Fabrication of Diatomite/Silicalite-1 Composites and Their Property for VOCs Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Dt/S-1 Composites
2.2.1. Synthesis of S-1 Zeolite Seeds
2.2.2. Pre-Modification of Dt
2.2.3. Seeds-Assisted Synthesis of Dt/S-1 Composites
2.3. Characterization of Adsorbents
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Characterization of the Adsorbents
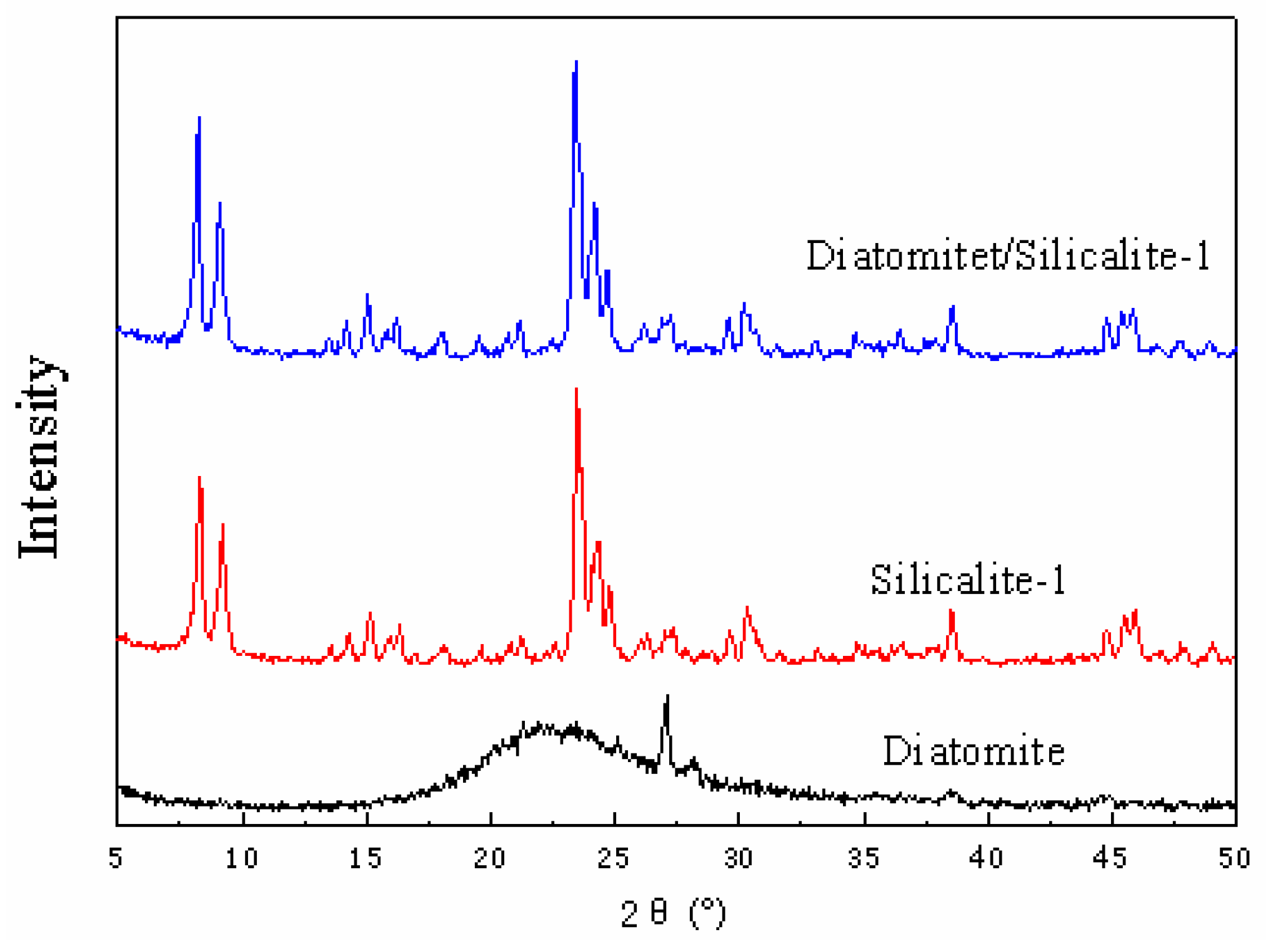
3.1.1. XRD
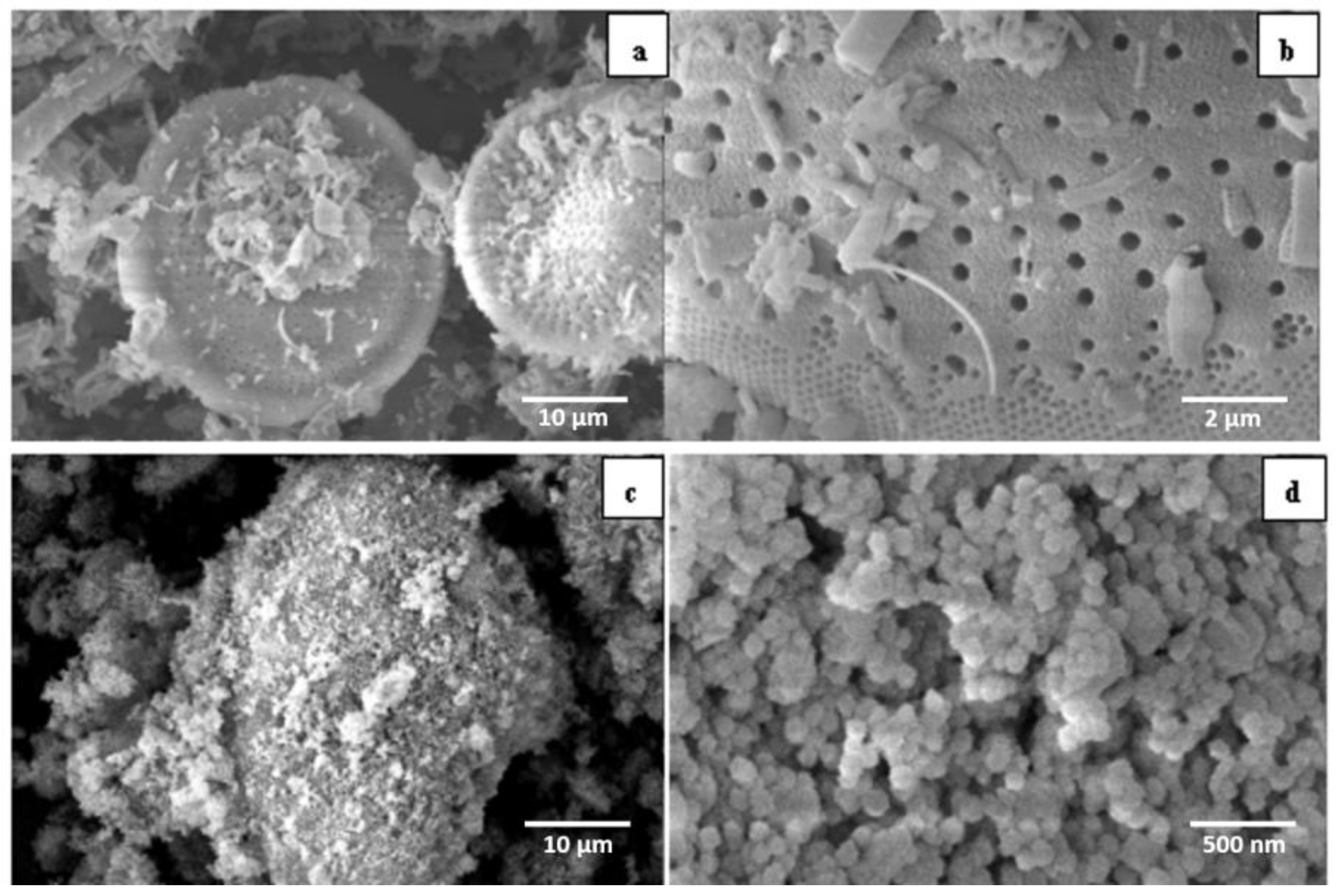
3.1.2. SEM
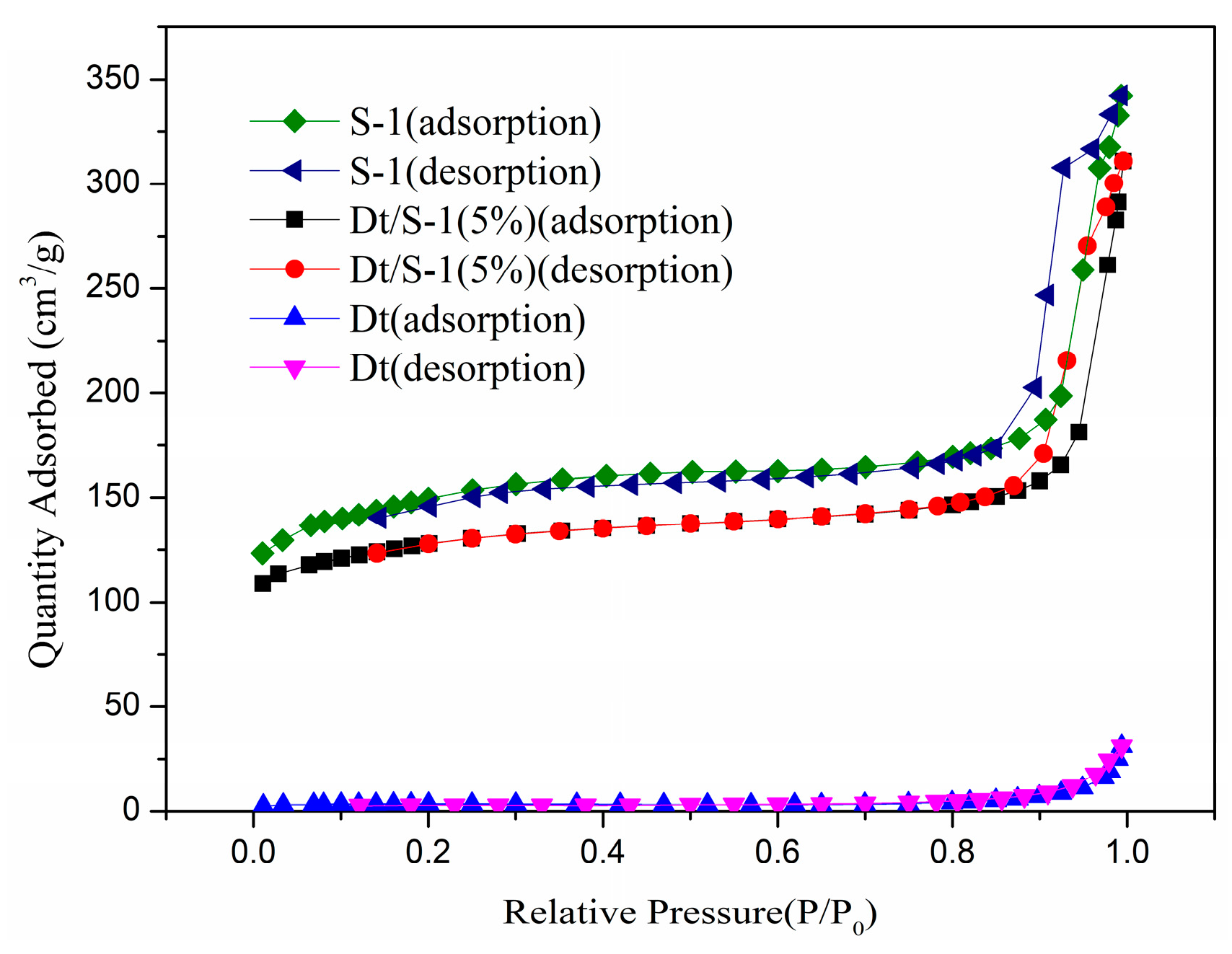
3.1.3. BET
3.2. VOC Adsorption Capacity Tests
3.2.1. Effects of Seeded Zeolite Contents and Hydrothermal Conditions
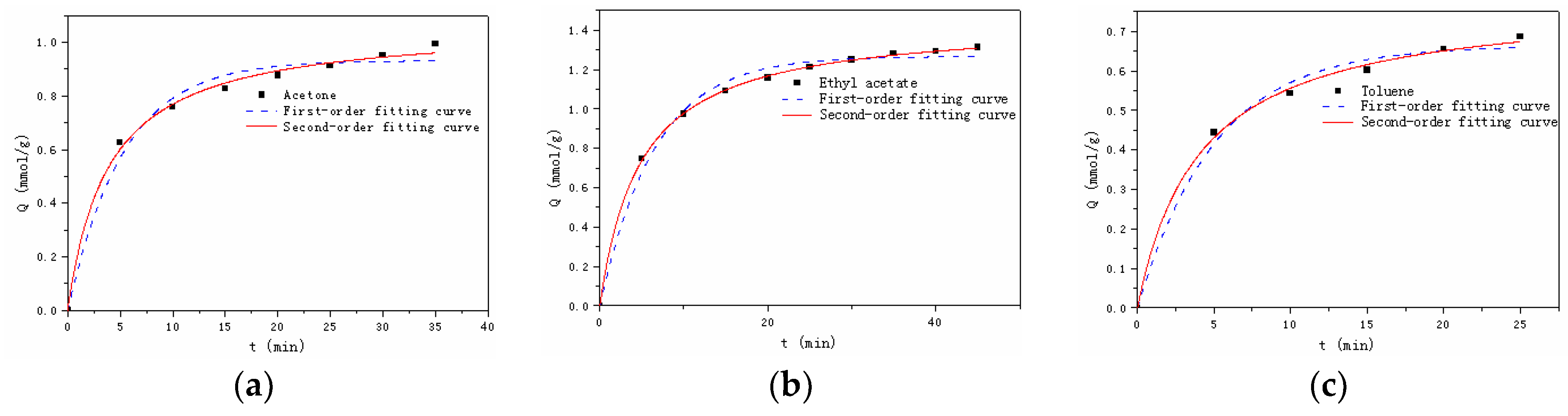
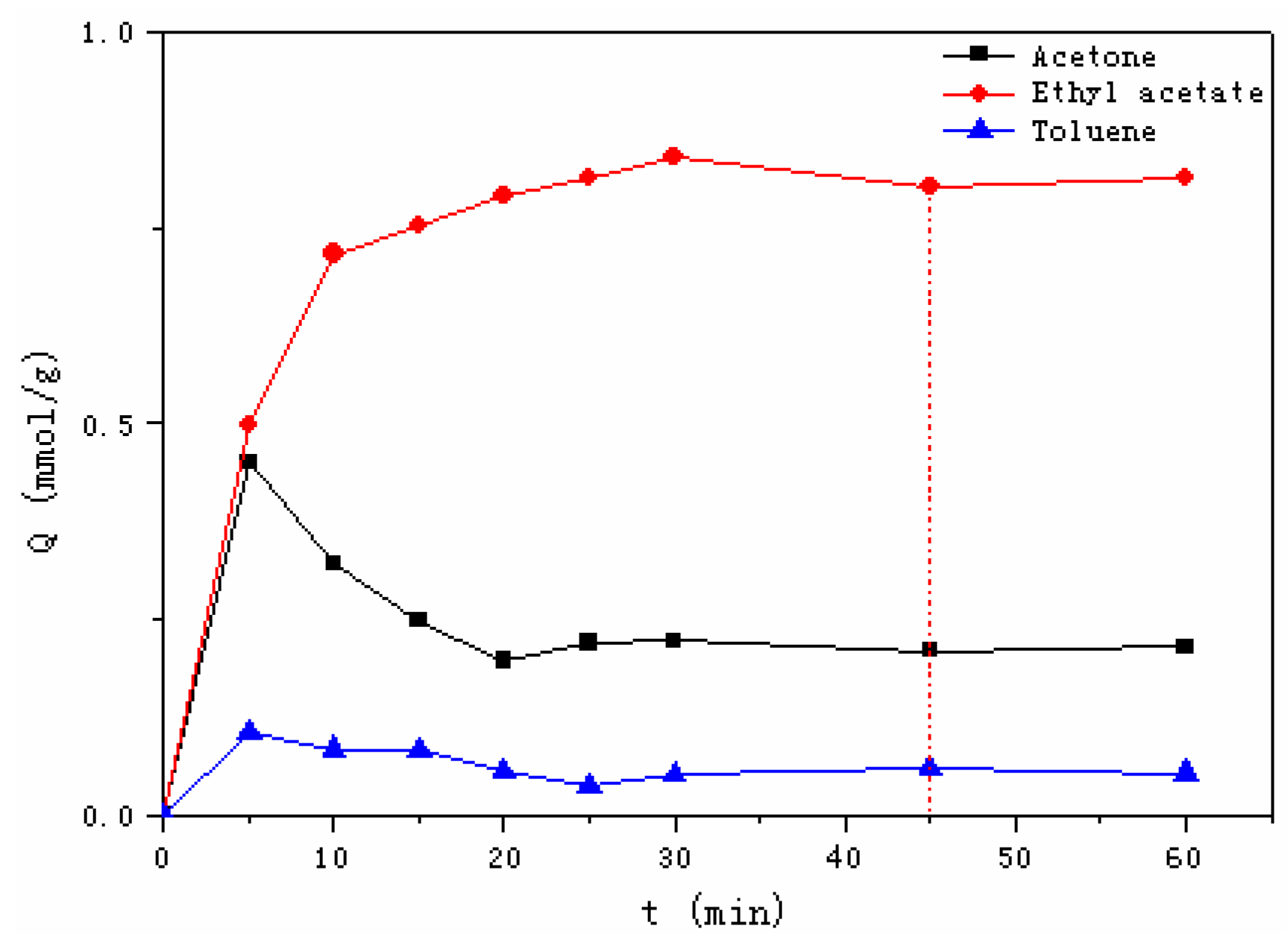
3.2.2. Adsorption Kinetics
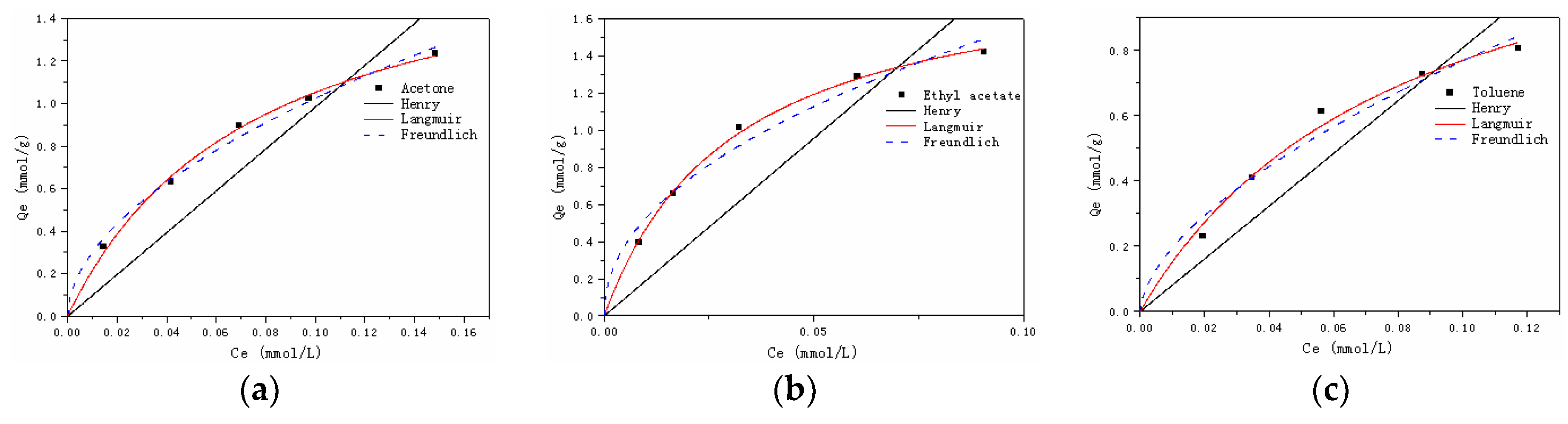
3.2.3. Adsorption Isotherms
3.2.4. Adsorption Selectivity
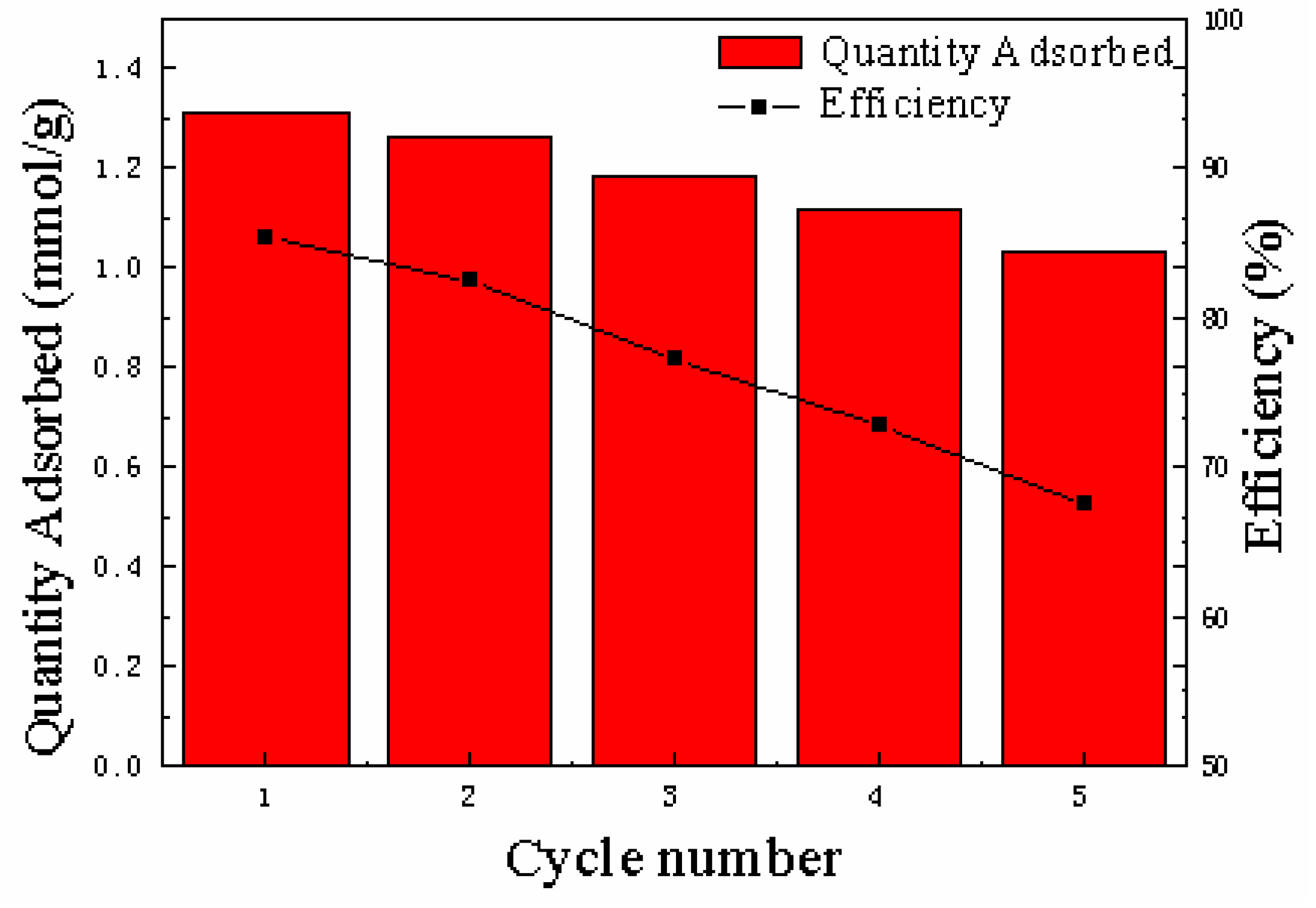
3.2.5. Regeneration of the Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, K.J.; Ahn, H.G. The effect of pore structure of zeolite on the adsorption of VOCs and their desorption properties by microwave heating. Microporous Mesoporous Mater. 2012, 152, 78–83. [Google Scholar] [CrossRef]
- Brown, S.K.; Sim, M.R.; Abramson, M.J.; Gray, C.N. Concentrations of Volatile Organic Compounds in Indoor Air—A Review. Indoor Air 1994, 4, 123–134. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K. Adsorption of aromatic hydrocarbons on highly siliceous MCM-41. Langmuir 2000, 16, 7031–7037. [Google Scholar] [CrossRef]
- Pires, J.; Carvalho, A.; de Carvalho, M.B. Adsorption of volatile organic compounds in Y zeolites and pillared clays. Microporous Mesoporous Mater. 2001, 43, 277–287. [Google Scholar] [CrossRef]
- Cheng, H.F.; Reinhard, M. Sorption of trichloroethylene in hydrophobic micropores of dealuminated Y zeolites and natural minerals. Environ. Sci. Technol. 2006, 40, 7694–7701. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Long, C.; Li, Q.; Qian, H.; Li, A.; Zhang, Q. Adsorption of trichloroethylene and benzene vapors onto hypercrosslinked polymeric resin. J. Hazard. Mater. 2009, 166, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Gaur, V.; Verma, N. Removal of volatile organic compound by activated carbon fiber. Carbon 2004, 42, 2949–2962. [Google Scholar] [CrossRef]
- Lordgooei, M.; Rood, M.J.; Rostam-Abadi, M. Modeling effective diffusivity of volatile organic compounds in activated carbon fiber. Environ. Sci. Technol. 2001, 35, 613–619. [Google Scholar] [CrossRef]
- Aizpuru, A.; Malhautier, L.; Roux, J.C.; Fanlo, J.L. Biofiltration of a mixture of volatile organic compounds on granular activated carbon. Biotechnol. Bioeng. 2003, 83, 479–488. [Google Scholar] [CrossRef]
- Cundy, C.S.; Cox, P.A. The hydrothermal synthesis of zeolites: history and development from the earliest days to the present time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef]
- Giannakopoulos, I.G.; Nikolakis, V. Recovery of hydrocarbons from mixtures containing C3H6, C3H8 and N2 using NaX membranes. J. Membr. Sci. 2007, 305, 332–337. [Google Scholar] [CrossRef]
- Farzaneh, A.; DeJaco, R.F.; Ohlin, L.; Holmgren, A.; Siepmann, J.I.; Grahn, M. Comparative Study of the Effect of Defects on Selective Adsorption of Butanol from Butanol/Water Binary Vapor Mixtures in Silicalite-1 Films. Langmuir 2017, 33, 8420–8427. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Lu, A.H.; Palkovits, R.; Schmidt, W.; Spliethoff, B.; Schuth, F. Hierarchically structured monolithic silicalite-1 consisting of crystallized nanoparticles and its performance in the Beckmann rearrangement of cyclohexanone oxime. J. Am. Chem. Soc. 2005, 127, 12595–12600. [Google Scholar] [CrossRef] [PubMed]
- Burggraaf, A.J.; Vroon, Z.A.E.P.; Keizer, K.; Verweij, H. Permeation of single gases in thin zeolite MFI membranes. J. Membr. Sci. 1998, 144, 77–86. [Google Scholar] [CrossRef]
- Gu, X.; Dong, J.; Nenoff, T.M.; Ozokwelu, D.E. Separation of p-xylene from multicomponent vapor mixtures using tubular MFI zeolite mmbranes. J. Membrane Sci. 2006, 280, 624–633. [Google Scholar] [CrossRef]
- Erdem, E.; Colgecen, G.; Donat, R. The removal of textile dyes by diatomite earth. J. Colloid Interface Sci. 2005, 282, 314–319. [Google Scholar] [CrossRef]
- Al-Degs, Y.; Khraisheh, M.A.M.; Tutunji, M.F. Sorption of lead ions on diatomite and manganese oxides modified diatomite. Water Res. 2001, 35, 3724–3728. [Google Scholar] [CrossRef]
- Xie, F.; Wu, F.; Liu, G.; Mu, Y.; Feng, C.; Wang, H.; Giesy, J.P. Removal of phosphate from eutrophic lakes through adsorption by in situ formation of magnesium hydroxide from diatomite. Environ. Sci. Technol. 2014, 48, 582–590. [Google Scholar] [CrossRef]
- Khraisheh, M.; Aldegs, Y.; McMinn, W. Remediation of wastewater containing heavy metals using raw and modified diatomite. Angew. Chem. Int. Ed. 2004, 99, 177–184. [Google Scholar] [CrossRef]
- Aderson, M.W.; Holms, S.M.; Hanif, N.; Cundy, C.S. Hierarchical pore structures through Diatom Zeolitization. Angew. Chem. Int. Ed. 2000, 39, 2707–2709. [Google Scholar] [CrossRef]
- Yuan, W.; Yuan, P.; Liu, D.; Deng, L.; Zhou, J.; Yu, W.; Chen, F. A hierarchically porous diatomite/silicalite-1 composite for benzene adsorption/desorption fabricated via a facile pre-modification in situ synthesis route. Chem. Eng. J. 2016, 294, 333–342. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, D.; Lin, Z.; He, H.; Wen, X.; Wang, L.; Deng, F. Influences of pretreatment temperature on the surface silylation of diatomaceous amorphous silica with trimethylchlorosilane. J. Non-Cryst. Solids 2006, 352, 3762–3771. [Google Scholar] [CrossRef]
- Panacek, A.; Balzerova, A.; Prucek, R.; Ranc, V.; Vecerova, R.; Husickova, V.; Pechoušek, J.; Filip, J.; Zbořil, R.; Kvítek, L. Preparation, characterization and antimicrobial efficiency of Ag/PDDA-diatomite nanocomposite. Colloids Surf. B 2013, 110, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Tan, D.Y.; Liu, K.K.; Yu, H.G.; Zhong, Y.H.; Yuan, A.H.; Yu, W.B.; He, H.P. Surface silylation of mesoporous/macroporous diatomite (diatomaceous earth) and its function in Cu(II) adsorption: The effects of heating pretreatment. Microporous Mesoporous Mater. 2013, 170, 9–19. [Google Scholar] [CrossRef]
- Holland, B.T.; Abrams, L.; Stein, A. Dual templating of macroporous silicates with zeolitic microporous frameworks. J. Am. Chem. Soc. 1999, 121, 4308–4309. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, D.; Fan, M.; Yang, D.; Zhu, R.; Ge, F.; Zhu, J.; He, H. Removal of hexavalent chromium [Cr(VI)] from aqueous solutions by the diatomite-supported/unsupported magnetite nanoparticles. J. Hazard. Mater. 2010, 173, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Liu, J. Surface modification of coconut shell based activated carbon for the improvement of hydrophobic VOC removal. J. Hazard. Mater. 2011, 192, 683–690. [Google Scholar] [CrossRef]
- Jhung, S.H.; Yoon, J.W.; Lee, J.S.; Chang, J.S. Low-temperature adsorption/storage of hydrogen on FAU, MFI, and MOR zeolites with various Si/Al ratios: effect of electrostatic fields and pore structures. Chemistry 2007, 13, 6502–6507. [Google Scholar] [CrossRef]
- Jahandar, L.M.; Atkinson, J.D.; Hashisho, Z.; Phillips, J.H.; Anderson, J.E.; Nichols, M. The role of beaded activated carbon’s surface oxygen groups on irreversible adsorption of organic vapors. J. Hazard. Mater. 2016, 317, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Gong, C.; Zhang, Z.; Yuan, G. Removal of VOCs by activated carbon microspheres derived from polymer: a comparative study. Adsorption 2015, 21, 333–341. [Google Scholar] [CrossRef]
- Fletcher, A.J.; Yüzak, Y.; Thomas, K.M. Adsorption and desorption kinetics for hydrophilic and hydrophobic vapors on activated carbon. Carbon 2006, 44, 989–1004. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Tsapatsis, M. Permeation of aromatic isomer vapors through oriented MFI-type membranes made by secondary growth. Chem. Mater. 1999, 11, 875–878. [Google Scholar] [CrossRef]
- Ali, S.; Wang, F.; Zubair Iqbal, M.; Ullah Shah, H.; Zafar, S. Hydrothermal synthesis, characterization and optical properties of SnS prismatic nanorods. Mater. Lett. 2017, 206, 22–25. [Google Scholar] [CrossRef]
- Song, W.; Justice, R.E.; Jones, C.A.; Grassian, V.H.; Larsen, S.C. Size-dependent properties of nanocrystalline silicalite synthesized with systematically varied crystal sizes. Langmuir 2004, 20, 4696–4702. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Huang, G.; Mu, S.; An, C.; Chen, X. Immobilization of phenanthrene onto gemini surfactant modified sepiolite at solid/aqueous interface: Equilibrium, thermodynamic and kinetic studies. Sci. Total Environ. 2017, 598, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Ashour, R.M.; El-sayed, R.; Abdel-Magied, A.F.; Abdel-khalek, A.A.; Ali, M.M.; Forsberg, K.; Uheidaa, A.; Muhammedf, M.; Duttaa, J. Selective separation of rare earth ions from aqueous solution using functionalized magnetite nanoparticles: kinetic and thermodynamic studies. Chem. Eng. J. 2017, 327, 286–296. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Y.; Liu, S.; Zeng, G.; Tan, X.; Huang, B.; Tang, X.; Wang, S.; Hua, Q.; Yan, Z. Competitive adsorption of Pb(II), Cd(II) and Cu(II) onto chitosan-pyromellitic dianhydride modified biochar. J Colloid Interface Sci. 2017, 506, 355–364. [Google Scholar] [CrossRef]
- Abo Markeb, A.; Alonso, A.; Sanchez, A.; Font, X. Adsorption process of fluoride from drinking water with magnetic core-shell Ce-Ti@Fe3O4 and Ce-Ti oxide nanoparticles. Sci. Total Environ. 2017, 598, 949–958. [Google Scholar] [CrossRef]
| Sample | Dt | S-1 | Dt/S-1 (5 wt%) |
|---|---|---|---|
| SBET (m2/g) | 10.0 | 532.0 | 398.8 |
| Vmicropore (cm3/g) | 0.005 | 0.170 | 0.131 |
| Vtotal (cm3/g) | 0.045 | 0.515 | 0.342 |
| Dt/S-1 (wt%) | S-1 | Dt | ||||
|---|---|---|---|---|---|---|
| Sample | 1 | 5 | 10 | 20 | ||
| Acetone | 0.82 ± 0.04 | 1.01 ± 0.06 | 1.02 ± 0.05 | 0.80 ± 0.04 | 1.01 ± 0.05 | 0.07 ± 0.002 |
| Ethyl acetate | 1.10 ± 0.05 | 1.31 ± 0.08 | 1.28 ± 0.08 | 0.92 ± 0.04 | 1.28 ± 0.08 | 0.06 ± 0.002 |
| Toluene | 0.62 ± 0.02 | 0.71 ± 0.03 | 0.69 ± 0.02 | 0.48 ± 0.01 | 0.72 ± 0.03 | 0.12 ± 0.005 |
| Sample | 90 °C | 100 °C | 110 °C |
|---|---|---|---|
| Acetone | 0.58 ± 0.02 | 1.01 ± 0.06 | 0.97 ± 0.05 |
| Ethyl acetate | 0.90 ± 0.05 | 1.31 ± 0.08 | 1.22 ± 0.07 |
| Toluene | 0.48 ± 0.02 | 0.71 ± 0.04 | 0.68 ± 0.03 |
| Sample | 3 Days | 4 Days | 5 Days |
|---|---|---|---|
| Acetone | 0.88 ± 0.05 | 1.01 ± 0.06 | 0.98 ± 0.05 |
| Ethyl acetate | 1.17 ± 0.06 | 1.31 ± 0.08 | 1.19 ± 0.07 |
| Toluene | 0.71 ± 0.04 | 0.71 ± 0.03 | 0.69 ± 0.04 |
| Model | Pseudo First-Order Model | Pseudo Second-Order Model | |||||
|---|---|---|---|---|---|---|---|
| Parameter | Qe (exp, mmol/g) | Qe (cal, mmol/g) | k1 | R2 | Qe(cal, mmol/g) | k2 | R2 |
| Acetone | 0.99 | 0.91 | 0.21 | 0.9874 | 1.04 | 0.27 | 0.9981 |
| Ethyl acetate | 1.31 | 1.22 | 0.17 | 0.9908 | 1.39 | 0.17 | 0.9995 |
| Toluene | 0.69 | 0.62 | 0.22 | 0.9948 | 0.75 | 0.35 | 0.9993 |
| Model | Henry | Langmuir | Freundhch | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | kH | R2 | kL | Qmax | R2 | kF | n | R2 |
| Acetone | 9.84 | 0.8250 | 13.24 | 1.85 | 0.9974 | 3.50 | 0.53 | 0.9933 |
| Ethyl acetate | 19.15 | 0.7028 | 32.45 | 1.93 | 0.9986 | 4.62 | 0.47 | 0.9787 |
| Toluene | 8.08 | 0.8601 | 12.07 | 1.41 | 0.9903 | 3.03 | 0.60 | 0.9743 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tian, T. Fabrication of Diatomite/Silicalite-1 Composites and Their Property for VOCs Adsorption. Materials 2019, 12, 551. https://doi.org/10.3390/ma12040551
Liu Y, Tian T. Fabrication of Diatomite/Silicalite-1 Composites and Their Property for VOCs Adsorption. Materials. 2019; 12(4):551. https://doi.org/10.3390/ma12040551
Chicago/Turabian StyleLiu, Yutong, and Tao Tian. 2019. "Fabrication of Diatomite/Silicalite-1 Composites and Their Property for VOCs Adsorption" Materials 12, no. 4: 551. https://doi.org/10.3390/ma12040551
APA StyleLiu, Y., & Tian, T. (2019). Fabrication of Diatomite/Silicalite-1 Composites and Their Property for VOCs Adsorption. Materials, 12(4), 551. https://doi.org/10.3390/ma12040551




