Bottom-Up Self-Assembled Supramolecular Structures Built by STM at the Solid/Liquid Interface
Abstract
1. Introduction
2. Molecular 2D Patterned Arrays
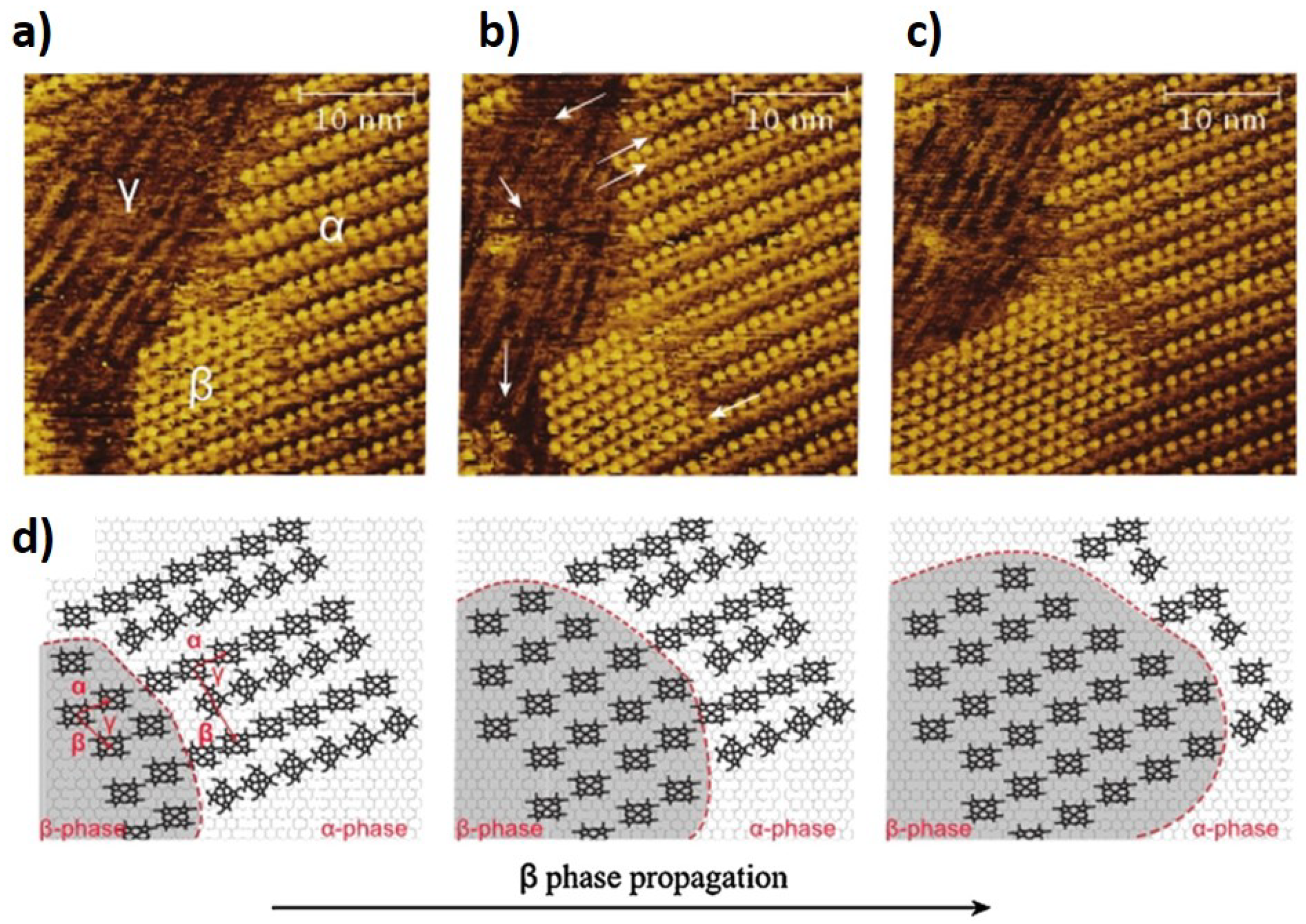
2.1. Polymorphism
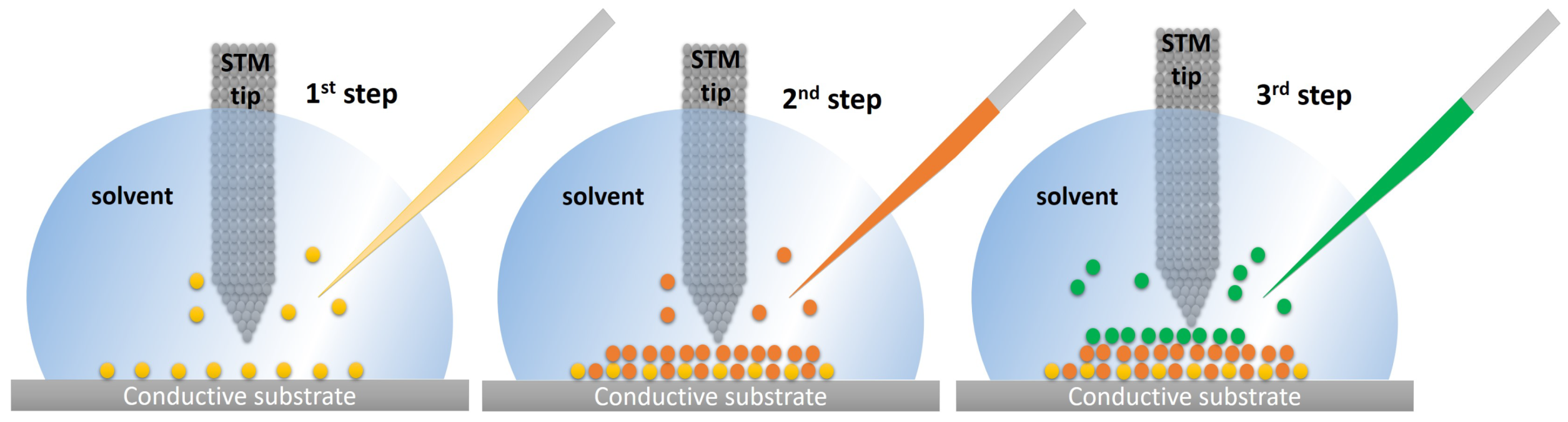
2.2. In Situ Synthesis
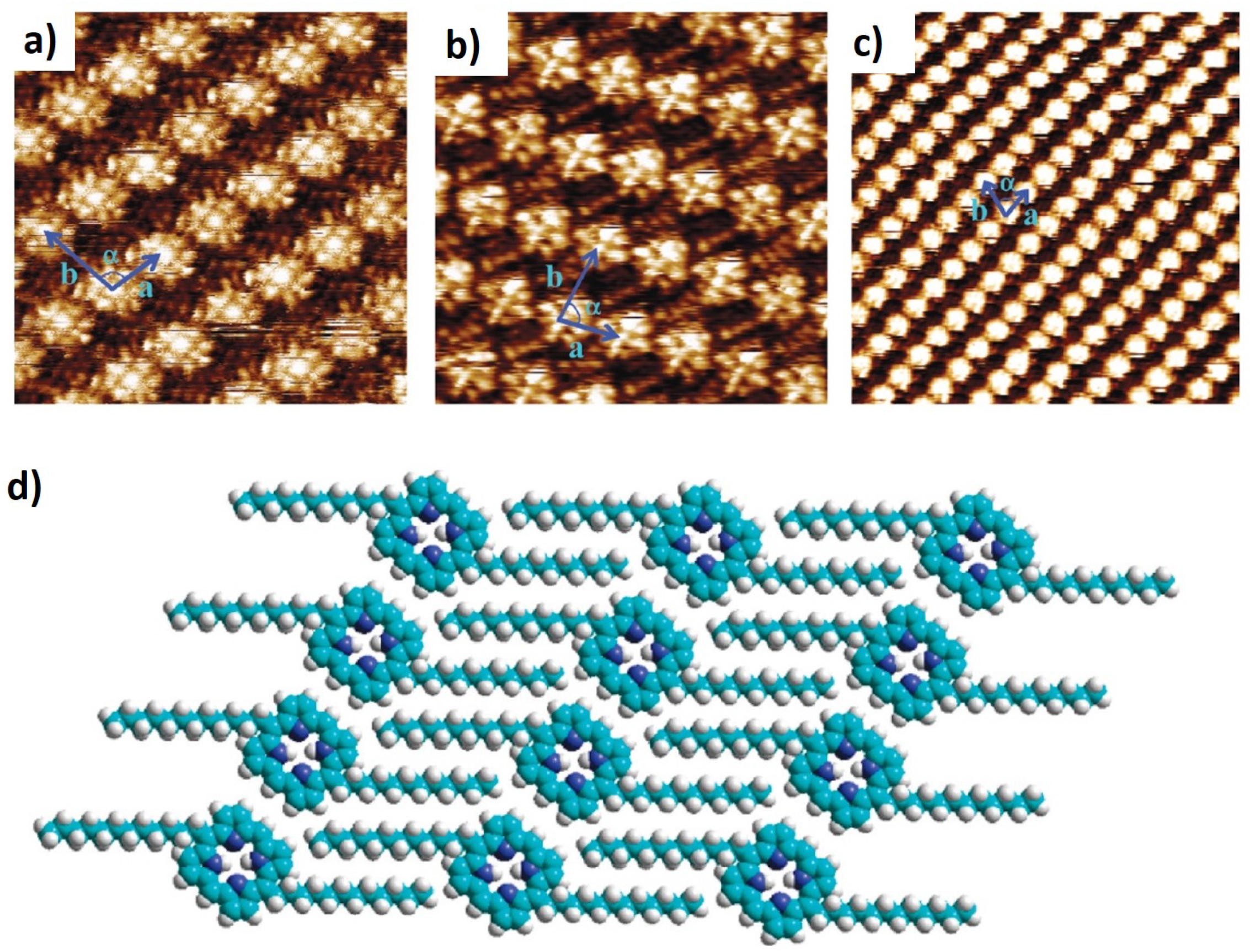
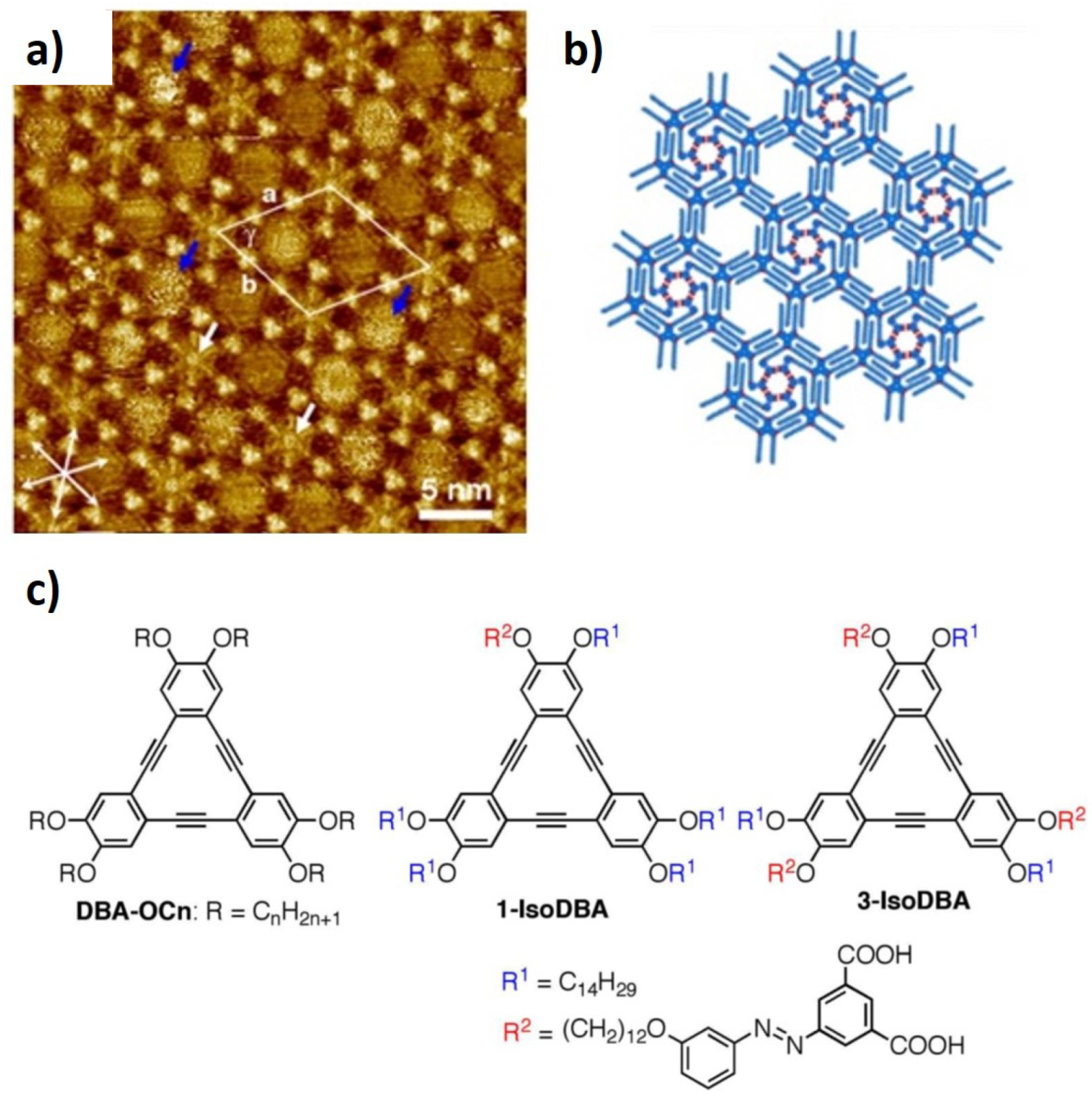
2.3. Nanoporous Networks
2.4. Host-Guest Systems
3. Molecular Machines
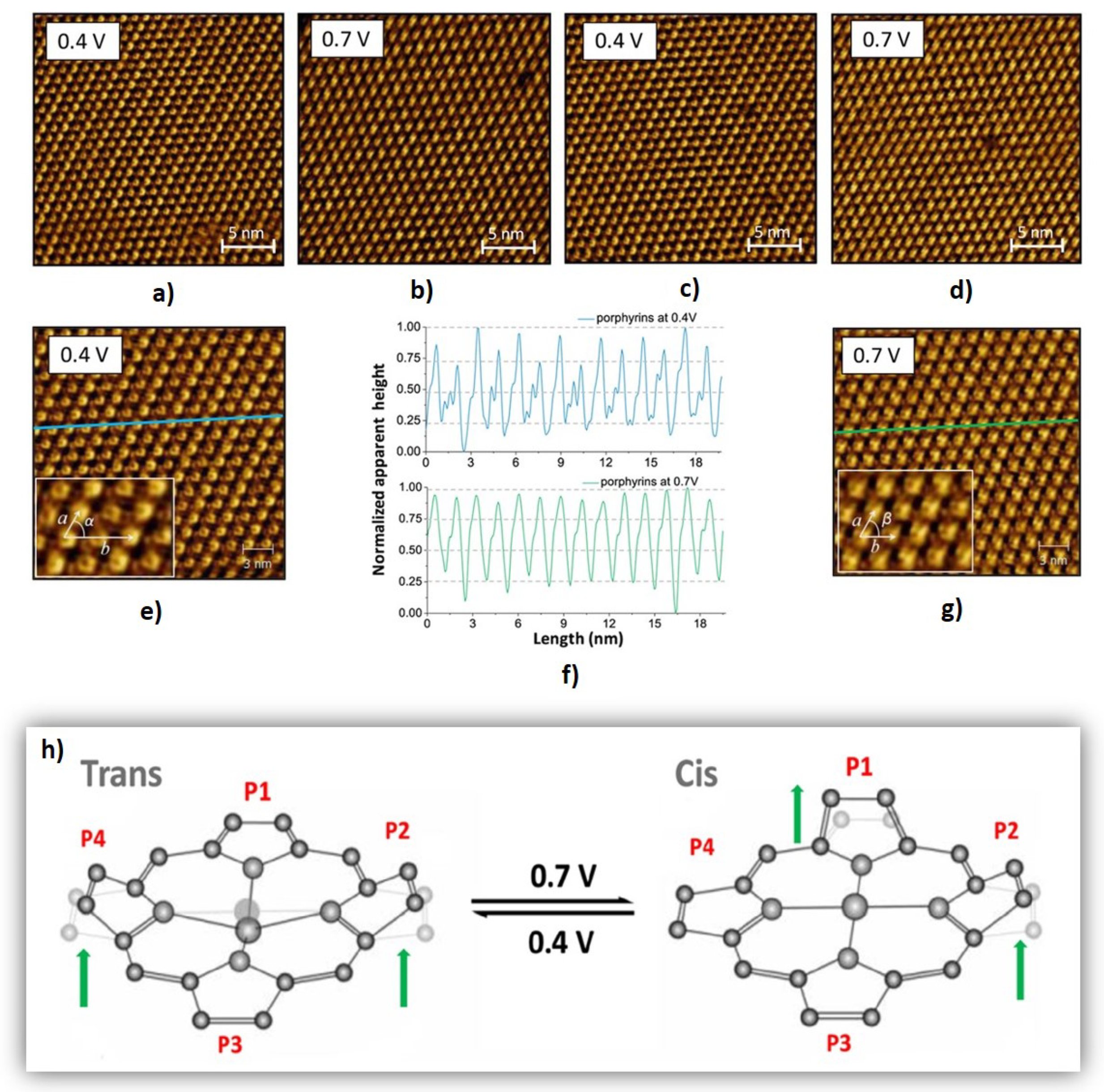
3.1. Molecular Switches
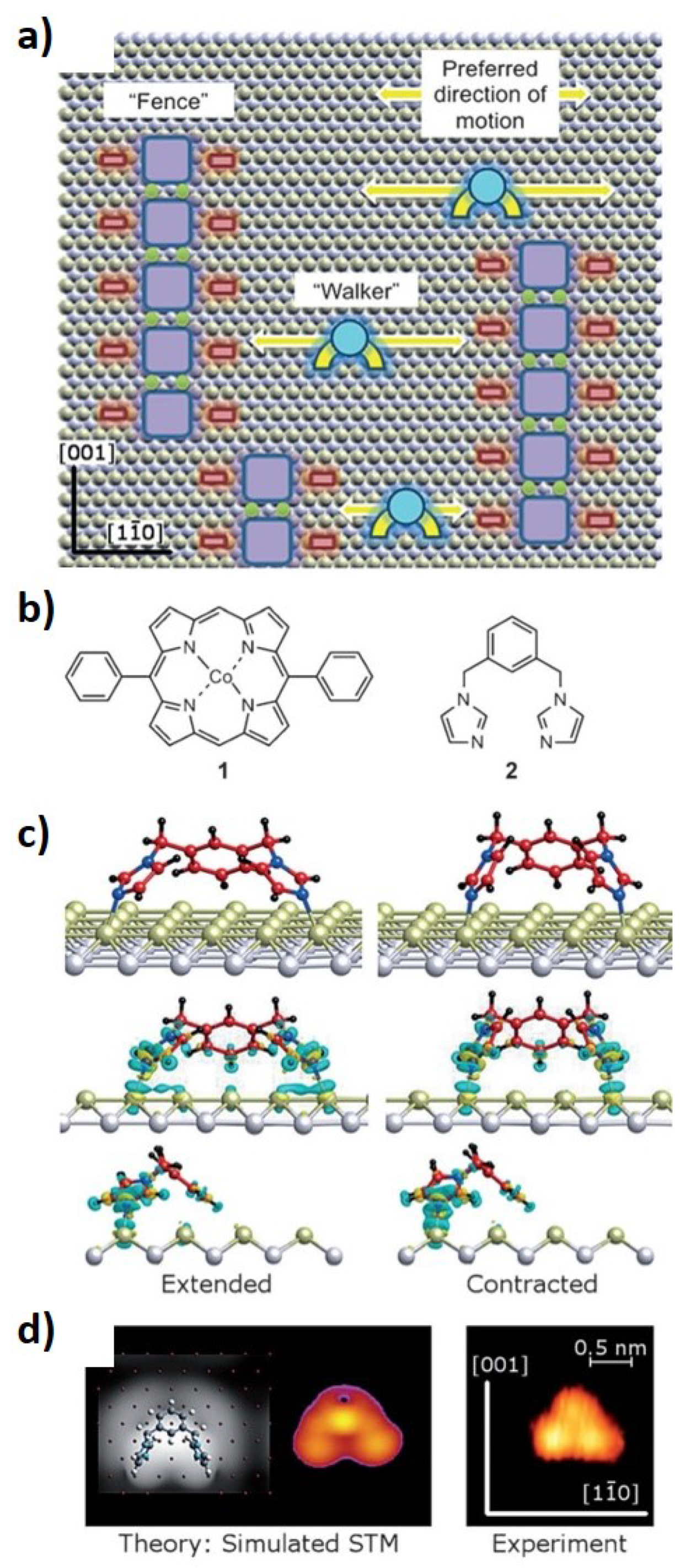
3.2. Molecular Motors
4. Molecular Wires
5. Perspectives
- to create unimolecular systems stable at room temperatures;
- to develop encapsulation methods while maintaining the integrity of the organic device.
Author Contributions
Funding
Conflicts of Interest
References
- Inácio, P.; Mestre, A.G.; Medeiros, M.C.R.; Asgarifar, S.; Elamine, Y.; Canudo, J.; Santos, J.; Bragança, J.; Morgado, J.; Biscarini, F.; et al. Bioelectrical Signal Detection Using Conducting Polymer Electrodes and the Displacement Current Method. IEEE Sens. J. 2017, 17, 3961–3966. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.K.; Ferreira, B.F.; Gomes, C.S.B.; Vila-Viçosa, D.; Charas, A.; Morgado, J.; Calhorda, M.; Maçanita, A.; Gomes, P. Violet-Blue emitting 2-(N-Alkylimino)pyrrolyl Organoboranes: Synthesis, Structure and Luminescent Properties. Dyes Pigments 2017, 140, 520–532. [Google Scholar] [CrossRef]
- Meng, L.; Zhang, Y.; Wan, X.; Li, C.; Zhang, X.; Wang, Y.; Ke, X.; Xiao, Z.; Ding, L.; Xia, R.; et al. Organic and solution-processed tandem solar cells with 17.3% efficiency. Science 2018, 361, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Farinhas, J.; Oliveira, R.; Hansson, R.H.; Ericsson, L.K.E.E.; Moons, E.M.; Morgado, J.; Charas, A. Efficient ternary organic solar cells based on immiscible blends. Org. Electron. 2017, 41, 130–136. [Google Scholar] [CrossRef]
- Afonso, M.; Morgado, J.; Alcácer, L. Inkjet printed organic electrochemical transistors with highly conducting polymer electrolytes. J. Appl. Phys. 2016, 120, 1–6. [Google Scholar] [CrossRef]
- Morgado, J.; Pereira, A.; Bragança, A.M.; Fernandes, S.; Freire, C.; Silvestre, A.; Pascoal Neto, C.; Alcácer, L. Self-standing chitosan films as dielectrics in organic thin-film transistors. Express Polym. Lett. 2013, 7, 960–965. [Google Scholar] [CrossRef]
- Ferreira, Q.; Alcácer, L.; Morgado, J. Stepwise preparation and characterization of molecular wires made of zinc octaethylporphyrin complexes bridged by 4,4′-bipyridine on HOPG. Nanotechnology 2011, 22, 435604. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, Q.; Bragança, A.M.; Alcácer, L.; Morgado, J. Conductance of well-defined porphyrin self-assembled molecular wires up to 14 nm in length. J. Phys. Chem. C 2014, 118, 7229–7234. [Google Scholar] [CrossRef]
- Eric, D.K. Engines of Creation, The coming Era of Nanotechnology; Springer Press: New York, NY, USA, 1986. [Google Scholar]
- Pedersen, C.J. The discovery of crown ethers (Noble Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1021–1027. [Google Scholar] [CrossRef]
- Cram, D.J. The design of molecular hosts, guests, and their complexes (Nobel lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 1009–1020. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry—Scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 1988, 27, 89–112. [Google Scholar] [CrossRef]
- Sauvage, J.P. From chemical topology to molecular machines (Nobel lecture). Angew. Chem. Int. Ed. 2017, 56, 11080–11093. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, J.F. Mechanically interlocked molecules (MIMs)—Molecular shuttles, switches, and machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef] [PubMed]
- Feringa, B.L. The art of building small: From molecular switches to motors (Nobel lecture). Angew. Chem. Int. Ed. 2017, 56, 11060–11078. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, J.; Seki, E.; Taguchi, T.; Asakawa, M.; Miyake, K. STM observation of labile axial ligands to zinc porphyrin at liquid/solid interface. Chem. Lett. 2007, 36, 740–741. [Google Scholar] [CrossRef]
- Ferreira, Q.; Bragança, A.; Moura, N.; Faustino, M.; Alcácer, L.; Morgado, J. Dynamics of porphyrin adsorption on highly oriented pyrolytic graphite monitored by scanning tunnelling microscopy at the liquid/solid interface. Appl. Surf. Sci. 2013, 273, 220–225. [Google Scholar] [CrossRef]
- Uemura, S.; Tanoue, R.; Yilmaz, N.; Ohira, A.; Kunitake, M. Molecular dynamics in two-dimensional supramolecular systems observed by STM. Materials 2010, 3, 4252–4276. [Google Scholar] [CrossRef]
- Ośmiałowski, B.; Kolehmainen, E.; Gawinecki, R.; Dobosz, R.; Kauppinen, R. Complexation of 2,6-Bis(acylamino)pyridines with Dipyridin-2-ylamine and 4,4-Dimethylpiperidine-2,6-dione. J. Phys. Chem. A 2010, 114, 12881–12887. [Google Scholar] [CrossRef]
- Ośmiałowski, B.; Kolehmainen, E.; Kowalska, M. 2-Acylamino-6-pyridones: Breaking of an Intramolecular Hydrogen Bond by Self-association and Complexation with Double and Triple Hydrogen Bonding Counterparts. Uncommon Steric Effect on Intermolecular Interactions. J. Org. Chem. 2012, 77, 1653–1662. [Google Scholar] [CrossRef]
- Gansäuer, A.; Kube, C.; Daasbjerg, K.; Sure, R.; Grimme, S.; Fianu, G.D.; Sadasivam, D.V.; Flowers, R.A. Substituent Effects and Supramolecular Interactions of Titanocene(III) Chloride: Implications for Catalysis in Single Electron Steps. J. Am. Chem. Soc. 2014, 136, 1663–1671. [Google Scholar] [CrossRef]
- Tresca, B.W.; Hansen, R.J.; Chau, C.V.; Hay, B.P.; Zakharov, L.N.; Haley, M.M.; Johnson, D.W. Substituent Effects in CH Hydrogen Bond Interactions: Linear Free Energy Relationships and Influence of Anions. J. Am. Chem. Soc. 2015, 137, 14959–14967. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Samorí, P.; Müllen, K.; Rabe, J.P. Molecular-Scale Tracking of the Self-Healing of Polycrystalline Monolayers at the Solid–Liquid Interface. Adv. Mater. 2004, 16, 1761–1765. [Google Scholar] [CrossRef]
- Palma, C.A.; Bonini, M.; Breiner, T.; Samorì, P. Supramolecular Crystal Engineering at the Solid–Liquid Interface from First Principles: Toward Unraveling the Thermodynamics of 2D Self-Assembly. Adv. Mater. 2009, 21, 1383–1386. [Google Scholar] [CrossRef]
- Kim, K.; Plass, K.E.; Matzger, A.J. Kinetic and thermodynamic forms of a two-dimensional crystal. Langmuir 2003, 19, 7149–7152. [Google Scholar] [CrossRef]
- Lei, S.; Tahara, K.; De Schryver, F.C.; Van der Auweraer, M.; Tobe, Y.; De Feyter, S. One building block, two different supramolecular surface-confined patterns: concentration in control at the solid–liquid interface. Angew. Chem. Int. Ed. 2008, 47, 2964–2968. [Google Scholar] [CrossRef] [PubMed]
- Gesquière, A.; Abdel-Mottaleb, M.M.; De Feyter, S.; De Schryver, F.C.; Sieffert, M.; Müllen, K.; Calderone, A.; Lazzaroni, R.; Brédas, J.L. Dynamics in Physisorbed Monolayers of 5-Alkoxy-isophthalic Acid Derivatives at the Liquid/Solid Interface Investigated by Scanning Tunneling Microscopy. Chem. Eur. J. 2000, 6, 3739–3746. [Google Scholar] [CrossRef]
- Sirtl, T.; Song, W.; Eder, G.; Neogi, S.; Schmittel, M.; Heckl, W.M.; Lackinger, M. Solvent-Dependent Stabilization of Metastable Monolayer Polymorphs at the Liquid–Solid Interface. ACS Nano 2013, 7, 6711–6718. [Google Scholar] [CrossRef] [PubMed]
- Jahanbekam, A.; Vorpahl, S.; Mazur, U.; Hipps, K. Temperature stability of three commensurate surface structures of coronene adsorbed on Au(111) from heptanoic acid in the 0 to 60 C range. J. Phys. Chem. C 2013, 117, 2914–2919. [Google Scholar] [CrossRef]
- Dienstmaier, J.F.; Mahata, K.; Walch, H.; Heckl, W.M.; Schmittel, M.; Lackinger, M. On the Scalability of Supramolecular Networks- High Packing Density vs Optimized Hydrogen Bonds in Tricarboxylic Acid Monolayers. Langmuir 2010, 26, 10708–10716. [Google Scholar] [CrossRef]
- Zhang, R.; Yan, Q.; Shen, Y.; Gan, L.; Zeng, Q.d.; Zhao, D.; Wang, C. Conformational polymorphism of multimeric perylene derivatives observed by using scanning tunneling microscopy. CrystEngComm 2011, 13, 5566–5570. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, E.; Liu, J.; Zhang, S.; Lin, Z.; Duan, X.; Heinz, H.; Huang, Y.; De Yoreo, J.J. Building two-dimensional materials one row at a time: Avoiding the nucleation barrier. Science 2018, 362, 1135–1139. [Google Scholar] [CrossRef] [PubMed]
- Romão, R.I.S.; Ferreira, Q.; Morgado, J.; Martinho, J.M.G.; Gonçalves da Silva, A.M.P.S. Microphase Separation in Mixed Monolayers of DPPG with a Double Hydrophilic Block Copolymer at the Air–Water Interface: A BAM, LSCFM, and AFM Study. Langmuir 2010, 26, 17165–17177. [Google Scholar] [CrossRef] [PubMed]
- Raposo, M.; Ferreira, Q.; Ribeiro, P. Modern Research and Educational Topics in Microscopy; Chapter A Guide for Atomic Force Microscopy Analysis of Soft-Condensed Matter; FORMATEX: Badajoz, Spain, 2007. [Google Scholar]
- Ferreira, Q.; Raposo, M.R.; Ribeiro, P. Villain’s fractal growth of poly[1-[4-(3-carboxy-4-hydroxyphenylazo) benzenesulfonamido]-1,2-ethanediyl, sodium salt] J-aggregates onto layer-by-layer films and its effect on film absorbance spectrum. J. Appl. Phys. 2013, 113, 243508–243515. [Google Scholar] [CrossRef]
- Ferreira, Q.; Bernardo, G.; Charas, A.; Alcácer, L.; Morgado, J. Polymer Light-Emitting Diode Interlayers’ Formation Studied by Current-Sensing Atomic Force Microscopy and Scaling Laws. J. Phys. Chem. C 2010, 114, 572–579. [Google Scholar] [CrossRef]
- Visser, J.; Katsonis, N.; Vicario, J.; Feringa, B.L. Two-dimensional molecular patterning by surface-enhanced zn-porphyrin coordination. Langmuir 2009, 25, 5980–5985. [Google Scholar] [CrossRef] [PubMed]
- Iritani, K.; Tahara, K.; De Feyter, S.; Tobe, Y. Host–guest chemistry in integrated porous space formed by molecular self-assembly at liquid-solid interfaces. Langmuir 2017, 33, 4601–4618. [Google Scholar] [CrossRef] [PubMed]
- Kudernac, T.; Lei, S.; Elemans, J.A.; De Feyter, S. Two-dimensional supramolecular self-assembly: nanoporous networks on surfaces. Chem. Soc. Rev. 2009, 38, 402–421. [Google Scholar] [CrossRef]
- Lackinger, M.; Griessl, S.; Heckl, W.M.; Hietschold, M.; Flynn, G.W. Self-assembly of trimesic acid at the liquid–solid interface a study of solvent-induced polymorphism. Langmuir 2005, 21, 4984–4988. [Google Scholar] [CrossRef]
- Lackinger, M.; Heckl, W.M. Carboxylic acids: versatile building blocks and mediators for two-dimensional supramolecular self-assembly. Langmuir 2009, 25, 11307–11321. [Google Scholar] [CrossRef]
- Tahara, K.; Nakatani, K.; Iritani, K.; De Feyter, S.; Tobe, Y. Periodic Functionalization of Surface-Confined Pores in a Two-Dimensional Porous Network Using a Tailored Molecular Building Block. ACS Nano 2016, 10, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Inukai, K.; Adisoejoso, J.; Yamaga, H.; Balandina, T.; Blunt, M.O.; De Feyter, S.; Tobe, Y. Tailoring Surface-Confined Nanopores with Photoresponsive Groups. Angew. Chem. Int. Ed. 2013, 52, 8373–8376. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Furukawa, S.; Uji-i, H.; Uchino, T.; Ichikawa, T.; Zhang, J.; Mamdouh, W.; Sonoda, M.; De Schryver, F.C.; De Feyter, S.; et al. Two-dimensional porous molecular networks of dehydrobenzo[12]annulene derivatives via alkyl chain interdigitation. J. Am. Chem. Soc. 2006, 128, 16613–16625. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Lei, S.; Adisoejoso, J.; De Feyter, S.; Tobe, Y. Supramolecular surface-confined architectures created by self-assembly of triangular phenylene-ethynylene macrocycles via van der Waals interaction. Chem. Commun. 2010, 46, 8507–8525. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Adisoejoso, J.; Inukai, K.; Lei, S.; Noguchi, A.; Li, B.; Vanderlinden, W.; De Feyter, S.; Tobe, Y. Harnessing by a diacetylene unit: a molecular design for porous two-dimensional network formation at the liquid/solid interface. Chem. Commun. 2014, 50, 2831–2833. [Google Scholar] [CrossRef] [PubMed]
- Schlickum, U.; Decker, R.; Klappenberger, F.; Zoppellaro, G.; Klyatskaya, S.; Ruben, M.; Silanes, I.; Arnau, A.; Kern, K.; Brune, H.; et al. Metal–Organic Honeycomb Nanomeshes with Tunable Cavity Size. Nano Lett. 2007, 7, 3813–3817. [Google Scholar] [CrossRef] [PubMed]
- Kühne, D.; Klappenberger, F.; Decker, R.; Schlickum, U.; Brune, H.; Klyatskaya, S.; Ruben, M.; Barth, J.V. High-Quality 2D Metal–Organic Coordination Network Providing Giant Cavities within Mesoscale Domains. J. Am. Chem. Soc. 2009, 131, 3881–3883. [Google Scholar] [CrossRef]
- Teyssandier, J.; De Feyter, S.; Mali, K.S. Host–guest chemistry in two-dimensional supramolecular networks. Chem. Commun. 2016, 52, 11465–11487. [Google Scholar] [CrossRef]
- Lei, S.; Tahara, K.; Adisoejoso, J.; Balandina, T.; Tobe, Y.; De Feyter, S. Towards two-dimensional nanoporous networks: crystal engineering at the solid–liquid interface. CrystEngComm 2010, 12, 3369–3381. [Google Scholar] [CrossRef]
- Griessl, S.J.; Lackinger, M.; Jamitzky, F.; Markert, T.; Hietschold, M.; Heckl, W.M. Incorporation and manipulation of coronene in an organic template structure. Langmuir 2004, 20, 9403–9407. [Google Scholar] [CrossRef]
- Balandina, T.; Tahara, K.; Sandig, N.; Blunt, M.O.; Adisoejoso, J.; Lei, S.; Zerbetto, F.; Tobe, Y.; De Feyter, S. Role of Substrate in Directing the Self-Assembly of Multicomponent Supramolecular Networks at the Liquid–Solid Interface. ACS Nano 2012, 6, 8381–8389. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Tahara, K.; Feng, X.; Furukawa, S.; De Schryver, F.C.; Müllen, K.; Tobe, Y.; De Feyter, S. Molecular clusters in two-dimensional surface-confined nanoporous molecular networks: Structure, rigidity, and dynamics. J. Am. Chem. Soc. 2008, 130, 7119–7129. [Google Scholar] [CrossRef]
- Lei, S.; Surin, M.; Tahara, K.; Adisoejoso, J.; Lazzaroni, R.; Tobe, Y.; Feyter, S.D. Programmable hierarchical three-component 2D assembly at a liquid–solid interface: recognition, selection, and transformation. Nano Lett. 2008, 8, 2541–2546. [Google Scholar] [CrossRef]
- Iritani, K.; Tahara, K.; Hirose, K.; De Feyter, S.; Tobe, Y. Construction of cyclic arrays of Zn-porphyrin units and their guest binding at the solid–liquid interface. Chem. Commun. 2016, 52, 14419–14422. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, D.; Mohnani, S.; Llanes-Pallas, A. Supramolecular chemistry at interfaces: molecular recognition on nanopatterned porous surfaces. Chem. Eur. J. 2009, 15, 7004–7025. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ghijsens, E.; Ivasenko, O.; Cao, H.; Noguchi, A.; Mali, K.S.; Tahara, K.; Tobe, Y.; De Feyter, S. Dynamic control over supramolecular handedness by selecting chiral induction pathways at the solution–solid interface. Nat. Chem. 2016, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E.; Moorthy, S.; Chio, W.I.K.; Lee, T.C. Artificial molecular and nanostructures for advanced nanomachinery. Chem. Commun. 2018, 54, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Franken, L.; Wei, Y.; Chen, J.; Boekema, E.J.; Zhao, D.; Stuart, M.C.; Feringa, B.L. Solvent mixing to induce molecular motor aggregation into bowl-shaped particles: underlying mechanism, particle nature and application to control motor behavior. J. Am. Chem. Soc. 2018, 140, 7860–7868. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S.; Sleven, J.; Binnemans, K.; De Feyter, S. Two-dimensional self-assembly and phase behavior of an alkoxylated sandwich-type bisphthalocyanine and its phthalocyanine analogues at the liquid–solid interface. Langmuir 2006, 22, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Wesley; Brown, B.L.; Feringa. Making molecular machines work. Nat. Nanothechnol. 2006, 1, 25–35. [Google Scholar]
- Pathem, B.K.; Claridge, S.A.; Zheng, Y.B.; Weiss, P.S. Molecular switches and motors on surfaces. Annu. Rev. Phys. Chem. 2013, 64, 605–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.; Bragança, A.; Alcácer, L.; Morgado, J.; Figueiredo, M.; BIOUCAS-DIAS, J.; Ferreira, Q. Sparse-coding denoising applied to reversible conformational switching of a porphyrin self-assembled monolayer induced by scanning tunnelling microscopy. J. Microsc. 2018, 271, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, R.A.; Ter Wiel, M.K.; Pollard, M.M.; Vicario, J.; Koumura, N.; Feringa, B.L. Unidirectional molecular motor on a gold surface. Nature 2005, 437, 1337. [Google Scholar] [CrossRef] [PubMed]
- Lensen, D.; Elemans, J.A. Artificial molecular rotors and motors on surfaces: STM reveals and triggers. Soft Matter 2012, 8, 9053–9063. [Google Scholar] [CrossRef]
- Lu, S.; Huang, M.; Qin, Z.; Yu, Y.; Guo, Q.; Cao, G. Highly ordered molecular rotor matrix on a nanopatterned template: titanyl phthalocyanine molecules on FeO/Pt(111). Nanotechnology 2018, 29, 315301. [Google Scholar] [CrossRef] [PubMed]
- Graziano, G. Molecular motors in a tight spot. Nat. Rev. Chem. 2018, 2, 99. [Google Scholar] [CrossRef]
- Seldenthuis, J.S.; Prins, F.; Thijssen, J.M.; van der Zant, H.S. An all-electric single-molecule motor. ACS Nano 2010, 4, 6681–6686. [Google Scholar] [CrossRef] [PubMed]
- Otsuki, J.; Komatsu, Y.; Kobayashi, D.; Asakawa, M.; Miyake, K. Rotational libration of a double-decker porphyrin visualized. J. Am. Chem. Soc. 2010, 132, 6870–6871. [Google Scholar] [CrossRef] [PubMed]
- Nirmalraj, P.N.; Thompson, D.; Riel, H.E. Capturing the embryonic stages of self-assembly-design rules for molecular computation. Sci. Rep. 2015, 5, 10116. [Google Scholar] [CrossRef] [PubMed]
- Haq, S.; Wit, B.; Sang, H.; Floris, A.; Wang, Y.; Wang, J.; Pérez-García, L.; Kantorovitch, L.; Amabilino, D.B.; Raval, R. A small molecule walks along a surface between porphyrin fences that are assembled in situ. Angew. Chem. Int. Ed. 2015, 54, 7101–7105. [Google Scholar] [CrossRef] [PubMed]
- Nirmalraj, P.; La Rosa, A.; Thompson, D.; Sousa, M.; Martin, N.; Gotsmann, B.; Riel, H. Fingerprinting electronic molecular complexes in liquid. Sci. Rep. 2016, 6, 19009. [Google Scholar] [CrossRef] [PubMed]
- Puigmartí-Luis, J.; Saletra, W.J.; González, A.; Amabilino, D.B.; Pérez-García, L. Bottom-up assembly of a surface-anchored supramolecular rotor enabled using a mixed self-assembled monolayer and pre-complexed components. Chem. Commun. 2014, 50, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Castelvecchi, D. Drivers gear up for world’s first nanocar race. Nat. News 2017, 544, 278. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Mori, T.; Nakanishi, W. Nano Trek Beyond: Driving Nanocars/Molecular Machines at Interfaces. Chem. Asian J. 2018, 13, 1266–1278. [Google Scholar] [CrossRef] [PubMed]
- Rapenne, G.; Joachim, C. The first nanocar race. Nat. Rev. Mater. 2017, 2, 17040. [Google Scholar] [CrossRef]
- Arrhenius, T.S.; Blanchard-Desce, M.; Dvolaitzky, M.; Lehn, J.M.; Malthete, J. Molecular devices: caroviologens as an approach to molecular wires-synthesis and incorporation into vesicle membranes. Proc. Natl. Acad. Sci. USA 1986, 83, 5355–5359. [Google Scholar] [CrossRef]
- Li, L.; Lo, W.Y.; Cai, Z.; Zhang, N.; Yu, L. Proton-triggered switch based on a molecular transistor with edge-on gate. Chem. Sci. 2016, 7, 3137–3141. [Google Scholar] [CrossRef]
- Brooke, R.J.; Szumski, D.S.; Vezzoli, A.; Higgins, S.J.; Nichols, R.J.; Schwarzacher, W. Dual Control of Molecular Conductance through pH and Potential in Single-Molecule Devices. Nano Lett. 2018, 18, 1317–1322. [Google Scholar] [CrossRef]
- Nacci, C.; Ample, F.; Bleger, D.; Hecht, S.; Joachim, C.; Grill, L. Conductance of a single flexible molecular wire composed of alternating donor and acceptor units. Nat. Commun. 2015, 6, 7397. [Google Scholar] [CrossRef]
- Tricoli, A.; Nasiri, N.; De, S. Wearable and miniaturized sensor technologies for personalized and preventive medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Yokota, T.; Zalar, P.; Kaltenbrunner, M.; Jinno, H.; Matsuhisa, N.; Kitanosako, H.; Tachibana, Y.; Yukita, W.; Koizumi, M.; Someya, T. Ultraflexible organic photonic skin. Sci. Adv. 2016, 2, e1501856. [Google Scholar] [CrossRef] [PubMed]
- Lipomi, D.J.; Bao, Z. Stretchable and ultraflexible organic electronics. MRS Bull. 2017, 42, 93–97. [Google Scholar] [CrossRef]
- Rogers, J.A. Wearable electronics: Nanomesh on-skin electronics. Nat. Nanotechnol. 2017, 12, 839. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, K.; Jeong, U. Strategies for stretchable polymer semiconductor layers. MRS Bull. 2017, 42, 98–102. [Google Scholar] [CrossRef]
- Lee, S.; Reuveny, A.; Reeder, J.; Lee, S.; Jin, H.; Liu, Q.; Yokota, T.; Sekitani, T.; Isoyama, T.; Abe, Y.; et al. A transparent bending-insensitive pressure sensor. Nat. Nanotechnol. 2016, 11, 472. [Google Scholar] [CrossRef] [PubMed]
- Merces, L.; de Oliveira, R.F.; Gomes, H.L.; Bufon, C.C.B. The role of the electrode configuration on the electrical properties of small-molecule semiconductor thin-films. Org. Electron. 2017, 49, 107–113. [Google Scholar] [CrossRef]
- Martínez-Domingo, C.; Conti, S.; Terés, L.; Gomes, H.L.; Ramon, E. Novel flexible inkjet-printed Metal-Insulator-Semiconductor organic diode employing silver electrodes. Org. Electron. 2018, 62, 335–341. [Google Scholar] [CrossRef]
- Rocha, P.R.; Schlett, P.; Kintzel, U.; Mailänder, V.; Vandamme, L.K.; Zeck, G.; Gomes, H.L.; Biscarini, F.; De Leeuw, D.M. Electrochemical noise and impedance of Au electrode/electrolyte interfaces enabling extracellular detection of glioma cell populations. Sci. Rep. 2016, 6, 34843. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta (BBA) Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Stefani, D.; Perrin, M.; Gutiérrez-Cerón, C.; Aragonès, A.C.; Labra-Muñoz, J.; Carrasco, R.D.; Matsushita, Y.; Futera, Z.; Labuta, J.; Ngo, T.H.; et al. Mechanical Tuning of Through-Molecule Conductance in a Conjugated Calix[4]pyrrole. ChemistrySelect 2018, 3, 6473–6478. [Google Scholar] [CrossRef]
- Ruiz, M.P.; Aragonès, A.C.; Camarero, N.; Vilhena, J.; Ortega, M.; Zotti, L.A.; Pérez, R.; Cuevas, J.C.; Gorostiza, P.; Díez-Pérez, I. Bioengineering a single-protein junction. J. Am. Chem. Soc. 2017, 139, 15337–15346. [Google Scholar] [CrossRef]
- Cardigos, J.; Ferreira, Q.; Crisóstomo, S.; Moura-Coelho, N.; Cunha, J.P.; Pinto, L.A.; Ferreira, J.T. Nanotechnology-Ocular Devices for Glaucoma Treatment: A Literature Review. Curr. Eye Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, M.; Artés, J.M.; Sarasso, V.; Carminati, M.; Díez-Pérez, I.; Sanz, F.; Gorostiza, P. Differential electrochemical conductance imaging at the nanoscale. Small 2017, 13, 1700958. [Google Scholar] [CrossRef] [PubMed]
- Sepunaru, L.; Pecht, I.; Sheves, M.; Cahen, D. Solid-state electron transport across azurin: From a temperature-independent to a temperature-activated mechanism. J. Am. Chem. Soc. 2011, 133, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sepunaru, L.; Amdursky, N.; Cohen, S.R.; Pecht, I.; Sheves, M.; Cahen, D. Temperature and force dependence of nanoscale electron transport via the Cu protein azurin. ACS Nano 2012, 6, 10816–10824. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lovrincic, R.; Sepunaru, L.; Li, W.; Vilan, A.; Pecht, I.; Sheves, M.; Cahen, D. Insights into solid-state electron transport through proteins from inelastic tunneling spectroscopy: The case of azurin. ACS Nano 2015, 9, 9955–9963. [Google Scholar] [CrossRef]
- Ouyang, C.; Hashimoto, K.; Tsuji, H.; Nakamura, E.; Majima, Y. Coherent Resonant Electron Tunneling at 9 and 300 K through a 4.5 nm Long, Rigid, Planar Organic Molecular Wire. ACS Omega 2018, 3, 5125–5130. [Google Scholar] [CrossRef]
- Steinmann, V.; Moro, L. Encapsulation requirements to enable stable organic ultra-thin and stretchable devices. J. Mater. Res. 2018, 33, 1925–1936. [Google Scholar] [CrossRef]
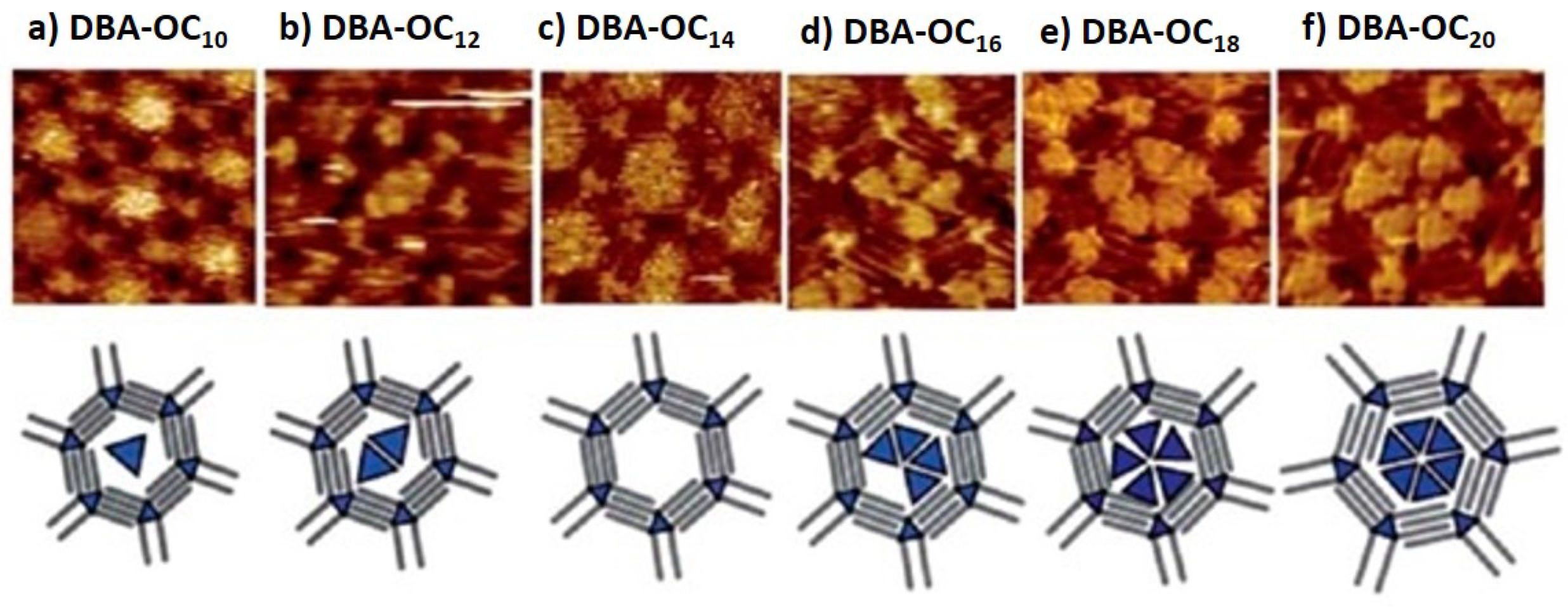
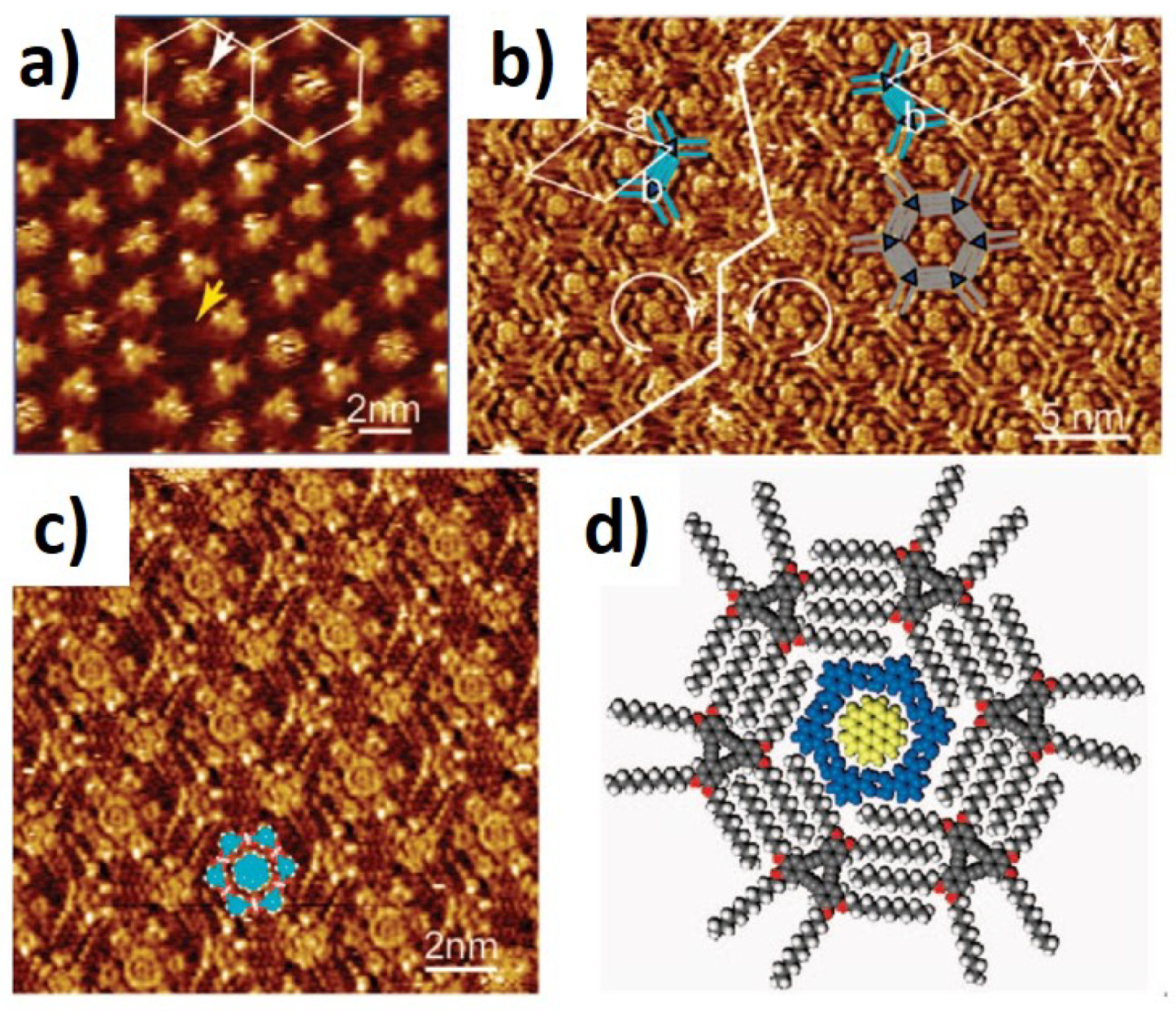

| Nanoporous Self-Assembled Networks | |||
|---|---|---|---|
| Bonding between Building Blocks | Building Blocks | Range of Pore | References |
| Hydrogen bonds | TMA | 1–2.8 nm | [41,42] |
| van der Waals interactions | DBA | 1.6–4.7 nm | [43,44,46,50] |
| Metal-Ligand coordination bonds | Dicycanooligophenylene | 4.2–6.7 nm | [48,49] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, Q.; Delfino, C.L.; Morgado, J.; Alcácer, L. Bottom-Up Self-Assembled Supramolecular Structures Built by STM at the Solid/Liquid Interface. Materials 2019, 12, 382. https://doi.org/10.3390/ma12030382
Ferreira Q, Delfino CL, Morgado J, Alcácer L. Bottom-Up Self-Assembled Supramolecular Structures Built by STM at the Solid/Liquid Interface. Materials. 2019; 12(3):382. https://doi.org/10.3390/ma12030382
Chicago/Turabian StyleFerreira, Quirina, Catarina L. Delfino, Jorge Morgado, and Luís Alcácer. 2019. "Bottom-Up Self-Assembled Supramolecular Structures Built by STM at the Solid/Liquid Interface" Materials 12, no. 3: 382. https://doi.org/10.3390/ma12030382
APA StyleFerreira, Q., Delfino, C. L., Morgado, J., & Alcácer, L. (2019). Bottom-Up Self-Assembled Supramolecular Structures Built by STM at the Solid/Liquid Interface. Materials, 12(3), 382. https://doi.org/10.3390/ma12030382





