Effects of the Particle Size of BaTiO3 Fillers on Fabrication and Dielectric Properties of BaTiO3/Polymer/Al Films for Capacitor Energy-Storage Application
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Setup
2.2. Fabrication of BaTiO3/Polymer/Al Films
2.3. Characterization and Dielectric Property Test
3. Results and Discussion
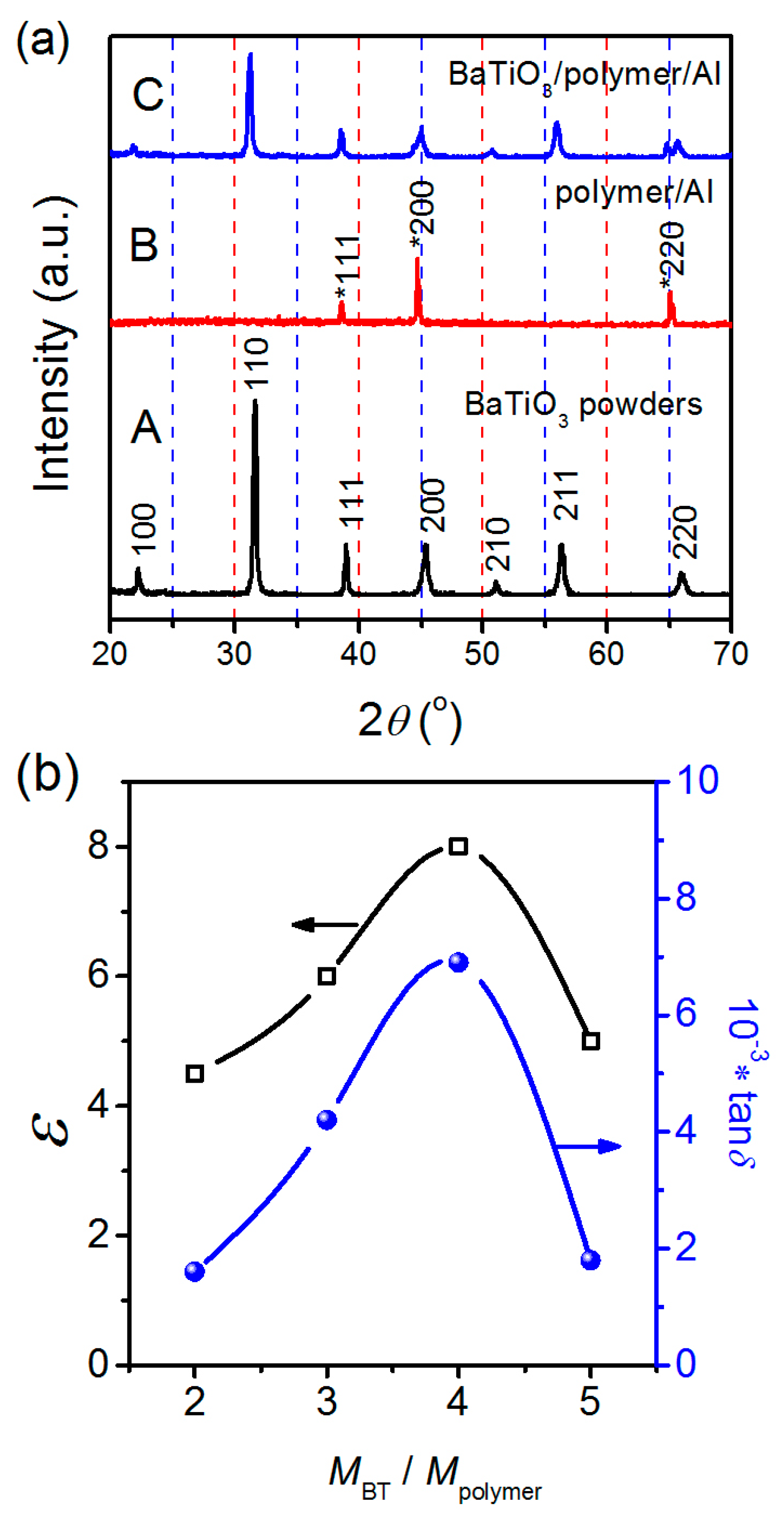
3.1. Fabrication of BPA Composite Films
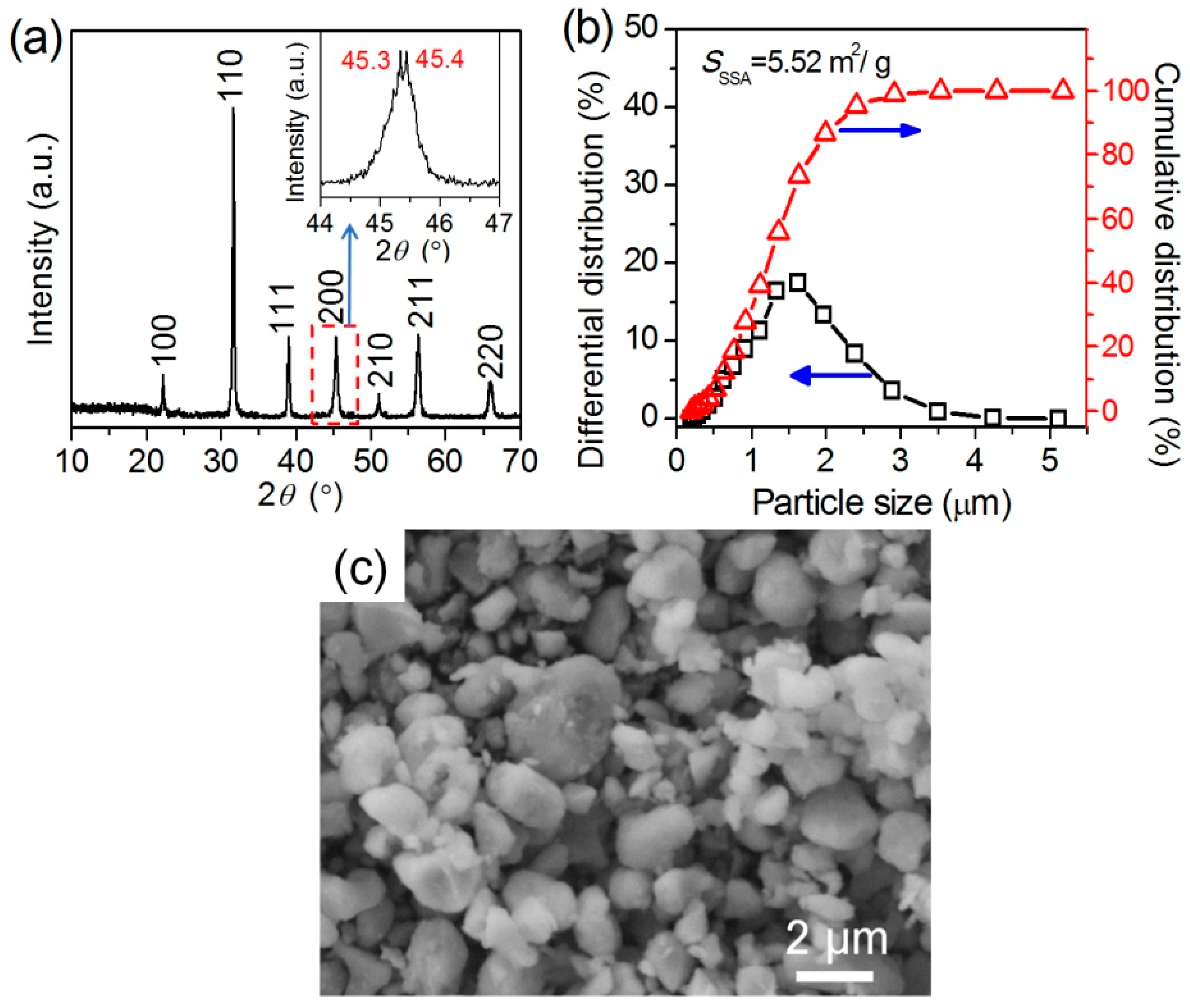
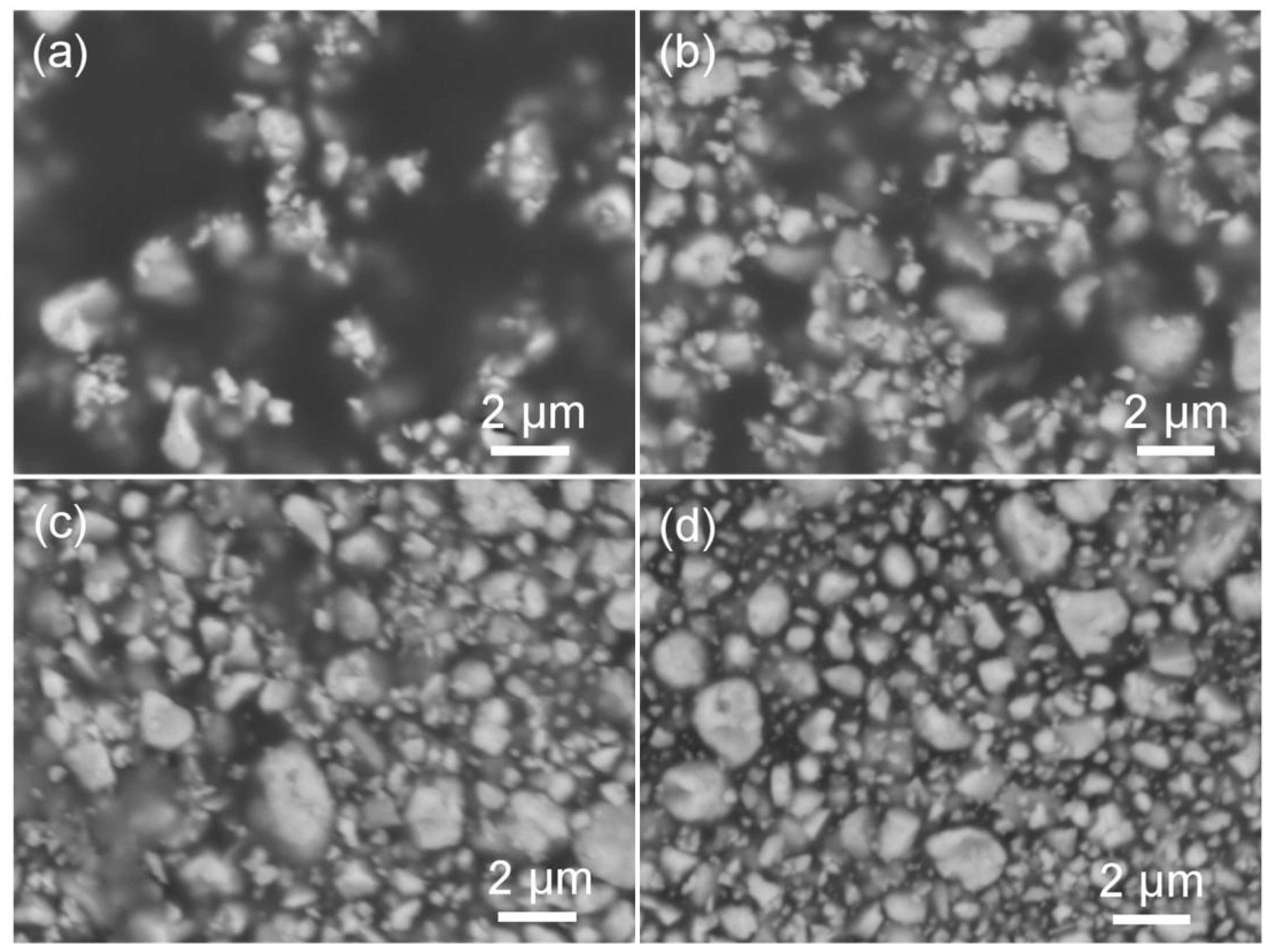
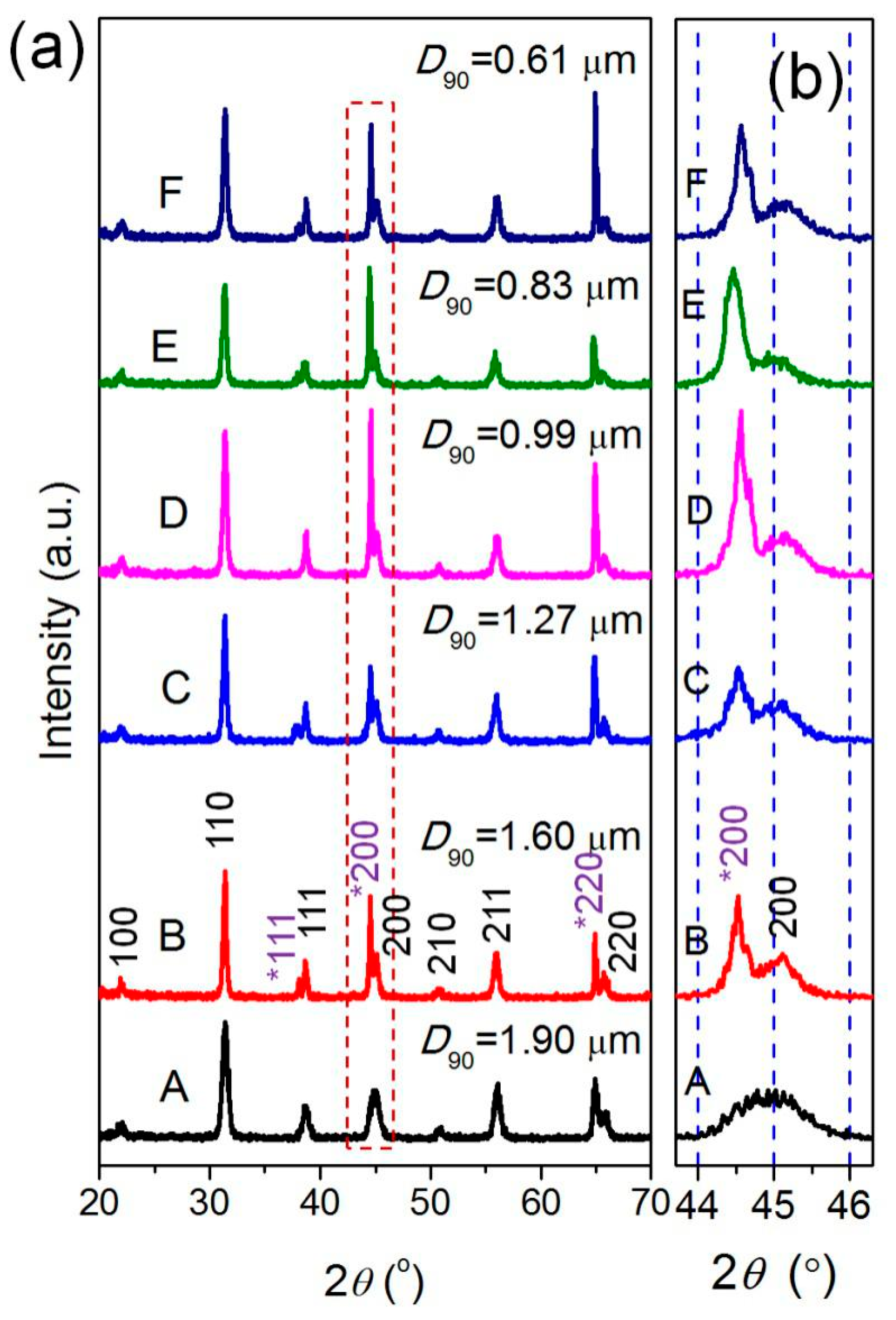
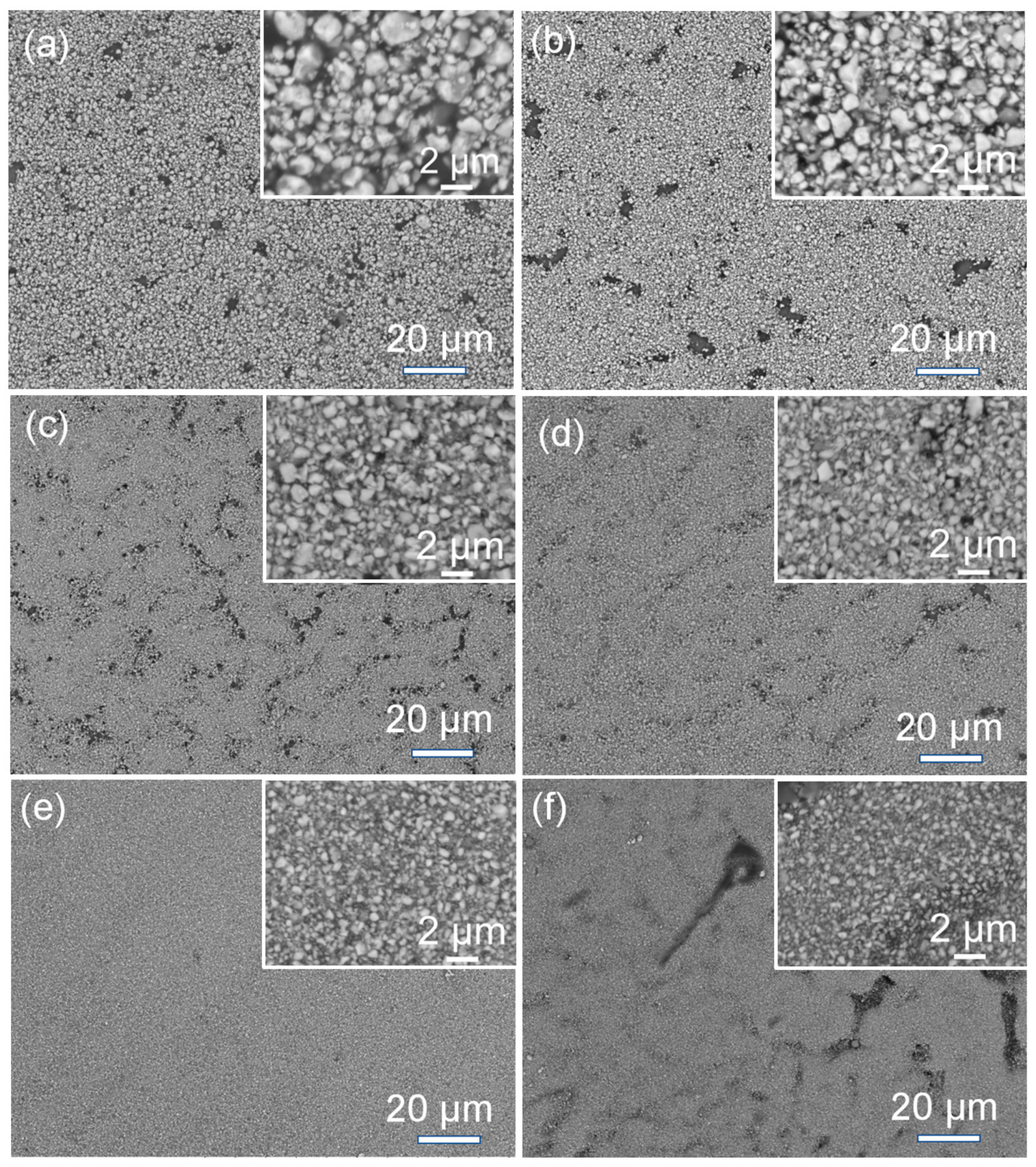
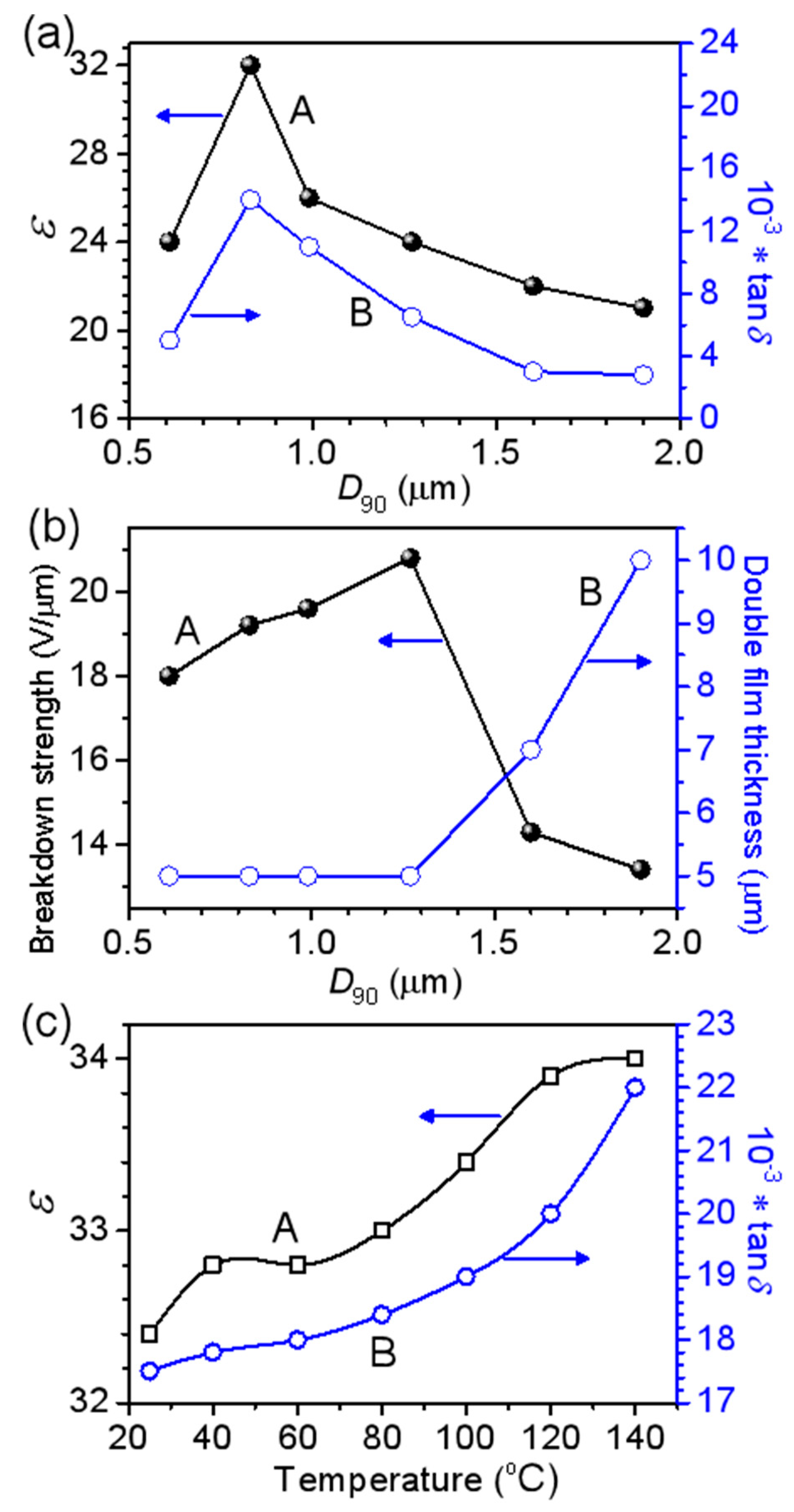
3.2. BaTiO3/Polymer/Al Composite Films with Pristine BaTiO3 Powders
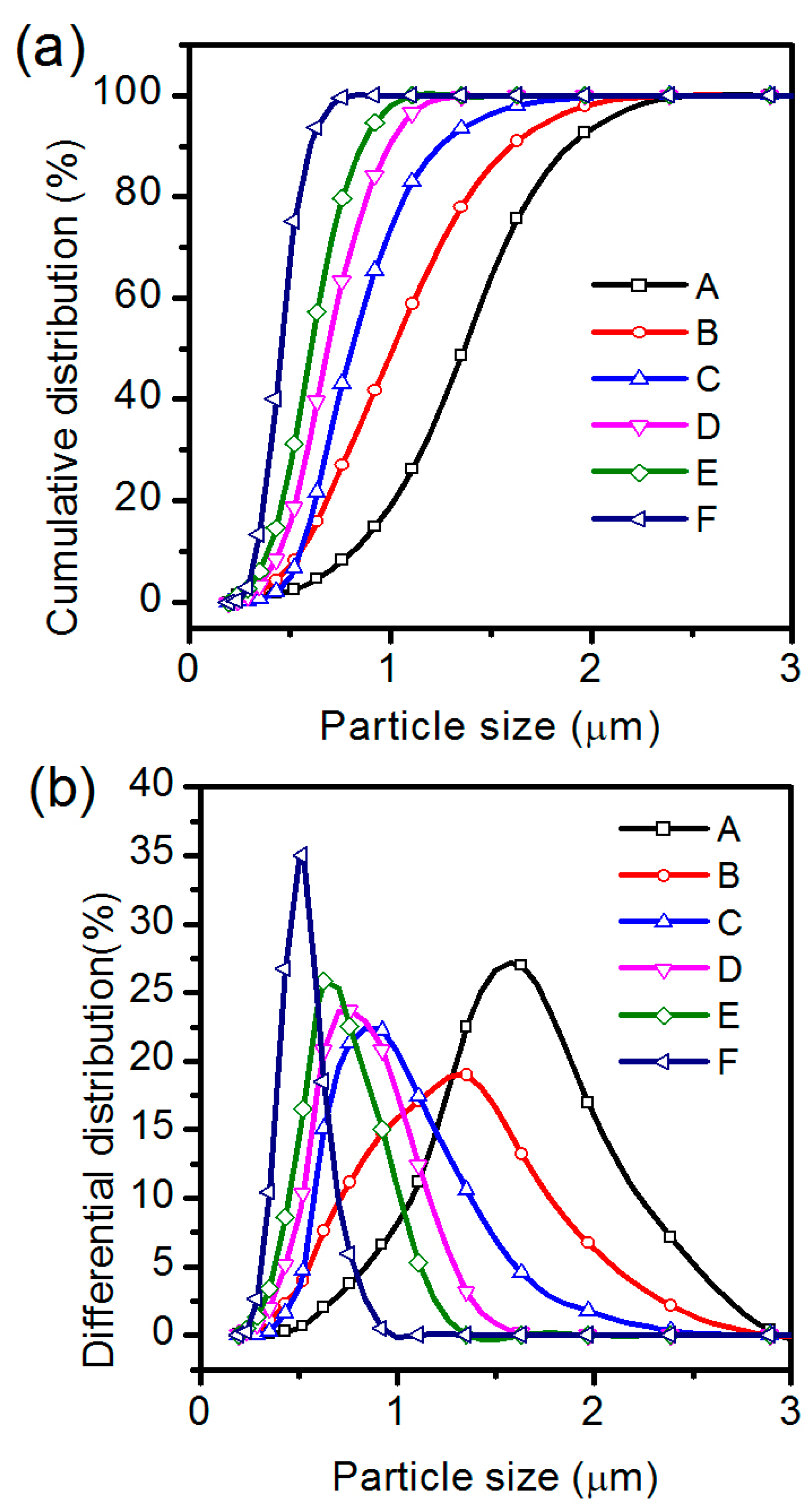
3.3. BaTiO3/Polymer/Al Composite Films with Ultra-Fine BaTiO3 Powders
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shin, D.; Kwon, C.H.; Ko, Y.B.; Lee, S.W.; Lee, B.; Cho, J. Stitchable supercapacitors with high energy density and high rate capability using metal nanoparticle-assembled cotton threads. J. Mater. Chem. A 2018, 6, 20421–20432. [Google Scholar] [CrossRef]
- Pourmousavian, N.; Kuo, F.W.; Siriburanon, T.; Babaic, M.; Staszewski, R.B. A 0.5 V 1.6 mW 2.4 GHz fractional-N all-digital PLL for bluetooth LE with PVT-insensitive TDC using switched-capacitor doubler in 28 nm CMOS. IEEE J. Solid State Circuits 2018, 53, 2572–2583. [Google Scholar] [CrossRef]
- Hu, C.; Chen, M.; Li, H.; Zhang, Z.; Jiao, Y.; Shao, H. An accurate on-site calibration system for electronic voltage transformers using a standard capacitor. Meas. Sci. Technol. 2018, 29, 055901. [Google Scholar] [CrossRef]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Pedro, G.R. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Q.; Yuan, N.; Tang, J. A review on flexible and transparent energy storage system. Materials 2018, 11, 2280. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Greenbaum, S.G. Polymer capacitor dielectrics for high temperature applications. ACS Appl. Mater. Interfaces 2018, 10, 29189–29218. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Peng, H.; Zeng, R.; Hu, Y.; Ni, K. A fundamental frequency sorting algorithm for capacitor voltage balance of modular multilevel converter with low-frequency carrier phase shift modulation. IEEE J. Em. Sel. Top. Power Electron. 2018, 6, 1595–1604. [Google Scholar] [CrossRef]
- Araga, Y.; Nagata, M.; Miura, N.; Lkeda, H.; Kikuchi, K. Superior decoupling capacitor for three-dimensional LSI with ultrawide communication bus. Jpn. J. Appl. Phys. 2017, 56, 04CC05. [Google Scholar] [CrossRef]
- Schunemann, B.; Schael, U.; Rauer, G.; Wickert, M.; Cunrath, R.; Nau, S. Rapid recharging power supply for multiple-hit electrical armor. In Proceedings of the 17th International Symposium on Electromagnetic Launch Technology, La Jolla, CA, USA, 7–11 July 2014; pp. 1–6. [Google Scholar]
- Rezzak, D.; Boudjerda, N. Management and control strategy of a hybrid energy source fuel cell/supercapacitor in electric vehicles. Int. Trans. Electr. Energy Syst. 2017, 27, e2308. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J. Implementation methodology of powertrain for series-hybrid military vehicles applications equipped with hybrid energy storage. Energy 2017, 120, 229–240. [Google Scholar] [CrossRef]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Khan, A.H.; Ghosh, S.; Pradhan, B.; Dalui, A.; Shrestha, L.K.; Acharya, S.; Ariga, K. Two-dimensional (2D) nanomaterials towards electrochemical nanoarchitectonics in energy-related applications. Bull. Chem. Soc. Jpn. 2017, 90, 627–648. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef] [PubMed]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Woerner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M.; Weber, S.; Ocakoglu, K.; Erdem, E. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef]
- Sengottaiyan, C.; Jayavel, R.; Bairi, P.; Shrestha, R.G.; Ariga, K.; Shrestha, L.K. Cobalt oxide/reduced graphene oxide composite with enhanced electrochemical supercapacitance performance. Bull. Chem. Soc. Jpn. 2017, 90, 955–962. [Google Scholar] [CrossRef]
- Zhang, K.; Ying, G.; Liu, L.; Ma, F.; Su, L.; Zhang, C.; Wu, D.; Wang, X.; Zhou, Y. Three-dimensional porous Ti₃C₂Tx-NiO composite electrodes with enhanced electrochemical performance for supercapacitors. Materials 2019, 12, 188. [Google Scholar] [CrossRef]
- Zhou, M.; Liang, R.; Zhou, Z.; Dong, X. Novel BaTiO3-based lead-free ceramic capacitors featuring high energy storage density, high power density and excellent stability. J. Mater. Chem. C 2018, 6, 8528–8537. [Google Scholar] [CrossRef]
- Hu, P.; Sun, W.; Fan, M.; Qian, J.; Jiang, J.; Dan, Z.; Lin, Y.; Nan, C.W.; Li, M.; Shen, Y. Large energy density at high-temperature and excellent thermal stability in polyimide nanocomposite contained with small loading of BaTiO3 nanofibers. Appl. Surf. Sci. 2018, 458, 743–750. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.; Na, B.; Lv, R.; Liu, H.; Li, W.; Zhou, H. Enhanced dielectric thermal stability and permittivity of flexible composite films based on BaTiO3 nanoparticles highly filled PVDF/PAN blend nanofibrous membranes. Polym. Compos. 2018, 39, E1841–E1848. [Google Scholar] [CrossRef]
- Bi, M.; Hao, Y.; Zhang, J.; Lei, M.; Bi, K. Particle size effect of BaTiO3 nanofillers on the energy storage performance of polymer nanocomposites. Nanoscale 2017, 9, 16386–16395. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Pan, Z.; Zhai, J.; Zhang, G.; Liu, Z.; Liu, Y. High-energy-density with polymer nanocomposites containing of SrTiO3 nanofibers for capacitor application. Compos. Part A Appl. Sci. Manuf. 2018, 109, 48–54. [Google Scholar] [CrossRef]
- Guan, S.; Li, H.; Zhao, S.; Guo, L. The surface modification of BaTiO3 and its effects on the microstructure and electrical properties of BaTiO3/silicone rubber composites. J. Vinyl Addit. Technol. 2018, 24, 288–294. [Google Scholar] [CrossRef]
- Luo, S.; Shen, Y.; Yu, S.; Wan, Y.; Liao, W.H.; Sun, R.; Wong, C.P. Construction of a 3D-BaTiO3 network leading to significantly enhanced dielectric permittivity and energy storage density of polymer composites. Energy Environ. Sci. 2017, 10, 137–144. [Google Scholar] [CrossRef]
- Airimioaei, M.; Buscaglia, M.T.; Tredici, I.; Anselmi-Tamburini, U.; Ciomaga, C.E.; Curecheriu, L.; Bencan, A.; Buscaglia, V.; Mitoseriu, L. SrTiO3-BaTiO3 nanocomposites with temperature independent permittivity and linear tunability fabricated using field-assisted sintering from chemically synthesized powders. J. Mater. Chem. C 2017, 5, 9028–9036. [Google Scholar] [CrossRef]
- Hu, P.; Gao, S.; Zhang, Y.; Zhang, L.; Wang, C. Surface modified BaTiO3 nanoparticles by titanate coupling agent induce significantly enhanced breakdown strength and larger energy density in PVDF nanocomposite. Compos. Sci. Technol. 2018, 156, 109–116. [Google Scholar] [CrossRef]
- Chen, S.; Lv, X.; Han, X.; Luo, H.; Bowen, C.R.; Zhang, D. Significantly improved energy density of BaTiO3 nanocomposites by accurate interfacial tailoring using a novel rigid-fluoro-polymer. Polym. Chem. 2018, 9, 548–557. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, W.; Fu, T.; Liu, J.; Zhang, Z.; Wang, S. Achieving high energy density and low loss in PVDF/BST nanodielectrics with enhanced structural homogeneity. ACS Appl. Mater. Interfaces 2018, 10, 29038–29047. [Google Scholar] [CrossRef]
- Fu, J.; Hou, Y.; Zheng, M.; Zhu, M. Comparative study of dielectric properties of the PVDF composites filled with spherical and rod-like BaTiO3 derived by molten salt synthesis method. J. Mater. Sci. 2018, 53, 7233–7248. [Google Scholar] [CrossRef]
- Zhu, P.; Sun, R.; Wong, C. Facile Synthesis of BaTiO3 Nanorods and Their Shape Effects on the Dielectric Constants of Polymer Composites. In Proceedings of the IEEE 63rd Electronic Components and Technology Conference, Las Vegas, NV, USA, 28–31 May 2013; pp. 1071–1075. [Google Scholar]
- Gao, J.; Shi, H.; Dong, H.; Zhang, R.; Chen, D. Factors influencing formation of highly dispersed BaTiO3 nanospheres with uniform sizes in static hydrothermal synthesis. J. Nanopart. Res. 2015, 17, 286. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, F.; Xu, J.; Wang, L.; Zhang, Q.; Kong, J. Fabrication and dielectric properties of Ba0.6Sr0.4TiO3 / acrylonitrile-butadiene-styrene resin composites. J. Mater. Sci. Mater. Electron. 2017, 28, 8960–8968. [Google Scholar] [CrossRef]
- Nootsuwan, N.; Plungpongpan, K.; Wattanathana, W.; Koonsaeng, N.; Laobuthee, A.; Manuspiya, H. Dielectric and mechanical properties of poly(butylene succinate) thin film composites incorporated with barium strontium titanate powder. Integr. Ferroelectr. 2016, 174, 155–166. [Google Scholar] [CrossRef]
- Chi, Q.; Gao, Z.; Zhang, C.; Cui, Y.; Dong, J.; Wang, X.; Lei, Q. Dielectric properties of sandwich-structured BaTiO3/polyimide hybrid films. J. Mater. Sci. - Mater. Electron. 2017, 28, 15142–15148. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, J.; Yu, Y.; Jiang, W.; Zhang, Z. Enhancing breakdown strength and energy storage performance of PVDF-based nanocomposites by adding exfoliated boron nitride. Appl. Surf. Sci. 2018, 440, 1150–1158. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, X. Improved dielectric properties and energy density of PVDF composites using PVP engineered BaTiO3 nanoparticles. Korean J. Chem. Eng. 2018, 35, 1570–1576. [Google Scholar] [CrossRef]
- He, D.; Wang, Y.; Chen, X.; Deng, Y. Core-shell structured BaTiO3@Al2O3 nanoparticles in polymer composites for dielectric loss suppression and breakdown strength enhancement. Compos. Part A Appl. Sci. Manuf. 2017, 93, 137–143. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, H.; Chen, C.; Zhou, K.; Zhang, D. Improved energy density and dielectric properties of P(VDF-HFP) composites with TiO2 nanowire clusters. J. Electroceram. 2018, 40, 65–71. [Google Scholar] [CrossRef]
- Huang, Q.; Luo, H.; Chen, C.; Zhou, X.; Zhou, K.; Zhang, D. Enhanced energy density in P(VDF-HFP) nanocomposites with gradient dielectric fillers and interfacial polarization. J. Alloys Compd. 2017, 696, 1220–1227. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. Enhanced energy density and thermostability in polyimide nanocomposites containing core-shell structured BaTiO3@SiO2 nanofibers. Appl. Surf. Sci. 2017, 426, 437–445. [Google Scholar] [CrossRef]
- Zheng, M.S.; Zheng, Y.T.; Zha, J.W.; Yang, Y.; Han, P.; Wen, Y.Q.; Dang, Z.M. Improved dielectric, tensile and energy storage properties of surface rubberized BaTiO3/polypropylene nanocomposites. Nano Energy 2018, 48, 144–151. [Google Scholar] [CrossRef]
- Wang, J.; Long, Y.; Sun, Y.; Zhang, X.; Yang, H.; Lin, B. Fabrication and enhanced dielectric properties of polyimide matrix composites with core-shell structured CaCu3Ti4O12@TiO2 nanofibers. J. Mater. Sci. Mater. Electron. 2018, 29, 7842–7850. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Feng, C.; Zeng, Q. Improved dielectric properties of surface modified BaTiO3/polyimide composite films. Microelectron. Eng. 2016, 154, 17–21. [Google Scholar] [CrossRef]

| Fillers | Polymer Matrix | Dielectric Constant | Dielectric Loss | Breakdown Strength | Ref. |
|---|---|---|---|---|---|
| BT microparticles | Resin | 32 | 0.014 | 20.8 V/μm | This work |
| BT nanoparticles (60 nm) | In suit prepared polyimide | 3.85 | 0.0024 | 334.26 kV/mm | [35] |
| PDA coated BT nanoparticles (100 nm)/BN nanosheets | Poly(vinylidene fluoride-chlorotrifluoroe thylene) | 11.7 | 0.10 | 425 MV/m | [36] |
| PVP coated BT nanoparticles (100 nm) | Poly(vinylidene fluoride) | 80.4 | 0.085 | 240 kV/mm | [37] |
| BT@Al2O3 nanoparticles (150 nm) | Poly(vinylidene fluoride) | 17.5 | 0.02 | 312 kV/mm | [38] |
| Sphere-like TiO2 nanowire clusters | Poly(vinylidene fluoride-co-hexafluoropylene) | 11.9 | 0.048 | 160 kV/mm | [39] |
| Dopamine modified urchin-like hierarchical structure of BT particles @ TiO2 nanowires | Poly(vinylidene fluoride-co-hexafluoropylene) | 14.7 | 0.0037 | 240 kV/mm | [40] |
| Dopamine-coated BT@SiO2 nanofiber | In suit prepared polyimide | 5.05 | 0.0225 | 346 kV/mm | [41] |
| Ethylene propylene diene monomer coated BT nanoparticles (<100 nm) | Polypropylene | 5.8 | / | 370 MV/m | [42] |
| CaCu3Ti4O12@TiO2 nanofibers | In suit prepared polyimide | 5.85 | 0.025 | 236 kV/mm | [43] |
| BT modified with 2-phosphonobutane-1,2,4-tricarboxylic acid | In suit prepared polyimide | 23.5 | 0.00942 | 80 MV/m | [44] |
| BT/TH-615 acrylic-acrylate-amide copolymer | In suit prepared polyimide | 20.3 | 0.00571 | 73 MV/m | [44] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Li, T.; Xu, Y.; Sun, C.; Yang, Z.; Zhu, D.; Chen, D. Effects of the Particle Size of BaTiO3 Fillers on Fabrication and Dielectric Properties of BaTiO3/Polymer/Al Films for Capacitor Energy-Storage Application. Materials 2019, 12, 439. https://doi.org/10.3390/ma12030439
Gu L, Li T, Xu Y, Sun C, Yang Z, Zhu D, Chen D. Effects of the Particle Size of BaTiO3 Fillers on Fabrication and Dielectric Properties of BaTiO3/Polymer/Al Films for Capacitor Energy-Storage Application. Materials. 2019; 12(3):439. https://doi.org/10.3390/ma12030439
Chicago/Turabian StyleGu, Lulu, Tao Li, Yongjun Xu, Chenghua Sun, Zhenyu Yang, Deliang Zhu, and Deliang Chen. 2019. "Effects of the Particle Size of BaTiO3 Fillers on Fabrication and Dielectric Properties of BaTiO3/Polymer/Al Films for Capacitor Energy-Storage Application" Materials 12, no. 3: 439. https://doi.org/10.3390/ma12030439
APA StyleGu, L., Li, T., Xu, Y., Sun, C., Yang, Z., Zhu, D., & Chen, D. (2019). Effects of the Particle Size of BaTiO3 Fillers on Fabrication and Dielectric Properties of BaTiO3/Polymer/Al Films for Capacitor Energy-Storage Application. Materials, 12(3), 439. https://doi.org/10.3390/ma12030439





