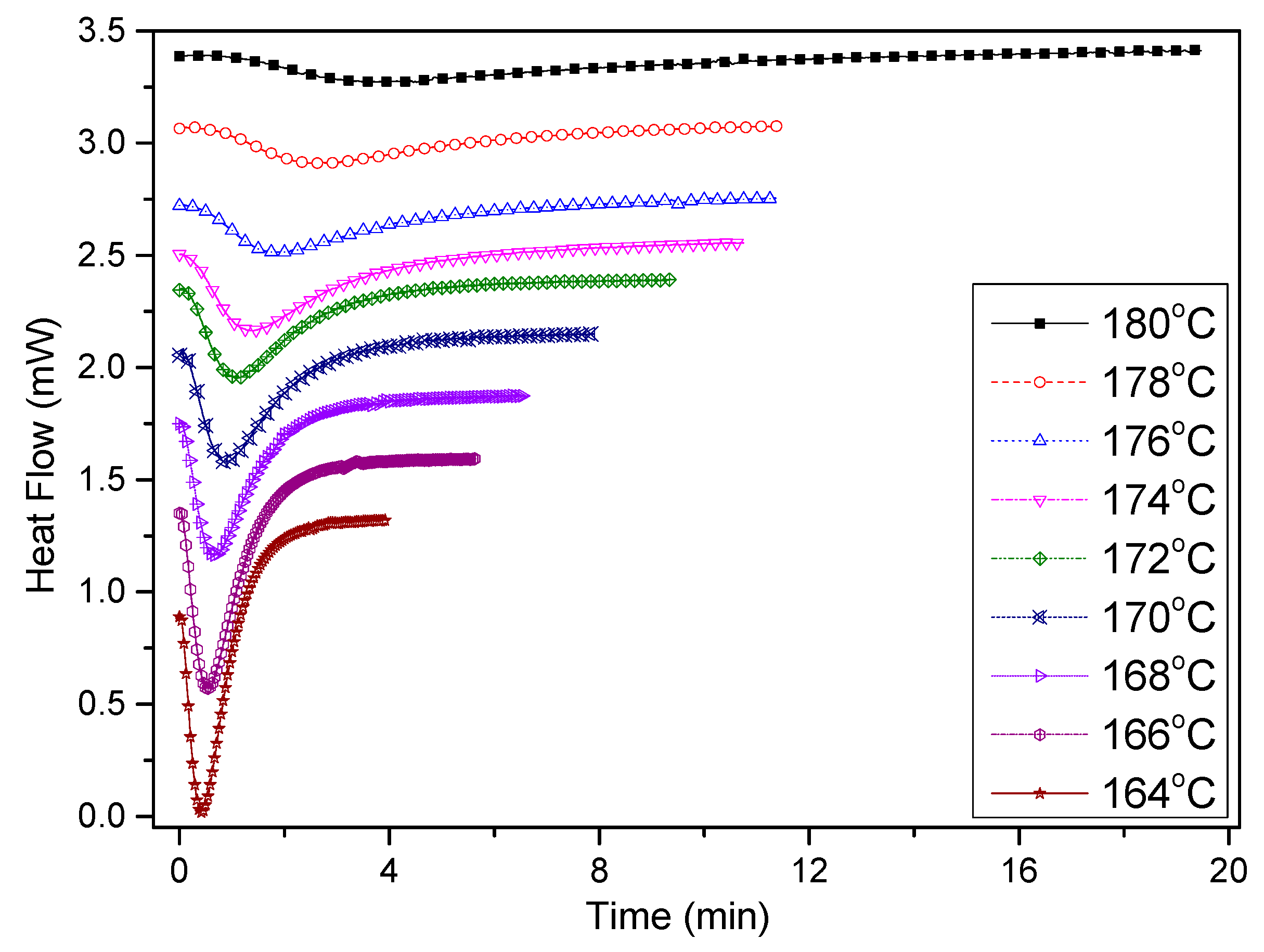
3.2.1. Isothermal Crystallization Kinetics Analyses
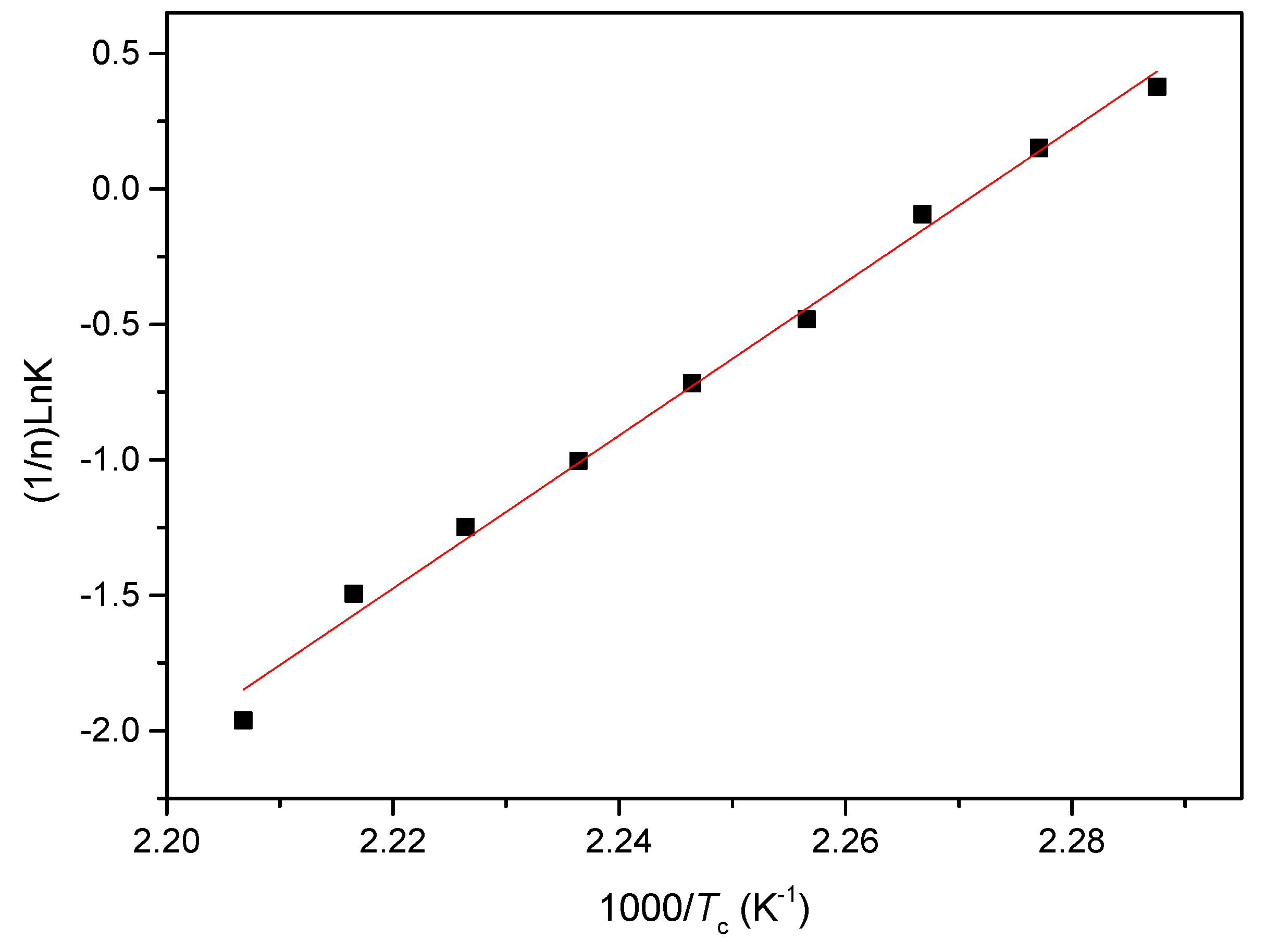
3.2.2. Isothermal Crystallization Activation Energy of PCCE/6MDBS
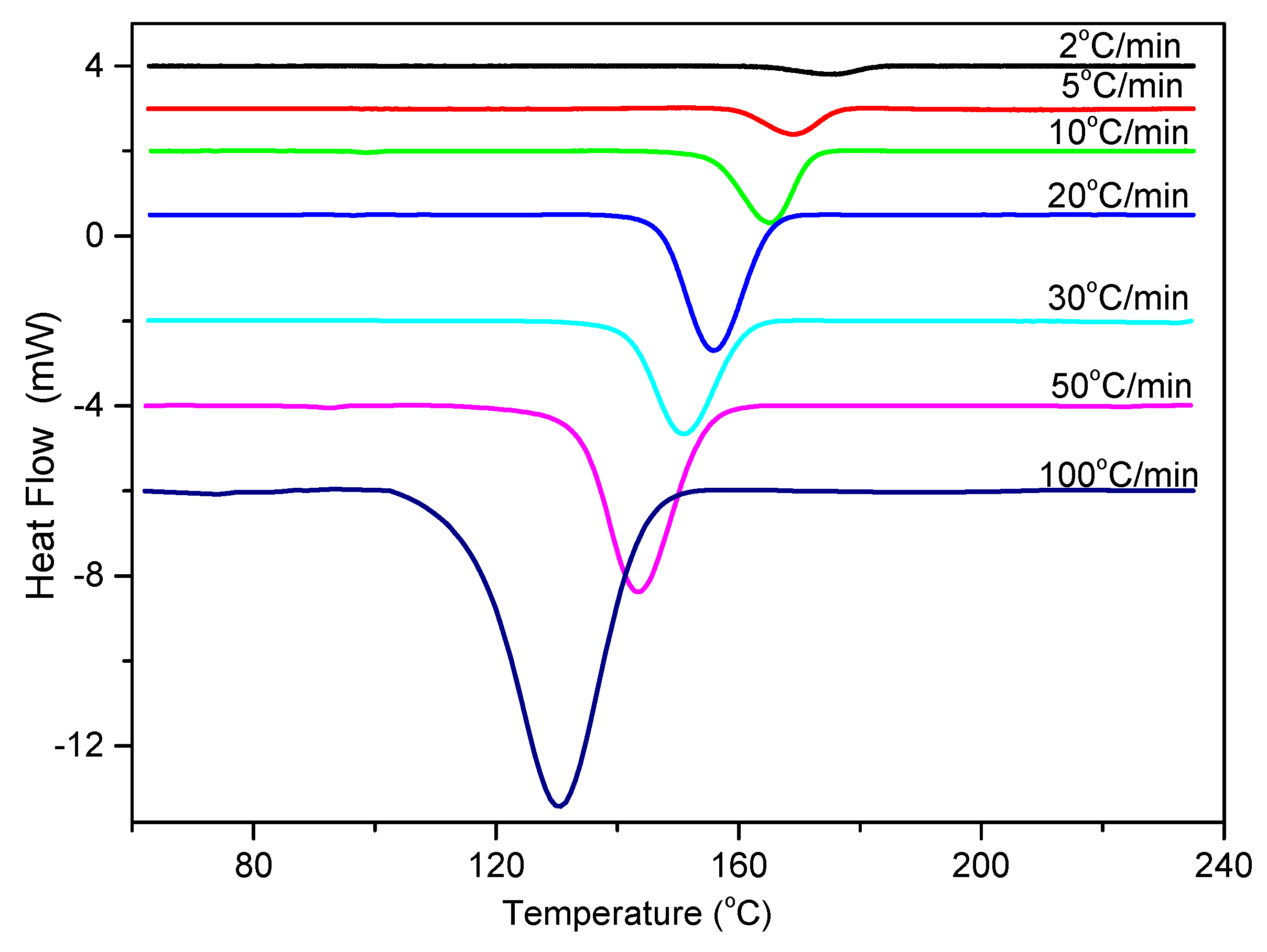
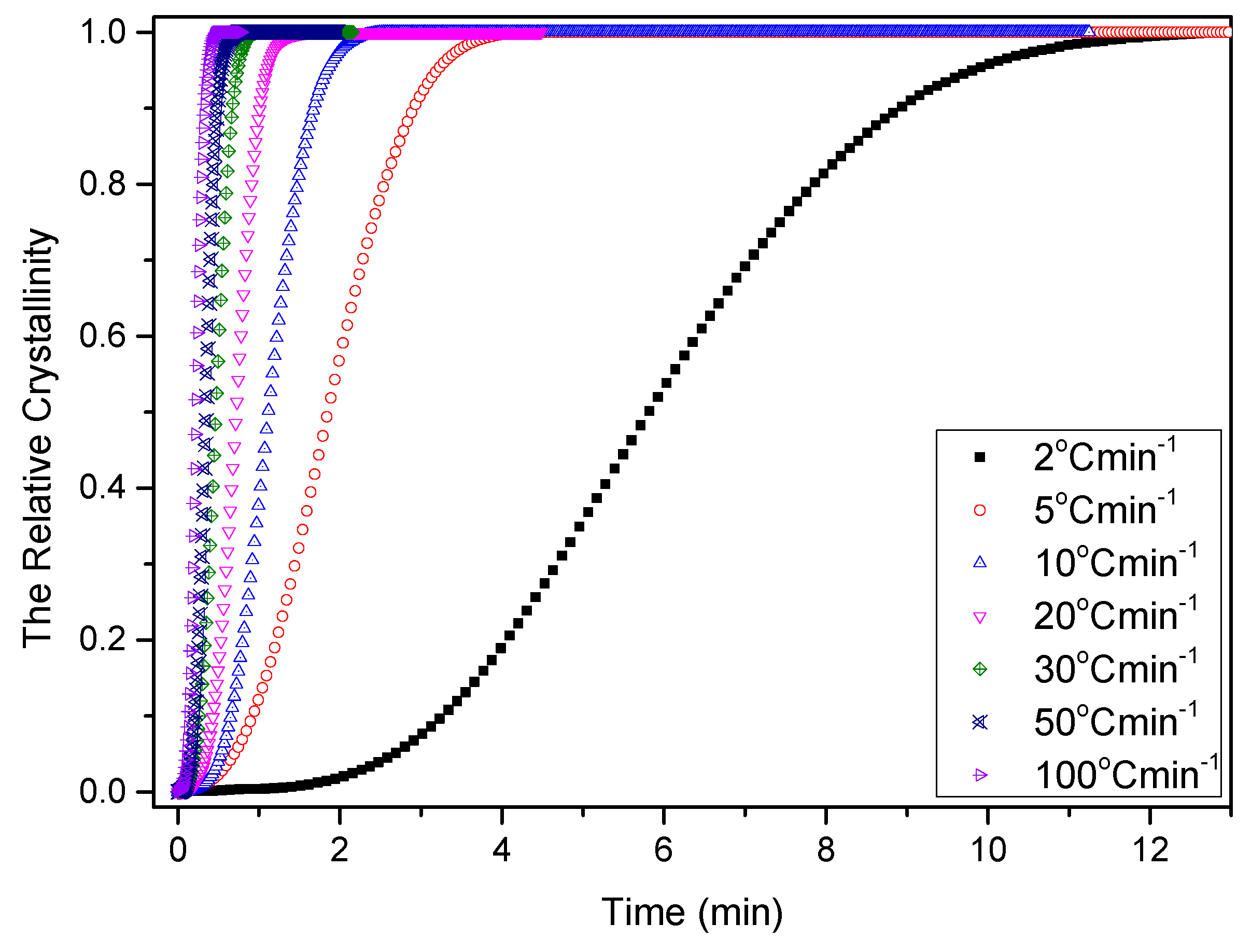
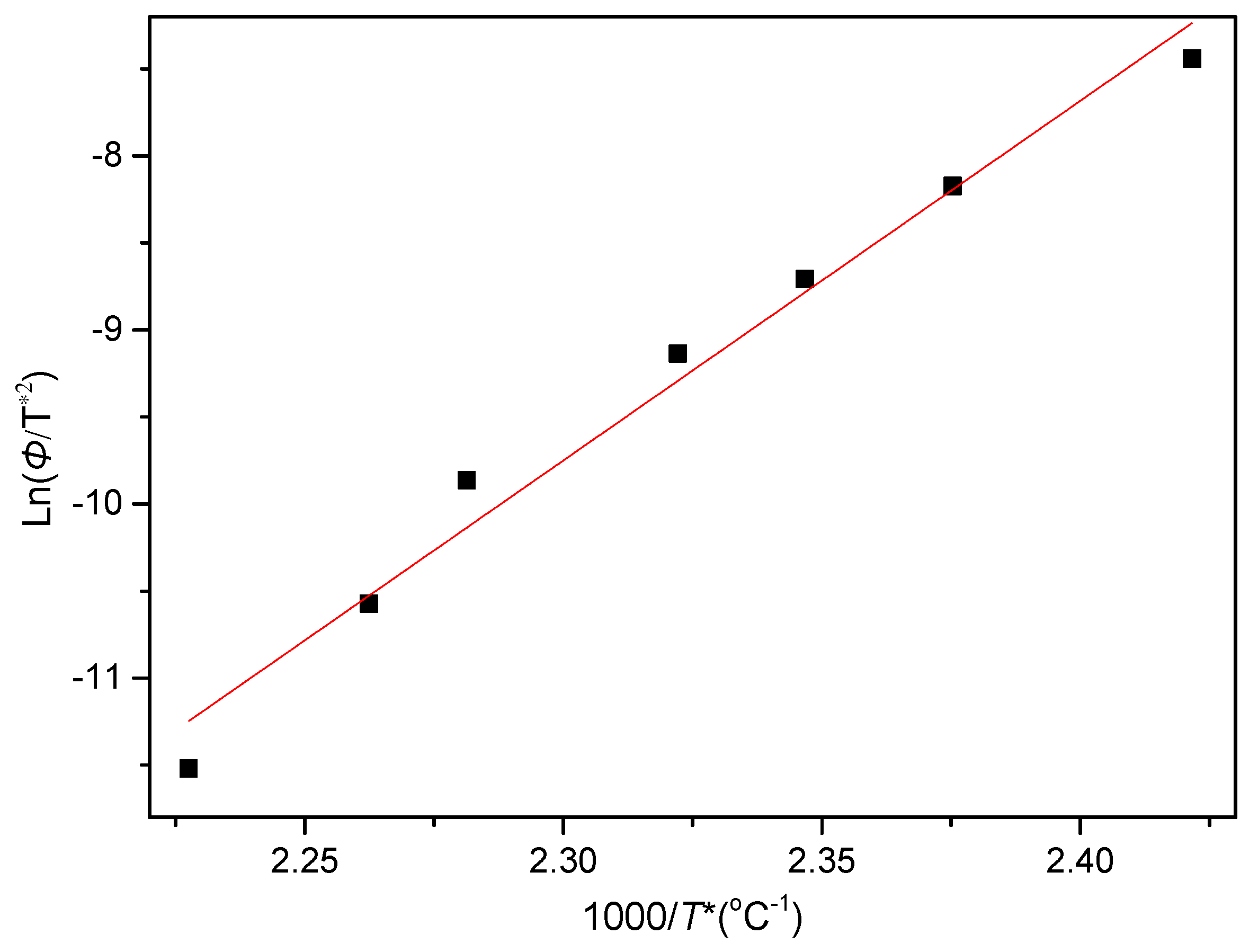
3.2.3. Nonisothermal Crystallization Activation Energy of PCCE/6MDBS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Strobl, G. Crystallization and Melting of Bulk Polymers: New Observations, Conclusions and a Thermodynamic Scheme. Prog. Polym. Sci. 2006, 31, 398–442. [Google Scholar] [CrossRef]
- Zhu, P.W.; Edward, G. Distribution of Shish-Kebab Structure of Isotactic Polypropylene under Shear in the Presence of Nucleating Agent. Macromolecules 2004, 37, 2658–2660. [Google Scholar] [CrossRef]
- Pantani, R.; Coccorullo, I.; Speranza, V.; Titomanlio, G. Modeling of Morphology Evolution in the Injection Molding Process of Thermoplastic Polymers. Prog. Polym. Sci. 2005, 30, 1185–1222. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Shen, C.Y.; Turng, L.S.; Liu, C.T.; Li, Q. Computational Modeling and Numerical Simulation of Flow-Induced Crystallization Kinetics During Injection Molding of Polyethylene Terephthalate. J. Reinf. Plast. Comp. 2010, 29, 76–85. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Lu, S.; Wang, G.L.; Shi, F.J. Modeling and Numerical Simulation of Injection Molding of Semi-crystalline Polymer Isotactic Polypropylene. J. Comput. Theor. Nanosci. 2012, 9, 1364–1367. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Wu, W.B.; Zou, J.; Turng, L.S. Dual-scale Modeling and Simulation of Film Casting of Isotactic Polypropylene. J. Plast. Film Sheet 2016, 32, 239–271. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Su, B.; Wu, H.H. Effect of Cold-drawn Fibers on the Self-reinforcement of the PP/LDPE Composites. J. Mater. Eng. Perform. 2017, 26, 4072–4082. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Su, B.; Turng, L.S. Influence of Processing Conditions on Morphological Structure and Ductility of Water-Foamed Injection Molded PP/LDPE Blended Parts. Cell. Polym. 2017, 36, 51–74. [Google Scholar] [CrossRef]
- Svoboda, P.; Zeng, C.; Wang, H.; Lee, J.; Tomasko, D.L. Morphology and Mechanical Properties of Polypropylene/organoclay Nanocomposites. J. Appl. Polym. Sci. 2002, 85, 1562–1570. [Google Scholar] [CrossRef]
- Pozsgay, A.; Frater, T.; Papp, L.; Sajo, I.; Pukanszky, B. Nucleating Effect of Montmorillonite Nanoparticles in Polypropylene. J. Macromol. Sci. Part B 2002, 41, 1249–1265. [Google Scholar] [CrossRef]
- Nowackia, R.; Monasseb, B.; Piorkowskaa, E.; Galeskia, A.; Haudin, J.M. Spherulite Nucleation in Isotactic Polypropylene Based Nanocomposites with Montmorillonite under Shear. Polymer 2004, 45, 4877–4892. [Google Scholar] [CrossRef]
- Qian, J.; Nie, K. Non-isothermal Crystallization of PP/Nano-SiO2 Composites. J. Appl. Polym. Sci. 2004, 91, 1013–1019. [Google Scholar] [CrossRef]
- Kim, J.Y.; Han, S.; Kim, D.K.; Kim, S.H. Crystallization Kinetics and Nucleation Activity of Silica Nanoparticle-filled Poly(ethylene 2,6-naphthalate). Compos. Part A-Appl. S. 2009, 40, 45–53. [Google Scholar] [CrossRef]
- Kim, S.H.; Ahn, S.H.; Hirai, T. Mechanical Reinforcement and Crystallization Behavior of Poly(ethylene 2,6-naphthalate) Nanocomposites Induced by Modified Carbon Nanotube. Polymer 2003, 44, 5625–5634. [Google Scholar] [CrossRef]
- Miltner, H.E.; Grossiord, N.; Lu, K.; Loos, J.; Koning, C.E.; Van Mele, B. Isotactic Polypropylene/carbon nanotube Composites Prepared by Latex Technology Thermal Analysis of Carbon Nanotube-induced Nucleation. Macromolecular 2008, 41, 5753–5762. [Google Scholar] [CrossRef]
- Fiorentino, B.; Fulchiron, R.; Duchet-Rumeau, J.; Bounor-Legaré, V.; Majesté, J.C. Controlled Shear-induced Molecular Orientation and Crystallization in Polypropylene-talc Microcomposites – Effects of the Talc Nature. Polymer 2013, 54, 2764–2775. [Google Scholar] [CrossRef]
- Yousfi, M.; Livi, S.; Dumas, A.; Roux, C.L.; Crepin-Leblond, J.; Greenhill-Hooper, M.; Duchet-Rumeau, J. Use of New Synthetic Talc as Reinforcing Nanofillers for Polypropylene and Polyamide 6 Systems: Thermal and Mechanical Properties. J. Coll. Interf. Sci. 2013, 403, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.G.; Su, B.; Turng, L.S. Mechanical Properties, Fiber Orientation, and Length Distribution of Glass Fiber-Reinforced Polypropylene Parts: Influence of Water-Foaming Technology. Polym. Compos. 2018, 39, 4386–4399. [Google Scholar]
- Su, B.; Zhou, Y.G. Influence of Foaming Technology on Fiber Breakage in Long Fiber-Reinforced Composites Foamed Parts. Plast. Rubber Compos. 2017, 46, 365–374. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Su, B.; Turng, L.S. Depositing- Induced Effects of Isotactic Polypropylene and Polycarbonate Composites During Fused Deposition Modeling. Rapid Prototyping J. 2017, 23, 869–880. [Google Scholar] [CrossRef]
- Chen, W.Y.; Yang, Y.J.; Lee, C.H.; Shen, A.Q. Confinement Effects on the Self-assembly of 1,3:2,4-Di-p-methylbenzylidene Sorbitol based Organogel. Langmuir 2008, 24, 10432–10436. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.C.; Wu, C.H. Studies on the Self-assembly of Neat DBS and DBS/PPG Organogels. J. Appl. Polym. Sci. 2010, 115, 1113–1119. [Google Scholar] [CrossRef]
- Shepard, T.A.; Delsorbo, C.R.; Louth, R.M.; Walborn, J.L.; Norman, D.A.; Harvey, N.G.; Spontak, R.J. Self-Organization and Polyolefin Nucleation Efficacy of 1,3-2,4-Di-p-Methylbenzylidene Sorbitol. J. Polym. Sci. Part B 1997, 35, 2617–2628. [Google Scholar] [CrossRef]
- Lipp, J.; Shuster, M.; Terry, A.E.; Cohen, Y. Fibril Formation of 1,3:2,4-Di(3,4-dimethylbenzylidene) Sorbitol in Polymer Melts. Polym. Eng. Sci. 2008, 48, 705–710. [Google Scholar] [CrossRef]
- Zhou, Y.G.; Wu, W.B.; Lu, G.Y.; Zou, J. Isothermal and Non-isothermal Crystallization Kinetics and Predictive Modeling in the Solidification of Poly(cyclohexylene dimethylene cyclohexanedicarboxylate) Melt. J. Elastomers Plast. 2017, 49, 132–156. [Google Scholar] [CrossRef]
- Piorkowska, E.; Galeski, A.; Haudin, J.M. Critical Assessment of Overall Crystallization Kinetics Theories and Predictions. Prog. Polym. Sci. 2006, 31, 549–575. [Google Scholar] [CrossRef]
- Avrami, M. Kinetics of Phase Change. I. General Theory. J. Chem. Phys. 1939, 7, 1103–1112. [Google Scholar] [CrossRef]
- Cui, X.; Qing, S.; Yan, D. Isothermal and Non-isothermal Crystallization Kinetics of Novel Odd-odd Polyamide 9 11. Eur. Polym. J. 2005, 41, 3060–3068. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, Q.; Wang, Y.; Zhang, C.; Mo, Z.; Cao, S. Melting Behaviors, Isothermal and Mon-isothermal Crystallization Kinetics of Nylon 1212. Polymer 2003, 44, 2537–2545. [Google Scholar] [CrossRef]
- Cebe, P.; Hong, S.D. Crystallization Behaviour of Poly(ether-ether-ketone). Polymer 1986, 27, 1183–1192. [Google Scholar] [CrossRef]
- Patel, R.M.; Spruiell, L.J.E. Crystallization Kinetics during Polymer Processing-Analysis of Available Approaches for Process Modeling. Polym. Eng. Sci. 1991, 31, 730–738. [Google Scholar] [CrossRef]
- Chan, T.W.; Isayev, A.I. Quiescent Polymer Crystallization: Modeling and Measurements. Polym. Eng. Sci. 1994, 34, 461–471. [Google Scholar] [CrossRef]
- Hammami, A.; Spruiell, J.E.; Mehrotra, A.K. Quiescent Nonisothermal Crystallization Kinetics of Isotactic Polypropylenes. Polym. Eng. Sci. 1995, 35, 797–804. [Google Scholar] [CrossRef]
- Mubarak, Y.; Harkin-Jones, E.M.A.; Martin, P.J.; Ahmad, M. Modeling of Non-isothermal Crystallization Kinetics of Isotactic Polypropylene. Polymer 2001, 42, 3171–3182. [Google Scholar] [CrossRef]
- Kissinger, H.E. Variation of Peak Temperature with Heating Rate in Differential Thermal Analysis. J. Res. Nat. Bur. Stand. Section A Phys. Chem. 1956, 57, 217–221. [Google Scholar] [CrossRef]
- Wang, W.X.; Zhou, S.; Xin, Z.; Shi, Y.Q.; Zhao, S.C.; Meng, X. Preparation and Foaming Mechanism of Foamable Polypropylene based on Self-assembled Nanofibrils from Sorbitol Nucleating Agents. J. Mater. Sci. 2016, 51, 788–796. [Google Scholar] [CrossRef]
- Liu, S.J.; Yu, W.; Zhou, C.X. Molecular Self-Assembly Assisted Liquid-Liquid Phase Separation in Ultrahigh Molecular Weight Polyethylene/Liquid Paraffin/Dibenzylidene Sorbitol Ternary Blends. Macromolecules 2013, 46, 6309–6318. [Google Scholar] [CrossRef]
- Bai, T.; Dong, B.; Xiao, M.; Liu, H.; Wang, N.; Wang, Y.M. Polystyrene Foam with High Cell Density and Small Cell Size by Compression-Injection Molding and Core Back Foaming Technique: Evolution of Cells in Cavity. Macromol. Mater. Eng. 2018, 303, 1800110. [Google Scholar] [CrossRef]
- Ania, F.; Baltá-Calleja, F.J.; Flores, A.; Michler, G.H.; Scholtyssek, S.; Khariwala, D.; Hiltner, A.; Baer, E.; Rong, L.; Hsiao, B.S. Nanostructure and Crystallization Phenomena in Multilayered Films of Alternating iPP and PA6 Semicrystalline Polymers. Eur. Polym. J. 2012, 48, 86–96. [Google Scholar] [CrossRef]
- Hu, G.H.; Hoppe, S.; Feng, L.F.; Fonteix, C. Nano-scale Phenomena and Applications in Polymer Processing. Chem. Eng. Sci. 2007, 62, 3528–3537. [Google Scholar] [CrossRef]
- Su, B.; Zhou, Y.G.; Wu, H.H. Influence of Mechanical Properties of PP/LDPE Nanocomposites: Compatibility and Crystallization. Nanomater. Nanotechnol. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Furlan, L.G.; Ferreira, C.I.; Castel, C.D.; Santos, K.S.; Mello, A.C.E.; Liberman, S.A.; Oviedo, M.A.S.; Mauler, R.S. Effect of Processing Conditions on the Mechanical and Thermal Properties of High-impact Polypropylene Nanocomposites. Mat. Sci. Eng. A 2011, 528, 6715–6718. [Google Scholar] [CrossRef]
- Xu, B.P.; Liu, Y.; He, L.; Turng, L.S.; Liu, C.T. Effect of Centerline Distance on Mixing of a Non-Newtonian Fluid in a Cavity with Asymmetric Rotors. Phys. Fluids 2019, 31, 1–18. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, B.; Zhou, Y.-G. Improvement of Transparencies and Mechanical Properties of Poly(cyclohexylene dimethylene cyclohexanedicarboxylate) Parts Using a Compounding Nucleating Agent to Control Crystallization. Materials 2019, 12, 563. https://doi.org/10.3390/ma12040563
Su B, Zhou Y-G. Improvement of Transparencies and Mechanical Properties of Poly(cyclohexylene dimethylene cyclohexanedicarboxylate) Parts Using a Compounding Nucleating Agent to Control Crystallization. Materials. 2019; 12(4):563. https://doi.org/10.3390/ma12040563
Chicago/Turabian StyleSu, Bei, and Ying-Guo Zhou. 2019. "Improvement of Transparencies and Mechanical Properties of Poly(cyclohexylene dimethylene cyclohexanedicarboxylate) Parts Using a Compounding Nucleating Agent to Control Crystallization" Materials 12, no. 4: 563. https://doi.org/10.3390/ma12040563
APA StyleSu, B., & Zhou, Y.-G. (2019). Improvement of Transparencies and Mechanical Properties of Poly(cyclohexylene dimethylene cyclohexanedicarboxylate) Parts Using a Compounding Nucleating Agent to Control Crystallization. Materials, 12(4), 563. https://doi.org/10.3390/ma12040563