IPS e.max for All-Ceramic Restorations: Clinical Survival and Success Rates of Full-Coverage Crowns and Fixed Partial Dentures
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Baseline Data
3.2. Non-Recommended Uses and Restoration Subtypes
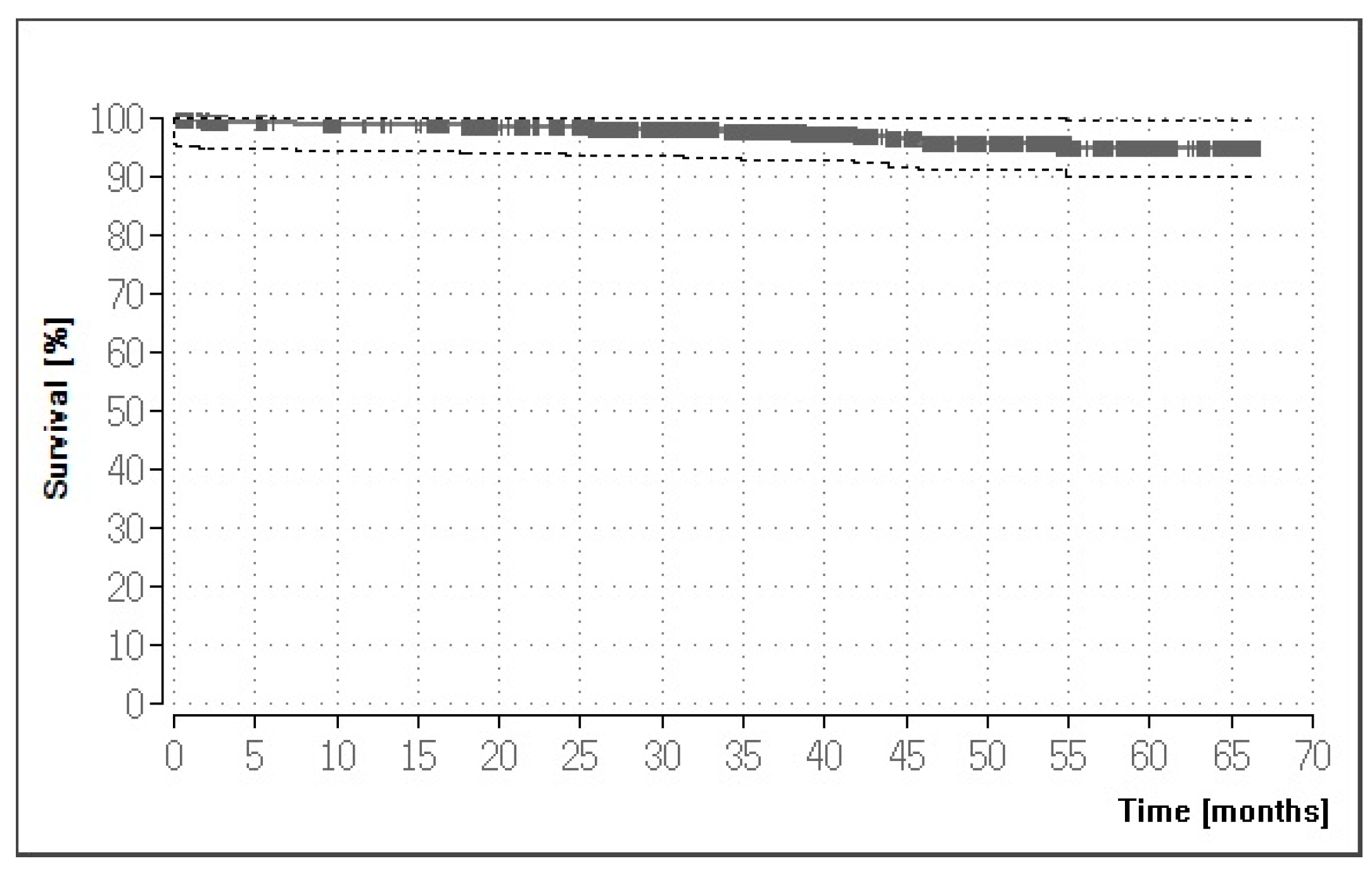
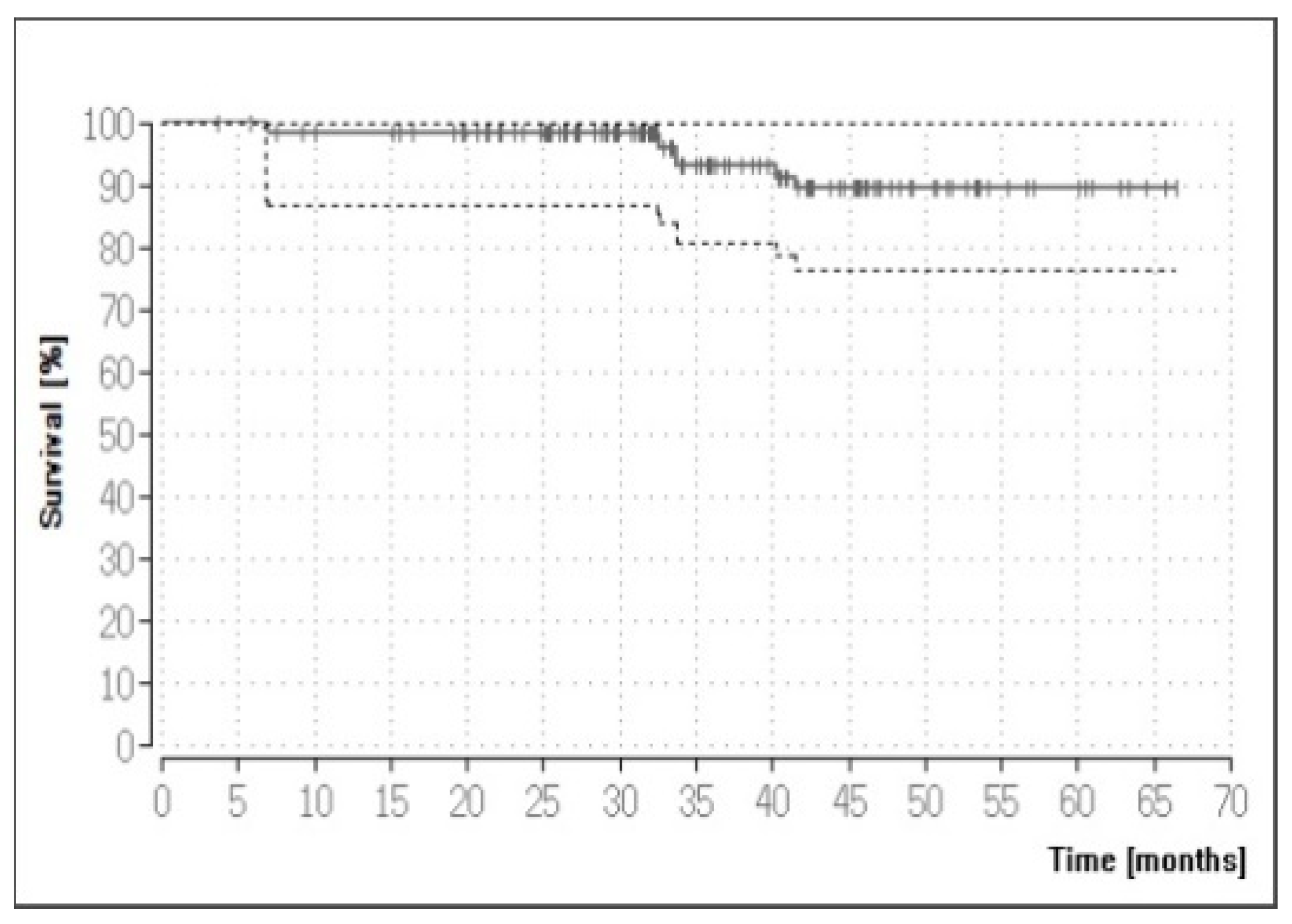
3.3. Overall Success and Survival
3.4. Results for the Crown Restorations
3.5. Results for the FPD Restorations
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Tang, X.; Tang, C.; Su, H.; Luo, H.; Nakamura, T.; Yatani, H. The effects of repeated heat-pressing on the mechanical properties and microstructure of IPS e.max Press. J. Mech. Behav. Biomed. Mater. 2014, 40, 390–396. [Google Scholar] [CrossRef]
- Guess, P.C.; Zavanelli, R.A.; Silva, N.R.; Bonfante, E.A.; Coelho, P.G.; Thompson, V.P. Monolithic CAD/CAM lithium disilicate versus veneered Y-TZP crowns: Comparison of failure modes and reliability after fatigue. Int. J. Prosthodont. 2010, 23, 434–442. [Google Scholar]
- Rosentritt, M.; Hahnel, S.; Engelhardt, F.; Behr, M.; Preis, V. In vitro performance and fracture resistance of CAD/CAM-fabricated implant supported molar crowns. Clin. Oral. Investig. 2017, 21, 1213–1219. [Google Scholar] [CrossRef]
- Ke, J.; He, F.; Ye, J. Enhancing the bioactivity of yttria-stabilized tetragonal zirconia ceramics via grain-boundary activation. ACS Appl. Mater. Interfaces 2017, 9, 16015–16025. [Google Scholar] [CrossRef]
- Pieger, S.; Salman, A.; Bidra, A.S. Clinical outcomes of lithium disilicate single crowns and partial fixed dental prostheses: A systematic review. J. Prosthet. Dent. 2014, 112, 22–30. [Google Scholar] [CrossRef]
- Fasbinder, D.J.; Dennison, J.B.; Heys, D.; Neiva, G. A clinical evaluation of chairside lithium disilicate CAD/CAM crowns: A two-year report. J. Am. Dent. Assoc. 2010, 141 (Suppl. 2), 10S–14S. [Google Scholar] [CrossRef]
- Kern, M.; Sasse, M.; Wolfart, S. Ten-year outcome of three-unit fixed dental prostheses made from monolithic lithium disilicate ceramic. J. Am. Dent. Assoc. 2012, 143, 234–240. [Google Scholar] [CrossRef]
- Monaco, C.; Rosentritt, M.; Llukacej, A.; Baldissara, P.; Scotti, R. Marginal adaptation, gap width, and fracture strength of teeth restored with different all-ceramic vs metal ceramic crown systems: An in vitro study. Eur. J. Prosthodont. Restor. Dent. 2016, 24, 130–137. [Google Scholar] [CrossRef]
- Etman, M.K.; Woolford, M.J. Three-year clinical evaluation of two ceramic crown systems: A preliminary study. J. Prosthet. Dent. 2010, 103, 80–90. [Google Scholar] [CrossRef]
- Cortellini, D.; Canale, A. Bonding lithium disilicate ceramic to feather-edge tooth preparations: A minimally invasive treatment concept. J. Adhes. Dent. 2012, 14, 7–10. [Google Scholar] [CrossRef]
- Gehrt, M.; Wolfart, S.; Rafai, N.; Reich, S.; Edelhoff, D. Clinical results of lithium-disilicate crowns after up to 9 years of service. Clin. Oral Investig. 2013, 17, 275–284. [Google Scholar] [CrossRef]
- Rauch, A.; Reich, S.; Schierz, O. Chair-side generated posterior monolithic lithium disilicate crowns: Clinical survival after 6 years. Clin. Oral. Investig. 2017, 21, 2083–2089. [Google Scholar] [CrossRef]
- Reich, S.; Schierz, O. Chair-side generated posterior lithium disilicate crowns after 4 years. Clin. Oral. Investig. 2013, 17, 1765–1772. [Google Scholar] [CrossRef]
- Fabbri, G.; Zarone, F.; Dellificorelli, G.; Cannistraro, G.; De Lorenzi, M.; Mosca, A.; Sorrentino, R. Clinical evaluation of 860 anterior and posterior lithium disilicate restorations: Retrospective study with a mean follow-up of 3 years and a maximum observational period of 6 years. Int. J. Periodontics Restor. Dent. 2014, 34, 165–177. [Google Scholar] [CrossRef]
- Reich, S.; Endres, L.; Weber, C.; Wiedhahn, K.; Neumann, P.; Schneider, O.; Rafai, N.; Wolfart, S. Three-unit CAD/CAM-generated lithium disilicate FDPs after a mean observation time of 46 months. Clin. Oral Investig. 2014, 18, 2171–2178. [Google Scholar] [CrossRef]
- Simeone, P.; Gracis, S. Eleven-year retrospective survival study of 275 veneered lithium disilicate single crowns. Int. J. Periodontics Restor. Dent. 2015, 35, 685–694. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, J.; Gao, J.; Guo, J.; Li, L.; Zhao, Y.; Zhang, S. Clinical outcomes of different types of tooth-supported bilayer lithium disilicate all-ceramic restorations after functioning up to 5 years: A retrospective study. J. Dent. 2016, 51, 56–61. [Google Scholar] [CrossRef]
- Kern, M.; Beuer, F.; Frankenberger, R.; Kohal, R.J.; Kunzelmann, K.H.; Mehl, A.; Pospiech, P.; Reiss, B. Vollkeramik auf einen Blick; Arbeitsgemeinschaft für Keramik in der Zahnheilkunde e.V.: Esslingen, Germany, 2015. [Google Scholar]
- Zhang, Z.; Yi, Y.; Wang, X.; Guo, J.; Li, D.; He, L.; Zhang, S. A comparative study of progressive wear of four dental monolithic, veneered glass-ceramics. J. Mech. Behav. Biomed. Mater. 2017, 74, 111–117. [Google Scholar] [CrossRef]
- Ivoclar Vivadent. Available online: http://www.ivoclarvivadent.com/en/p/all/products/all-ceramics/ips-emax-technicians/ips-emax-press (accessed on 7 December 2018).
- Ivoclar Vivadent. Available online: http://www.ivoclarvivadent.com/en/p/all/all-ceramics/ips-emax-system-technicians/ips-emax-cad/ips-emax-cad-monolithic-solutions (accessed on 7 December 2018).
- Miura, S.; Kasahara, S.; Yamauchi, S.; Okuyama, Y.; Izumida, A.; Aida, J.; Egusa, H. Clinical evaluation of zirconia-based all-ceramic single crowns: An up to 12-year retrospective cohort study. Clin. Oral Investig. 2018, 22, 697–706. [Google Scholar] [CrossRef]
- Clausen, J.O.; Abou Tara, M.; Kern, M. Dynamic fatigue and fracture resistance of non-retentive all-ceramic full-coverage molar restorations. Influence of ceramic material and preparation design. Dent. Mater. 2010, 26, 533–538. [Google Scholar] [CrossRef]
- Obermeier, M.; Ristow, O.; Erdelt, K.; Beuer, F. Mechanical performance of cement- and screw-retained all-ceramic single crowns on dental implants. Clin. Oral Investig. 2018, 22, 981–991. [Google Scholar] [CrossRef]
- Aldegheishem, A.; Ioannidis, G.; Att, W.; Petridis, H. Success and survival of various types of all-ceramic single crowns: A critical review and analysis of studies with a mean follow-up of 5 years or longer. Int. J. Prosthodont. 2017, 30, 168–181. [Google Scholar] [CrossRef]
- Blatz, M.B.; Sadan, A.; Kern, M. Resin-ceramic bonding: A review of the literature. J. Prosthet. Dent. 2003, 89, 268–274. [Google Scholar] [CrossRef]
- Hooshmand, T.; Rostami, G.; Behroozibakhsh, M.; Fatemi, M.; Keshvad, A.; van Noort, R. Interfacial fracture toughness of different resin cements bonded to a lithium disilicate glass ceramic. J. Dent. 2012, 40, 139–145. [Google Scholar] [CrossRef]
- Lekesiz, H. Reliability estimation for single-unit ceramic crown restorations. J. Dent. Res. 2014, 93, 923–928. [Google Scholar] [CrossRef]
- Pospiech, P. All-ceramic crowns: Bonding or cementing? Clin. Oral Investig. 2002, 6, 189–197. [Google Scholar] [CrossRef]
- Martins, M.E.; Leite, F.P.; Queiroz, J.R.; Vanderlei, A.D.; Reskalla, H.N.; Ozcan, M. Does the ultrasonic cleaning medium affect the adhesion of resin cement to feldspathic ceramic? J. Adhes. Dent. 2012, 14, 507–509. [Google Scholar] [CrossRef]
- Esquivel-Upshaw, J.F.; Young, H.; Jones, J.; Yang, M.; Anusavice, K.J. Four-year clinical performance of a lithia disilicate-based core ceramic for posterior fixed partial dentures. Int. J. Prosthodont. 2008, 21, 155–160. [Google Scholar]
- Huettig, F.; Gehrke, U.P. Early complications and performance of 327 heat-pressed lithium disilicate crowns up to five years. J. Adv. Prosthodont. 2016, 8, 194–200. [Google Scholar] [CrossRef]
- Fedorowicz, Z.; Carter, B.; de Souza, R.F.; Chaves, C.A.; Nasser, M.; Sequeira-Byron, P. Single crowns versus conventional fillings for the restoration of root filled teeth. Cochrane Database Syst. Rev. 2012, CD009109. [Google Scholar] [CrossRef]
- Toman, M.; Toksavul, S. Clinical evaluation of 121 lithium disilicate all-ceramic crowns up to 9 years. Quintessence Int. 2015, 46, 189–197. [Google Scholar] [CrossRef]
- Weiß, C. Basiswissen Medizinische Statistik; Springer Medizin Verlag: Heidelberg, Germany, 2010. [Google Scholar]
- Kistler, S.; Pospiech, P.; Frasch, C.; Gernet, W. Clinical performance of Empress 2 lithium-disilicate-glassceramic posterior crowns: Results up to two years. J. Dent. Res. 2000, 79, 172. [Google Scholar]
- Cooper, L.F.; Stanford, C.; Feine, J.; McGuire, M. Prospective assessment of CAD/CAM zirconia abutment and lithium disilicate crown restorations: 2.4 year results. J. Prosthet. Dent. 2016, 116, 33–39. [Google Scholar] [CrossRef]
- Dogan, D.O.; Gorler, O.; Mutaf, B.; Ozcan, M.; Eyuboglu, G.B.; Ulgey, M. Fracture resistance of molar crowns fabricated with monolithic all-ceramic CAD/CAM materials cemented on titanium abutments: An in vitro study. J. Prosthodont. 2017, 26, 309–314. [Google Scholar] [CrossRef]
| Materials | n | Distribution |
|---|---|---|
| Total | 1058 | 100.00% |
| IPS e.max Press | 861 | 81.38% |
| Zirconia framework + IPS e.max ZirPress | 87 | 8.22% |
| IPS e.max CAD | 50 | 4.73% |
| Zirconia framework + IPS e.max Ceram | 30 | 2.84% |
| IPS e.max Press + IPS e.max Ceram | 27 | 2.55% |
| IPS e.max Ceram | 3 | 0.28% |
| Patients | Evaluable Restorations | ||
| Total (n) | 368 | Total (n) | 1058 |
| Female/male (n) | 206/162 | Full-coverage crowns (n) | 922 |
| Mean age (years) | 57.84 | Maxilla (n) | 540 |
| Cementation | Mandible (n) | 382 | |
| Total (n) | 1058 | Fixed partial dentures (n) | 136 |
| Adhesive (n) | 564 | Maxilla (n) | 69 |
| Conventional (n) | 494 | Mandible (n) | 67 |
| Restorations | n | Distribution |
|---|---|---|
| All restorations | 1058 | 100.00% |
| Tooth-supported single crowns | 615 | 58.13% |
| Implant-supported single crowns | 156 | 14.74% |
| Tooth-supported splinted crowns | 126 | 11.91% |
| Tooth-supported FPDs | 83 | 7.84% |
| Implant-supported FPDs | 44 | 4.16% |
| Other 1 | 21 | 1.98% |
| Implant-supported splinted crowns | 13 | 1.23% |
| Restorations | n | Survival |
|---|---|---|
| Cumulative (all restorations) | 1058 | 94.22% |
| All full-coverage crowns | 922 | 94.90% |
| Recommended uses | 807 | 94.51% |
| Lithium-disilicate crowns 1 | 768 | 94.69% |
| Veneered zirconia-based crowns | 39 | 100.00% |
| Non-recommended uses | 115 | 94.95% |
| All fixed partial dentures | 136 | 89.44% |
| Recommended uses | 93 | 88.47% |
| Lithium-disilicate FDPs 1 | 43 | 90.58% |
| Veneered zirconia-based FDPs | 50 | 90.06% |
| Non-recommended uses | 43 | 95.35% |
| Causes of Failure | n | Distribution |
|---|---|---|
| All full-coverage crowns | 922 | 100.00% |
| Total number of failures | 27 | 2.93% |
| Fracture of the restoration | 5 | 0.54% |
| Apical osteitis | 5 | 0.54% |
| Loss of retention | 4 | 0.43% |
| Hypersensitivity | 4 | 0.43% |
| Pre-prosthetic core fracture | 3 | 0.33% |
| Chipping | 2 | 0.22% |
| Root fracture | 2 | 0.22% |
| Loss of implant | 1 | 0.11% |
| Secondary caries | 1 | 0.11% |
| Causes of Failure | n | Distribution |
|---|---|---|
| All fixed partial dentures (FPDs) | 136 | 100.00% |
| Total number of failures | 8 | 5.88% |
| Endodontic complications | 3 | 2.21% |
| Ceramic chipping/fracture | 2 | 1.47% |
| Root fracture | 2 | 1.47% |
| Preprosthetic core fracture | 1 | 0.74% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandt, S.; Winter, A.; Lauer, H.-C.; Kollmar, F.; Portscher-Kim, S.-J.; Romanos, G.E. IPS e.max for All-Ceramic Restorations: Clinical Survival and Success Rates of Full-Coverage Crowns and Fixed Partial Dentures. Materials 2019, 12, 462. https://doi.org/10.3390/ma12030462
Brandt S, Winter A, Lauer H-C, Kollmar F, Portscher-Kim S-J, Romanos GE. IPS e.max for All-Ceramic Restorations: Clinical Survival and Success Rates of Full-Coverage Crowns and Fixed Partial Dentures. Materials. 2019; 12(3):462. https://doi.org/10.3390/ma12030462
Chicago/Turabian StyleBrandt, Silvia, Anna Winter, Hans-Christoph Lauer, Fritz Kollmar, Soo-Jeong Portscher-Kim, and Georgios E. Romanos. 2019. "IPS e.max for All-Ceramic Restorations: Clinical Survival and Success Rates of Full-Coverage Crowns and Fixed Partial Dentures" Materials 12, no. 3: 462. https://doi.org/10.3390/ma12030462
APA StyleBrandt, S., Winter, A., Lauer, H.-C., Kollmar, F., Portscher-Kim, S.-J., & Romanos, G. E. (2019). IPS e.max for All-Ceramic Restorations: Clinical Survival and Success Rates of Full-Coverage Crowns and Fixed Partial Dentures. Materials, 12(3), 462. https://doi.org/10.3390/ma12030462






