Electromagnetic Properties of Carbon Gels
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Carbon Gels
2.2. Characterisation of Carbon Gels
3. Results and Discussion
Porosity of Carbon Gels
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, T.; Liu, Z.; Li, H. Heteroatom doping modified mesoporous carbon derived from ZIF-8 for capacitive deionization with enhanced salt removal rate. Sep. Purif. Technol. 2020, 231, 115918. [Google Scholar] [CrossRef]
- Cai, T.W.; Zhou, M.; Ren, D.Y.; Ham, G.S.; Guan, S.Y. Highly ordered mesoporous phenol-formaldehyde carbon as supercapacitor electrode material. J. Power Sources 2013, 231, 197–202. [Google Scholar] [CrossRef]
- Song, T.; Yang, Y. Metal nanoparticles supported on biomass-derived hierarchical porous heteroatom-doped carbonfrom bamboo shoots: Design, synthesis and applications. Chem. Rec. 2019, 19, 1283–1301. [Google Scholar] [CrossRef] [PubMed]
- Peluso, S.; Gargiulo, N.; Aprea, P.; Pepe, F.; Caputo, D. Nanoporous materials as H2S adsorbents for biogas purification: A reviw. Sep. Purif. Rev. 2019, 48, 78–89. [Google Scholar] [CrossRef]
- Celzard, A.; Fierro, V.; Amaral-Labat, G. Adsorption by Carbon Gels. In Novel Carbon Adsorbents; Tascon, J.M.D., Ed.; Elsevier: Oxford, UK, 2012; pp. 207–244. [Google Scholar]
- Arenillas, A.; Angel Menéndez, J.; Reichenauer, G.; Celzard, A.; Fierro, V.; Maldonado Hodar, F.J.; Bailon-Garcia, E.; Job, N. Organic and Carbon Gels Derived from Biosourced Polyphenols; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Arenillas, A.; Rey-Raap, N.; Angel Menéndez, J. Carbon Gels and Their Applications: A Review of Patents. In Submicron Porous Materials; Bettotti, P., Ed.; Springer: Cham, Switzerland, 2017; pp. 25–52. [Google Scholar]
- Liu, Q.L.; Zhang, D.; Fan, T.X. Electromagnetic wave absorption properties of porous carbon Co nanocomposites. Appl. Phys. Lett. 2008, 93, 013110. [Google Scholar] [CrossRef]
- Letellier, M.; Macutkevic, J.; Kuzhir, P.; Banys, J.; Fierro, V.; Celzard, A. Electromagnetic properties of model vitreous carbon foam. Carbon 2017, 122, 217–227. [Google Scholar] [CrossRef]
- Lettelier, M.; Macutkevic, J.; Paddubskaya, A.; Klochkov, A.; Kuzhir, P.; Banys, J.; Fierro, V.; Celzard, A. Microwave dielectric properties of tannin-based carbon foams. Ferroelectrics 2015, 479, 119–126. [Google Scholar] [CrossRef]
- Letellier, M.; Macutkevic, J.; Paddubskaya, A.; Pliushch, A.; Kuzhir, P.; Ivanov, M.; Banys, J.; Pizzi, A.; Fierro, V.; Celzard, A. Tannin-based carbon foams for electromagnetic applications. IEEE Trans. Electromagn. Compat. 2015, 57, 989–995. [Google Scholar] [CrossRef]
- Gonzalez, M.; Baselga, J.; Porueto, J. High porosity scaffold composites of graphene and carbon nanotubes as microwave absorbing materials. J. Mater. Chem. C 2016, 4, 8575–8582. [Google Scholar] [CrossRef]
- Ma, Y.Z.; Yin, X.W.; Li, Q. Effects of heat treatment temperature on microstructure and electromagnetic properties of ordered mesoporous carbon. Trans. Nonferrous Met. Soc. China 2013, 23, 1652–1660. [Google Scholar] [CrossRef]
- Wang, J.C.; Xiang, C.S.; Liu, Q.; Pan, Y.B.; Guo, J.K. Ordered mesoporous carbon/fused silica composites. Adv. Funct. Mater. 2008, 18, 2995–3002. [Google Scholar] [CrossRef]
- Job, N.; Thery, A.; Pirard, R.; Marien, J.; Kocon, L.; Rouzaud, J.N.; Beguin, F.; Pirard, J.P. Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 2005, 43, 2481–2494. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. Impact of depressurizing rate on the porosity of aerogels. Microporous Mesoporous Mater. 2012, 152, 240–245. [Google Scholar] [CrossRef]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Celzard, A. The use of tannin for preparing carbon gels. Part II. Carbon cryogels. Carbon 2011, 49, 2785–2794. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Stein, N.; Boulanger, C.; Pizzi, A.; Celzard, A. Pore structure and electrochemical performances of tannin-based carbon cryogels. Biomass Bioenergy 2012, 39, 274–282. [Google Scholar] [CrossRef]
- Amaral-Labat, G.; Szczurek, A.; Fierro, V.; Celzard, A. Unique bimodal carbon xerogels from soft templating of tannin. Mater. Chem. Phys. 2015, 149, 193–201. [Google Scholar] [CrossRef]
- Grigas, J. Microwave Dielectric Spectroscopy of Ferroelectric and Related Materials; Gordon and Breach Science Publishing, OPA: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Szczurek, A.; Amaral-Labat, G.; Fierro, V.; Pizzi, A.; Masson, E.; Celzard, A. The use of tannin for preparing carbon gels. Part, I. Carbon aerogels. Carbon 2011, 49, 2773–2784. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, H.; Chen, Y.; Li, Z.; Zhang, D.; Zeng, G.; Huang, Y.; Wang, W.; Wu, Q.; Zhi, C. The electromagnetic property and microwave absorption of wormhole-like mesoporous carbons with different surface areas. J. Mater. Sci. 2016, 51, 9723–9731. [Google Scholar] [CrossRef]
- Nuzhnyy, D.; Savinov, M.; Bovtun, V.; Kempa, M.; Petzelt, J.; Mayoral, B.; McNally, T. Broad-band conductivity and dielectric spectroscopy of composites of multiwalled carbon nanotubes and poly(ethylene terephthalate) around their low percolation threshold. Nanotechnology 2013, 24, 055707. [Google Scholar] [CrossRef]
- Deptuck, D.; Harison, J.P.; Zavadski, P. Measurement of elasticity and electrical conductivity of a three-dimensional percolation system. Phys. Rev. Lett. 1985, 54, 913–916. [Google Scholar] [CrossRef]
- Halperin, B.I.; Feng, S.; Sen, P.N. Differences between lattice and continuum percolation transport exponents. Phys. Rev. Lett. 1985, 54, 2391. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Pan, W.; Shi, S.L. Electrical conductivity and dielectric properties of multiwalled carbon nanotube and alumina composites. Appl. Phys. Lett. 2006, 89, 133122. [Google Scholar] [CrossRef]
- Szczurek, A.; Fierro, V.; Plyushch, A.; Macutkevic, J.; Kuzhir, P.; Celzard, A. Structure and electromagnetic properties of cellular glassy carbon monoliths with controlled cell size. Materials 2018, 11, 709. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Yin, X.; Cheng, L.; Ren, S.; Li, Z. Effect of core shell microspheres as pore forming agent on the properties of porous alumina ceramics. Mater. Des. 2017, 113, 384–390. [Google Scholar] [CrossRef]
- Penn, S.J.; Alford, N.M.; Templeton, A.; Wang, X.; Xu, M.; Riece, M.; Schrapel, K. Effect of porosity and grain size on the microwave dielectric properties of sintered alumina. J. Am. Ceram. Soc. 1997, 80, 1885–18888. [Google Scholar] [CrossRef]
- Liu, J.; Duan, C.G.; Yin, W.G.; Mei, W.N.; Smith, R.W.; Hardy, J.R. Large dielectric constant and Maxwell-Wagner relaxation in Bi2/3Cu3Ti4O12. Phys. Rev. B 2004, 70, 144106. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevic, J.; Svirskas, S.; Banys, K.; Plausinaitiene, V.; Bychanok, D.; Maksimenko, S.A.; Selskis, A.; Sokal, A.; Lapko, K.N.; et al. Silicon carbide/phosphate ceramics composite for electromagnetic shielding applications whiskers vs. particles. Appl. Phys. Lett. 2019, 114, 183105. [Google Scholar] [CrossRef]

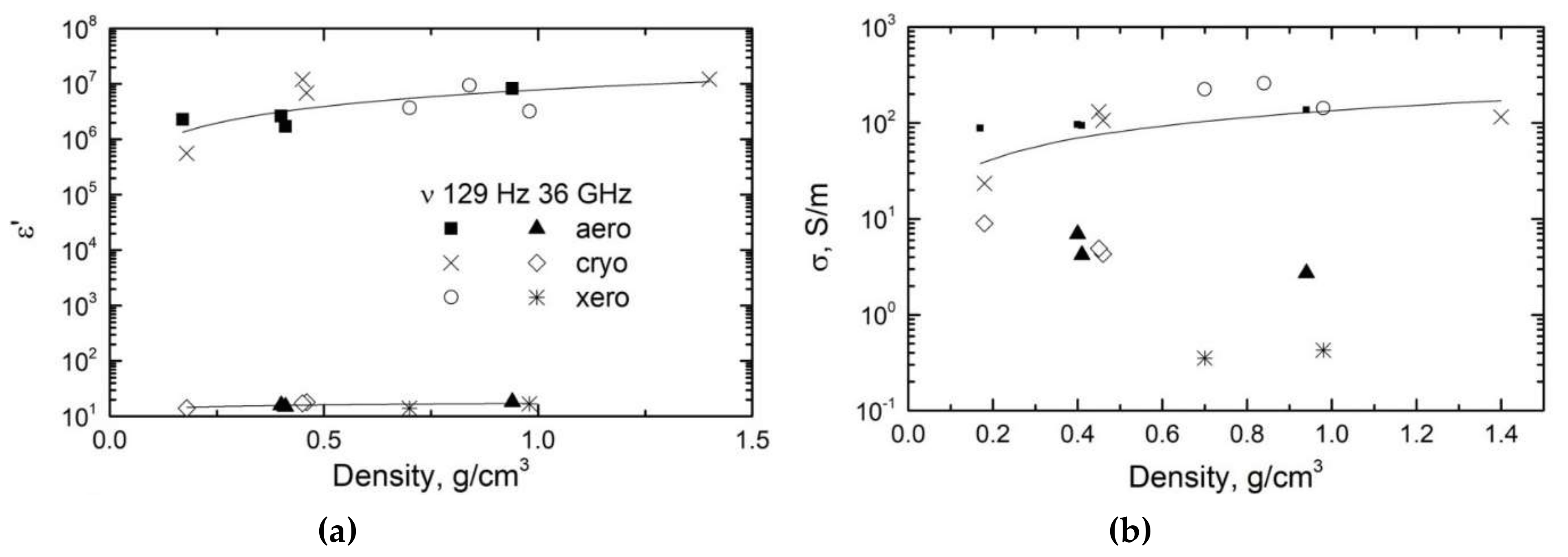
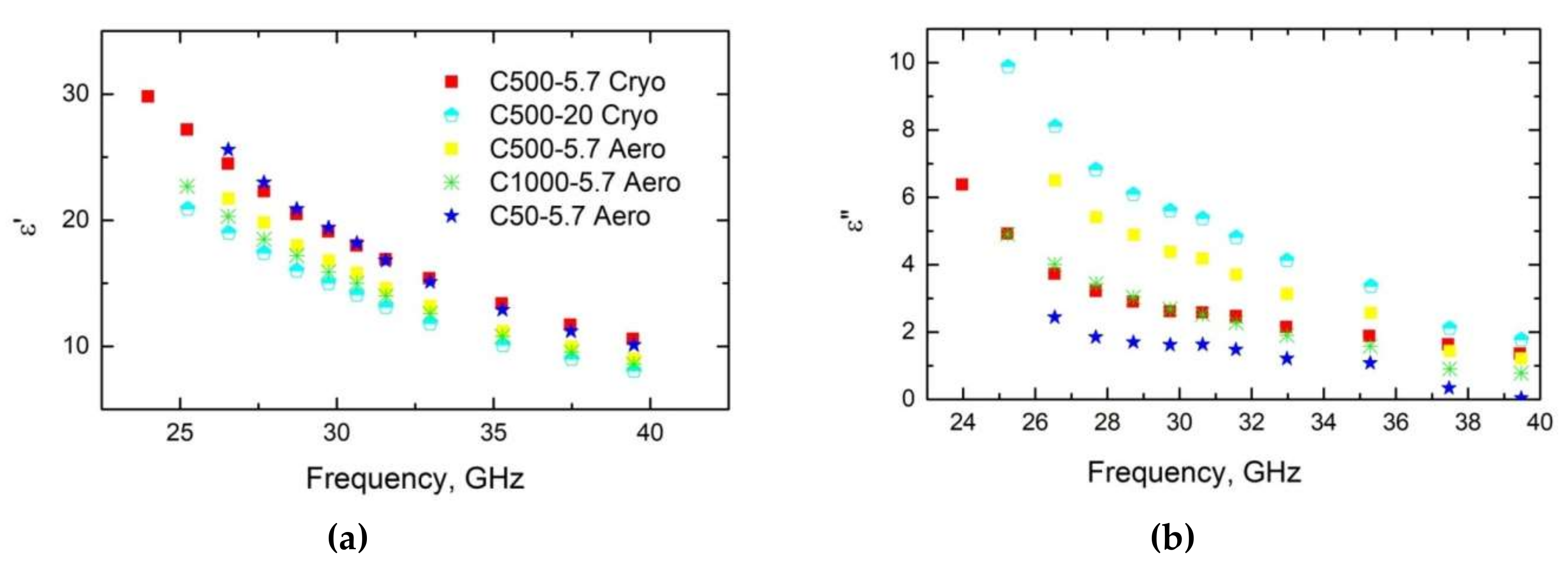
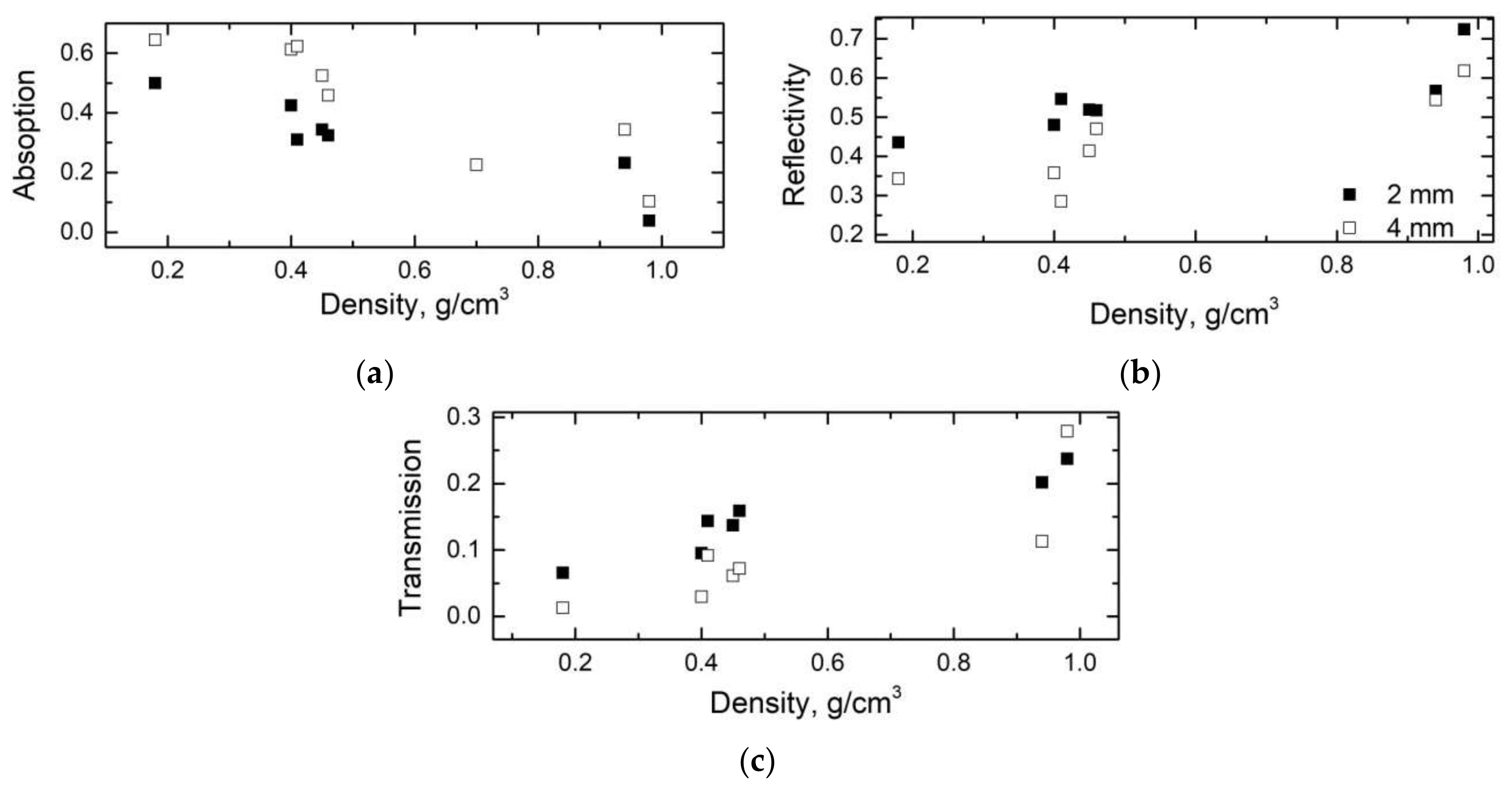

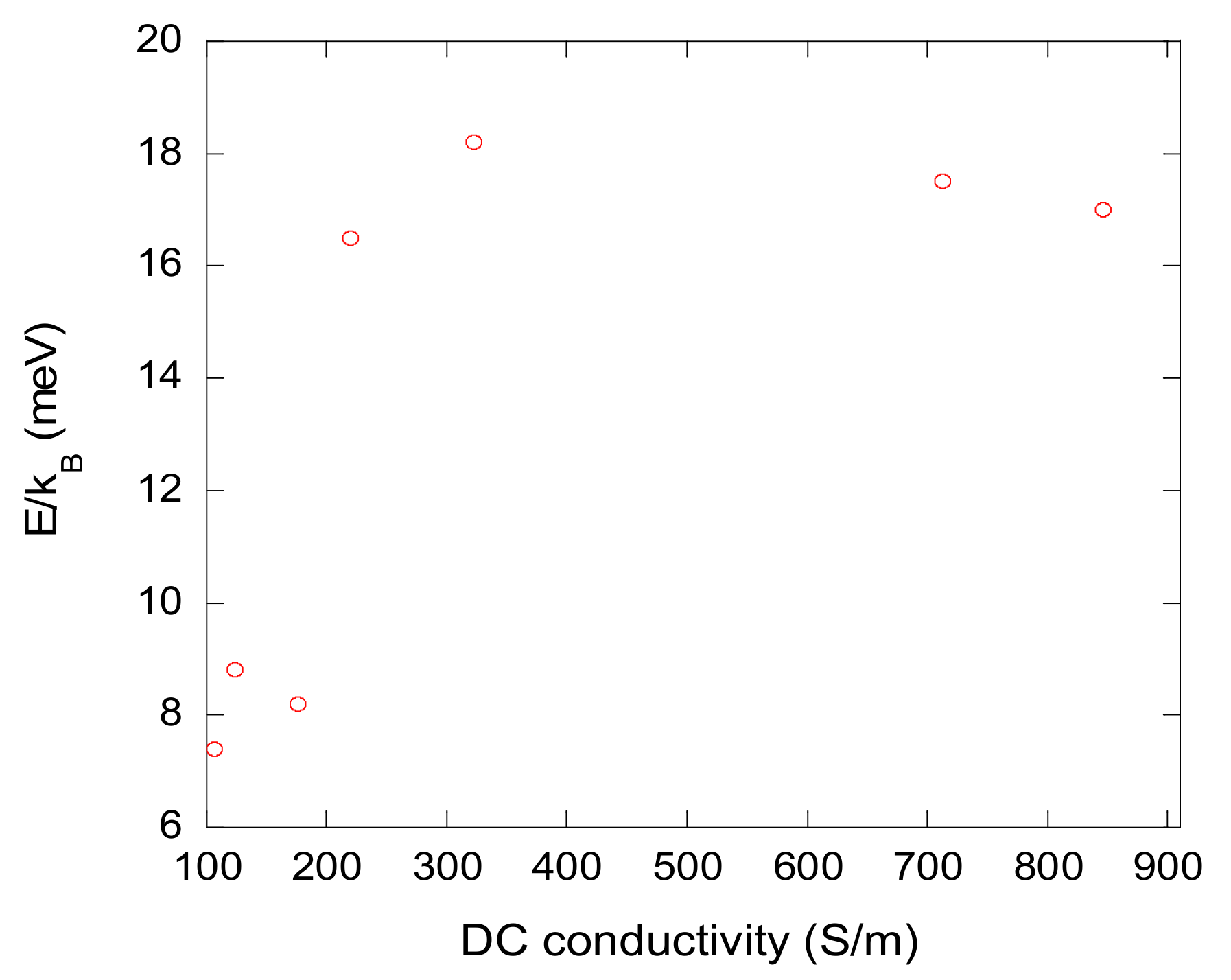
| Sample | Bulk Density, ρb (g/cm3) | Skeletal Density, ρs (g/cm3) | Maximum Pore Size, dmax (nm) | Total Porosity, Φ (%) |
|---|---|---|---|---|
| C1000-5.7 XERO | 0.70 | 2.07 | 40 | 66.2 |
| C500-5.7 XERO | 0.98 | 2.21 | 16 | 55.7 |
| C500-20 XERO | 0.84 | 2.10 | 20 | 60.0 |
| C1000-5.7 CRYO | 0.45 | 2.00 | 60 | 77.5 |
| C500-5.7 CRYO | 0.46 | 2.12 | 38 | 78.3 |
| C50-5.7 CRYO | 1.40 | 1.93 | 4 | 27.5 |
| C500-20 CRYO | 0.18 | 2.19 | 100 | 91.8 |
| C1000-5.7 AERO | 0.41 | 2.02 | 60 | 79.7 |
| C500-5.7 AERO | 0.40 | 2.03 | 40 | 80.3 |
| C50-5.7 AERO | 0.94 | 2.00 | 8 | 53.0 |
| C500-20 AERO | 0.17 | 2.16 | 70 | 92.1 |
| k or l | t or s | Comment |
|---|---|---|
| 15.87 | 0.99 | ε’ at 129 Hz |
| 4.9 | 0.716 | σ at 129 Hz |
| 2.84 | 0.094 | ε’ at 30 GHz |
| Sample | σ0, S/m | E/kB, K (meV) |
|---|---|---|
| C1000-5.7 AERO | 124 | 102 (8.8) |
| C500-5.7 AERO | 107 | 86 (7.4) |
| C1000-5.7 CRYO | 176 | 95 (8.2) |
| C500-20 CRYO | 713 | 191 (17.5) |
| C1000-5.7 XERO | 846 | 197 (17.0) |
| C500-5.7 XERO | 323 | 211 (18.2) |
| C500-5.7 CRYO | 220 | 191 (16.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Gutiérrez, J.; Palaimiene, E.; Macutkevic, J.; Banys, J.; Kuzhir, P.; Schaefer, S.; Fierro, V.; Celzard, A. Electromagnetic Properties of Carbon Gels. Materials 2019, 12, 4143. https://doi.org/10.3390/ma12244143
Castro-Gutiérrez J, Palaimiene E, Macutkevic J, Banys J, Kuzhir P, Schaefer S, Fierro V, Celzard A. Electromagnetic Properties of Carbon Gels. Materials. 2019; 12(24):4143. https://doi.org/10.3390/ma12244143
Chicago/Turabian StyleCastro-Gutiérrez, Jimena, Edita Palaimiene, Jan Macutkevic, Juras Banys, Polina Kuzhir, Sébastien Schaefer, Vanessa Fierro, and Alain Celzard. 2019. "Electromagnetic Properties of Carbon Gels" Materials 12, no. 24: 4143. https://doi.org/10.3390/ma12244143
APA StyleCastro-Gutiérrez, J., Palaimiene, E., Macutkevic, J., Banys, J., Kuzhir, P., Schaefer, S., Fierro, V., & Celzard, A. (2019). Electromagnetic Properties of Carbon Gels. Materials, 12(24), 4143. https://doi.org/10.3390/ma12244143










