Effect of Sodium Hexametaphosphate and Trisodium Phosphate on Dispersion of Polycarboxylate Superplasticizer
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.1.1. Cement
2.1.2. Polycarboxylate Superplasticizer
2.1.3. Phosphates
2.2. Test Methods
2.2.1. Rheology
2.2.2. Adsorption Amount
2.2.3. Adsorption Layer
- I0 denoted the initial photoelectron intensity;
- I(b) represented the photoelectron intensity after the photoelectron went through the adsorption layer;
- meant the thickness of adsorption layer, nm;
- Ek was the photoelectron kinetic energy after the photoelectron went through the adsorption layer, eV;
- Eb was the electron binding energy, eV.
2.2.4. Hydration Heat
2.2.5. Phase Analysis
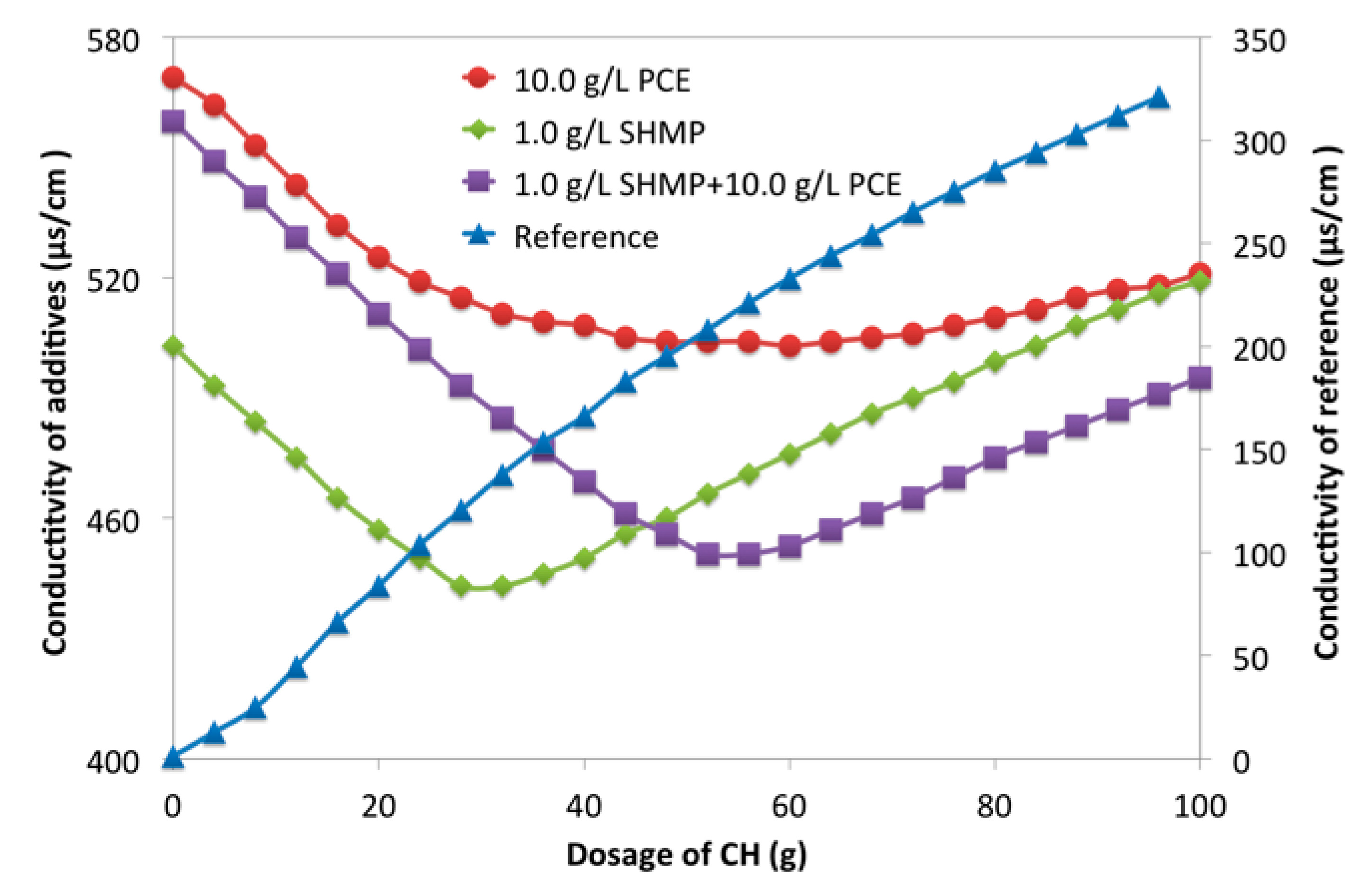
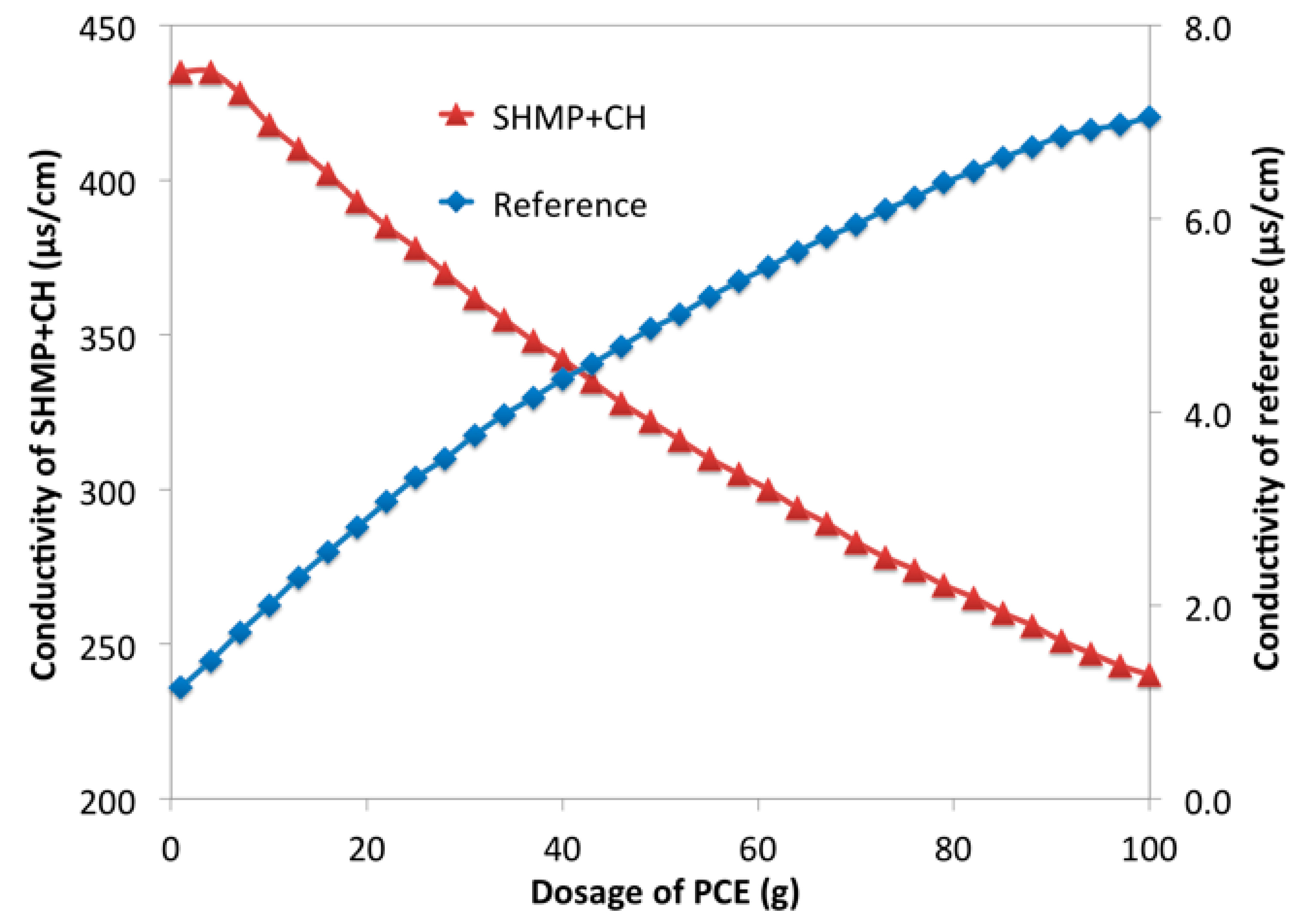
2.2.6. Conductivity Measurement
3. Results and Discussions
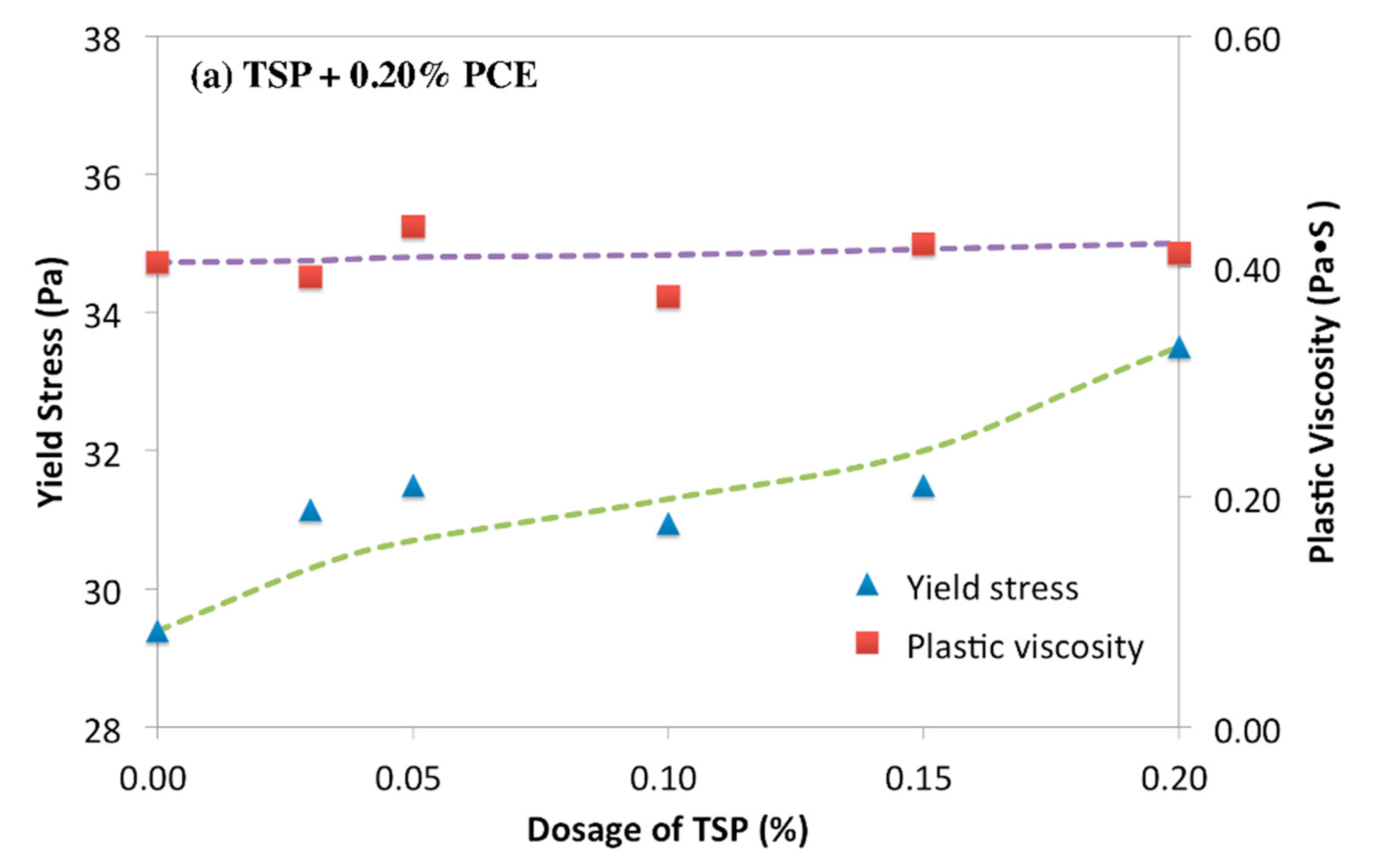
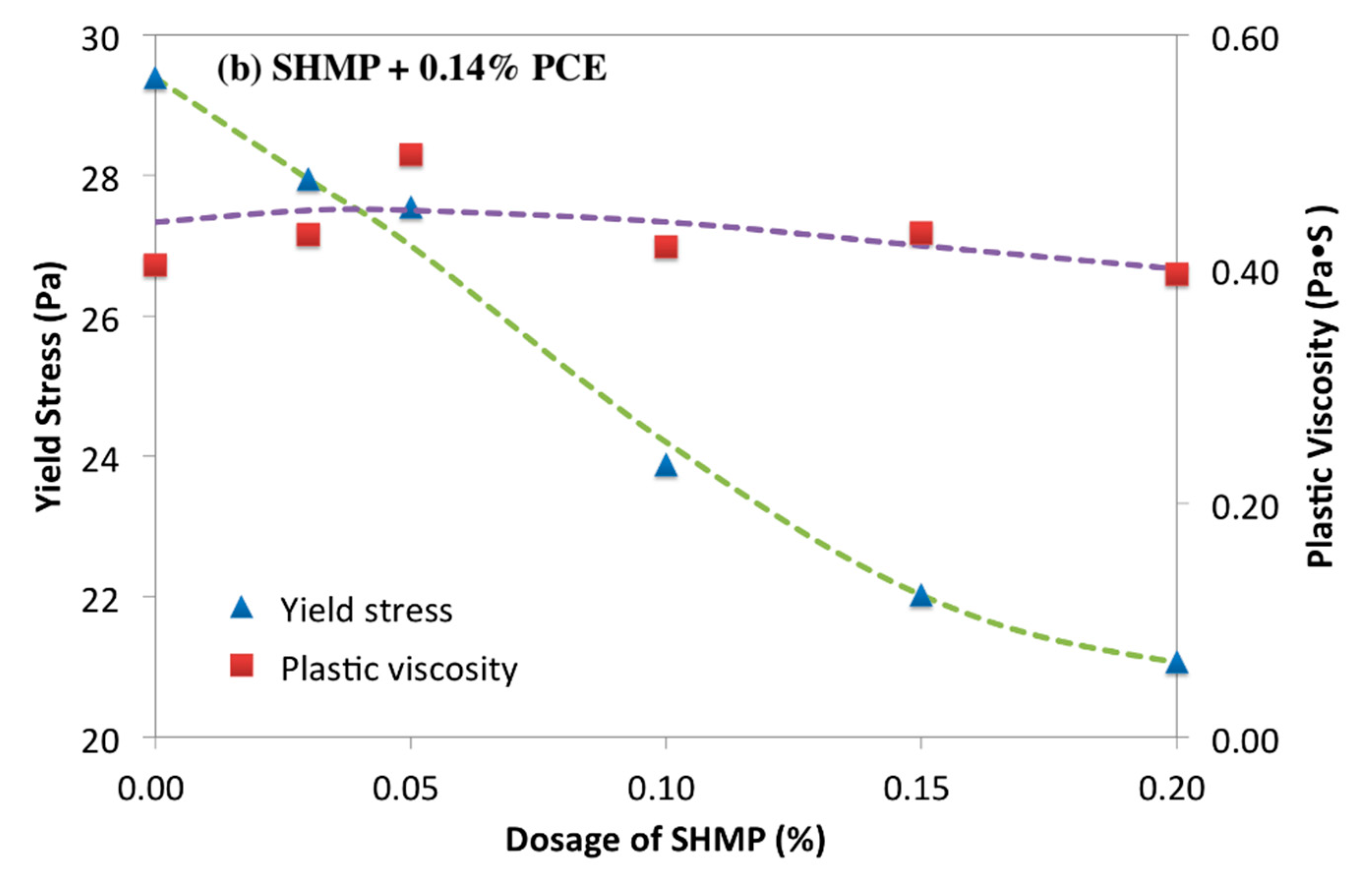
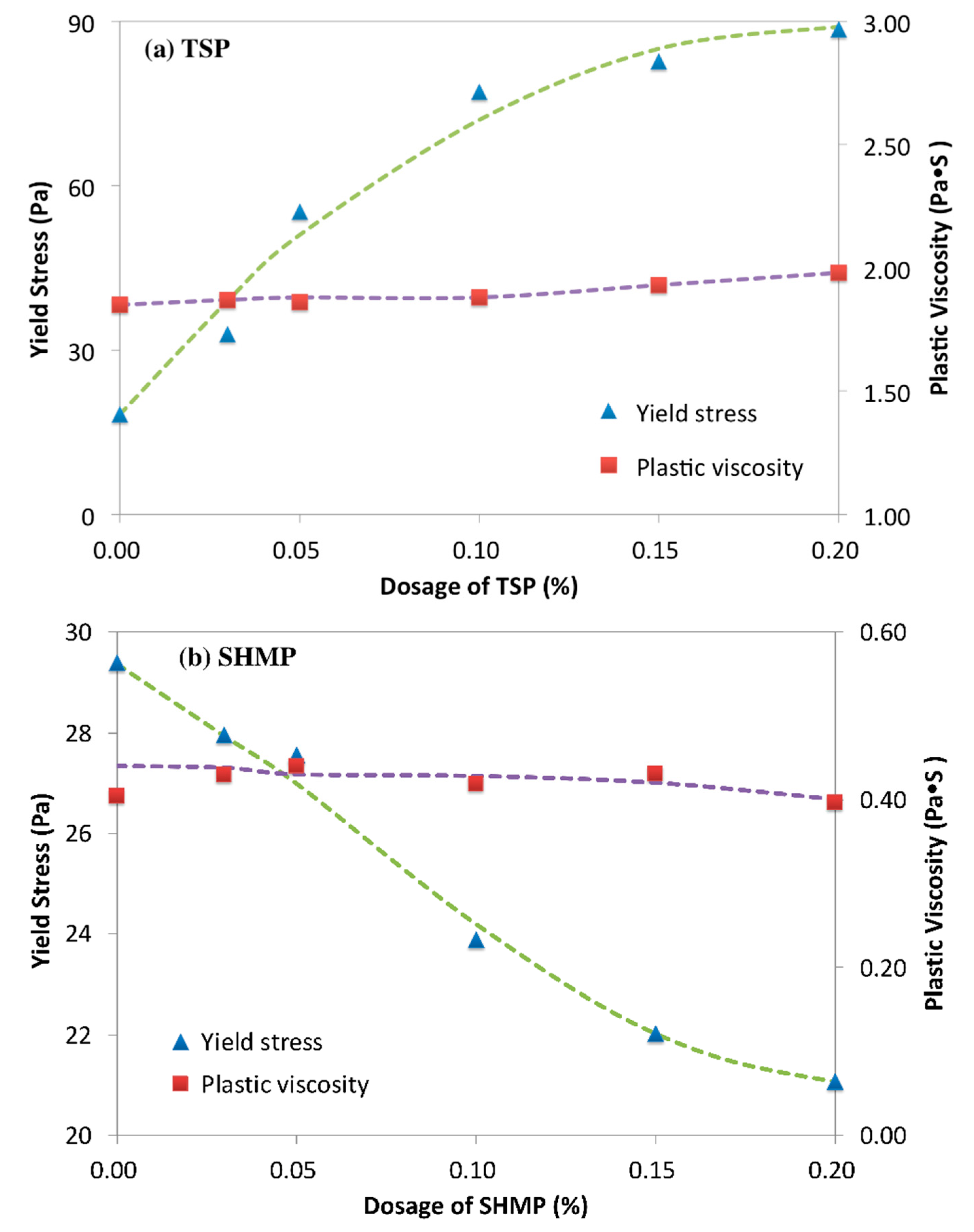
3.1. Rheology of Cement Paste Plasticized by PCE-Phosphate
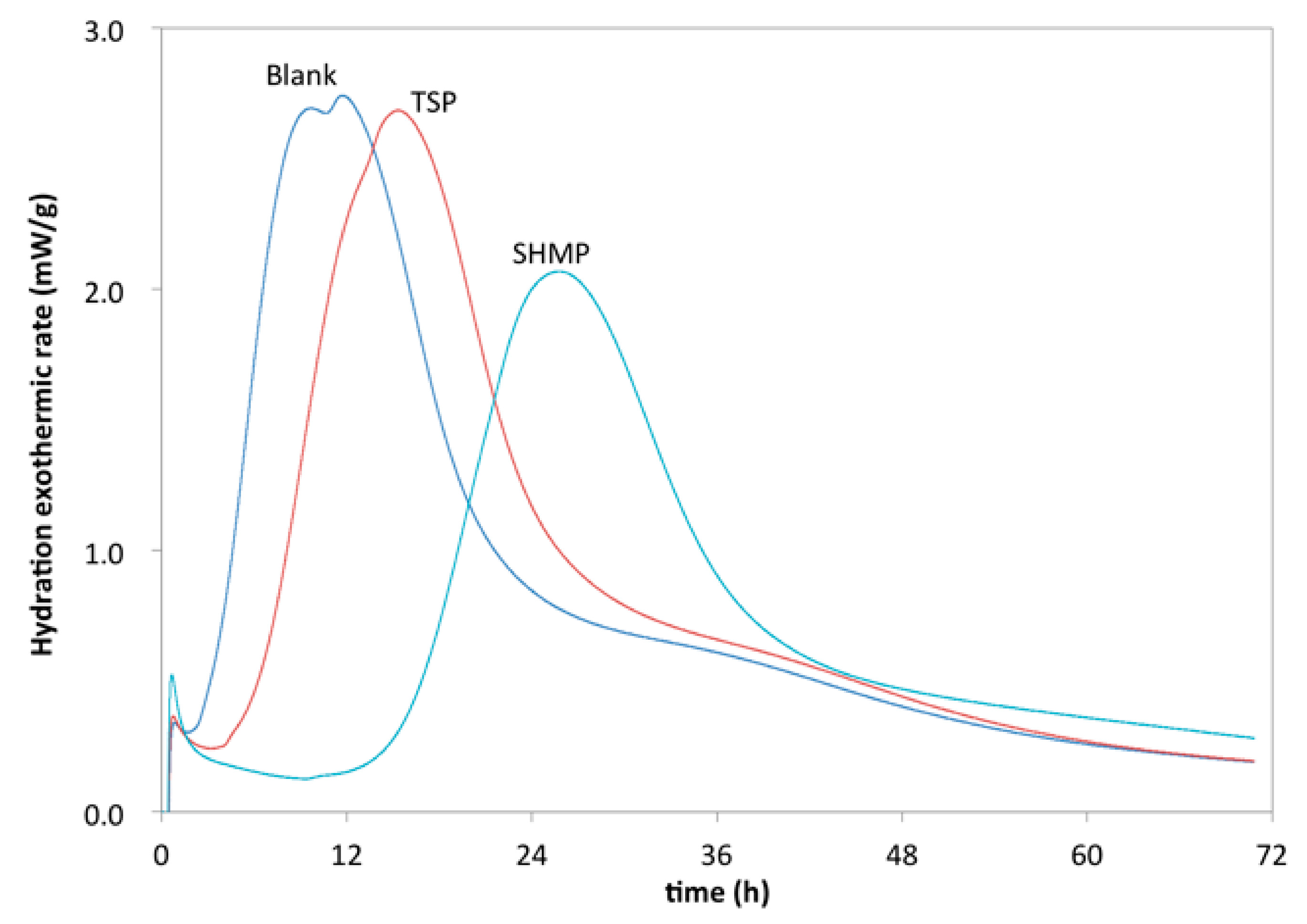
3.2. Retarding Effect of Phosphates on Cement Paste
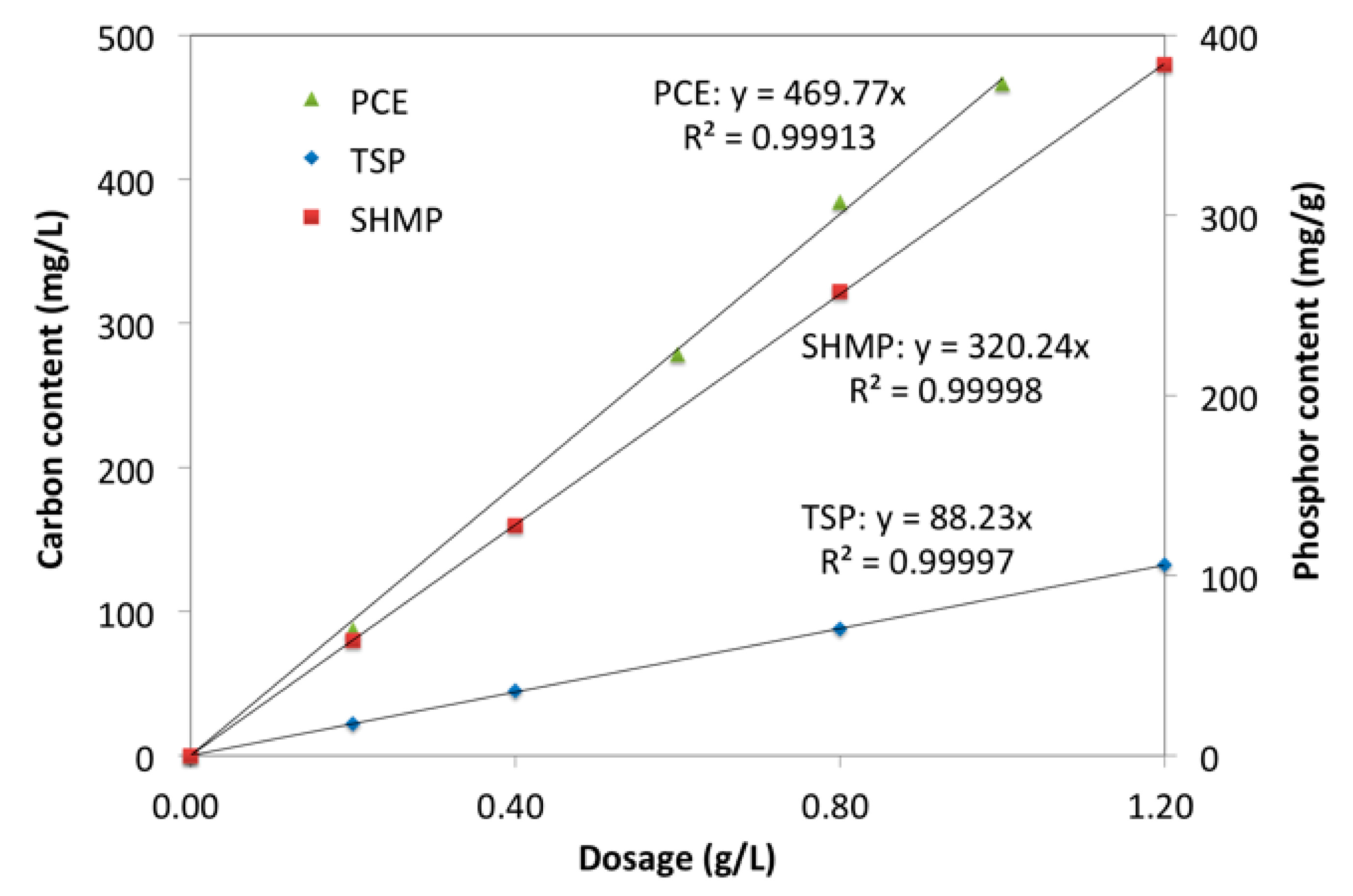
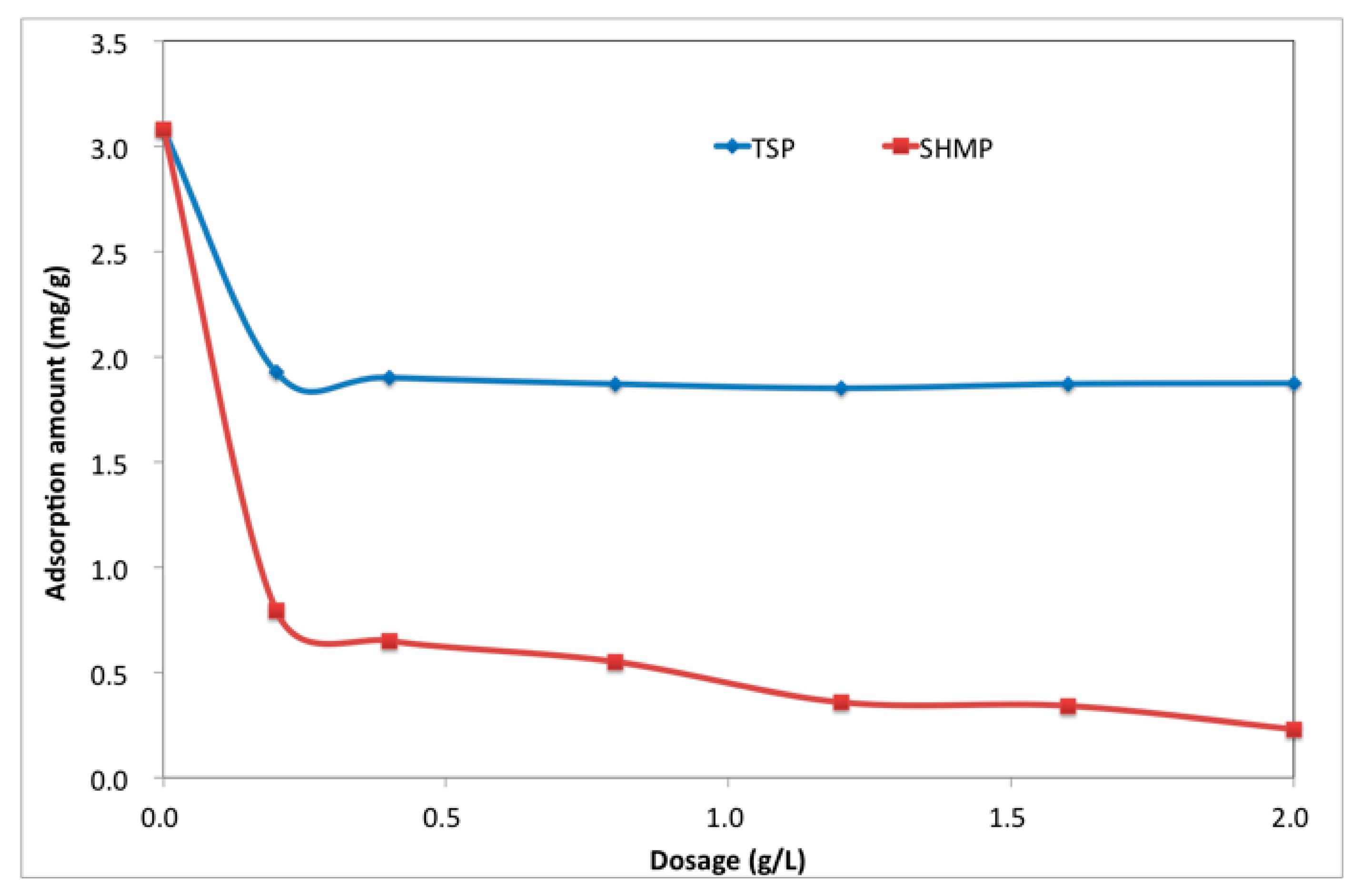
3.3. Adsorption Amount
3.4. Thickness of Adsorption Layer
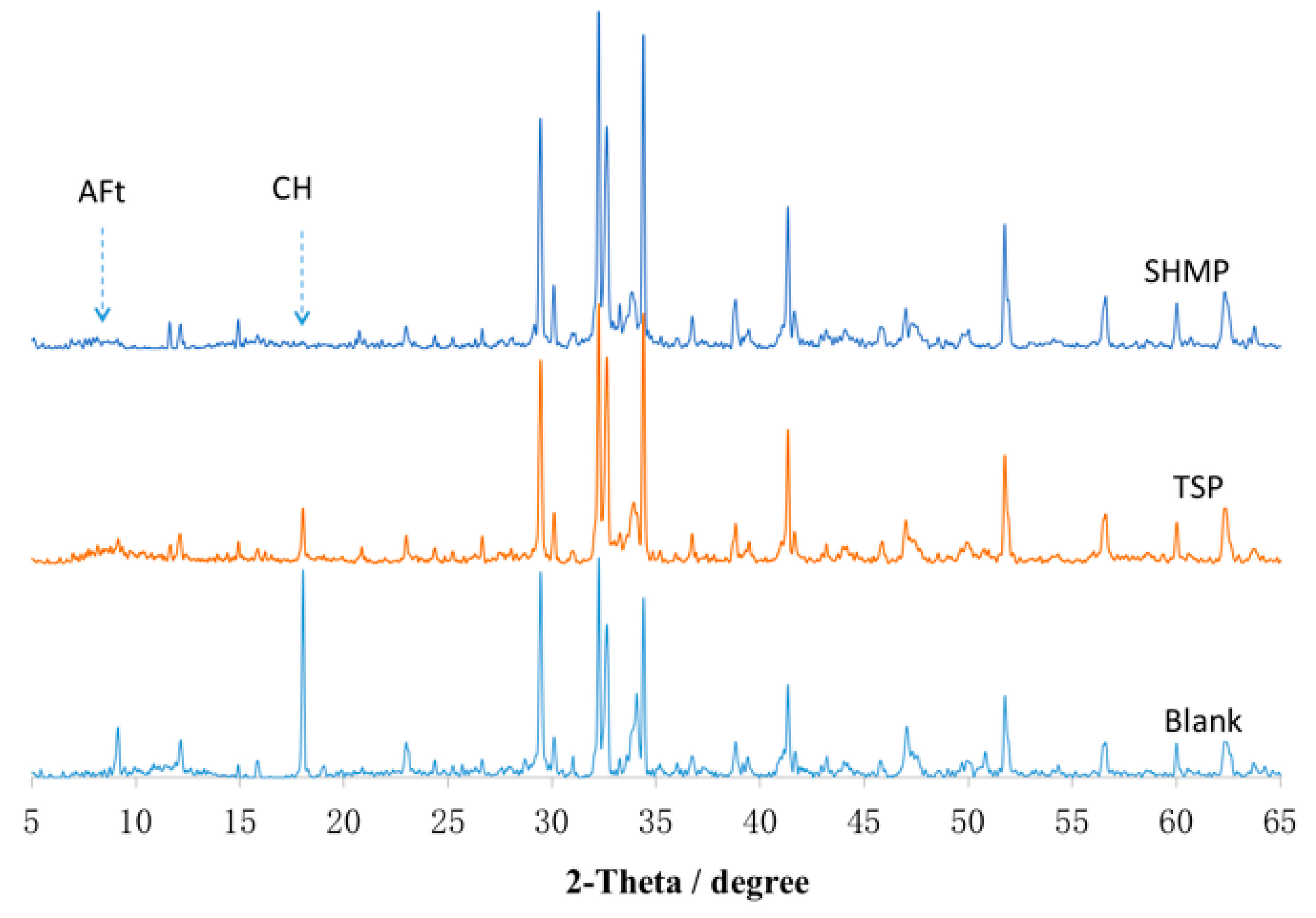
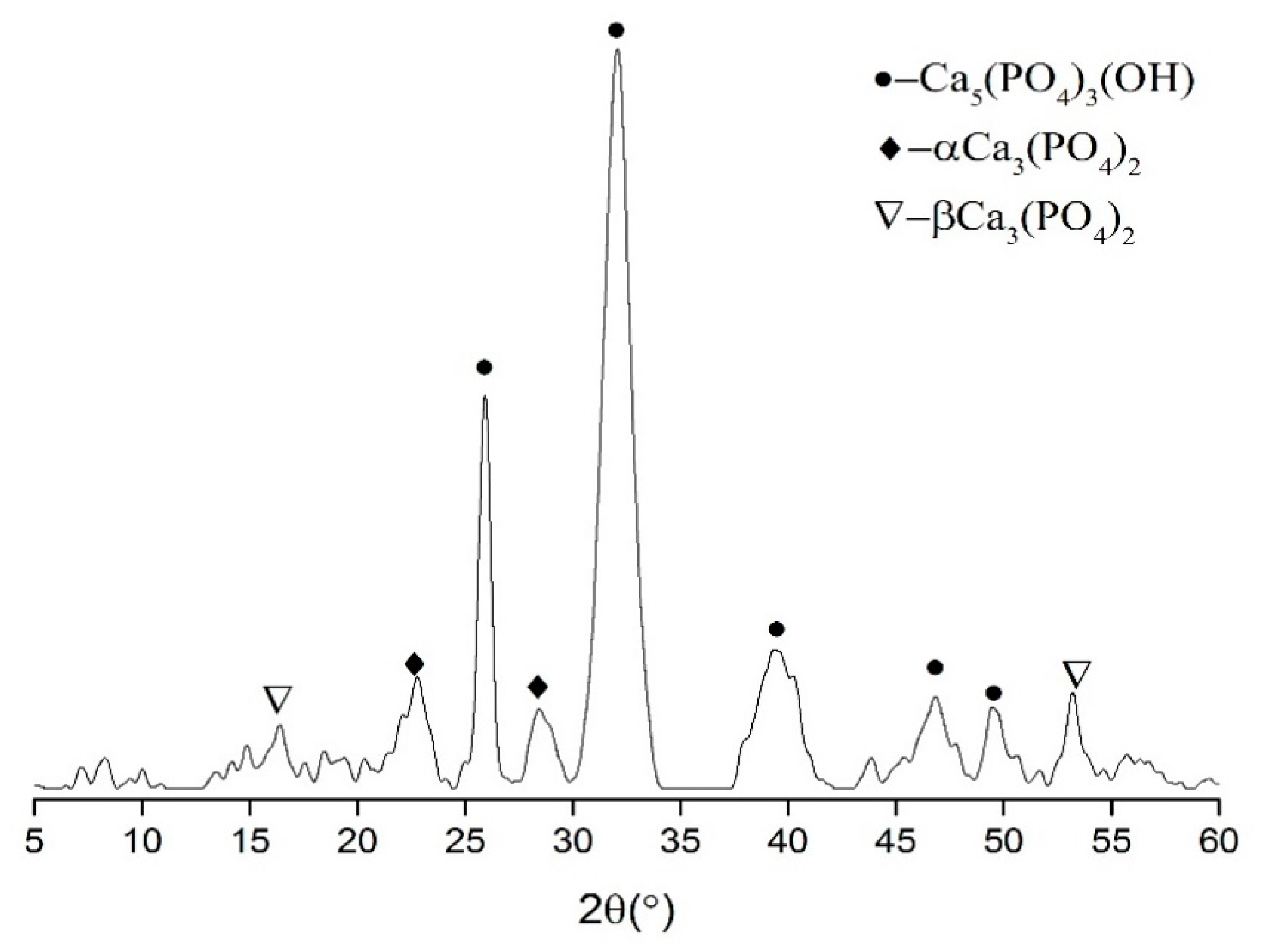
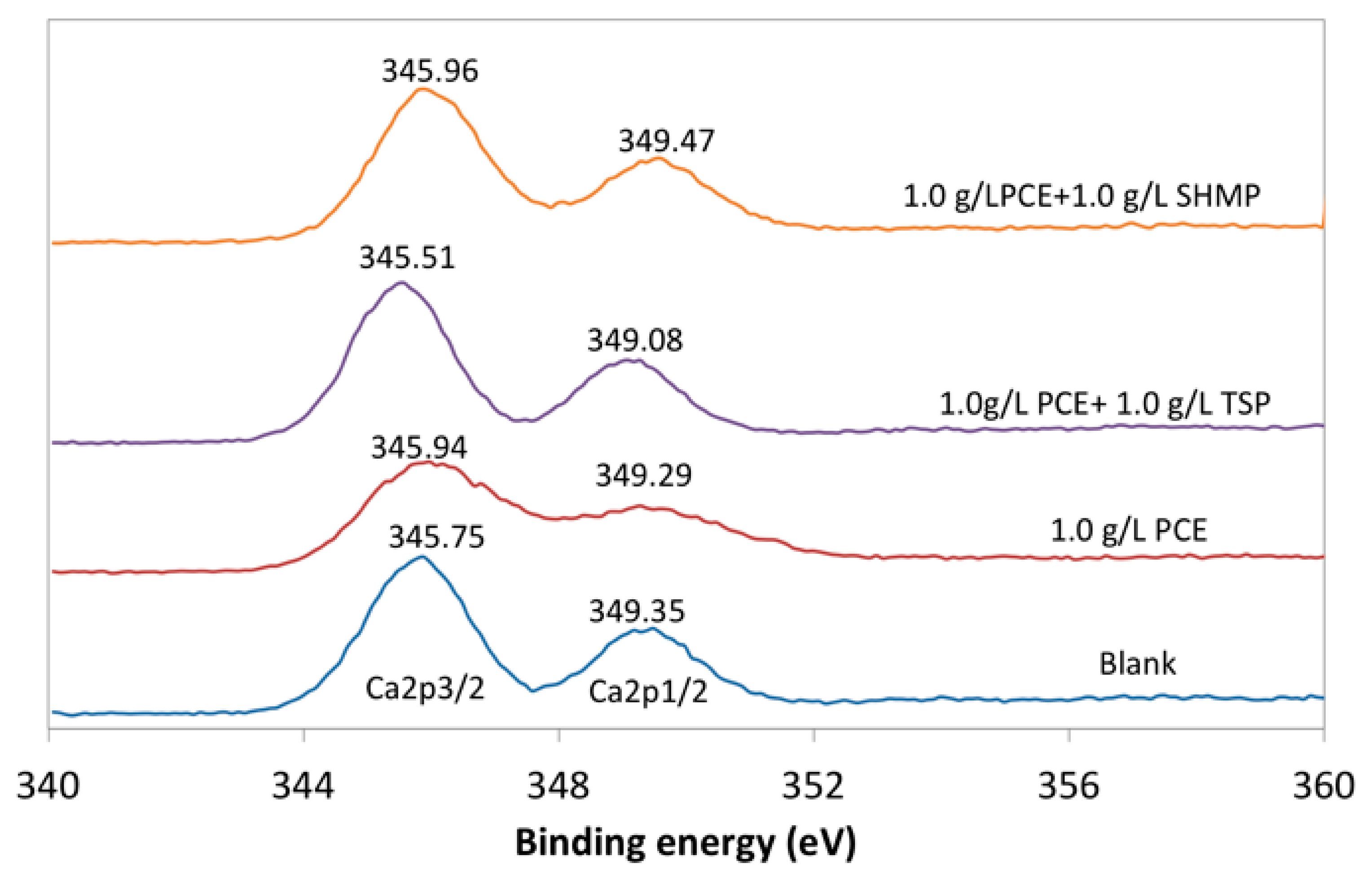
3.5. Composition of Adsorption Layer
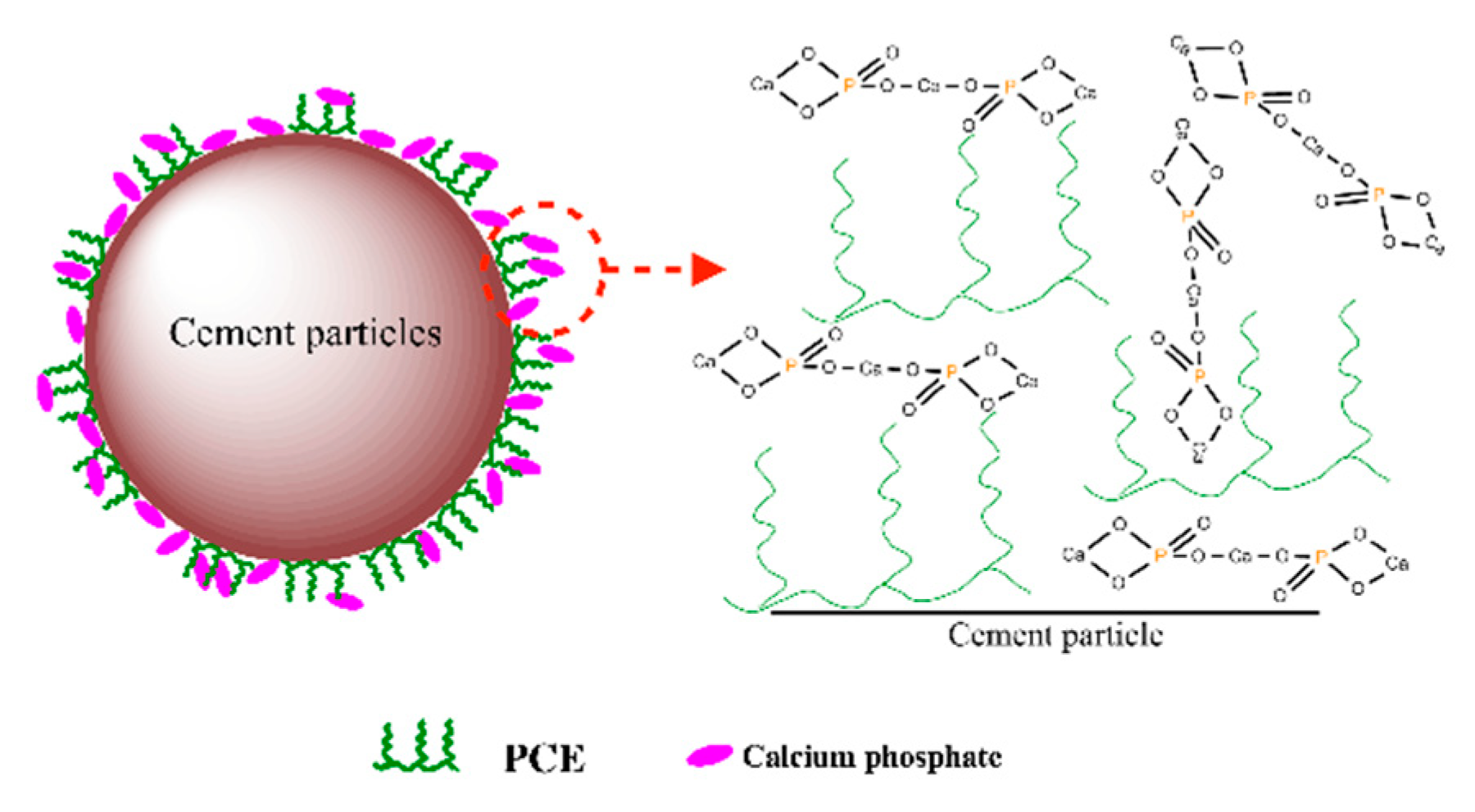
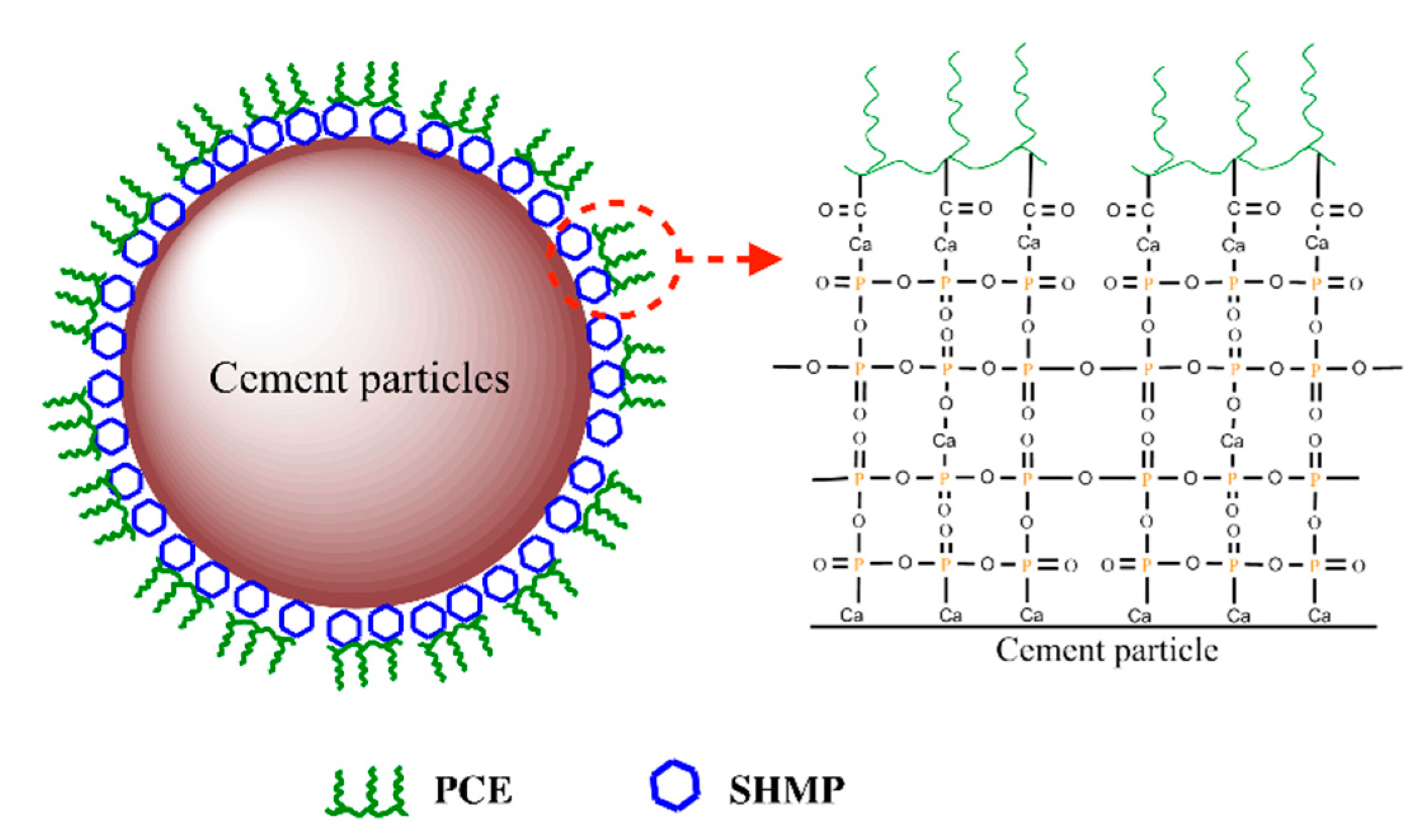
3.6. Dispersion Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferraris, C.F.; Obla, K.H.; Hill, R. The influence of mineral admixtures on the rheology of cement paste and concrete. Cem. Concr. Res. 2001, 31, 245–255. [Google Scholar] [CrossRef]
- Peng, J.; Qu, J.; Zhang, J.; Chen, M.; Wan, T. Adsorption characteristics of water-reducing agents on gypsum surface and its effect on the rheology of gypsum plaster. Cem. Concr. Res. 2005, 35, 527–531. [Google Scholar] [CrossRef]
- Hanehara, S.; Yamada, K. Rheology and early age properties of cement systems. Cem. Concr. Res. 2008, 38, 175–195. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Z.; Zou, D.; Zhou, A.; Du, J. Strength enhancement of recycled aggregate pervious concrete using a cement paste redistribution method. Cem. Concr. Res. 2019, 122, 72–82. [Google Scholar] [CrossRef]
- Senff, L.; Labrincha, J.A.; Ferreira, V.M.; Hotza, D.; Repette, W.L. Effect of nano-silica on rheology and fresh properties of cement pastes and mortars. Constr. Build. Mater. 2009, 23, 2487–2491. [Google Scholar] [CrossRef]
- Feys, D.; De Schutter, G.; Khayat, K.H.; Verhoeven, R. Changes in rheology of self-consolidating concrete induced by pumping. Mater. Struct. 2016, 49, 4657–4677. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Gu, B.; Zhang, T.; Chen, P.; Li, H.; Mei, J. Effect of aluminum sulfate on the hydration of Portland cement, tricalcium silicate and tricalcium aluminate. Constr. Build. Mater. 2020, 232. [Google Scholar] [CrossRef]
- Ng, S.; Justnes, H. Influence of plasticizers on the rheology and early heat of hydration of blended cements with high content of fly ash. Cem. Concr. Compos. 2016, 65, 41–54. [Google Scholar] [CrossRef]
- Ghahari, S.A.; Mohammadi, A.; Ramezanianpour, A.A. Performance assessment of natural pozzolan roller compacted concrete pavements. Case Stud. Constr. Mater. 2017, 7, 82–90. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A.; Ghahari, S.A.; Esmaeili, M. Effect of combined carbonation and chloride ion ingress by an accelerated test method on microscopic and mechanical properties of concrete. Constr. Build. Mater. 2014, 58, 138–146. [Google Scholar] [CrossRef]
- Shannag, M.J. High strength concrete containing natural pozzolan and silica fume. Cem. Concr. Compos. 2000, 22, 399–406. [Google Scholar] [CrossRef]
- Tan, H.; Li, M.; He, X.; Su, Y.; Zhang, J.; Pan, H.; Yang, J.; Wang, Y. Preparation for micro-lithium slag via wet grinding and its application as accelerator in Portland cement. J. Clean. Prod. 2019, 119528. [Google Scholar] [CrossRef]
- Kong, F.-R.; Pan, L.-S.; Wang, C.-M.; Zhang, D.-L.; Xu, N. Effects of polycarboxylate superplasticizers with different molecular structure on the hydration behavior of cement paste. Constr. Build. Mater. 2016, 105, 545–553. [Google Scholar] [CrossRef]
- Plank, J.; Sakai, E.; Miao, C.W.; Yu, C.; Hong, J.X. Chemical admixtures—Chemistry, applications and their impact on concrete microstructure and durability. Cem. Concr. Res. 2015, 78, 81–99. [Google Scholar] [CrossRef]
- Felekoğlu, B.; Sarıkahya, H. Effect of chemical structure of polycarboxylate-based superplasticizers on workability retention of self-compacting concrete. Constr. Build. Mater. 2008, 22, 1972–1980. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Gauckler, L. Adsorption of polyelectrolytes and its influence on the rheology, zeta potential, and microstructure of various cement and hydrate phases. J. Colloid Interface Sci. 2008, 323, 301–312. [Google Scholar] [CrossRef]
- Lesage, K.; Cizer, Ö.; Desmet, B.; Vantomme, J.; De Schutter, G.; Vandewalle, L. Plasticising mechanism of sodium gluconate combined with PCE. Adv. Cem. Res. 2015, 27, 163–174. [Google Scholar] [CrossRef]
- Wu, Y.; He, T.; Song, X.; Liang, G. Effect of sodium gluconate on polynaphthalene sulfonate adsorption. Adv. Cem. Res. 2011, 23, 249–254. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Zou, F.; Jian, S.; Ma, B.; Zhi, Z. Effect of borax on rheology of calcium sulphoaluminate cement paste in the presence of polycarboxylate superplasticizer. Constr. Build. Mater. 2017, 139, 277–285. [Google Scholar] [CrossRef]
- Bessaies-Bey, H.; Baumann, R.; Schmitz, M.; Radler, M.; Roussel, N. Organic admixtures and cement particles: Competitive adsorption and its macroscopic rheological consequences. Cem. Concr. Res. 2016, 80, 1–9. [Google Scholar] [CrossRef]
- Tan, H.; Huang, J.; Ma, B.; Li, X. Effect of superplasticiser and sodium tripolyphosphate on fluidity of cement paste. Mag. Concr. Res. 2014, 66, 1194–1200. [Google Scholar] [CrossRef]
- Li, G.; He, T.; Hu, D.; Huang, R.; Shi, C. Effects of retarders on the fluidity of pastes containingβ-naphthalenesulfonic acid-based superplasticiser. Adv. Cem. Res. 2012, 24, 203–210. [Google Scholar] [CrossRef]
- Ma, S.; Li, W.; Zhang, S.; Ge, D.; Yu, J.; Shen, X. Influence of sodium gluconate on the performance and hydration of Portland cement. Constr. Build. Mater. 2015, 91, 138–144. [Google Scholar] [CrossRef]
- Zhang, G.; Li, G.; Li, Y. Effects of superplasticizers and retarders on the fluidity and strength of sulphoaluminate cement. Constr. Build. Mater. 2016, 126, 44–54. [Google Scholar] [CrossRef]
- Nocuń-Wczelik, W.; Czapik, P. Use of calorimetry and other methods in the studies of water reducers and set retarders interaction with hydrating cement paste. Constr. Build. Mater. 2013, 38, 980–986. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, X. Correlations of the dispersing capability of NSF and PCE types of superplasticizer and their impacts on cement hydration with the adsorption in fresh cement pastes. Cem. Concr. Res. 2015, 69, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Kong, X.-M.; Lu, Z.-B.; Lu, Z.-C.; Hou, S.-S. Effects of the charge characteristics of polycarboxylate superplasticizers on the adsorption and the retardation in cement pastes. Cem. Concr. Res. 2015, 67, 184–196. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Ma, B.; Li, X.; Gu, B. Adsorbing Behavior of Polycarboxylate Superplasticizer in the Presence of Ester Group in Side Chain. J. Disper. Sci. Technol. 2017, 38, 743–749. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Yang, Y.; Wang, X.; Zhang, Q.; Zhao, H.; Ran, Q.; Liu, J. Rheological Properties of Cement Pastes with Polycarboxylate Superplasticizers of Varied Backbone Stiffness. J. Mater. Civ. Eng. 2019, 31, 04019092. [Google Scholar] [CrossRef]
- Ran, Q.; Somasundaran, P.; Miao, C.; Liu, J.; Wu, S.; Shen, J. Effect of the length of the side chains of comb-like copolymer dispersants on dispersion and rheological properties of concentrated cement suspensions. J. Colloid Interface Sci. 2009, 336, 624–633. [Google Scholar] [CrossRef]
- Bey, H.B.; Hot, J.; Baumann, R.; Roussel, N. Consequences of competitive adsorption between polymers on the rheological behaviour of cement pastes. Cem. Concr. Compos. 2014, 54, 17–20. [Google Scholar] [CrossRef]
- Li, G.; He, T.; Hu, D.; Shi, C. Effects of two retarders on the fluidity of pastes plasticized with aminosulfonic acid-based superplasticizers. Constr. Build. Mater. 2012, 26, 72–78. [Google Scholar] [CrossRef]
- Plank, J.; Winter, C. Competitive adsorption between superplasticizer and retarder molecules on mineral binder surface. Cem. Concr. Res. 2008, 38, 599–605. [Google Scholar] [CrossRef]
- Tan, H.; Zou, F.; Ma, B.; Guo, Y.; Li, X.; Mei, J. Effect of competitive adsorption between sodium gluconate and polycarboxylate superplasticizer on rheology of cement paste. Constr. Build. Mater. 2017, 144, 338–346. [Google Scholar] [CrossRef]
- Zou, F.; Tan, H.; Guo, Y.; Ma, B.; He, X.; Zhou, Y. Effect of sodium gluconate on dispersion of polycarboxylate superplasticizer with different grafting density in side chain. J. Ind. Eng. Chem. 2017, 55, 91–100. [Google Scholar] [CrossRef]
- Lv, S.; Gao, R.; Cao, Q.; Li, D.; Duan, J. Preparation and characterization of poly-carboxymethyl-β-cyclodextrin superplasticizer. Cem. Concr. Res. 2012, 42, 1356–1361. [Google Scholar] [CrossRef]
- Tan, H.; Zou, F.; Ma, B.; Liu, M.; Li, X.; Jian, S. Effect of sodium tripolyphosphate on adsorbing behavior of polycarboxylate superplasticizer. Constr. Build. Mater. 2016, 126, 617–623. [Google Scholar] [CrossRef]
- Tan, H.; Zou, F.; Liu, M.; Ma, B.; Guo, Y.; Jian, S. Effect of the Adsorbing Behavior of Phosphate Retarders on Hydration of Cement Paste. J. Mater. Civ. Eng. 2017, 29, 04017088. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, B.; Zhi, Z.; Tan, H.; Liu, M.; Jian, S.; Guo, Y. Effect of polyacrylic acid emulsion on fluidity of cement paste. Colloids Surf. A Physicochem. Eng. Asp. 2017, 535, 139–148. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, X.; Lu, Z.; Jansen, D.; Pakusch, J.; Wang, S. Pore structure of hardened cement paste containing colloidal polymers with varied glass transition temperature and surface charges. Cem. Concr. Compos. 2019, 95, 154–168. [Google Scholar] [CrossRef]
- Tian, H.; Kong, X.; Sun, J.; Wang, D.; Huang, C. Fluidizing effects of polymers with various anchoring groups in cement pastes and their sensitivity to environmental temperatures. J. Appl. Polym. Sci. 2019, 136, 47494. [Google Scholar] [CrossRef]
- Tian, H.; Kong, X.; Su, T.; Wang, D. Comparative study of two PCE superplasticizers with varied charge density in Portland cement and sulfoaluminate cement systems. Cem. Concr. Res. 2019, 115, 43–58. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Cai, Y. Effect of highly carboxylated colloidal polymers on cement hydration and interactions with calcium ions. Cem. Concr. Res. 2018, 113, 140–153. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Zhang, C.; Xing, F.; Zhang, Y. Effect of colloidal polymers with different surface properties on the rheological property of fresh cement pastes. Colloids Surf A Physicochem. Eng. Asp. 2017, 520, 154–165. [Google Scholar] [CrossRef]
- Kong, X.; Pakusch, J.; Jansen, D.; Emmerling, S.; Neubauer, J.; Goetz-Neuhoeffer, F. Effect of polymer latexes with cleaned serum on the phase development of hydrating cement pastes. Cem. Concr. Res. 2016, 84, 30–40. [Google Scholar] [CrossRef]
- Ren, Q.; Jiang, Z.; Li, H.; Zhu, X.; Chen, Q. Fresh and hardened properties of self-compacting concrete using silicon carbide waste as a viscosity-modifying agent. Constr. Build. Mater. 2019, 200, 324–332. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; He, X.; Guo, Y.; Deng, X.; Su, Y.; Yang, J.; Wang, Y. Utilization of lithium slag by wet-grinding process to improve the early strength of sulphoaluminate cement paste. J. Clean. Prod. 2018, 205, 536–551. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; He, X. Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume fly ash cement system. Constr. Build. Mater. 2019, 194, 350–359. [Google Scholar] [CrossRef]
- Ahmad, M.R.; Chen, B.; Haque, M.A.; Ali Shah, S.F. Development of a sustainable and innovant hygrothermal bio-composite featuring the enhanced mechanical properties. J. Clean. Prod. 2019, 229, 128–143. [Google Scholar] [CrossRef]
- Yousefi Oderji, S.; Chen, B.; Ahmad, M.R.; Shah, S.F.A. Fresh and hardened properties of one-part fly ash-based geopolymer binders cured at room temperature: Effect of slag and alkali activators. J. Clean. Prod. 2019, 225, 1–10. [Google Scholar] [CrossRef]
- Chen, P.; Ma, B.; Tan, H.; Liu, X.; Zhang, T.; Qi, H.; Peng, Y.; Yang, Q.; Wang, J. Effects of amorphous aluminum hydroxide on chloride immobilization in cement-based materials. Constr. Build. Mater. 2020, 231, 117171. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; Li, H.; Xu, L.; Ren, Q.; Li, L. Effect of triethanolamine hydrochloride on the performance of cement paste. Constr. Build. Mater. 2019, 200, 218–225. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, C.; Li, H.; Jiang, Z.; Gong, Z.; Yang, X.; Chen, Q. Utilization of the black tea powder as multifunctional admixture for the hemihydrate gypsum. J. Clean. Prod. 2019, 210, 231–237. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Huang, J.; Ma, B.; Qi, C.; Chaoliang, L. Effect of competitive adsorption between polycarboxylate superplasticiser and sodium tripolyphosphate on cement paste fluidity. Adv. Cem. Res. 2015, 27, 593–600. [Google Scholar] [CrossRef]
- AzariJafari, H.; Kazemian, A.; Ahmadi, B.; Berenjian, J.; Shekarchi, M. Studying effects of chemical admixtures on the workability retention of zeolitic Portland cement mortar. Constr. Build. Mater. 2014, 72, 262–269. [Google Scholar] [CrossRef]
- Zhang, D.-F.; Ju, B.-Z.; Zhang, S.-F.; He, L.; Yang, J.-Z. The study on the dispersing mechanism of starch sulfonate as a water-reducing agent for cement. Carbohyd. Polym. 2007, 70, 363–368. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 1999; Volume 15, p. 848. [Google Scholar]
- Lupu, C.; Arvidson, R.S.; Luttge, A.; Barron, A.R. Phosphonate mediated surface reaction and reorganization: Implications for the mechanism controlling cement hydration inhibition. Chem. Commun. 2005, 2354–2356. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem. Concr. Res. 2009, 39, 1–5. [Google Scholar] [CrossRef]
- Zhang, Q.; Ran, Q.; Zhao, H.; Shu, X.; Yang, Y. Effect of counterions on comb-like polycarboxylate conformation in aqueous solutions. J. Disper. Sci. Technol. 2017, 38, 721–728. [Google Scholar] [CrossRef]
- Ran, Q.; Qiao, M.; Liu, J. Influence of Ca2+ on the performance of poly(acrylic acid)-g-poly(ethylene glycol) comb-like copolymers in cement suspensions. Iran. Polym. J. 2014, 23, 663–669. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Ma, B.; Gu, B.; Zou, F. Effect of sodium gluconate on clay tolerance of polycarboxylate superplasticiser. Adv. Cem. Res. 2017, 29, 278–286. [Google Scholar] [CrossRef]
- Tan, H.; Gu, B.; Jian, S.; Ma, B.; Guo, Y.; Zhi, Z. Improvement of Polyethylene Glycol in Compatibility with Polycarboxylate Superplasticizer and Poor-Quality Aggregates Containing Montmorillonite. J. Mater. Civ. Eng. 2017, 29, 04017131. [Google Scholar] [CrossRef]
- Tan, H.; Gu, B.; Guo, Y.; Ma, B.; Huang, J.; Ren, J.; Zou, F.; Guo, Y. Improvement in compatibility of polycarboxylate superplasticizer with poor-quality aggregate containing montmorillonite by incorporating polymeric ferric sulfate. Constr. Build. Mater. 2018, 162, 566–575. [Google Scholar] [CrossRef]
- Tan, H.; Guo, Y.; Ma, B.; Huang, J.; Gu, B.; Zou, F. Effect of Sodium Tripolyphosphate on Clay Tolerance of Polycarboxylate Superplasticizer. KSCE J. Civ. Eng. 2018, 22, 2934–2941. [Google Scholar] [CrossRef]
- Zhang, Q.; Ran, Q.; Zhao, H.; Shu, X.; Yang, Y.; Zhou, H.; Liu, J. pH-induced conformational changes of comb-like polycarboxylate investigated by experiment and simulation. Colloid Polym. Sci. 2016, 294, 1705–1715. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; Guo, Y.; Ma, B.; Jian, S.; He, X.; Zhi, Z.; Liu, X. Improvement in fluidity loss of magnesia phosphate cement by incorporating polycarboxylate superplasticizer. Constr. Build. Mater. 2018, 165, 887–897. [Google Scholar] [CrossRef]
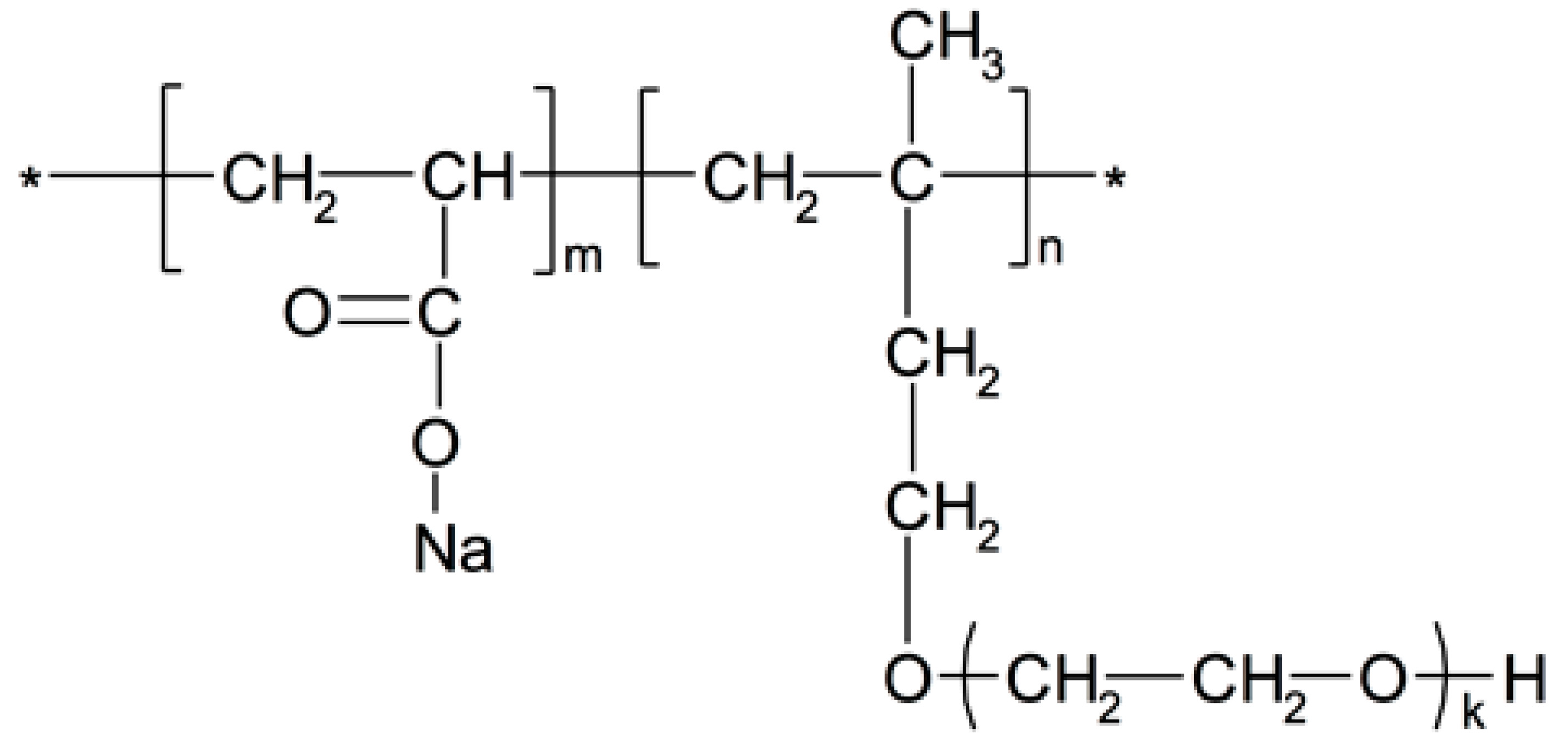
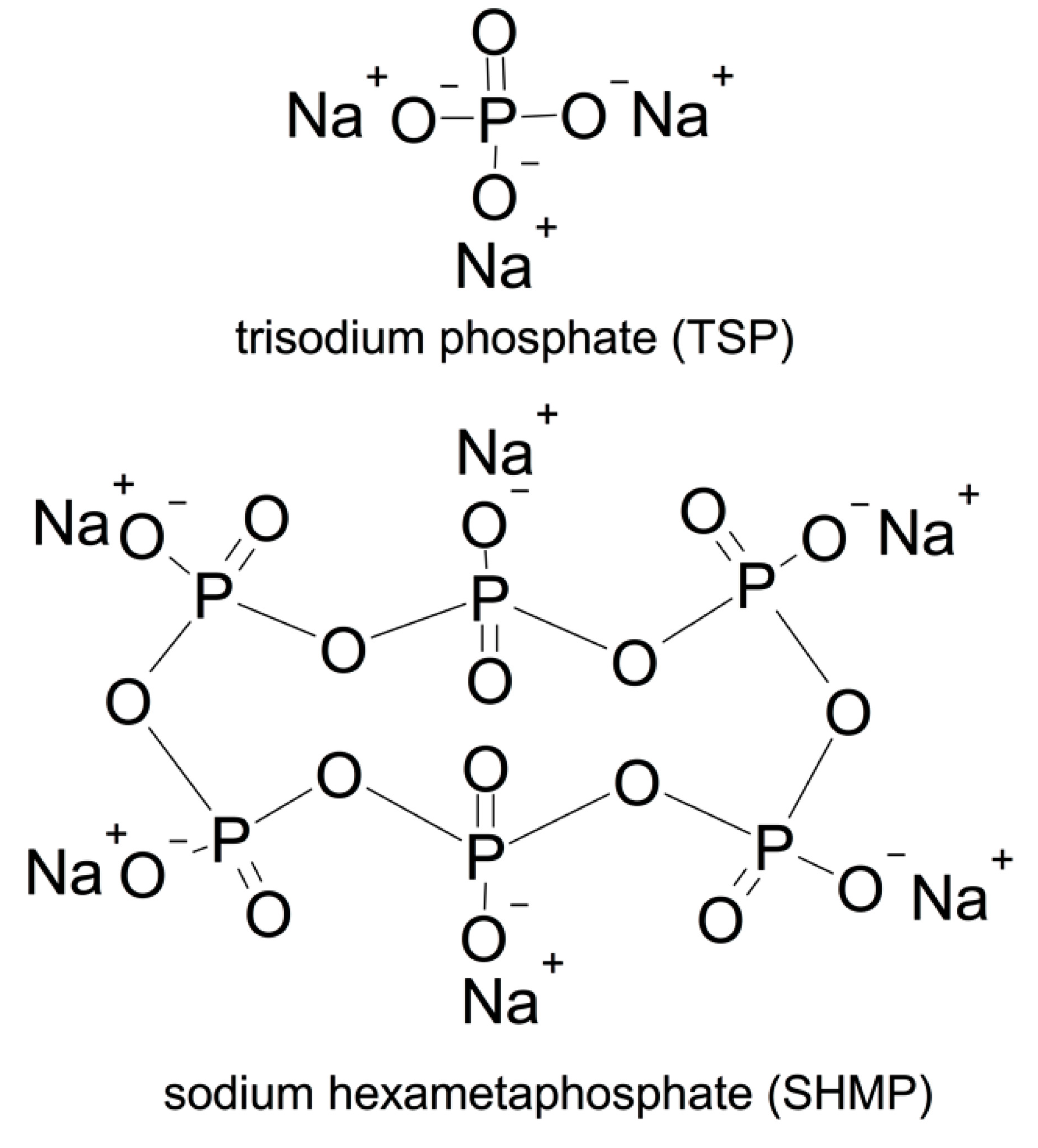


| LOI. | SiO2 | Al2O3 | Fe2O3 | SO3 | CaO | MgO | K2O | Na2O | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Cement | wt% | 3.72 | 22.79 | 7.03 | 3.14 | 3.76 | 55.05 | 2.90 | 0.57 | 0.14 |
| Water Reducing Ratio (%) | Density (g/cm3) | pH Value | Solid Content (%) |
|---|---|---|---|
| 32.1 | 1.076 | 7.0 | 40.0 |
| PCE (g/L) | TSP (g/L) | SHMP (g/L) | |
|---|---|---|---|
| Single-component system | 1.00 | ||
| 0–2.0 | |||
| 0–2.0 | |||
| Binary-component system | 1.00 | 0–2.0 | |
| 1.00 | 0–2.0 |
| Blank | TSP + PCE | SHMP + PCE | PCE | |
|---|---|---|---|---|
| hν(eV) | 1486.60 | 1486.60 | 1486.60 | 1486.60 |
| Eb (eV) | 101.98 | 101.97 | 101.97 | 101.97 |
| Height Counts | 1470.72 | 749.95 | 774.44 | 1013.12 |
| FWHM (eV) | 2.72 | 2.03 | 2.00 | 2.62 |
| I0 | 4000.36 | |||
| I(b) | 1522.40 | 1548.88 | 2654.37 | |
| I/I0 | 0.3805 | 0.3872 | 0.6635 | |
| Ek | 1384.63 | 1384.63 | 1384.62 | |
| λ(Ek) | 4.0932 | 4.0932 | 4.0932 | |
| Adsorption layer (b, nm) | 3.95 | 3.88 | 1.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Liu, H.; Liu, J.; Tong, R. Effect of Sodium Hexametaphosphate and Trisodium Phosphate on Dispersion of Polycarboxylate Superplasticizer. Materials 2019, 12, 4190. https://doi.org/10.3390/ma12244190
Zhang Y, Liu H, Liu J, Tong R. Effect of Sodium Hexametaphosphate and Trisodium Phosphate on Dispersion of Polycarboxylate Superplasticizer. Materials. 2019; 12(24):4190. https://doi.org/10.3390/ma12244190
Chicago/Turabian StyleZhang, Yan, Huaqing Liu, Jialong Liu, and Ruiming Tong. 2019. "Effect of Sodium Hexametaphosphate and Trisodium Phosphate on Dispersion of Polycarboxylate Superplasticizer" Materials 12, no. 24: 4190. https://doi.org/10.3390/ma12244190
APA StyleZhang, Y., Liu, H., Liu, J., & Tong, R. (2019). Effect of Sodium Hexametaphosphate and Trisodium Phosphate on Dispersion of Polycarboxylate Superplasticizer. Materials, 12(24), 4190. https://doi.org/10.3390/ma12244190




