Removal of Cadmium from Aqueous Solutions by Saccharomyces cerevisiae–Alginate System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Microorganism
2.2. Cadmium Solutions
2.3. Synthesis of the S. cerevisiae–Alginate System
2.4. Characterization and Microstructural Analysis
2.4.1. Particle Size Distribution
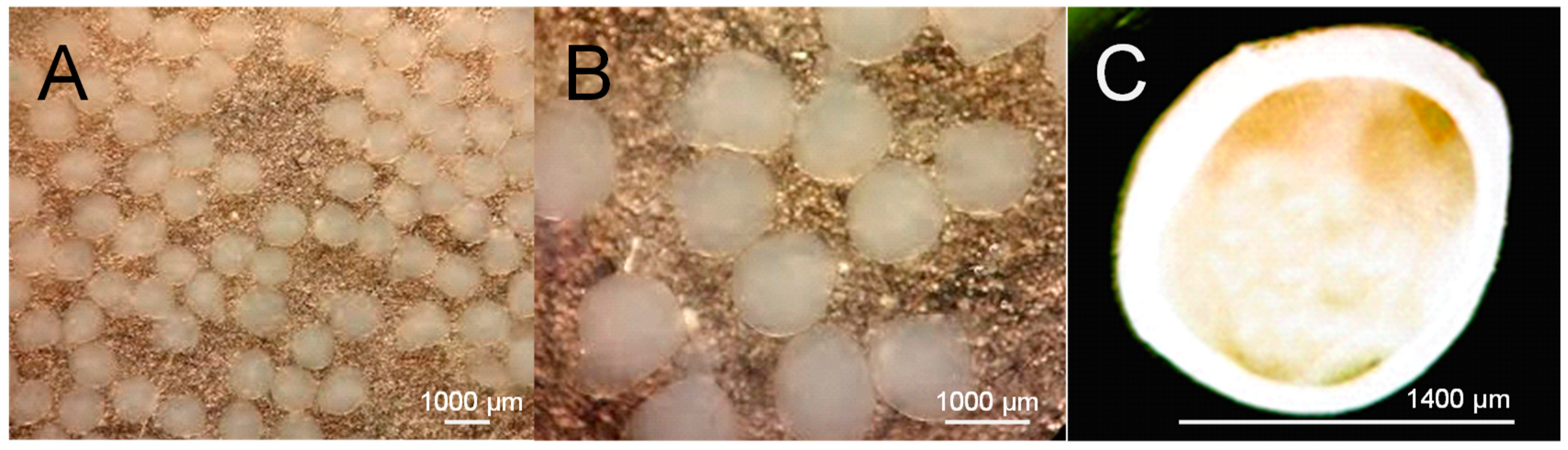
2.4.2. Optical Microscopy Characterization
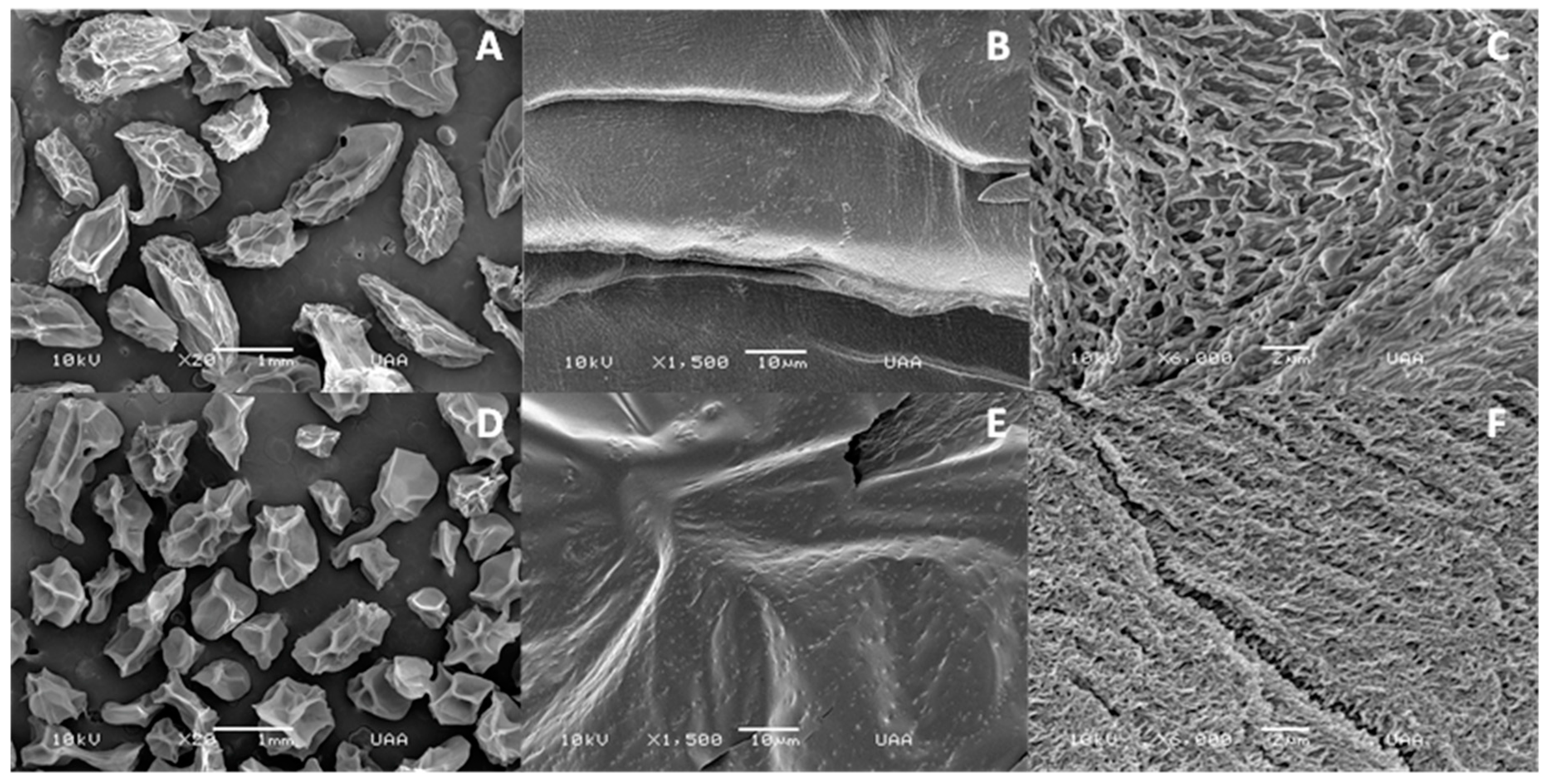
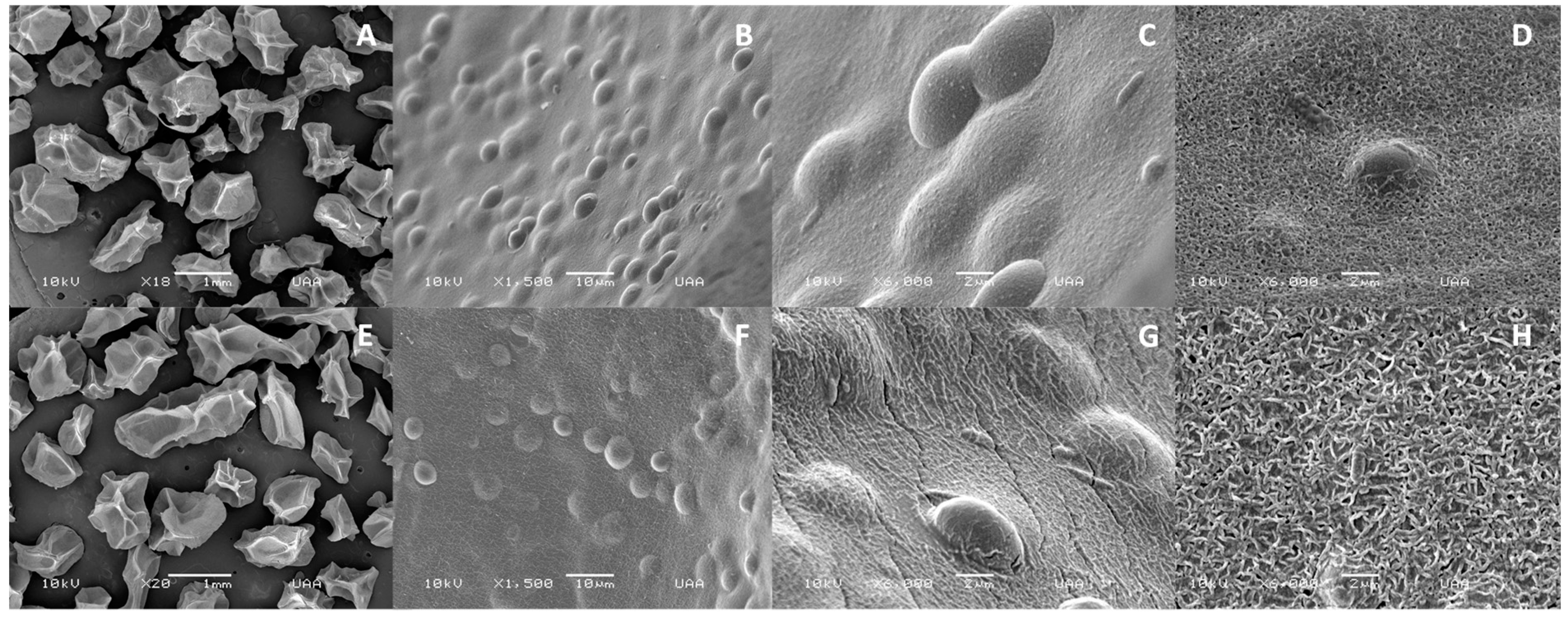
2.4.3. Microstructural Analysis
2.4.4. Surface Charge and Moisture Content
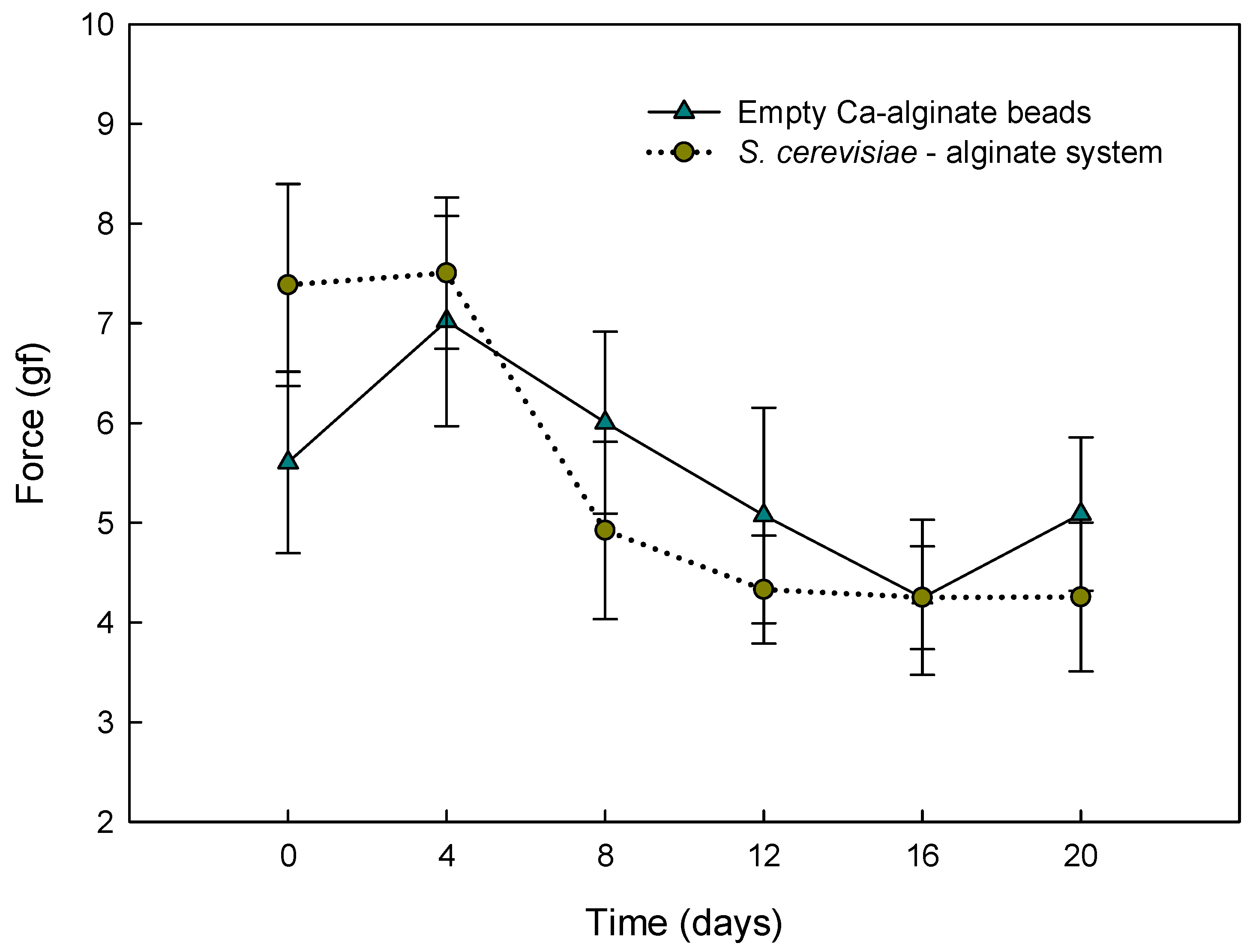
2.5. Texture Analysis during Storage
2.6. Batch Biosorption of Cd2+ by S. cerevisiae–Alginate System
2.7. Fixed-Bed Column Biosorption
2.8. Fourier-Transform Infrared (FTIR) Spectroscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization and Microstructural Analysis
3.2. Texture Analysis during Storage
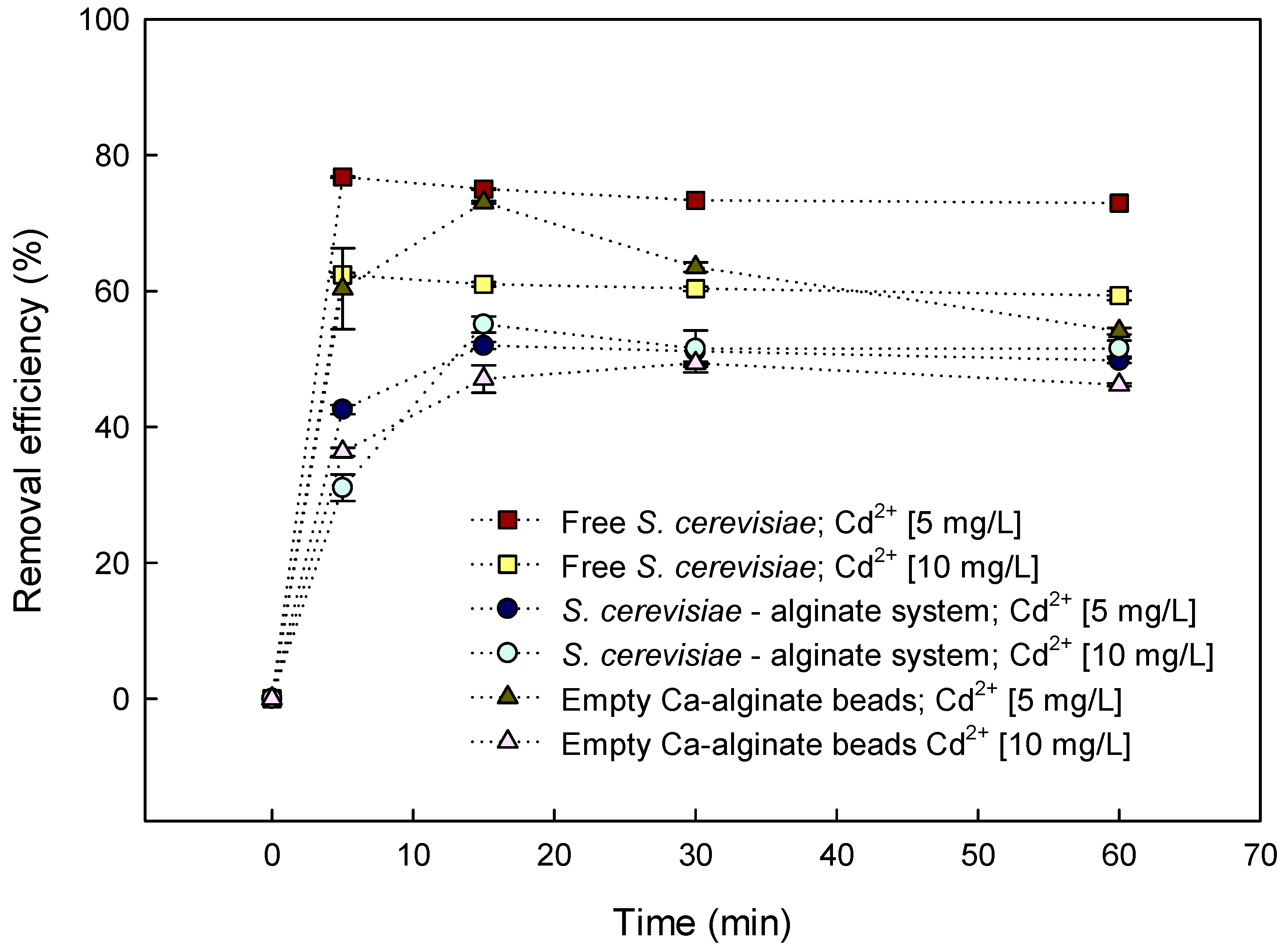
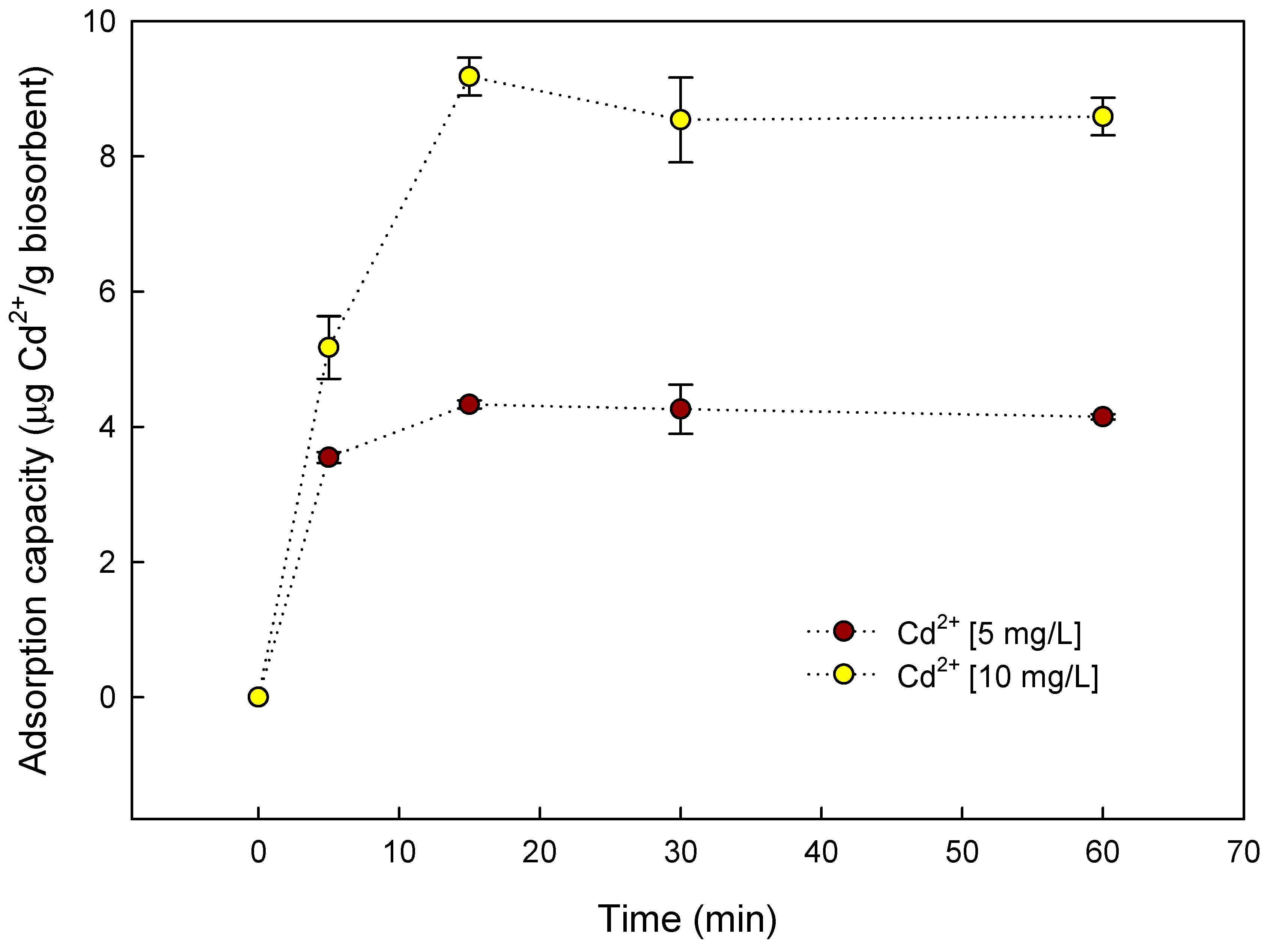
3.3. Batch Biosorption of Cd2+ by S. cerevisiae–Alginate System
3.4. Fixed-Bed Column Biosorption
3.5. Desorption of Cd2+ from Fixed-Bed Column
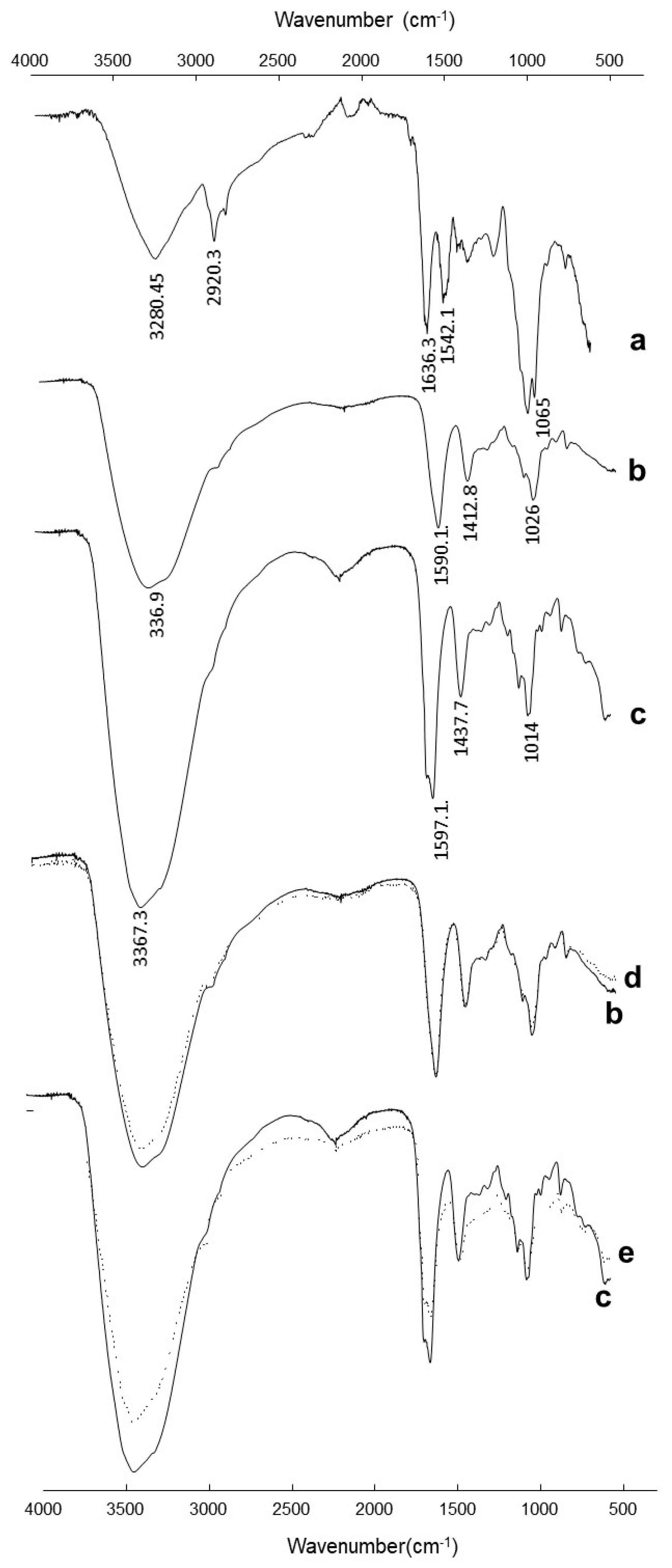
3.6. FTIR Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sarkar, A.; Ravindran, G.; Krishnamurthy, V.; Campus, K.K.B.G. A brief review on the effect of cadmium toxicity: From cellular to organ level. Int. J. Biotechnol. Res. 2013, 3, 17–36. [Google Scholar]
- WHO. Cadmium in Drinking Water. Background Document for Preparation of WHO Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- IARC. Agents Classified by the IARC Monographs; IARC: Lyon, France, 2018; Volume 1–122. [Google Scholar]
- USEPA. Drinking Water Standards and Health Advisories; USEPA: Washington, DC, USA, 2018. [Google Scholar]
- Purkayastha, D.; Mishra, U.; Biswas, S. A comprehensive review on Cd(II) removal from aqueous solution. J. Water Process Eng. 2014, 2, 105–128. [Google Scholar] [CrossRef]
- Kayode, A.A.A.; Babayemi, J.O.; Abam, E.O.; Kayode, O.T. Occurrence and health implications of high concentrations of cadmium and arsenic in drinking water sources in selected towns of Ogun State, South West, Nigeria. J. Toxicol. Environ. Heal. Sci. 2011, 3, 385–391. [Google Scholar] [CrossRef]
- Central Pollution Control Board, C.P.C.B. Cadmium an Environment Toxicant. Available online: http://cpcb.nic.in/upload/Newsletters/Newsletters_61_CADMIUM-AnEnvironmentToxicant-March-2007.pdf (accessed on 7 March 2019).
- Özer, A.; Özer, D. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: Determination of biosorption heats. J. Hazard. Mater. 2003, 100, 219–229. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Rao, K.; Mohapatra, M.; Anand, S.; Venkateswarlu, P. Review on cadmium removal from aqueous solutions. Int. J. Eng. Sci. Technol. 2010, 2, 81–103. [Google Scholar] [CrossRef]
- Jung, W.; Jeon, B.-H.; Cho, D.-W.; Roh, H.-S.; Cho, Y.; Kim, S.-J.; Lee, D.S. Sorptive removal of heavy metals with nano-sized carbon immobilized alginate beads. J. Ind. Eng. Chem. 2014, 26, 364–369. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Matis, K.A.; Lazaridis, N.K. Removal of metal ions from simulated wastewater by Saccharomyces yeast biomass: Combining biosorption and flotation processes. Sep. Sci. Technol. 2001, 36, 349–365. [Google Scholar] [CrossRef]
- Das, N.; Vimala, R.; Karthika, P. Biosorption of heavy metals—An overview. Indian J. Biotechnol. 2008, 7, 159–169. [Google Scholar] [CrossRef]
- Pokethitiyook, P.; Poolpak, T. Biosorption of heavy metal from aqueous solutions. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 113–141. [Google Scholar] [CrossRef]
- Marques, P.; Pinheiro, H.M.; Rosa, M.F. Cd(II) removal from aqueous solution by immobilised waste brewery yeast in fixed-bed and airlift reactors. Desalination 2007, 214, 343–351. [Google Scholar] [CrossRef]
- Machado, M.D.; Soares, E.V.; Soares, H.M.V.M. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: Chemical speciation as a tool in the prediction and improving of treatment efficiency of real electroplating effluents. J. Hazard. Mater. 2010, 180, 347–353. [Google Scholar] [CrossRef]
- Amirnia, S.; Ray, M.B.; Margaritis, A. Heavy metals removal from aqueous solutions using Saccharomyces cerevisiae in a novel continuous bioreactor-biosorption system. Chem. Eng. J. 2015, 264, 863–872. [Google Scholar] [CrossRef]
- Ҫabuk, A.; Akar, T.; Tunali, S.; Gedikli, S. Biosorption of Pb(II) by industrial strain of Saccharomyces cerevisiae immobilized on the biomatrix of cone biomass of Pinus Nigra: Equilibrium and mechanism analysis. Chem. Eng. J. 2007, 131, 293–300. [Google Scholar] [CrossRef]
- Soares, E.V.; Soares, H.M.V.M. Bioremediation of industrial effluents containing heavy metals using brewing cells of Saccharomyces cerevisiae as a green technology: A review. Environ. Sci. Pollut. Res. 2012, 19, 1066–1083. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Mazzitelli, S.; Borgatti, M.; Breveglieri, G.; Gambari, R.; Nastruzzi, C. Encapsulation of eukaryotic cells in alginate microparticles: Cell signaling by TNF-Alpha through capsular structure of cystic fibrosis cells. J. Cell Commun. Signal. 2011, 5, 157–165. [Google Scholar] [CrossRef]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Le Deit, H.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitata. J. Hazard. Mater. 2006, 137, 1765–1772. [Google Scholar] [CrossRef]
- Nagy, B.; Tonk, S.; Cerasella, I.; Măicăneanu, A.; Majdik, C. Biosorption of cadmium ions by unmodified, microwave and ultrasound modified brewery and pure strain yeast biomass. Am. J. Anal. Chem. 2013, 4, 63–71. [Google Scholar] [CrossRef][Green Version]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Moreno-Rivas, S.C.; Armenta-Corral, R.I.; Frasquillo-Félix, M.C.; Lagarda-Díaz, I.; Vázquez-Moreno, L.; Montfort, G.R.C. Biosorción de cadmio en solución acuosa utilizando levadura de panadería (Saccharomyces cerevisiae). Rev. Mex. Ing. Quim. 2016, 15, 843–857. [Google Scholar]
- Karunasagar, D.; Balarama Krishna, M.V.; Rao, S.V.; Arunachalam, J. Removal and preconcentration of inorganic and methyl mercury from aqueous media using a sorbent prepared from the plant Coriandrum sativum. J. Hazard. Mater. 2005, 118, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, S. Microgel capsules tailored by droplet-based microfluidics. ChemPhysChem 2013, 14, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Nemethova, V.; Lacik, I.; Razga, F. Vibration technology for microencapsulation: The restrictive role of viscosity. J. Bioprocess. Biotech. 2015, 5, 2014–2016. [Google Scholar] [CrossRef]
- Aceval Arriola, N.D.; Mattos De Medeiros, P.; Schwinden Prudencio, E.; Olivera Müller, M.; Dias De Mello Castanho Amboni, R. Encapsulation of aqueous leaf extract of Stevia rebaudiana Bertoni with sodium alginate and its impact on phenolic content. Food Biosci. 2016, 13, 32–40. [Google Scholar] [CrossRef]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of probiotic Lactobacillus plantarum in dry microcapsules under accelerated storage conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef]
- Pajic-Lijakovic, I.; Levic, S.; Hadnadev, M.; Stevanovic-dajic, Z.; Radosevic, R.; Nedovic, V.; Bugarski, B. Structural changes of Ca-alginate beads caused by immobilized yeast cell growth. Biochem. Eng. J. 2015, 103, 32–38. [Google Scholar] [CrossRef]
- Putra, W.P.; Kamari, A.; Najiah, S.; Yusoff, M.; Ishak, C.F.; Mohamed, A.; Hashim, N.; Isa, I.M.; Putra, W.P. Biosorption of Cu(II), Pb(II) and Zn(II) ions from aqueous solutions using selected waste materials: Adsorption and characterisation studies. J. Encapsulation Adsorpt. Sci. 2014, 4, 25–35. [Google Scholar] [CrossRef]
- Lata, R.; Khowala, S. Effect of pretreatment on hexavalent chromium biosorption and multimetal biosorption efficiency of Termitomyces clypeatus biomass. Int. J. Integr. Sci. Innov. Technol. 2012, 1, 7–15. [Google Scholar]
- Gotoh, T.; Matsushima, K.; Kikuchi, K. Adsorption of Cu and Mn on covalently cross-linked alginate gel beads. Chemosphere 2004, 55, 57–64. [Google Scholar] [CrossRef]
- Mørch, Ä.A.; Donati, I.; Strand, B.L.; Skja, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef]
- Mallepally, R.R.; Bernard, I.; Marin, M.A.; Ward, K.R.; Mchugh, M.A. Superabsorbent alginate aerogels. J. Supercrit. Fluid. 2013, 79, 202–208. [Google Scholar] [CrossRef]
- Fan, J.; Shao, M.; Lai, L.; Liu, Y.; Xie, Z. Inhibition of autophagy contributes to the toxicity of cadmium telluride quantum dots in Saccharomyces cerevisiae. Int. J. Nanomed. 2016, 11, 3371–3383. [Google Scholar] [CrossRef]
- Panda, J.; Sarkar, P. Biosorption of Cr(VI) by calcium alginate-encapsulated Enterobacter aerogenes T2, in a semi-batch plug flow process. Water Air Soil Pollut. 2015, 226. [Google Scholar] [CrossRef]
- De Vos, P.; de Haan, B.J.; Kamps, J.A.A.; Faas, M.M.; Kitano, T. Zeta-potentials of alginate-PLL capsules: A predictive measure for biocompatibility? J. Biomed. Mater. Res. A 2007, 80, 813–819. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef]
- Thippeswamy, B.; Shivakumar, C.K.; Krishnappa, M. Study on heavy metals biosorption ability of Saccharomyces cerevisiae. Int. J. Biol. Res. 2014, 2, 106–115. [Google Scholar] [CrossRef][Green Version]
- Ibañez, J.P.; Umetsu, Y. Potential of protonated alginate beads for heavy metals uptake. Hydrometallurgy 2002, 64, 89–99. [Google Scholar] [CrossRef]
- Moya, M.L.; Morley, M.; Khanna, O.; Opara, E.C.; Brey, E.M. Stability of alginate microbead properties in vitro. J. Mater. Sci. Mater. Med. 2012, 23, 903–912. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Paredes-Juarez, G.A.; Niclou, S.P.; de Vos, P. Factors influencing the mechanical stability of alginate beads applicable for immunoisolation of mammalian cells. J. Mech. Behav. Biomed. 2014, 37, 196–208. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface chemical functional groups modification of porous carbon. Recent Pat. Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Mustapha, S.; Ndamitso, D.T.S.M.M.; Sumaila, M.B.E.A. Adsorption isotherm, kinetic and thermodynamic studies for the removal of Pb(II), Cd(II), Zn(II) and Cu(II) ions from aqueous solutions ising Albizia lebbeck pods. Appl. Water Sci. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Ferraz, A.I.; Tavares, T.; Teixeira, J.A. Cr(III) removal and recovery from Saccharomyces cerevisiae. Chem. Eng. J. 2004, 105, 11–20. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J. Uranium removal by novel graphene oxide-immobilized Saccharomyces cerevisiae gel beads. J. Environ. Radioactiv. 2016, 162–163, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Stirk, W.A.; Van Staden, J. Desorption of cadmium and the reuse of brown seaweed derived products as biosorbents. Bot. Mar. 2002, 45, 9–16. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Mohammed, A.A.; Al-musawi, T.J. Competitive biosorption of lead, cadmium, copper, and arsenic ions using algae. Environ. Sci. Pollut. Res. Int. 2013, 3011–3023. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Yang, N.; Wang, R.; Rao, P.; Yan, L.; Zhang, W.; Wang, J.; Chai, F. The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu(II) from metal mixtures. Crystals 2019, 9, 255. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, J.; Xue, R.; Yang, Y. Spectroscopic characterization of Au3+ biosorption by waste biomass of Saccharomyces cerevisiae. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 761–765. [Google Scholar] [CrossRef]
| Bead Type | Freshly Synthetized Bed Size (mm) | Moisture Content (%) | Surface Charge (mV) | After Lyophilization | |
|---|---|---|---|---|---|
| Bead Size (mm) | Pore Size (µm) | ||||
| Empty Ca-alginate | 1.51 ± 0.02 a,* | 98.1 ± 0.1 a | −14.3 ± 0.7 a | 1.49 ± 0.07 a | 0.66 ± 0.04 a |
| Empty Ca-alginate exposed to Cd2+ | N/D | N/D | 7.5 ± 1.0 c | 0.89 ± 0.06 c | 0.28 ± 0.01 b |
| S. cerevisiae encapsulated | 1.62 ± 0.02 b | 96.0 ± 0.1 b | −29.3 ± 1.5 b | 1.37 ± 0.05 a,b | 0.12 ± 0.01 c |
| S. cerevisiae encapsulated exposed to Cd2+ | N/D | N/D | 2.7 ± 0.6 d | 1.15 ± 0.05 b,c | 0.10 ± 0.01 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno Rivas, S.C.; Armenta Corral, R.I.; Frasquillo Félix, M.d.C.; Islas Rubio, A.R.; Vázquez Moreno, L.; Ramos-Clamont Montfort, G. Removal of Cadmium from Aqueous Solutions by Saccharomyces cerevisiae–Alginate System. Materials 2019, 12, 4128. https://doi.org/10.3390/ma12244128
Moreno Rivas SC, Armenta Corral RI, Frasquillo Félix MdC, Islas Rubio AR, Vázquez Moreno L, Ramos-Clamont Montfort G. Removal of Cadmium from Aqueous Solutions by Saccharomyces cerevisiae–Alginate System. Materials. 2019; 12(24):4128. https://doi.org/10.3390/ma12244128
Chicago/Turabian StyleMoreno Rivas, Silvia Carolina, Rosa Idalia Armenta Corral, María del Carmen Frasquillo Félix, Alma Rosa Islas Rubio, Luz Vázquez Moreno, and Gabriela Ramos-Clamont Montfort. 2019. "Removal of Cadmium from Aqueous Solutions by Saccharomyces cerevisiae–Alginate System" Materials 12, no. 24: 4128. https://doi.org/10.3390/ma12244128
APA StyleMoreno Rivas, S. C., Armenta Corral, R. I., Frasquillo Félix, M. d. C., Islas Rubio, A. R., Vázquez Moreno, L., & Ramos-Clamont Montfort, G. (2019). Removal of Cadmium from Aqueous Solutions by Saccharomyces cerevisiae–Alginate System. Materials, 12(24), 4128. https://doi.org/10.3390/ma12244128






