Enhanced Wear Performance of Cu-Carbon Nanotubes Composite Coatings Prepared by Jet Electrodeposition
Abstract
1. Introduction
2. Experimental Procedures
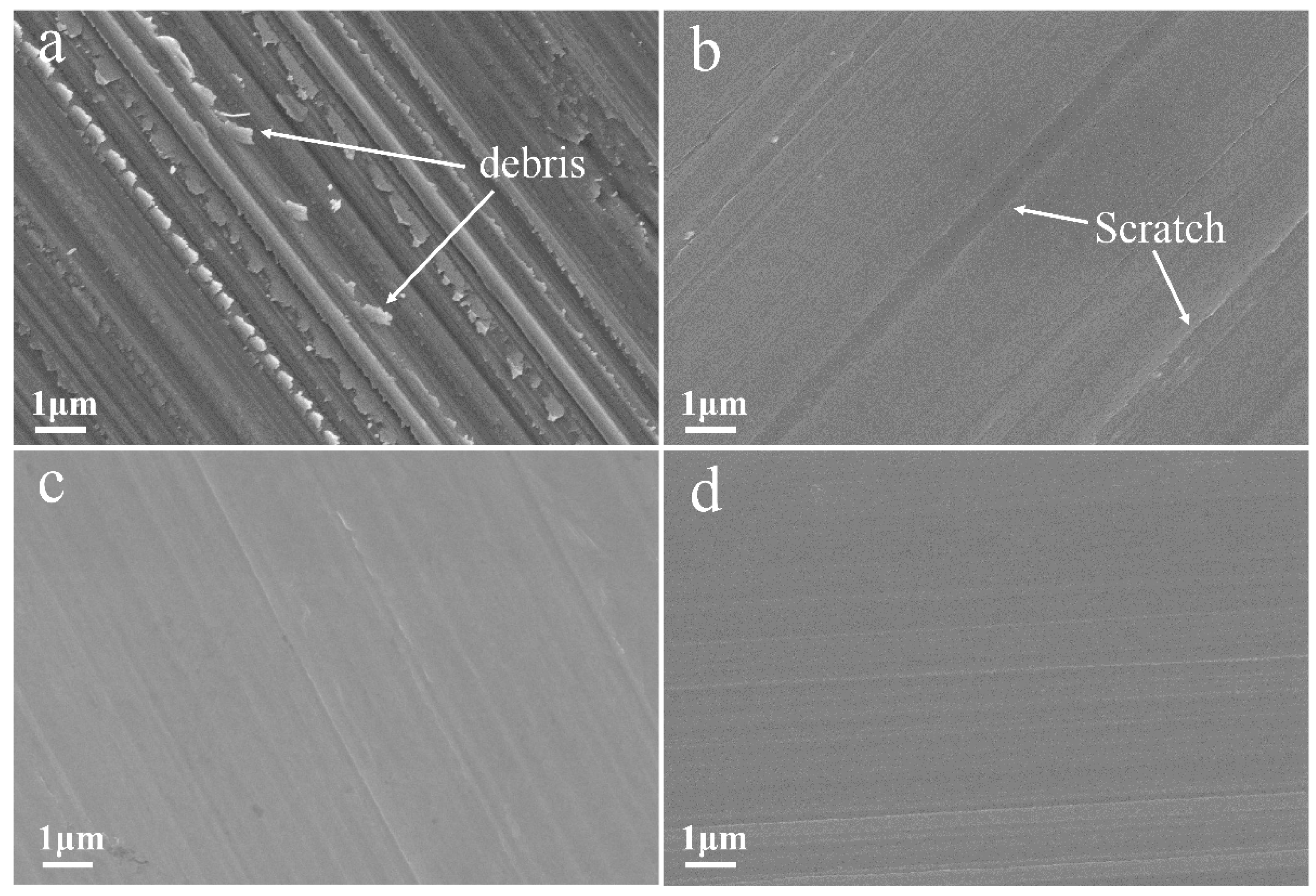
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, Y.H.; Mohamed, A.M.A. Electrodeposition of Nanostructured Nickel-Ceramic Composite Coatings: A review. Int. J. Electrochem. Sci. 2014, 9, 1942–1963. [Google Scholar]
- Hovestad, A.; Janessen, L.J.J. Electrochemical codeposition of inert particles in a metallic matrix. J. Appl. Electrochem. 1995, 25, 519–527. [Google Scholar] [CrossRef]
- Walsh, F.C.; Leon, C.P. A review of the electrodeposition of metal matrix composite coatings by inclusion of particles in a metal layer: An established and diversifying technology. Trans. IMF 2014, 92, 83–98. [Google Scholar] [CrossRef]
- Zhu, J.H.; Liu, L.; Zhao, H.J.; Shen, B.; Hu, W.B. Microstructure and performance of electroformed Cu/nano-SiC composite. Mater. Des. 2007, 28, 1958–1962. [Google Scholar] [CrossRef]
- Zhu, J.H.; Hu, G.H.; Shen, B.; Hu, W.B.; Ding, W.J. Study on composite electroforming of Cu/SiCp composites. Mater. Lett. 2004, 58, 1634–1637. [Google Scholar] [CrossRef]
- Efe, G.C.; Altinsoy, I.; Yener, T.; Ipek, M.; Zeytin, S.; Bindal, C. Characterization of cemented Cu matrix composites reinforced with SiC. Vacuum 2010, 85, 643–647. [Google Scholar] [CrossRef]
- Lajevardi, S.A.; Shahrabi, T. Effects of pulse electrodeposition parameters on the properties of Ni–TiO2 nanocomposite coatings. Appl. Surf. Sci. 2010, 256, 6775–6781. [Google Scholar] [CrossRef]
- Semboshi, S.; Sakamoto, Y.; Inoue, H.; Iwase, A.; Masahashi, N. Electroforming of oxide-nanoparticle-reinforced copper-matrix composite. J. Mater. Res. 2015, 30, 521–527. [Google Scholar] [CrossRef]
- Ramalingam, S.; Muralidharan, V.S.; Subramania, A. Electrodeposition and characterization of Cu-TiO2 nanocomposite coatings. J. Solid State Electrochem. 2009, 13, 1777–1783. [Google Scholar] [CrossRef]
- Ning, D.; Zhang, A.; Murtaza, M.; Wu, H. Effect of surfactants on the electrodeposition of Cu-TiO2 composite coatings prepared by jet electrodeposition. J. Alloys Compd. 2019, 777, 1245–1250. [Google Scholar] [CrossRef]
- Arai, S.; Endo, M. Carbon nanofiber–copper composite powder prepared by electrodeposition. Electrochem. Commun. 2003, 5, 797–799. [Google Scholar] [CrossRef]
- Ramalingam, S.; Balakrishnan, K.; Shanmugasamy, S.; Subramania, A. Electrodeposition and characterisation of Cu–MWCNTs nanocomposite coatings. Surf. Eng. 2016, 33, 369–374. [Google Scholar] [CrossRef]
- Mandal, P.; Mondal, S.C. Investigation of Electro-Thermal property for Cu-MWCNT composite coating on anodized 6061 aluminium alloy. Appl. Surf. Sci. 2018, 454, 138–147. [Google Scholar] [CrossRef]
- Arai, S.; Suwa, Y.; Endo, M. Cu/Multiwalled Carbon Nanotube Composite Films Fabricated by Pulse-Reverse Electrodeposition. J. Electrochem. Soc. 2011, 158, D49–D53. [Google Scholar] [CrossRef]
- Arai, S.; Kato, A. Mechanism for Codeposition of Multiwalled Carbon Nanotubes with Copper from Acid Copper Sulfate Bath. J. Electrochem. Soc. 2015, 160, D380–D385. [Google Scholar] [CrossRef]
- Arai, S.; Saito, T.; Endo, M. Cu–MWCNT Composite Films Fabricated by Electrodeposition. J. Electrochem. Soc. 2010, 157, D147–D153. [Google Scholar] [CrossRef]
- Berçot, P.; Peña-Muñoz, E.; Pagetti, J. Electrolytic composite Ni-PTFE coatings: An adaptation of Guglielmi’s model for the phenomena of incorporation. Surf. Coat. Technol. 2002, 157, 282–289. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam mechanics: Elasticity, strength, and toughness of nanorods and nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- An, Z.L.; Toda, M.; Ono, T. Comparative investigation into surface charged multi-walled carbon nanotubes reinforced Cu nanocomposites for interconnect applications. Compos. Part B Eng. 2016, 95, 137–143. [Google Scholar] [CrossRef]
- Yang, Y.L.; Wang, Y.D.; Ren, Y.; He, C.S.; Deng, J.N.; Nan, J.; Chen, J.G.; Zuo, L. Single-walled carbon nanotube-reinforced copper composite coatings prepared by electrodeposition under ultrasonic field. Mater. Lett. 2008, 62, 47–50. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, P.; Chen, X.; Li, W.; Liu, X. The influence of electro-deposition parameters on conductivity and morphology of structurally uniform MWCNTs/Cu composite films. Mater. Res. Express. 2018, 5, 065604. [Google Scholar] [CrossRef]
- Long, X.; Bai, Y.L.; Algarni, M.; Choi, Y.; Chen, Q.F. Study on the strengthening mechanisms of Cu/CNT nano-composites. Mater. Sci. Eng. A 2015, 645, 347–356. [Google Scholar] [CrossRef]
- Feng, Y.; Mcguire, G.E.; Shenderova, O.A.; Ke, H.; Burkett, S.L. Fabrication of copper/carbon nanotube composite thin films by periodic pulse reverse electroplating using nanodiamond as a dispersing agent. Thin Solid Films 2016, 615, 116–121. [Google Scholar] [CrossRef]
- Mandal, P.; Mondal, S.C. Investigation of electro-thermal property of Cu-MWCNT-coated 316L stainless steel. Surf. Eng. 2017, 34, 697–704. [Google Scholar] [CrossRef]
- Zhou, M.; Mai, Y.; Ling, H.; Chen, F.; Lian, W.; Jie, X. Electrodeposition of CNTs/copper composite coatings with enhanced tribological performance from a low concentration CNTs colloidal solution. Mater. Res. Bull. 2018, 97, 537–543. [Google Scholar] [CrossRef]
- Chen, X.C.; Peng, J.C.; Li, X.Q.; Deng, F.M.; Wang, J.X.; Li, W.Z. Tribological behavior of carbon nanotubes—Reinforced nickel matrix composite coatings. J. Mater. Sci. Lett. 2001, 20, 2057–2060. [Google Scholar] [CrossRef]
- Chen, W.X.; Tu, J.P.; Wang, L.Y.; Gan, H.Y.; Xu, Z.D.; Zhang, X.B. Tribological application of carbon nanotubes in a metal-based composite coating and composites. Carbon 2003, 41, 215–222. [Google Scholar] [CrossRef]
- Thiemig, D.; Osborne, S.J.; Sweet, W.S.; Talbot, J.B. Electroplating of Copper-Alumina Nanocomposite Films with an Impinging Jet Electrode. ECS Trans. 2008, 11, 35–44. [Google Scholar]
- Osborne, S.J.; Sweet, W.S.; Vecchio, K.S.; Talbot, J.B. Electroplating of Copper–Alumina Nanocomposite Films with an Impinging Jet Electrode. J. Electrochem. Soc. 2007, 154, D394–D399. [Google Scholar] [CrossRef]
- Takeuchi, H.; Tsenekawa, Y.; Okumiya, M. Formation of Compositionally Graded Ni-P Deposits Containing SiC Particles by Jet Electroplating. Mater. Trans. JIM 1997, 38, 43–48. [Google Scholar] [CrossRef]
- Wang, Y.H.; Qiu, L.D.; Qiu, M.B.; Tian, Z.J.; Liu, X.; Zhuo, W. Jet Electrodeposition of Ni-SiO2 Nanocomposite Coatings with Online Friction and Its Performance. J. Electrochem. Soc. 2016, 163, D579–D584. [Google Scholar] [CrossRef]
- Sabri, M.; Sarabi, A.A.; Kondelo, S.M.N. The effect of sodium dodecyl sulfate surfactant on the electrodeposition of Ni-alumina composite coatings. Mater. Chem. Phys. 2012, 136, 566–569. [Google Scholar] [CrossRef]
- Guglielmi, N. Kinetics of the Deposition of Inert Particles from Electrolyte Baths. J. Electrochem. Soc. 1972, 119, 1009–1012. [Google Scholar] [CrossRef]
- Maharana, H.S.; Basu, A. Effects of Different Surfactants on Structural, Tribological and Electrical Properties of Pulsed Electro-Codeposited Cu-ZrO2 Composite Coatings. J. Mater. Eng. Perform. 2018, 27, 1854–1865. [Google Scholar] [CrossRef]

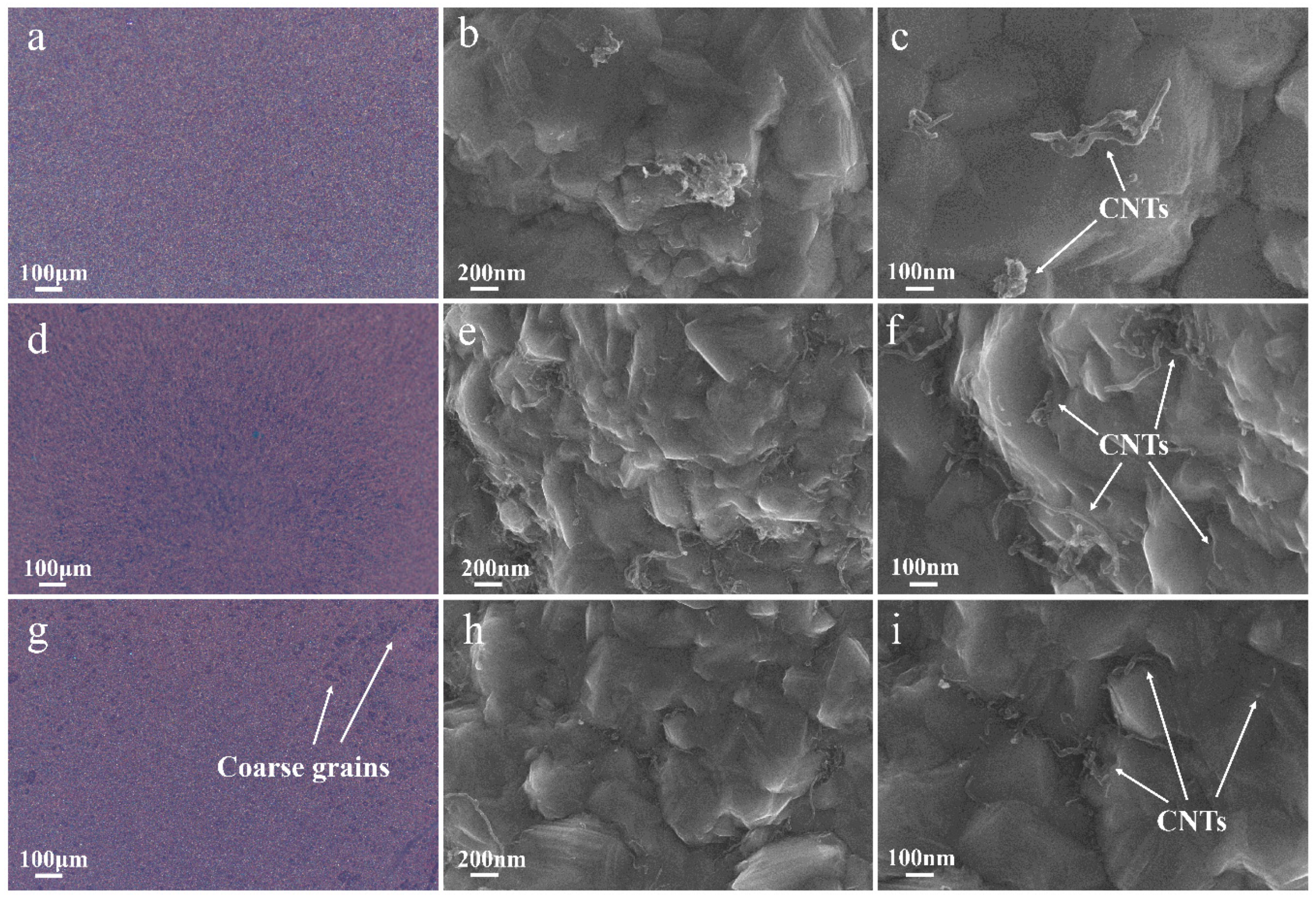
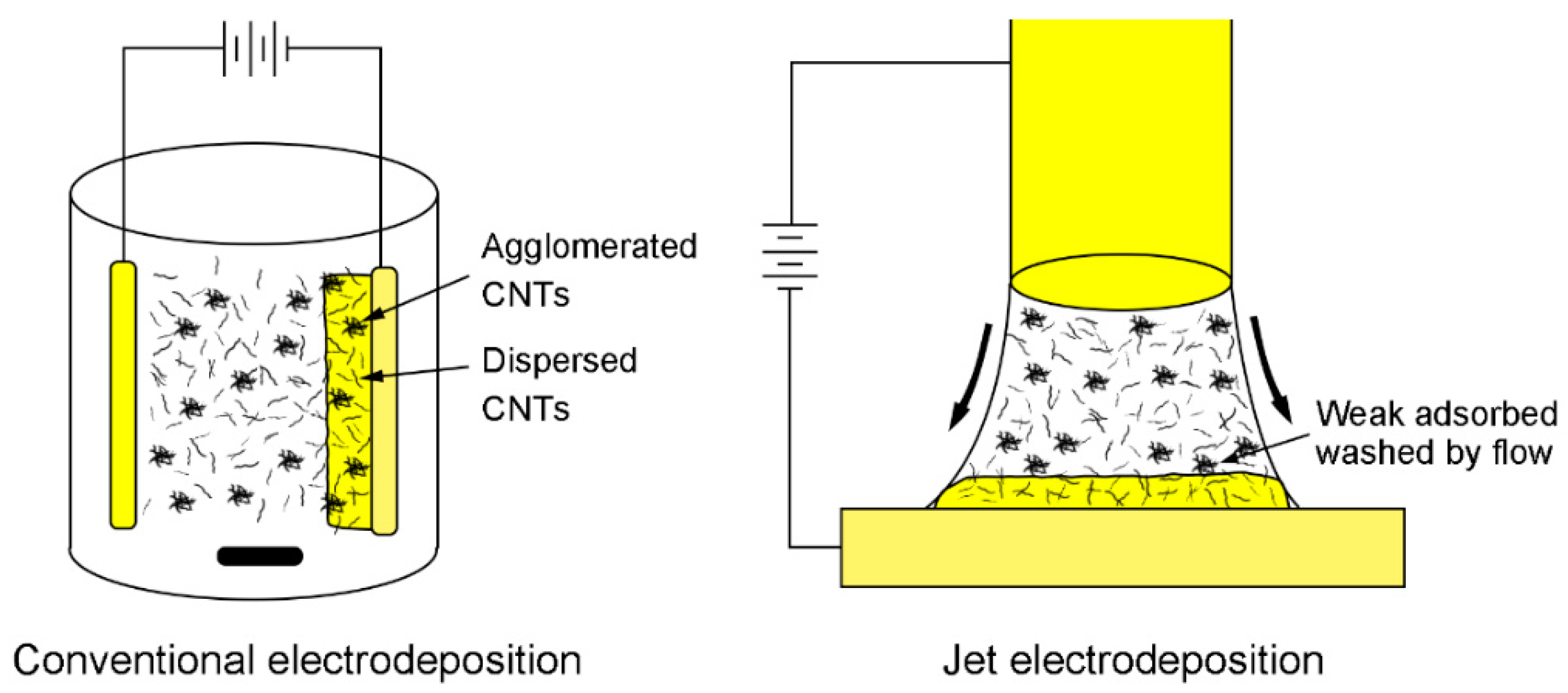
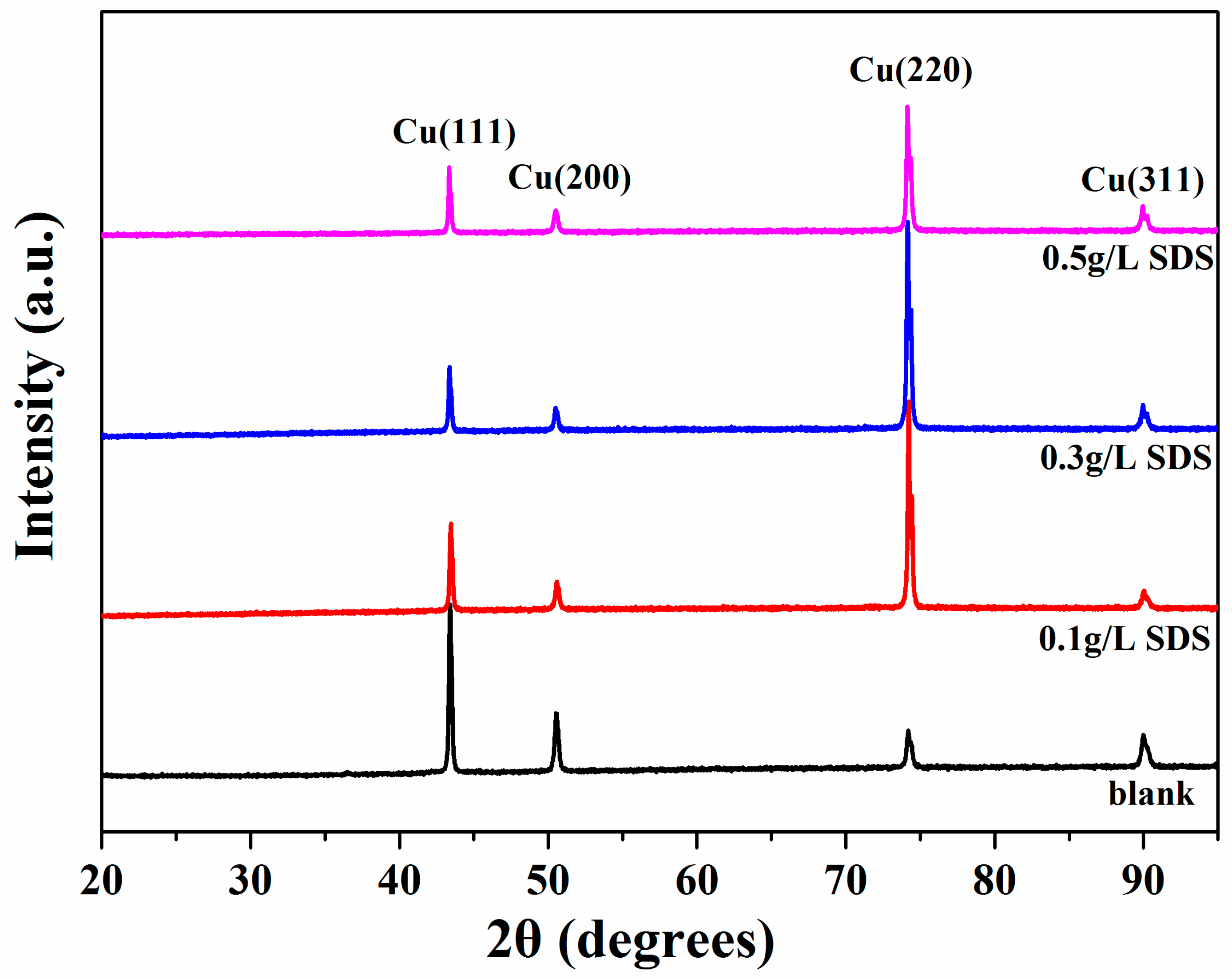
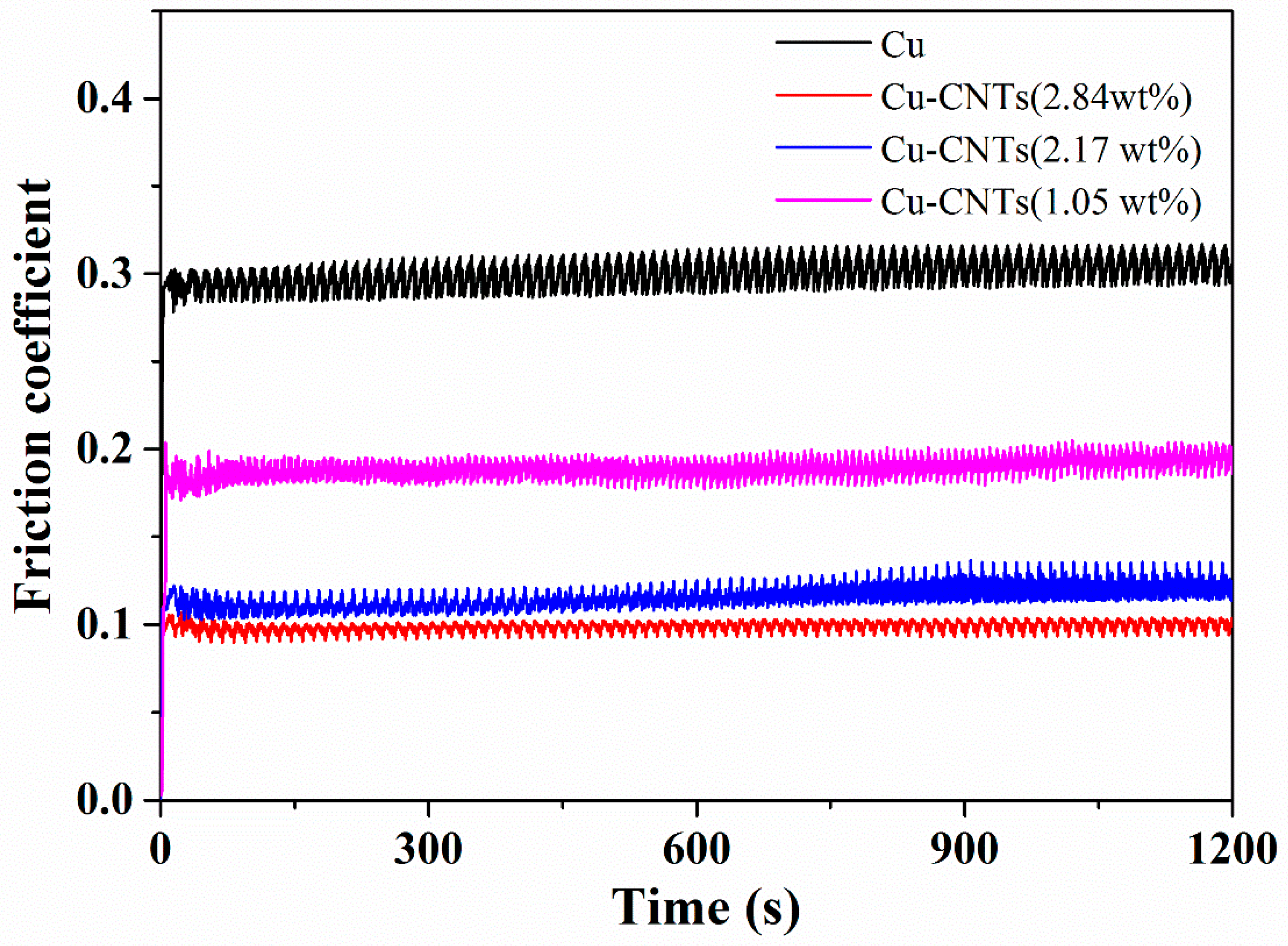
| SDS Concentration (g/L) | Zeta Potential (mV) | CNT Content (wt %) | Microhardness (Hv) |
|---|---|---|---|
| 0 | −6.1 ± 2.1 | - | - |
| 0.1 | −12.8 ± 1.1 | 1.05 | 127 |
| 0.3 | −16.8 ± 1.5 | 2.84 | 155 |
| 0.5 | −24.5 ± 2.4 | 2.17 | 147 |
| CNTs Content (wt %) | Microhardness (Hv) | Friction Coefficient | Wear Weight Loss (mg/Nm) |
|---|---|---|---|
| 0 | 85 | 0.28 | 8.9 × 10−2 |
| 1.05 | 127 | 0.18 | 4.5 × 10−2 |
| 2.17 | 147 | 0.12 | 2.2 × 10−2 |
| 2.84 | 155 | 0.11 | 1.3 × 10−2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, D.; Zhang, A.; Wu, H. Enhanced Wear Performance of Cu-Carbon Nanotubes Composite Coatings Prepared by Jet Electrodeposition. Materials 2019, 12, 392. https://doi.org/10.3390/ma12030392
Ning D, Zhang A, Wu H. Enhanced Wear Performance of Cu-Carbon Nanotubes Composite Coatings Prepared by Jet Electrodeposition. Materials. 2019; 12(3):392. https://doi.org/10.3390/ma12030392
Chicago/Turabian StyleNing, Dongdong, Ao Zhang, and Hui Wu. 2019. "Enhanced Wear Performance of Cu-Carbon Nanotubes Composite Coatings Prepared by Jet Electrodeposition" Materials 12, no. 3: 392. https://doi.org/10.3390/ma12030392
APA StyleNing, D., Zhang, A., & Wu, H. (2019). Enhanced Wear Performance of Cu-Carbon Nanotubes Composite Coatings Prepared by Jet Electrodeposition. Materials, 12(3), 392. https://doi.org/10.3390/ma12030392






