Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Sample Preparation
2.1.1. Material Preparation
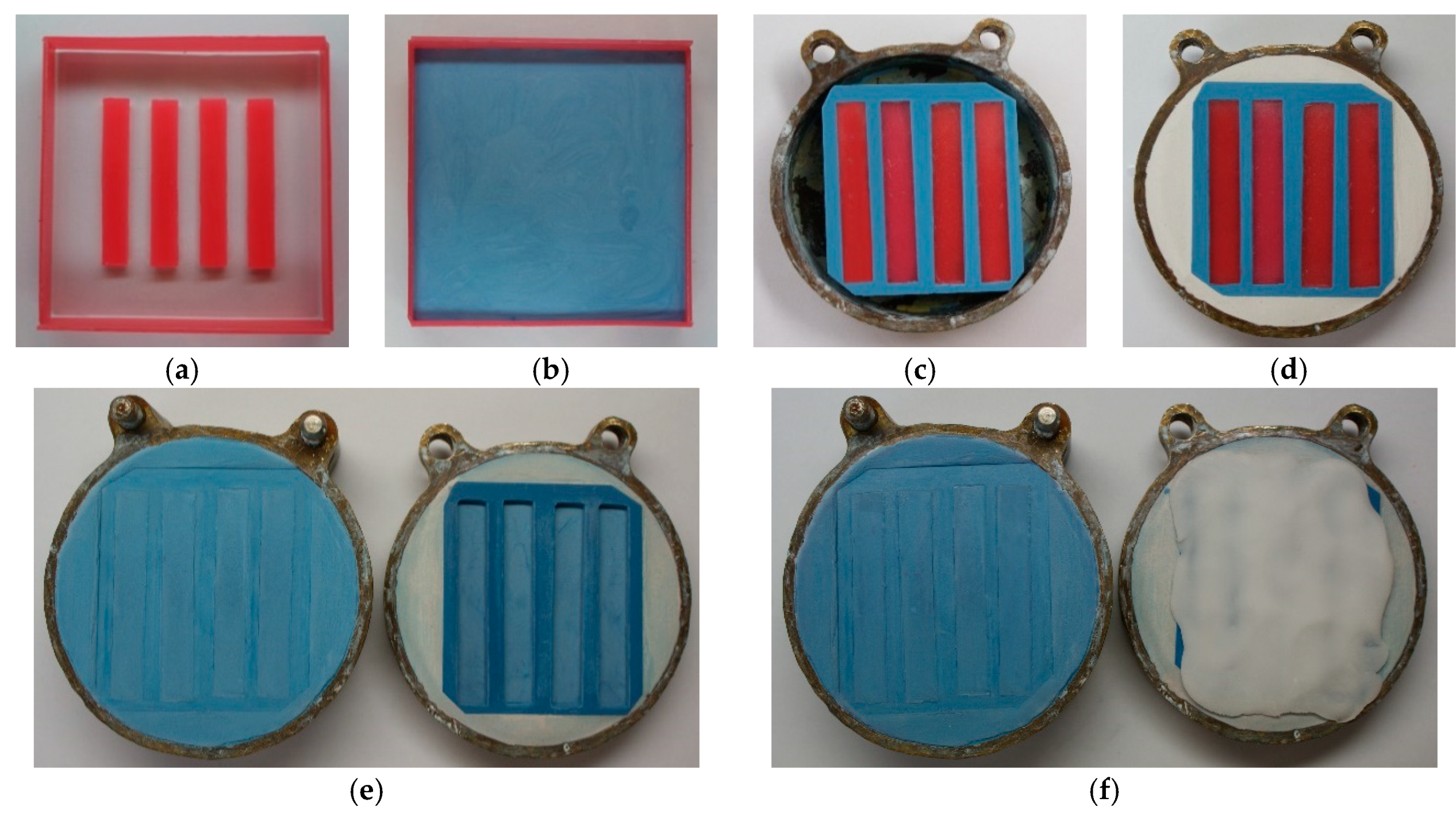
2.1.2. Sample Preparation
2.2. Methods
2.2.1. Flexural Properties
2.2.2. Impact Strength
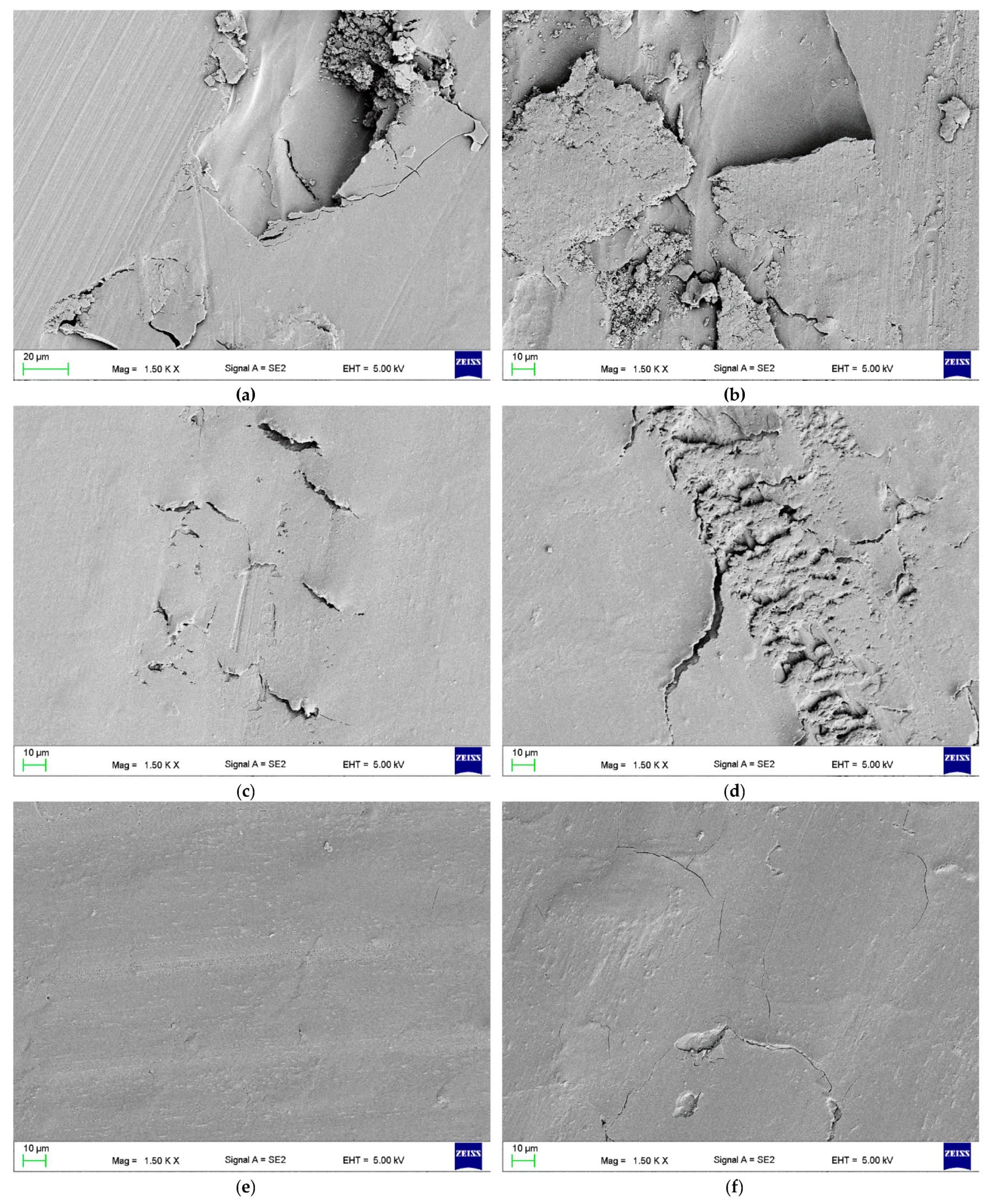
2.2.3. Fracture Analysis
2.2.4. Hardness
2.2.5. Wear Resistance
2.2.6. Sorption and Solubility
2.2.7. Statistical Analyses
3. Results
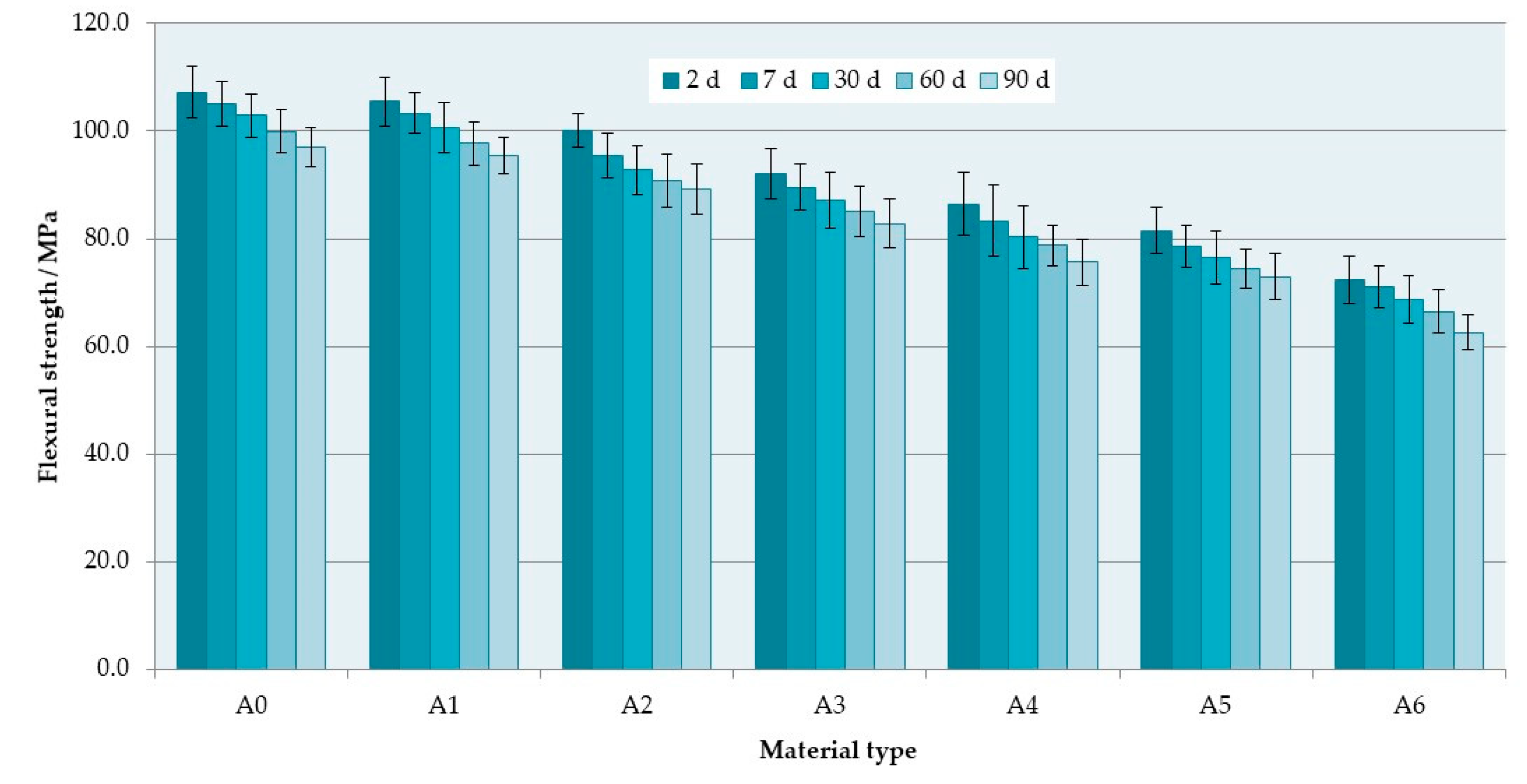
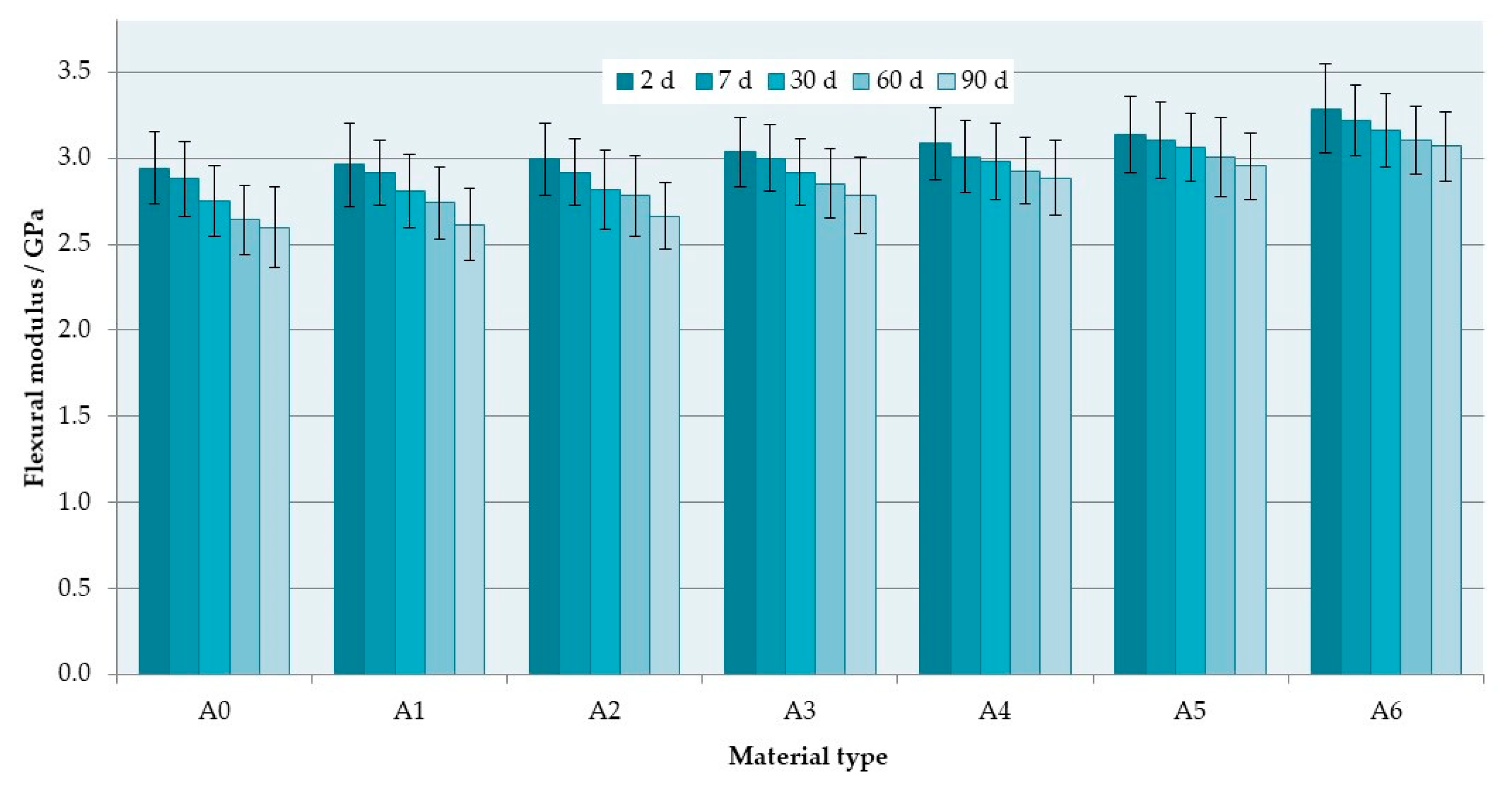
3.1. Flexural Properties
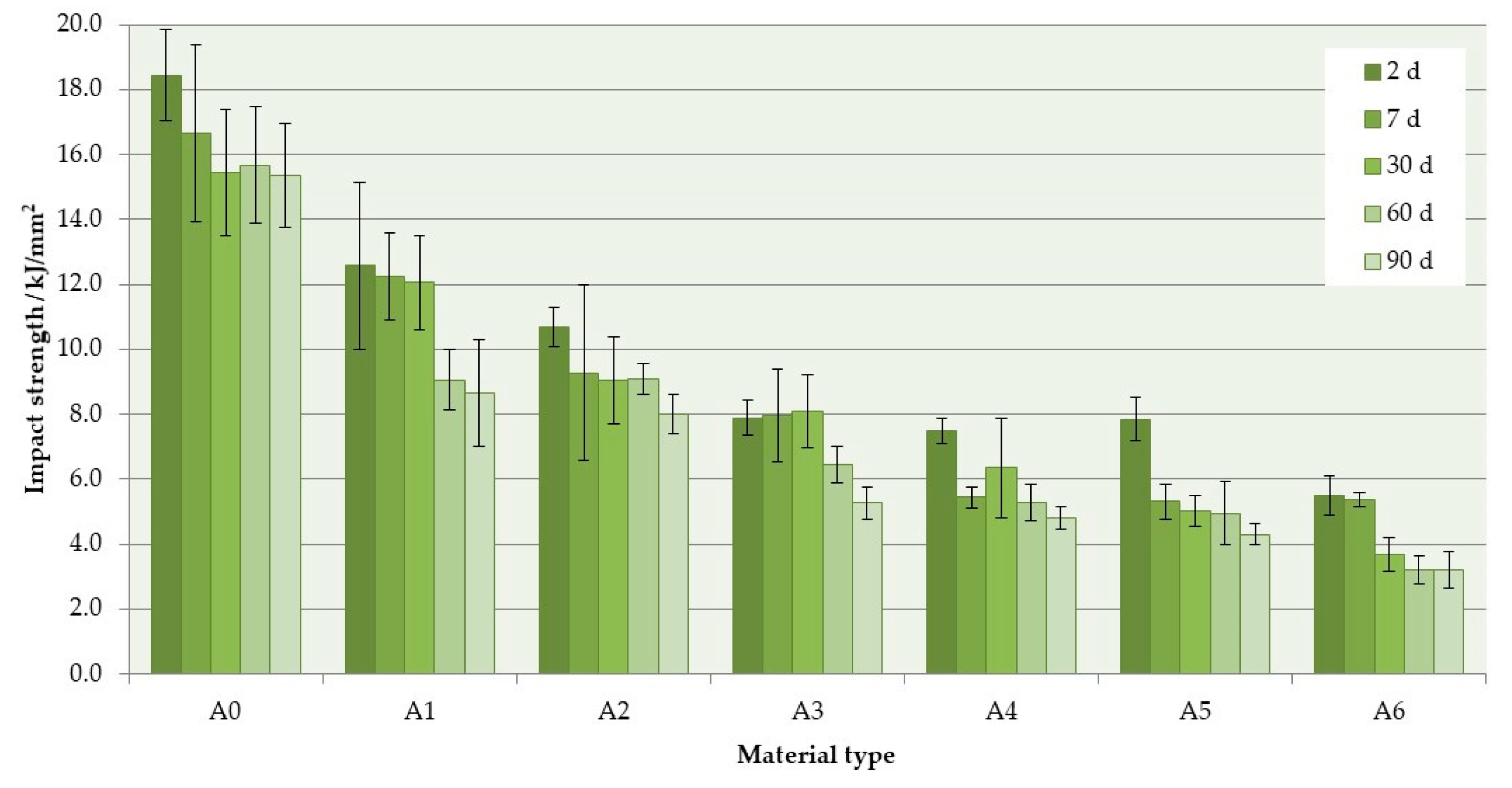
3.2. Impact Strength
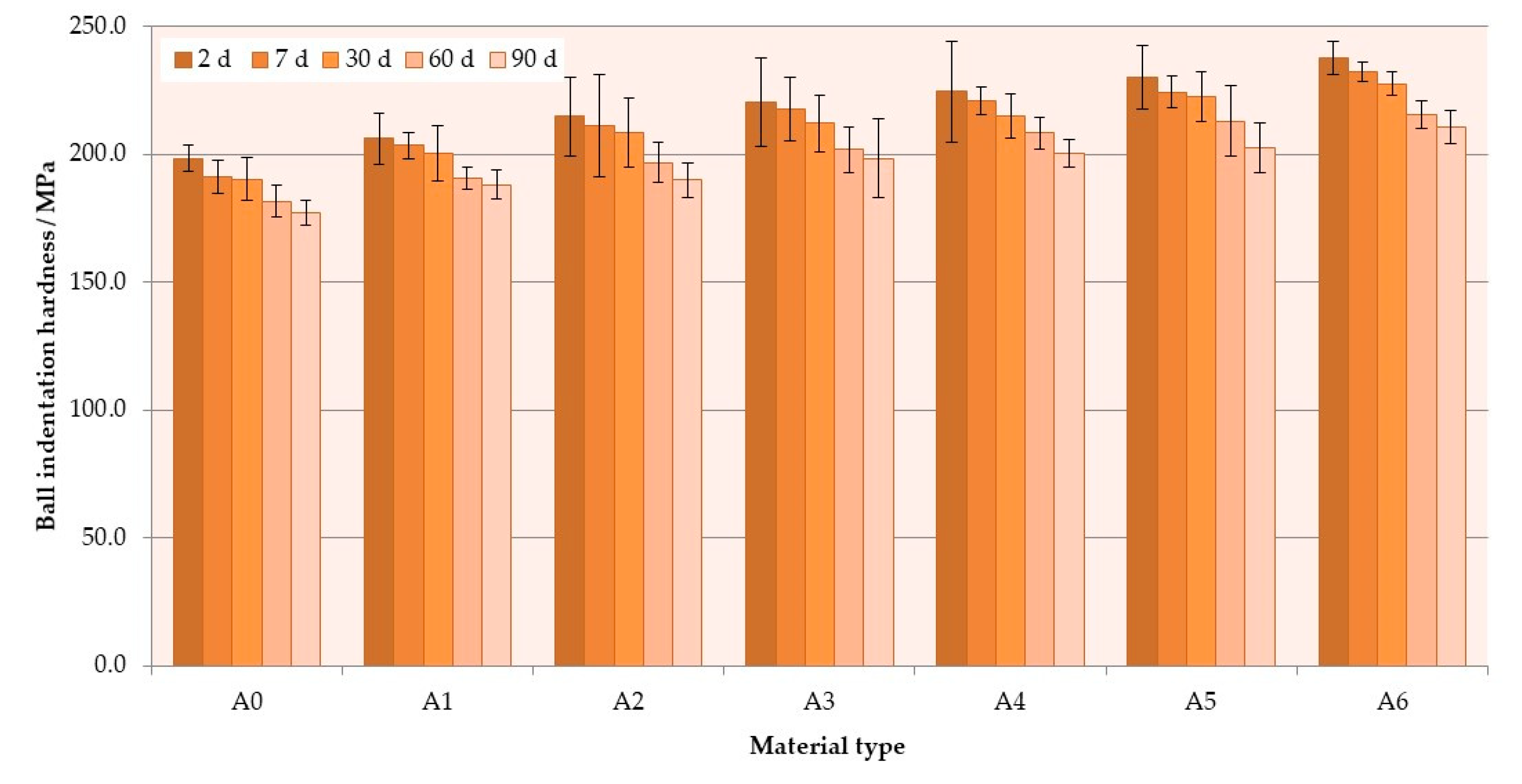
3.3. Hardness
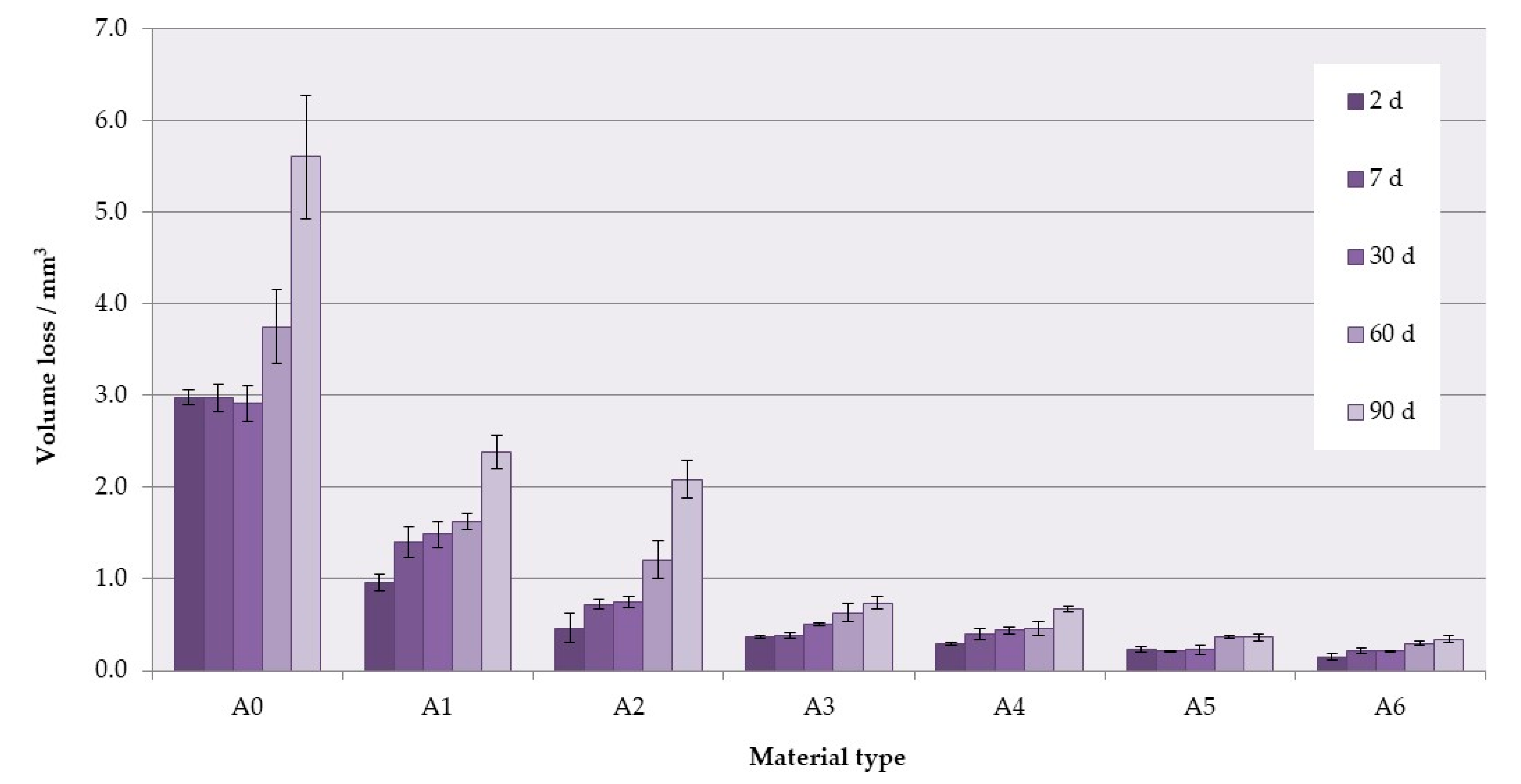
3.4. Wear Resistance
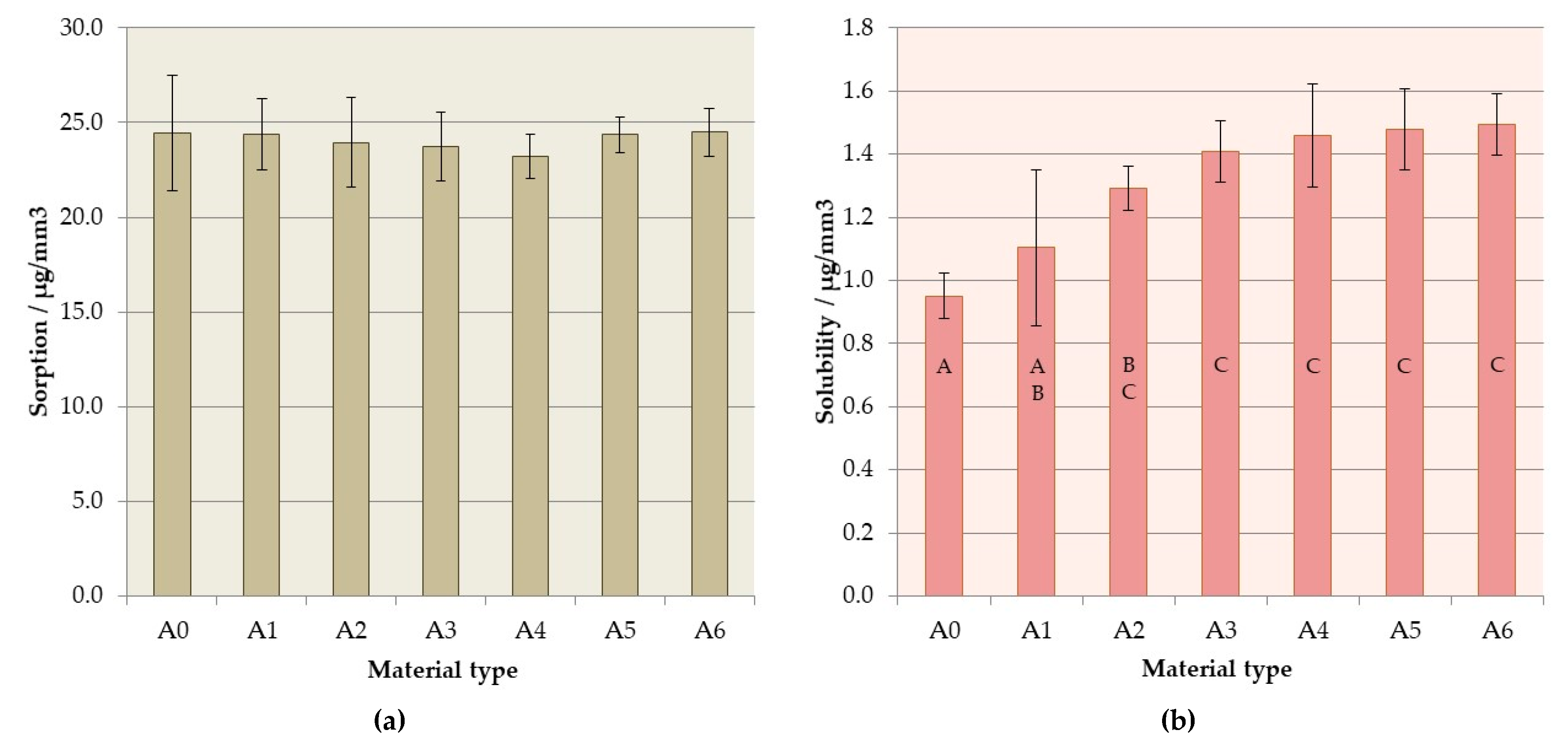
3.5. Sorption and Solubility
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dye, B.; Thornton-Evans, G.; Li, X.; Iafolla, T. Dental caries and tooth loss in adults in the United States, 2011–2012. NCHS Data Brief 2015, 197, 1–7. [Google Scholar]
- Peltzer, K.; Hewlett, S.; Yawson, A.E.; Moynihan, P.; Preet, R.; Wu, F.; Guo, G.; Arokiasamy, P.; Snodgrass, J.J.; Chatterji, S.; et al. Prevalence of loss of all teeth (edentulism) and associated factors in older adults in China, Ghana, India, Mexico, Russia and South Africa. Int. J. Environ. Res. Public Health 2014, 11, 11308–11324. [Google Scholar] [CrossRef] [PubMed]
- Kosuru, K.R.V.; Devi, G.; Grandhi, V.; Prasan, K.K.; Yasangi, M.K.; Dhanalakshmi, M. Denture care practices and perceived denture status among complete denture wearers. J. Int. Soc. Prev. Community Dent 2017, 7, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Piampring, P. Problems with complete dentures and related factors in patients in rajavithi hospital from 2007 to 2012. J. Med. Assoc. Thai. 2016, 99, 182–187. [Google Scholar]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.P.; Harty, D.W.S.; Knox, K.W. Candida-associated denture stoma titis. Aetiology and management: A review. Part 2. Oral diseases caused by candida species. Aust. Dent. J. 1998, 43, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef]
- Al-Dwairi, Z.N. Isolation of candida species from the oral cavity and fingertips of complete and partial dentures wearers. J. Dent. Health Oral Disord. Ther. 2014, 1, 00015. [Google Scholar] [CrossRef][Green Version]
- Uzel, N.G.; Teles, F.R.; Teles, R.P.; Song, X.Q.; Torresyap, G.; Socransky, S.S.; Haffajee, A.D. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J. Clin. Periodontol. 2011, 38, 612–620. [Google Scholar] [CrossRef]
- Evren, B.A.; Uludamar, A.; Işeri, U.; Ozkan, Y.K. The association between socioeconomic status, oral hygiene practice, denture stomatitis and oral status in elderly people living different residential homes. Arch. Gerontol. Geriatr. 2011, 53, 252–257. [Google Scholar] [CrossRef]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Spiechowicz, E.; Mierzwińska-Nastalska, E. Fungal Infections of Oral Cavity, 1st ed.; Med Tour Press International: Warszawa, Poland, 1998. [Google Scholar]
- Dniluk, T.; Fiedoruk, K.; Ściepuk, M. Aerobic bacteria in the oral cavity of patients with removable dentures. Adv. Med Sci. 2006, 51, 86–90. [Google Scholar]
- Preshaw, P.M.; Walls, A.W.G.; Jakubovics, N.S.; Moynihan, P.J.; Jepson, N.J.A.; Loewy, Z. Association of removable partial denture use with oral and systemic health. J. Dent. 2011, 39, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Niimi, M.; Firth, N.A.; Cannon, R.D. Antifungal drug resistance of oral fungi. Odontology 2010, 98, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Amin, W.M.; Al-Ali, M.H.; Salim, N.A.; Al-Tarawneh, S.K. A new form of intraoral delivery of antifungal drugs for the treatment of denture-induced oral candidosis. Eur. J. Dent. 2009, 3, 257–266. [Google Scholar] [CrossRef]
- Chandra, J.; Mukherjee, P.K.; Leidich, S.D.; Faddoul, F.F.; Hoyer, L.L.; Douglas, L.J.; Ghannoum, M.A. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef]
- Paranhos, H.D.; Peracini, A.; Pisani, M.X.; Oliveira, V.D.; Souza, R.F.; Silva-Lovato, C.H. Color stability, surface roughness and flexural strength of an acrylic resin submitted to simulated overnight immersion in denture cleansers. Braz. Dent. J. 2013, 24, 152–156. [Google Scholar] [CrossRef]
- Koray, M.; Ak, G.; Kurklu, E.; Issever, H.; Tanyeri, H.; Kulekci, G.; Guc, U. Fluconazole and/or hexetidine for management of oral candidiasis associated with denture-induced stomatitis. Oral Dis. 2005, 11, 309–313. [Google Scholar] [CrossRef]
- Carmen, S.; Michelangelo, P.; María, C.; Vincenzo, E.; Maurizio, B.; Lucio, M.; Agostino, G.; Massimo, P.; Rosario Serpico, S. Candida-associated denture stomatitis. Oral Med. Pathol. 2011, 16, 139–143. [Google Scholar]
- Richmond, R.; Macfarlane, T.V.; McCord, J.F. An evaluation of the surface changes in PMMA biomaterial formulations as a result of toothbrush/dentifrice abrasion. Dent. Mater. 2004, 20, 124–132. [Google Scholar] [CrossRef]
- Charman, K.M.; Fernandez, P.; Loewy, Z.; Middleton, A.M. Attachment of streptococcus oralis on acrylic substrates of varying roughness. Lett. Appl. Microbiol. 2009, 48, 472–477. [Google Scholar] [CrossRef]
- Kurt, A.; Erkose-Genc, G.; Uzun, M.; Sarı, T.; Isik-Ozkol, G. The effect of cleaning solutions on a denture base material: elimination of candida albicans and alteration of physical properties. J. Prosthodont. 2018, 27, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Köroğlu, A.; Şahin, O.; Dede, D.Ö.; Deniz, Ş.T.; Sever, N.K.; Özkan, S. Efficacy of denture cleaners on the surface roughness and candida albicans adherence of sealant agent coupled denture base materials. Dent. Mater. J. 2016, 35, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Hamada, N.; Kimoto, K.; Sawada, T.; Sawada, T.; Kumada, H.; Umemoto, T.; Toyoda, M. Antifungal Effect of acrylic resin containing apatite-coated TiO2 photocatalyst. Dent. Mater. J. 2007, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Bulad, K.; Taylor, R.L.; Verran, J.; McCord, J.F. Colonization and penetration of denture soft lining materials by candida albicans. Dent. Mater. 2004, 20, 167–175. [Google Scholar] [CrossRef]
- Rodger, G.; Taylor, R.L.; Pearson, G.J.; Verran, J. In vitro colonization of an experimental silicone by candida albicans. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 226–235. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Hallikerimath, R.B. An in-vitro evaluation of retention, colonization and penetration of commonly used denture lining materials by candida albicans. J. Clin. Diagn. Res. 2016, 10, 84–88. [Google Scholar] [CrossRef]
- Zhang, K.; Baras, B.; Lynch, C.D.; Weir, M.D.; Melo, M.A.S.; Li, Y.; Reynolds, M.A.; Bai, Y.; Wang, L.; Wang, S.; et al. Developing a new generation of therapeutic dental polymers to inhibit oral biofilms and protect teeth. Materials 2018, 11, 1747. [Google Scholar] [CrossRef]
- Gad, M.M.; Al-Thobity, A.M.; Shahin, S.Y.; Alsaqer, B.T.; Ali, A.A. Inhibitory effect of zirconium oxide nanoparticles on candida albicans adhesion to repaired polymethyl methacrylate denture bases and interim removable prostheses: a new approach for denture stomatitis prevention. Int. J. Nanomed. 2017, 12, 5409–5419. [Google Scholar] [CrossRef]
- Venkatesh Anehosur, G.; Kulkarni, R.D. Synthesis and determination of antimicrobial activity of visible light activated TiO2 nanoparticles with polymethyl methacrylate denture base resin against staphylococcus aureus. J. Gerontol. Geriatr. Res. 2012, 1, 1–8. [Google Scholar] [CrossRef]
- Totu, E.E.; Nechifor, A.C.; Nechifor, G.; Aboul-Enein, H.Y.; Cristache, C.M. Poly (methyl methacrylate) with TiO2 nanoparticles inclusion for stereolitographic complete denture manufacturing—The fututre in dental care for elderly edentulous patients? J. Dent. 2017, 59, 68–77. [Google Scholar] [CrossRef]
- Cierech, M.; Kolenda, A.; Grudniak, A.M.; Wojnarowicz, J.; Woźniak, B.; Gołaś, M.; Swoboda-Kopeć, E.; Łojkowski, W.; Mierzwińska-Nastalska, E. Significance of polymethylmethacrylate (PMMA) modification by zinc oxide nanoparticles for fungal biofilm formation. Int. J. Pharm. 2016, 510, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y. Characterization and bacterial anti-adherent effect on modified PMMA denture acrylic resin containing platinum nanoparticles. J. Adv. Prosthodont. 2014, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Torres, L.S.; Mendieta, I.; Nuñez-Anita, R.E.; Cajero-Juárez, M.; Castaño, V.M. Cytocompatible antifungal acrylic resin containing silver nanoparticles for dentures. Int. J. Nanomed. 2012, 7, 4777–4786. [Google Scholar]
- Fan, C.; Chu, L.; Rawls, H.R.; Norling, B.K.; Cardenas, H.L.; Whang, K. Development of an antimicrobial resin—A pilot study. Dent. Mater. 2011, 27, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Kurt, A.; Erkose-Genc, G.; Uzun, M.; Emrence, Z.; Ustek, D.; Isik-Ozkol, G. The antifungal activity and cytotoxicity of silver containing denture base material. Niger. J. Clin. Pract. 2017, 20, 290–295. [Google Scholar] [CrossRef]
- Chladek, G.; Basa, K.; Mertas, A.; Pakieła, W.; Żmudzki, J.; Bobela, E.; Król, W. Effect of storage in distilled water for three months on the antimicrobial properties of poly (methyl methacrylate) denture base material doped with inorganic filler. Materials 2016, 9, 328. [Google Scholar] [CrossRef]
- Meng, T.R.; Latta, M.A. Physical properties of four acrylic denture base resins. J. Contemp. Dent. Pract. 2005, 6, 93–100. [Google Scholar] [CrossRef]
- Oleiwi, J.K.; Hamad, Q.A. Studying the mechanical properties of denture base materials fabricated from polymer composite materials. Al-Khwarizmi Eng. J. 2018, 14, 100–111. [Google Scholar] [CrossRef]
- Choudhary, S. Complete denture fracture—a proposed classification system and its incidence in national capital region population: A survey. J. Indian Prosthodont. Soc. 2019, 19, 307–312. [Google Scholar] [CrossRef]
- Takamiya, A.S.; Monteiro, D.R.; Marra, J.; Compagnoni, M.A.; Barbosa, D.B. Complete denture wearing and fractures among edentulous patients treated in university clinics. Gerodontology 2012, 29, 728–734. [Google Scholar] [CrossRef]
- Shakir, S.; Jalil, H.; Khan, M.A.; Qayum, B.; Qadeer, A. Causes and types of denture fractures—A study. Pak. Oral Dent. J. 2017, 37, 634–637. [Google Scholar]
- de Freitas Pontes, K.M.; de Holanda, J.C.; Fonteles, C.S.R.; de Barros Pontes, C.; da Silva, C.H.L.; Paranhos, H.D.F.O. Effect of toothbrushes and denture brushes on heat-polymerized acrylic resins. Gen. Dent. 2016, 64, 49–53. [Google Scholar] [PubMed]
- Hamanaka, I.; Iwamoto, M.; Lassila, L.V.J.; Vallittu, P.K.; Takahashi, Y. Wear resistance of injection-molded thermoplastic denture base resins. Acta Biomater. Odontol. Scand. 2016, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kumar, L.; Sehgal, K.; Datta, K.; Pal, B. A Comparative evaluation of effect of reinforced autopolymerizing resin on the flexural strength of repaired heat-polymerized denture base resin before and after thermocycling. J. Int. Soc. Prev. Community Dent. 2017, 7, 99–106. [Google Scholar] [CrossRef]
- ISO 20795-1:2013. Dentistry—Base Polymers -Part 1: Denture Base Polymers; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- ISO 179-1:2010. Plastics—Determination of Charpy Impact Properties—Part 1: Non-instrumented Impact Test; ISO: Geneva, Switzerland, 2010. [Google Scholar]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J. A comparison of the flexural and impact strengths and flexural modulus of CAD/CAM and conventional heat-cured polymethyl methacrylate (PMMA). J. Prosthodont. 2018, 1–9. [Google Scholar] [CrossRef]
- Faot, F.; Panza, L.H.V.; Garcia, R.C.R.; Cury, A.D.B. Impact and flexural strength, and fracture morphology of acrylic resins with impact modifiers. In Proceedings of the Open Dentistry Journal, Open Dent. J. 2019, 3, 137. [Google Scholar] [CrossRef]
- ISO 2039-1:2001. Plastics—Determination of Hardness—Part 1: Ball Indentation Method; ISO: Geneva, Switzerland, 2001. [Google Scholar]
- ASTM G99—95a (2000). Test Method for Wear Testing with a Pin-on-Disk Apparatus; ASTM International: West Conshohocken, PA, USA, 2000. [Google Scholar]
- ISO/TS 14569-2:2001. Dental materials—Guidance on Testing of Wear—Part 2: Wear by Two—and/or Three Body Contact; ISO: Geneva, Switzerland, 2001. [Google Scholar]
- Jabłońska-Stencel, E.; Pakieła, W.; Mertas, A.; Bobela, E.; Kasperski, J.; Chladek, G. Effect of silver-emitting filler on antimicrobial and mechanical properties of soft denture lining material. Materials 2018, 11, 318. [Google Scholar] [CrossRef]
- Stencel, R.; Kasperski, J.; Pakieła, W.; Mertas, A.; Bobela, E.; Barszczewska-Rybarek, I.; Chladek, G. Properties of experimental dental composites containing antibacterial silver-releasing filler. Materials 2018, 11, 1031. [Google Scholar] [CrossRef]
- Matsuo, H.; Suenaga, H.; Takahashi, M.; Suzuki, O.; Sasaki, K.; Takahashi, N. Deterioration of polymethyl methacrylate dentures in the oral cavity. Dent. Mater. J. 2015, 34, 234–239. [Google Scholar] [CrossRef]
- Takahashi, Y.; Chai, J.; Kawaguchi, M. Equilibrium strengths of denture polymers subjected to long-term water immersion. Int. J. Prosthodont. 1999, 12, 348–352. [Google Scholar]
- Chandrahari, N.; Kumar, C.R.; Singh, M.; Singh, S. Comparison of fracture resistance of heat cure resins polymerized by conventional and microwave methods after immersion in artificial saliva. J. Contemp. Dent. Pract. 2019, 20, 71–77. [Google Scholar] [PubMed]
- Jagini, A.S.; Marri, T.; Jayyarapu, D.; Kumari, R.D. Effect of long-term immersion in water and artificial saliva on the flexural strength of two heat cure denture base resins. J. Contemp. Dent. Pract. 2019, 20, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA Denture Base Resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [PubMed]
- Bettencourt, A.F.; Neves, C.B.; de Almeida, M.S.; Pinheiro, L.M.; Oliveira, S.A.E.; Lopes, L.P.; Castro, M.F. Biodegradation of acrylic based resins: A review. Dent. Mater. 2010, 26, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.D.A.; Bertoldo, C.E.D.S.; Aguiar, F.H.B.; Lima, D.A.N.L.; Lovadino, J.R. Effects of mouthwashes on Knoop hardness and surface roughness of dental composites after different immersion times. Braz. Oral Res. 2011, 25, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Hirajima, Y.; Takahashi, H.; Minakuchi, S. Influence of a denture strengthener on the deformation of a maxillary complete denture. Dent. Mater. J. 2009, 28, 507–512. [Google Scholar] [CrossRef][Green Version]
- Diaz-Arnold, A.M.; Vargas, M.A.; Shaull, K.L.; Laffoon, J.E.; Qian, F. Flexural and fatigue strengths of denture base resin. J. Prosthet. Dent. 2008, 100, 47–51. [Google Scholar] [CrossRef]
- Ajaj-ALKordy, N.M.; Alsaadi, M.H. Elastic modulus and flexural strength comparisons of high-impact and traditional denture base acrylic resins. Saudi Dent. J. 2014, 26, 15–18. [Google Scholar] [CrossRef]
- Żmudzki, J.; Chladek, G.; Malara, P. Use of finite element analysis for the assessment of biomechanical factors related to pain sensation beneath complete dentures during mastication. J. Prosthet. Dent. 2018, 120, 934–941. [Google Scholar] [CrossRef]
- Żmudzki, J.; Chladek, G.; Kasperski, J. Biomechanical factors related to occlusal load transfer in removable complete dentures. Biomech. Model. Mechanobiol. 2015, 14, 679–691. [Google Scholar] [CrossRef]
- Ucar, Y.; Akova, T.; Aysan, I. Mechanical properties of polyamide versus different PMMA denture base materials. J. Prosthodont. 2012, 21, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Wadachi, J.; Sato, M.; Igarashi, Y. Evaluation of the rigidity of dentures made of injection-molded materials. Dent. Mater. J. 2013, 32, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Macura-Karbownik, A.; Chladek, G.; Żmudzki, J.; Kasperski, J. Chewing efficiency and occlusal forces in PMMA, acetal and polyamide removable partial denture wearers. Acta Bioeng. Biomech. 2016, 18, 137–144. [Google Scholar] [PubMed]
- Gunduz, O.; Unalan, F.; Dikbas, I. Comparison of the transverse strength of six acrylic denture resins. OHDMBSC 2010, 9, 21–24. [Google Scholar]
- Vojdani, M.; Bagheri, R.; Khaledi, A.A.R. Effects of aluminum oxide addition on the flexural strength, surface hardness, and roughness of heat-polymerized acrylic resin. J. Dent. Sci. 2012, 7, 238–244. [Google Scholar] [CrossRef]
- Chander, N.G.; Jayaraman, V.; Sriram, V. Comparison of ISO and ASTM standards in determining the flexural strength of denture base resin. Eur. Oral Res. 2019, 53, 137–140. [Google Scholar] [CrossRef]
- Barbosa, D.B.; de Souza, R.F.; Pero, A.C.; Marra, J.; Compagnoni, M.A. Flexural strength of acrylic resins polymerized by different cycles. J. Appl. Oral Sci. 2007, 15, 424–428. [Google Scholar] [CrossRef]
- Yunus, N.; Rashid, A.A.; Azmi, L.L.; Hassan, M.I.A. Some flexural properties of a nylon denture base polymer. J. Oral Rehabil. 2005, 32, 65–71. [Google Scholar] [CrossRef]
- Sato, S.; Cavalcante, M.R.S.; Orsi, I.A.; deParanhos, H.F.O.; Zaniquelli, O. Assessment of flexural strength and color alteration of heat-polymerized acrylic resins after simulated use of denture cleansers. Braz. Dent. J. 2005, 16, 124–128. [Google Scholar] [CrossRef][Green Version]
- Ali, I.L.; Yunus, N.; Abu-Hassan, M.I. Hardness, flexural strength, and flexural modulus comparisons of three differently cured denture base systems. J. Prosthodont. 2008, 17, 545–549. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yoshida, K.; Shimizu, H. Fracture resistance of maxillary complete dentures subjected to long-term water immersion. Gerodontology 2012, 29, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Santerre, J.P.; Shajii, L.; Leung, B.W. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit. Rev. Oral Biol. Med. 2001, 12, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Zappini, G.; Kammann, A.; Wachter, W. Comparison of fracture tests of denture base materials. J. Prosthet. Dent. 2003, 90, 578–585. [Google Scholar] [CrossRef]
- Faot, F.; Costa, M.A.; Del Bel Cury, A.A.; Rodrigues Garcia, R.C.M. Impact strength and fracture morphology of denture acrylic resins. J. Prosthet. Dent. 2006, 96, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Dikbas, I.; Gurbuz, O.; Unalan, F.; Koksal, T. Impact strength of denture polymethyl methacrylate reinforced with different forms of E-glass fibers. Acta Odontol. Scand. 2013, 71, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Arundati, R.; Patil, N.P. An investigation into the transverse and impact strength of a new indigenous high-impact denture base resin, DPI- tuff and its comparison with most commonly used two denture base resins. J. Indian Prosthodont. Soc. 2006, 6, 133–138. [Google Scholar] [CrossRef]
- Zidan, S.; Silikas, N.; Alhotan, A.; Haider, J.; Yates, J. Investigating the mechanical properties of ZrO2-impregnated PMMA nanocomposite for denture-based applications. Materials 2019, 12, 1344. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Zhang, X.-J.; Huang, Z.-L.; Zhu, B.-S.; Chen, R.-R. Hybrid effects of zirconia nanoparticles with aluminum borate whiskers on mechanical properties of denture base resin PMMA. Dent. Mater. J. 2014, 33, 141–146. [Google Scholar] [CrossRef]
- Ergun, G.; Sahin, Z.; Ataol, A.S. The effects of adding various ratios of zirconium oxide nanoparticles to poly(methyl methacrylate) on physical and mechanical properties. J. Oral Sci. 2018, 60, 304–315. [Google Scholar] [CrossRef]
- Ali, M.; Al Mgoter, I.N. Evaluation the effect of modified nano-fillers addition on some properties of heat cured acrylic denture base material. J. Baghdad Coll. Dent. 2011, 23, 23–29. [Google Scholar]
- Al-Harbi, F.A.; Abdel-Halim, M.S.; Gad, M.M.; Fouda, S.M.; Baba, N.Z.; AlRumaih, H.S.; Akhtar, S. Effect of nanodiamond addition on flexural strength, impact strength, and surface roughness of PMMA denture base: nanodiamond effect on PMMA denture base. J. Prosthodont. 2019, 28, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, A.; Lanari, S.; Santulli, C.; Pettinari, C. Use of almond shells and rice husk as fillers of poly(methyl methacrylate) (PMMA) composites. Materials 2017, 10, 872. [Google Scholar] [CrossRef] [PubMed]
- Somani, M.V.; Khandelwal, M.; Punia, V.; Sharma, V. The effect of incorporating various reinforcement materials on flexural strength and impact strength of polymethylmethacrylate: A meta-analysis. J. Indian Prosthodont. Soc. 2019, 19, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M.; Rahoma, A.; Abualsaud, R.; Al-Harbi, F.A.; Akhtar, S. Reinforcement of PMMA denture base material with a mixture of ZrO2 nanoparticles and glass fibers. Available online: https://www.hindawi.com/journals/ijd/2019/2489393/abs/ (accessed on 21 June 2019).
- Ozyilmaz, O.Y.; Akin, C. Effect of cleansers on denture base resins’ structural properties. J. Appl. Biomater. Funct. Mater. 2019, 17. [Google Scholar] [CrossRef] [PubMed]
- Al-Dwairi, Z.N.; Tahboub, K.Y.; Baba, N.Z.; Goodacre, C.J.; Özcan, M. A Comparison of the surface properties of CAD/CAM and conventional polymethylmethacrylate (PMMA). J. Prosthodont. 2019, 28, 452–457. [Google Scholar] [CrossRef]
- Unalan, F.; Dikbas, I. Effects of mica and glass on surface hardness of acrylic tooth material. Dent. Mater. J. 2007, 26, 545–548. [Google Scholar] [CrossRef][Green Version]
- Wu, W.; Mckinney, J.E. Influence of chemicals on wear of dental composites. J. Dent. Res. 1982, 1180–1183. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Chladek, G.; Basa, K.; Żmudzki, J.; Malara, P.; Nowak, A.J.; Kasperski, J. Influence of aging solutions on wear resistance and hardness of selected resin-based dental composites. Acta Bioeng. Biomech. 2016, 18, 43–52. [Google Scholar]
- Chladek, G.; Żmudzki, J.; Basa, K.; Pater, A.; Krawczyk, C.; Pakieła, W. Effect of silica filler on properties of PMMA resin. Arch. Mater. Sci. Eng. 2015, 71, 10. [Google Scholar]
- Jang, D.-E.; Lee, J.-Y.; Jang, H.-S.; Lee, J.-J.; Son, M.-K. Color stability, water sorption and cytotoxicity of thermoplastic acrylic resin for non metal clasp denture. J. Adv. Prosthodont. 2015, 7, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Lombardini, M.; Gaviati, S.; Chiesa, M. Evaluation of Vickers hardness and depth of cure of six composite resins photo-activated with different polymerization modes. J. Conserv. Dent. 2012, 15, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Dayan, C.; Kiseri, B.; Gencel, B.; Kurt, H.; Tuncer, N. Wear resistance and microhardness of various interim fixed prosthesis materials. J. Oral Sci. 2019, 61, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Suwannaroop, P.; Chaijareenont, P.; Koottathape, N.; Takahashi, H.; Arksornnukit, M. In vitro wear resistance, hardness and elastic modulus of artificial denture teeth. Dent. Mater. J. 2011, 30, 461–468. [Google Scholar] [CrossRef]
- Munshi, N.; Rosenblum, M.; Jiang, S.; Flinton, R. In vitro wear resistance of nano-hybrid composite denture teeth. J. Prosthodont. 2017, 26, 224–229. [Google Scholar] [CrossRef]
- Kamonwanon, P.; Yodmongkol, S.; Chantarachindawong, R.; Thaweeboon, S.; Thaweeboon, B.; Srikhirin, T. Wear resistance of a modified polymethyl methacrylate artificial tooth compared to five commercially available artificial tooth materials. J. Prosthet. Dent. 2015, 114, 286–292. [Google Scholar] [CrossRef]
- Yu, H.; Wegehaupt, F.J.; Wiegand, A.; Roos, M.; Attin, T.; Buchalla, W. Erosion and abrasion of tooth-colored restorative materials and human enamel. J. Dent. 2009, 37, 913–922. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Jurado, C.; Villalobos-Tinoco, J.; Barkmeier, W.; Fischer, N.; Takamizawa, T.; Latta, M.; Miyazaki, M. Wear resistance of indirect composite resins used for provisional restorations supported by implants. J. Adv. Prosthodont. 2019, 11, 232–238. [Google Scholar] [CrossRef]
- Tanoue, N.; Matsumura, H.; Atsuta, M. Wear and surface roughness of current prosthetic composites after toothbrush/dentifrice abrasion. J. Prosthet. Dent. 2000, 84, 93–97. [Google Scholar] [CrossRef]
- Oncescu Moraru, A.M.; Preoteasa, C.T.; Preoteasa, E. Masticatory function parameters in patients with removable dental prosthesis. J. Med. Life 2019, 12, 43–48. [Google Scholar] [CrossRef]
- Żmudzki, J.; Chladek, G.; Krawczyk, C. Relevance of tongue force on mandibular denture stabilization during mastication. J. Prosthodont. 2017, 28, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Morgan, T.D.; Wilson, M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J. Appl. Microbiol. 2001, 91, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Asar, N.V.; Albayrak, H.; Korkmaz, T.; Turkyilmaz, I. Influence of various metal oxides on mechanical and physical properties of heat-cured polymethyl methacrylate denture base resins. J. Adv. Prosthodont. 2013, 5, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Duraid, M.; Mudhaffar, M. Effect of modified zirconium oxide nano-fillers addition on some properties of heat cure acrylic denture base material. Restor. Dent. 2012, 24, 7. [Google Scholar]
- Pfeiffer, P.; Rosenbauer, E.-U. Residual methyl methacrylate monomer, water sorption, and water solubility of hypoallergenic denture base materials. J. Prosthet. Dent. 2004, 92, 72–78. [Google Scholar] [CrossRef]
- Miettinen, V.M.; Vallittu, P.K. Water sorption and solubility of glass fiber-reinforced denture polymethyl methacrylate resin. J. Prosthet. Dent. 1997, 77, 531–534. [Google Scholar] [CrossRef]
- Lin, B.A.; Jaffer, F.; Duff, M.D.; Tang, Y.W.; Santerre, J.P. Identifying enzyme activities within human saliva which are relevant to dental resin composite biodegradation. Biomaterials 2005, 26, 4259–4264. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J.P. Salivary esterase activity and its association with the biodegradation of dental composites. J. Dent. Res. 2004, 83, 22–26. [Google Scholar] [CrossRef]
- Rashid, H.; Sheikh, Z.; Vohra, F. Allergic effects of the residual monomer used in denture base acrylic resins. Eur. J. Dent. 2015, 9, 614–619. [Google Scholar] [CrossRef]
- Goiato, M.C.; Freitas, E.; dos Santos, D.; de Medeiros, R.; Sonego, M. Acrylic resin cytotoxicity for denture base-literature review. Adv. Clin. Exp. Med. 2015, 24, 679–686. [Google Scholar] [CrossRef]
- Cierech, M.; Wojnarowicz, J.; Kolenda, A.; Krawczyk-Balska, A.; Prochwicz, E.; Woźniak, B.; Łojkowski, W.; Mierzwińska-Nastalska, E. Zinc Oxide Nanoparticles cytotoxicity and release from newly formed PMMA–ZnO nanocomposites designed for denture bases. Nanomaterials 2019, 9, 1318. [Google Scholar] [CrossRef] [PubMed]
- Sokołowski, K.; Szynkowska, M.I.; Pawlaczyk, A.; Łukomska-Szymańska, M.; Sokołowski, J. The impact of nanosilver addition on element ions release form light-cured dental composite and compomer into 0.9% NaCl. Acta Biochim. Pol. 2014, 61, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Sleibi, A.; Tappuni, A.R.; Karpukhina, N.G.; Hill, R.G.; Baysan, A. A comparative evaluation of ion release characteristics of three different dental varnishes containing fluoride either with CPP-ACP or bioactive glass. Dent. Mater. 2019, 35, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Boeckler, A.F.; Morton, D.; Poser, S.; Dette, K.-E. Release of dibenzoyl peroxide from polymethyl methacrylate denture base resins: an in vitro evaluation. Dent. Mater. 2008, 24, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Ayman, A.-D. The residual monomer content and mechanical properties of CAD\CAM resins used in the fabrication of complete dentures as compared to heat cured resins. Electron. Physician 2017, 9, 4766–4772. [Google Scholar] [CrossRef]
- Nguyen, L.G.; Kopperud, H.M.; Øilo, M. Water sorption and solubility of polyamide denture base materials. Acta Biomater. Odontol. Scand. 2017, 3, 47–52. [Google Scholar] [CrossRef]


| Material Code | Concentration of Filler after Milling with Powder, % | Concentration of Filler in the Weight of The Liquid, % |
|---|---|---|
| A0 (Control) | 0 | 0 |
| A1 | 0.25 | 0.7 |
| A2 | 0.5 | 1.3 |
| A3 | 1 | 2.6 |
| A4 | 2 | 5.1 |
| A5 | 4 | 9.9 |
| A6 | 8 | 18.6 |
| Material Code | Storing Time /Days | ||||
|---|---|---|---|---|---|
| 2 | 7 | 30 | 60 | 90 | |
| (p ˂ 0.0001) | (p ˂ 0.0001) | (p ˂ 0.0001) | (p ˂ 0.0001) | (p ˂ 0.0001) | |
| A0 (p = 0.0076) | A; a | A; a | A; a,b | A; a,b | A; b |
| A1 (p = 0.0066) | A; a | A,B; a | A,B; a,b | A,B; a,b | A; b |
| A2 (p = 0.0062) | A,B; a | B,C; a,b | B,C; a,b | B,C; b | A,B; b |
| A3 (p = 0.0459) | B,C; a | C,D; a,b | C,D; a,b | C,D; a,b | B,C; b |
| A4 (p = 0.0427) | C,D; a | D,E; a,b | D,E; a,b | D,E; a,b | C,D; b |
| A5 (p = 0.0342) | D,E; a | E,F; a,b | E,F; a,b | E,F; a,b | D; b |
| A6 (p = 0.0084) | E; a | F; a | F; a,b | F; a,b | E; b |
| Material Code | Storing Time /Days | ||||
|---|---|---|---|---|---|
| 2 | 7 | 30 | 60 | 90 | |
| (p = 0.2185) | (p = 0.1563) | (p = 0.0447) | (p = 0.0237) | (p = 0.0076) | |
| A0 (p = 0.0848) | - | - | A | A | A |
| A1 (p = 0.1101) | - | - | A,B | A,B | A,B |
| A2 (p = 0.1662) | - | - | A,B | A,B | A,B |
| A3 (p = 0.3100) | - | - | A,B,C | A,B,C | B,C |
| A4 (p = 0.6143) | - | - | A,B,C | B,C | B,C,D |
| A5 (p = 0.6612) | - | - | B,C | C | C |
| A6 (p = 0.5290) | - | - | C | C | D |
| Material Code | Storing Time /Days | ||||
|---|---|---|---|---|---|
| 2 | 7 | 30 | 60 | 90 | |
| (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | |
| A0 (p = 0.1882) | A | A | A | A | A |
| A1 (p = 0.0084) | B; a | B; a,b | B; a,b | B; a,b | B; b |
| A2 (p = 0.1760) | B | B,C | C | B | B |
| A3 (p = 0.0017) | C,D; a | C,D; a | C; a | C; a,b | C,D; b |
| A4 (p = 0.0017) | D; a | D; a | C,D; a,b | C,D; b | D; b |
| A5 (p < 0.0001) | D; a | D; b | D; b | C,D; b | D; b |
| A6 (p < 0.0001) | D; a | D; a | D; b | D; b | D; b |
| Material Code | Storing Time /Days | ||||
|---|---|---|---|---|---|
| 2 | 7 | 30 | 60 | 90 | |
| (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | |
| A0 (p < 0.0001) | A; a | A; b | A; b | A; c | A; c |
| A1 (p < 0.0001) | A,B; a | B; a | A,B; a | A,B; b | A,B; b |
| A2 (p < 0.0001) | B,C; a | B,C; a | B,C; a,b | B,C; b,c | B,C; c |
| A3 (p < 0.0001) | B,C; a | C,D; a | C,D; a,b | C,D; b,c | C,D; c |
| A4 (p < 0.0001) | C,D; a | C,D; a | C,D; a,b | D,E; b,c | D; c |
| A5 (p < 0.0001) | C,D; a | D,E; a | D,E; a,b | E; b,c | D,E; c |
| A6 (p < 0.0001) | D; a | E; a,b | E; b | E; c | E; c |
| Material Code | Storing Time /Days | ||||
|---|---|---|---|---|---|
| 2 | 7 | 30 | 60 | 90 | |
| (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | (p < 0.0001) | |
| A0 (p < 0.0425) | A; a,b | A; a,b | A; a | A; b | A; c |
| A1 (p < 0.0001) | B; a | B; b | B; b | B; b | B; c |
| A2 (p < 0.0001) | C; a | C; a | C; a | B; b | B; c |
| A3 (p < 0.0001) | C,D; a | D; a | C,D; b | C; c | C; c |
| A4 (p < 0.0001) | D,E; a | D; a,b | D; b | C; b | C; c |
| A5 (p < 0.0001) | D,E; a | D; a | E; a | C; b | C; b |
| A6 (p < 0.0001) | E; a | D; b | E; b | C; c | C; c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chladek, G.; Pakieła, K.; Pakieła, W.; Żmudzki, J.; Adamiak, M.; Krawczyk, C. Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material. Materials 2019, 12, 4146. https://doi.org/10.3390/ma12244146
Chladek G, Pakieła K, Pakieła W, Żmudzki J, Adamiak M, Krawczyk C. Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material. Materials. 2019; 12(24):4146. https://doi.org/10.3390/ma12244146
Chicago/Turabian StyleChladek, Grzegorz, Katarzyna Pakieła, Wojciech Pakieła, Jarosław Żmudzki, Marcin Adamiak, and Cezary Krawczyk. 2019. "Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material" Materials 12, no. 24: 4146. https://doi.org/10.3390/ma12244146
APA StyleChladek, G., Pakieła, K., Pakieła, W., Żmudzki, J., Adamiak, M., & Krawczyk, C. (2019). Effect of Antibacterial Silver-Releasing Filler on the Physicochemical Properties of Poly(Methyl Methacrylate) Denture Base Material. Materials, 12(24), 4146. https://doi.org/10.3390/ma12244146









