Treatment of Diethyl Phthalate Leached from Plastic Products in Municipal Solid Waste Using an Ozone-Based Advanced Oxidation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Leachate Analysis
2.2. Synthetic Leachate Preparation
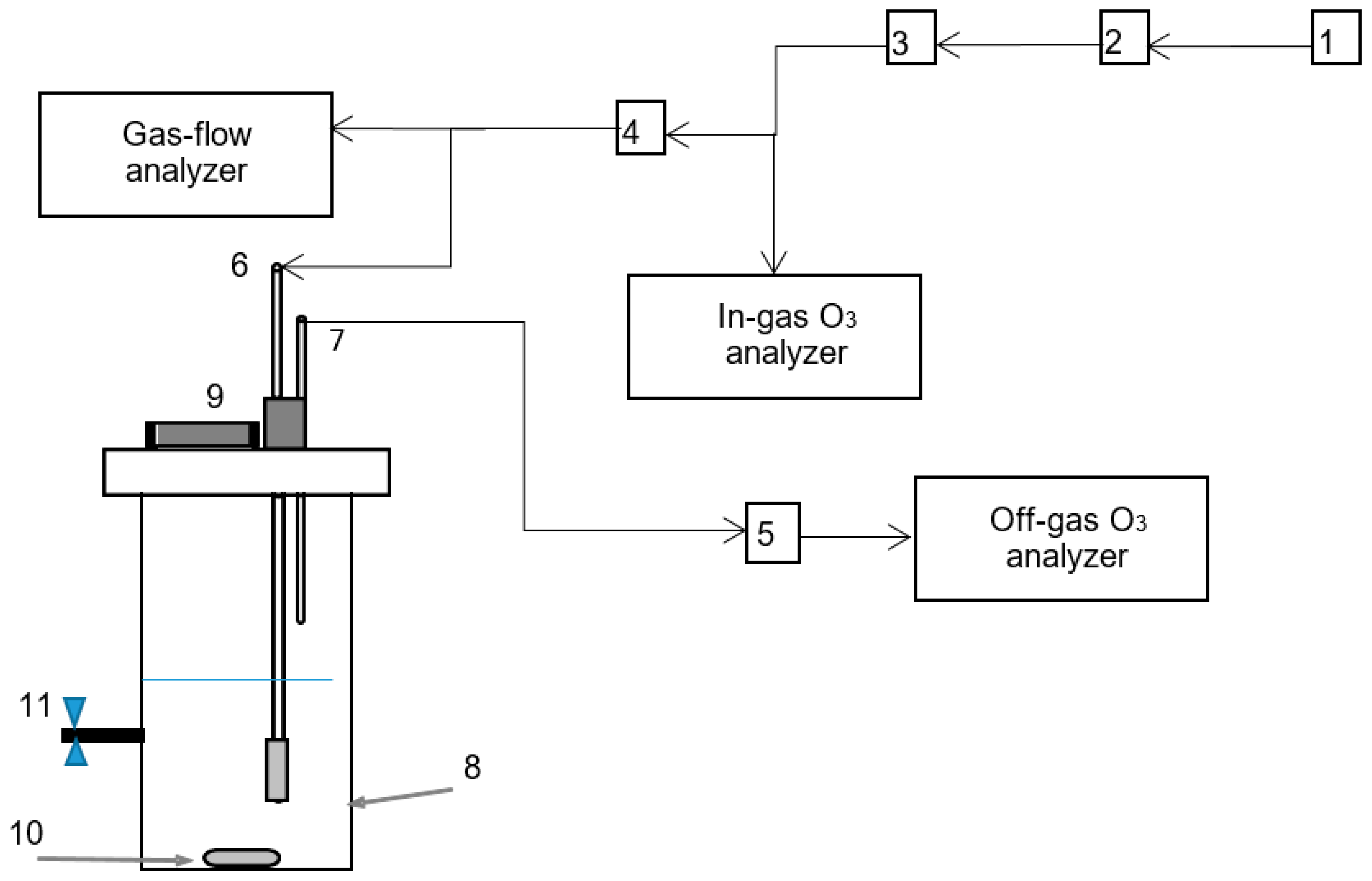
2.3. Experimental Setup and Procedure
2.4. High-Performance Liquid Chromatography (HPLC) Analysis
2.5. LC-MS Analysis
3. Results and Discussion
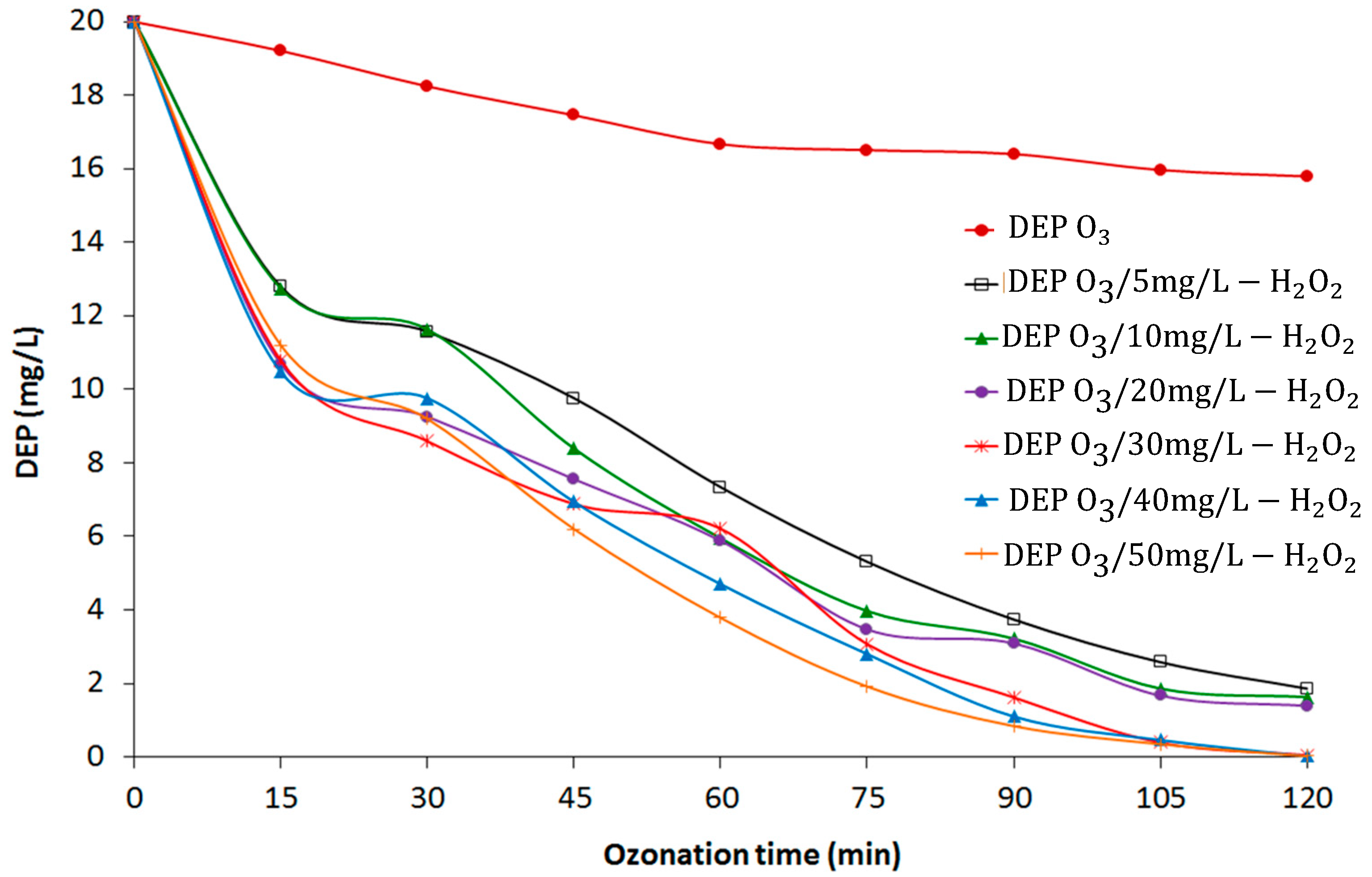
3.1. DEP Removal in O3 and O3/H2O2 Processes
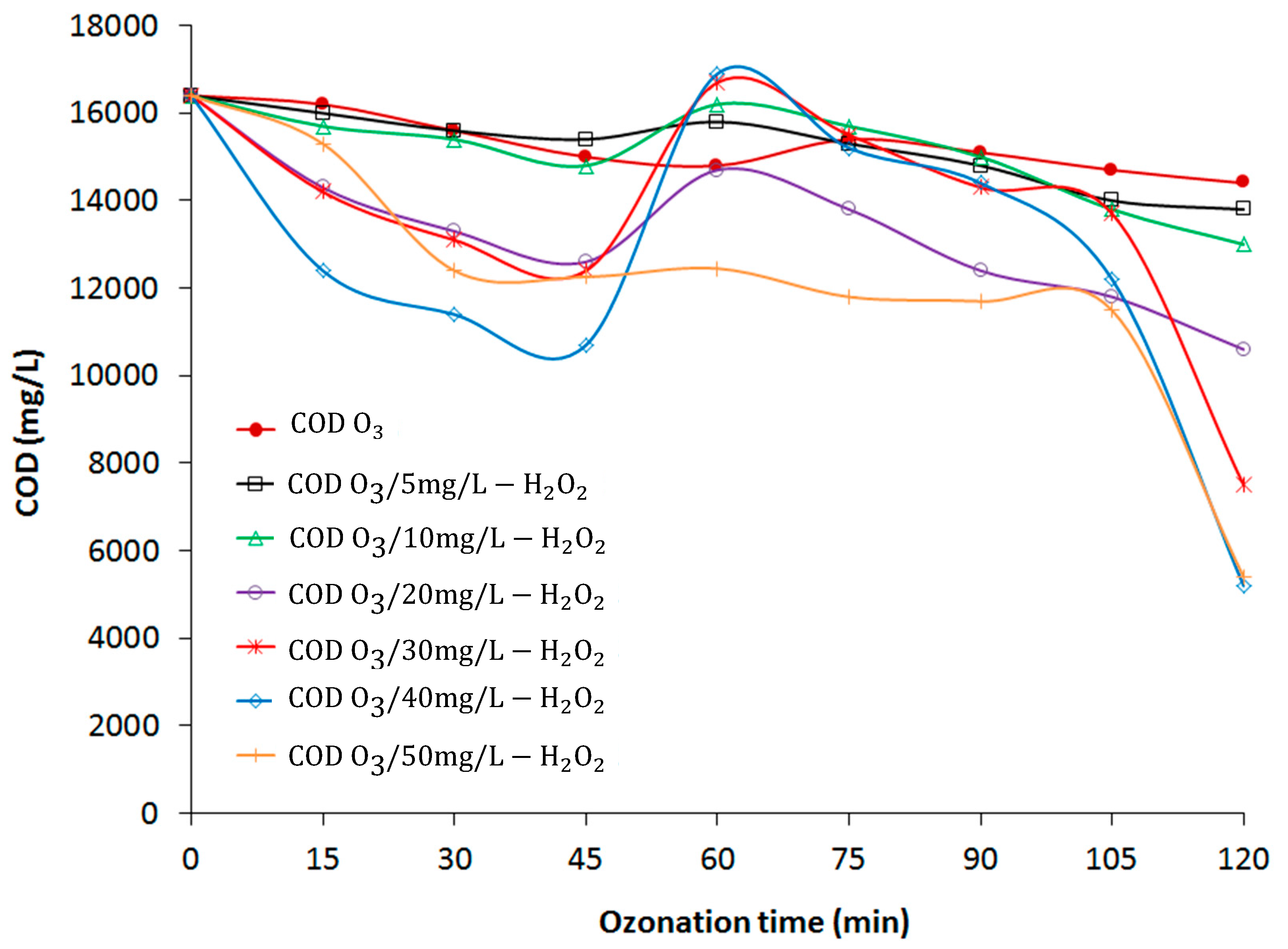
3.2. Effect of O3/H2O2 Process on COD, UVC, and pH
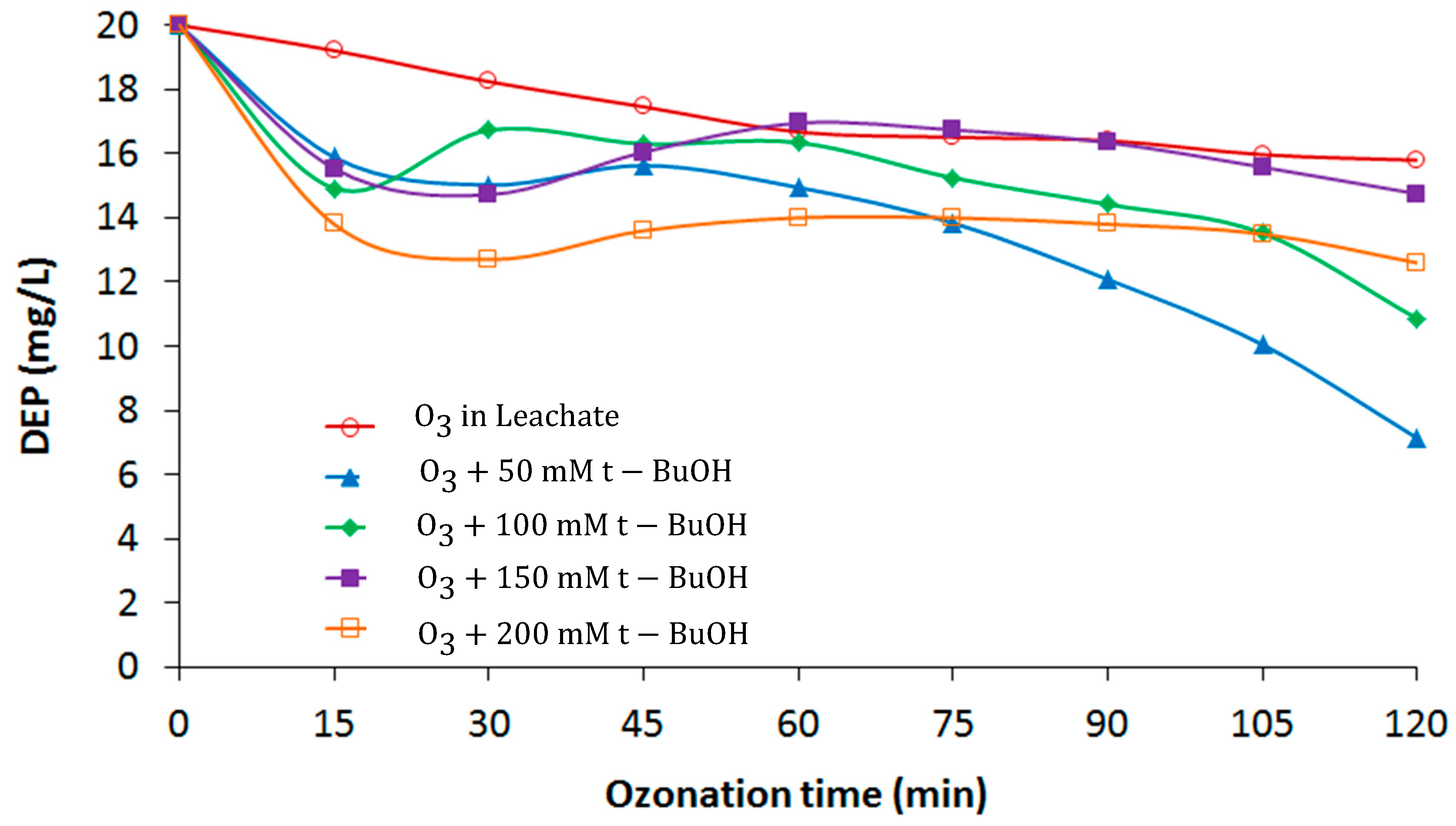
3.3. Effect of t-BuOH in the Kinetic Evaluation

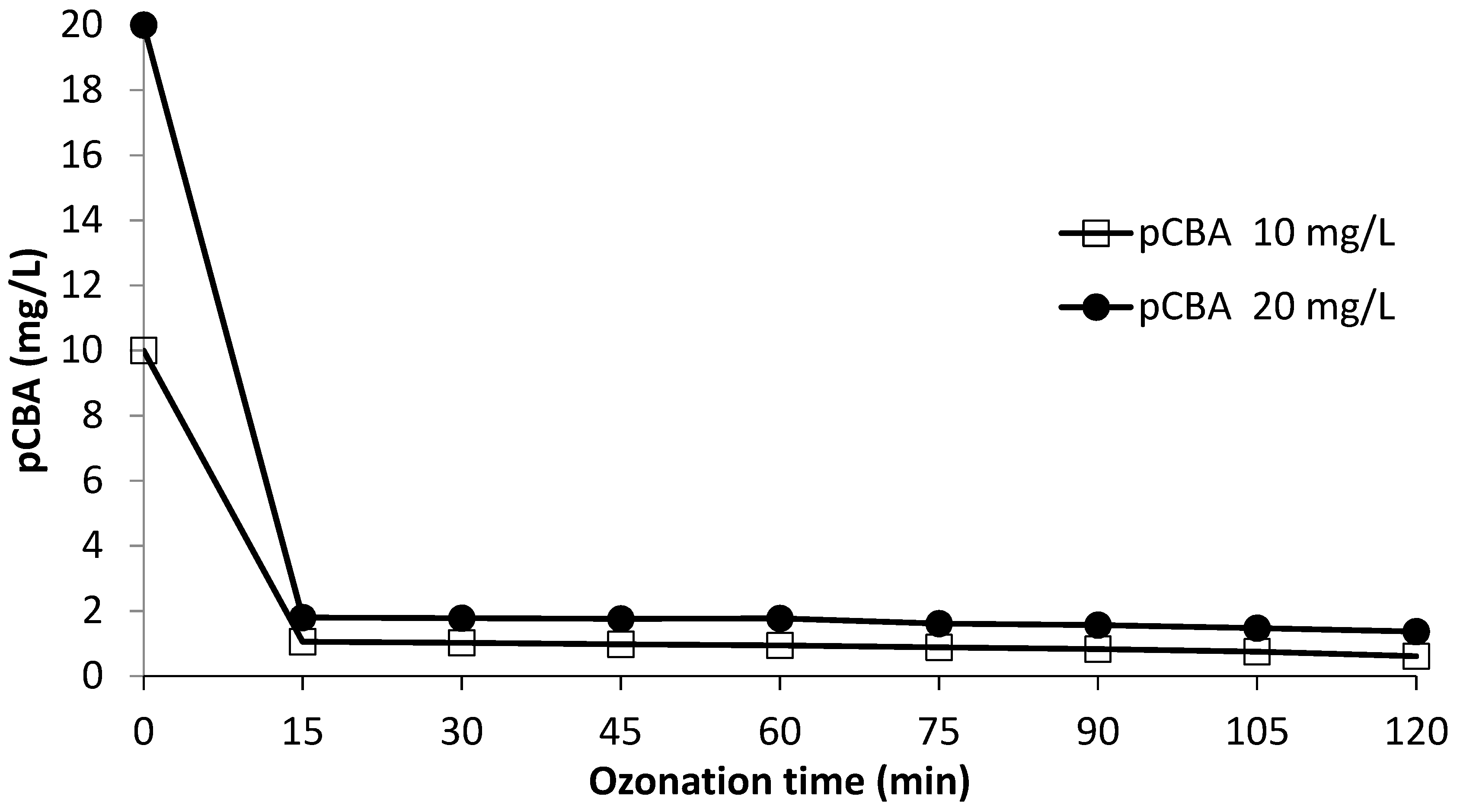
3.4. OH Radical Exposure
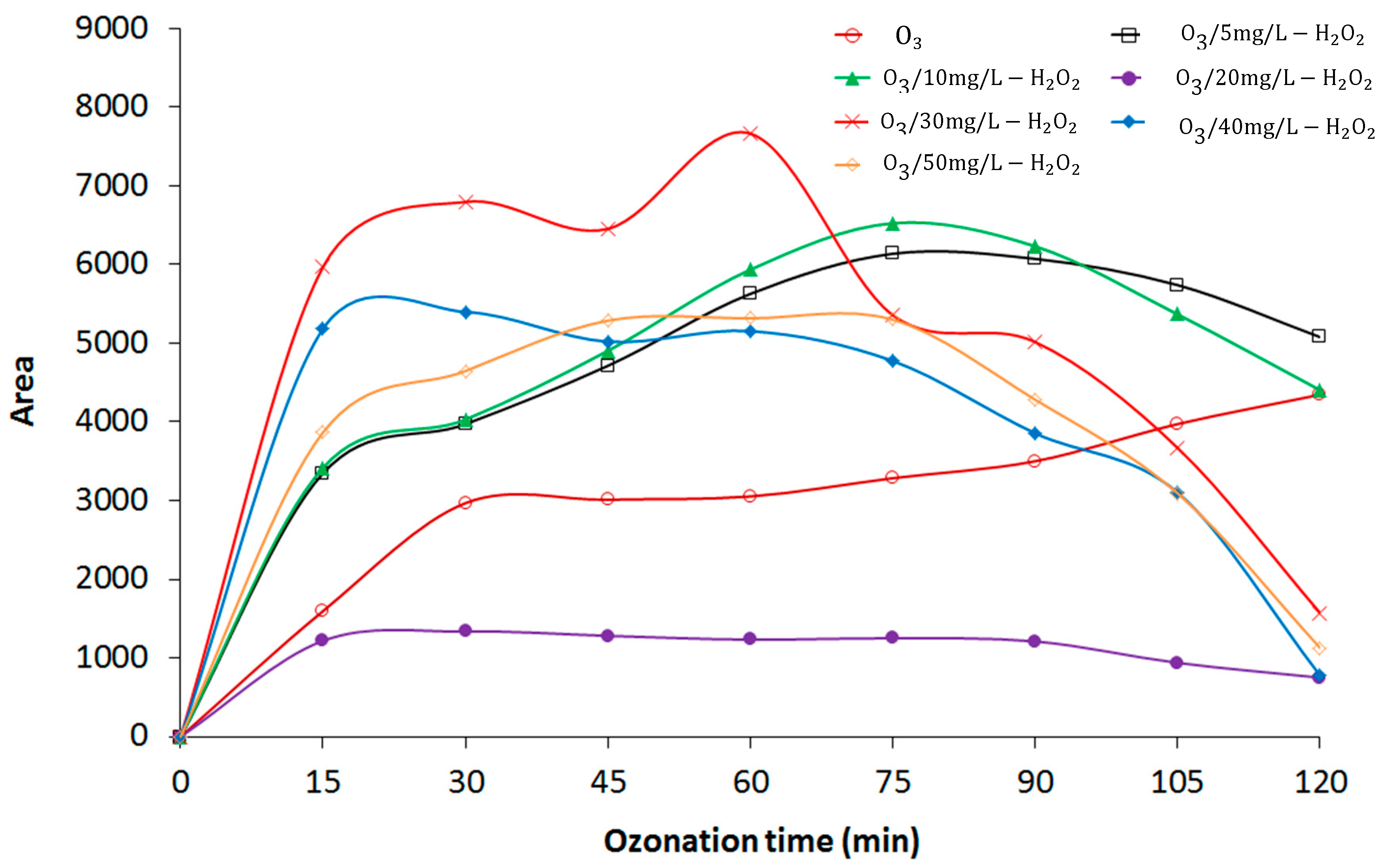
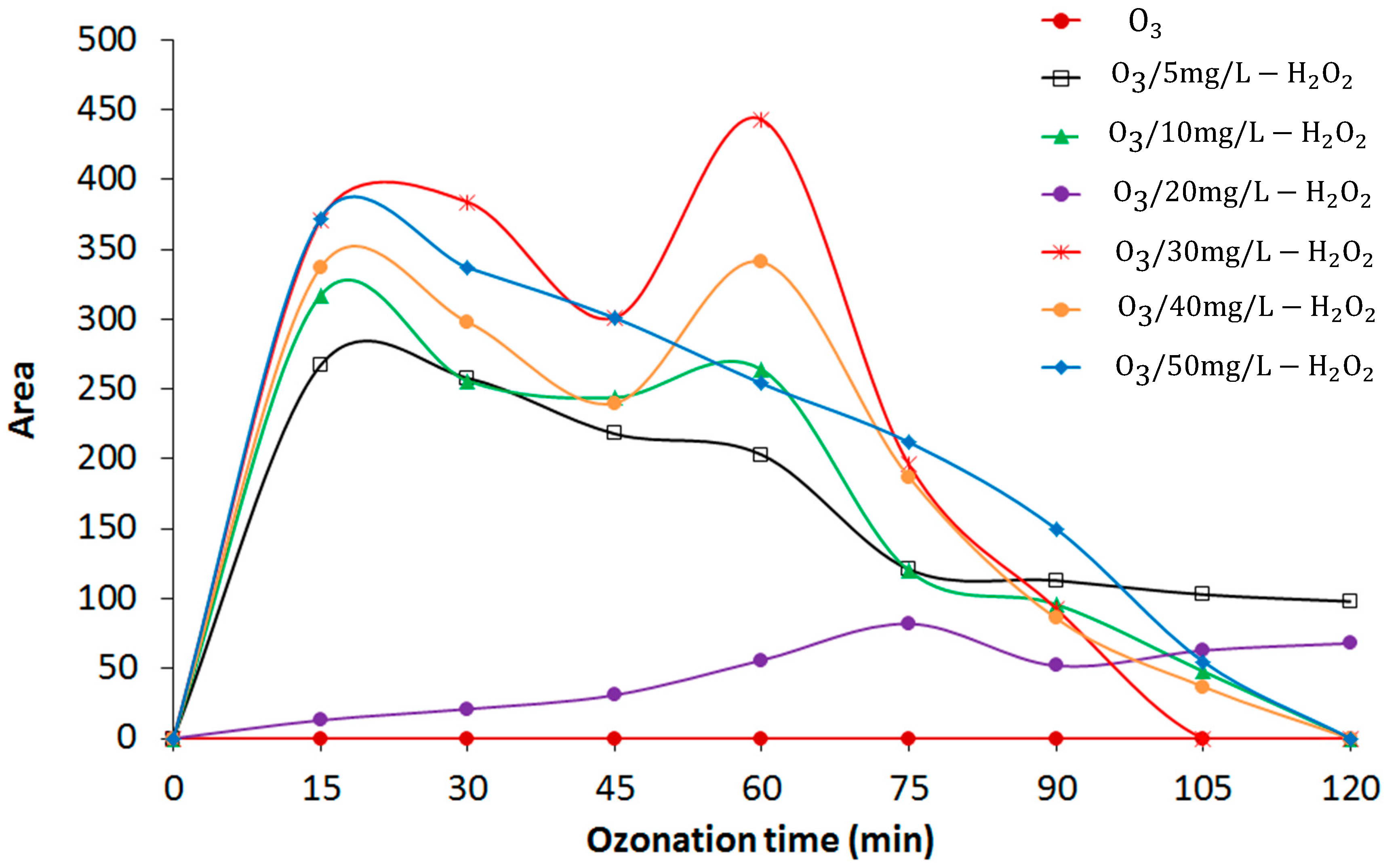
3.5. Formation of Intermediate Transformation Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Calabrò, P.S.; Gentili, E.; Meoni, C.; Orsi, S.; Komilis, D. Effect of the recirculation of a reverse osmosis concentrate on leachate generation: A case study in an Italian landfill. Waste Manag. 2018, 76, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shao, Y.; Gao, N.; Lu, X.; An, N. Degradation of diethyl phthalate (DEP) by UV/persulfate: An experiment and simulation study of contributions by hydroxyl and sulfate radicals. Chemosphere 2018, 193, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, P.; Sajjadi, B.; Abdul Aziz, A.R.; Daud, W.M.A.B.W. Ozone (O3) and sono (US) based advanced oxidation processes for the removal of color, COD and determination of electrical energy from landfill leachate. Sep. Purif. Technol. 2017, 172, 442–449. [Google Scholar] [CrossRef]
- Xu, B.; Gao, N.Y.; Sun, X.F.; Xia, S.J.; Rui, M.; Simonnot, M.O.; Causserand, C.; Zhao, J.F. Photochemical degradation of diethyl phthalate with UV/H2O2. J. Hazard. Mater. 2007, 139, 132–139. [Google Scholar] [CrossRef]
- Bilardi, S.; Calabrò, P.S.; Greco, R.; Moraci, N. Selective removal of heavy metals from landfill leachate by reactive granular filters. Sci. Total Environ. 2018, 644, 335–341. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Fatta, D.; Loizidou, M. Treatment of stabilized landfill leachate by physico-chemical and bio- oxidation processes. J. Environ. Sci. Heal. Part A Toxic/Hazard. Subst. Environ. Eng. 1998, 33, 651–670. [Google Scholar] [CrossRef]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment – A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Abu Amr, S.S.; Aziz, H.A.; Hossain, M.S.; Bashir, M.J.K. Simultaneous removal of COD and color from municipal landfill leachate using Ozone/Zinc sulphate oxidation process. Glob. Nest J. 2017, 19, 498–504. [Google Scholar]
- Abu Amr, S.; Aziz, H.A.; Bashir, M.; Aziz, S.Q.; Alslaibi, T. Comparison and Optimization of ozone—Based Advanced Oxidation Processes in The Treatment of Stabilized Landfill Leachate. J. Eng. Res. Technol. 2015, 2, 122–130. [Google Scholar] [CrossRef]
- Huang, W.B.; Chen, C.Y. Photocatalytic Degradation of Diethyl Phthalate (DEP) in water using TiO2. Water. Air. Soil Pollut. 2010, 207, 349–355. [Google Scholar] [CrossRef]
- Jung, Y.J.; Oh, B.S.; Kim, K.S.; Koga, M.; Shinohara, R.; Kang, J.W. The degradation of diethyl phthalate (DEP) during ozonation: Oxidation by-products study. J. Water Health 2010, 8, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Halim, A.A.; Abidin, N.N.Z.; Awang, N.; Ithnin, A.; Othman, M.S.; Wahab, M.I. Ammonia and COD removal from synthetic leachate using rice husk composite adsorbent. J. Urban Environ. Eng. 2011, 5, 24–31. [Google Scholar] [CrossRef]
- Mohan, S.; Gandhimathi, R. Removal of heavy metal ions from municipal solid waste leachate using coal fly ash as an adsorbent. J. Hazard. Mater. 2009, 169, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Peretz, R.; Gerchman, Y.; Mamane, H. Ozonation of tannic acid to model biomass pretreatment for bioethanol production. Bioresour. Technol. 2017, 241, 1060–1066. [Google Scholar] [CrossRef]
- Rodger, B.; Laura, B. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; pp. 1–101. ISBN 087553287X; 9780875532875. [Google Scholar]
- Tizaoui, C.; Bouselmi, L.; Mansouri, L.; Ghrabi, A. Landfill leachate treatment with ozone and ozone/hydrogen peroxide systems. J. Hazard. Mater. 2007, 140, 316–324. [Google Scholar] [CrossRef]
- Ozbelge, T.A.; Erol, F. Effects of pH, initiator, scavenger, and surfactant on the ozonation mechanism of an Azo Dye (Acid Red-151) in a batch reactor. Chem. Eng. Commun. 2009, 196, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Zucker, I.; Lester, Y.; Avisar, D.; Hübner, U.; Jekel, M.; Weinberger, Y.; Mamane, H. Influence of wastewater particles on ozone degradation of trace organic contaminants. Environ. Sci. Technol. 2015, 49, 301–308. [Google Scholar] [CrossRef]
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Elovitz, M.S.; von Gunten, U. Hydroxyl radical/ozone ratios during ozonation processes. I. the Rct concept. Ozone Sci. Eng. 1999, 21, 239–260. [Google Scholar] [CrossRef]
- Pi, Y.; Schumacher, J.; Jekel, M. The Use of para-Chlorobenzoic Acid (pCBA) as an Ozone/Hydroxyl Radical Probe Compound. Ozone Sci. Eng. 2005, 27, 431–436. [Google Scholar] [CrossRef]
- Wert, E.; Rosario-Ortiz, F.L.; Snyder, S.A. Using ultraviolet absorbance and color to assess pharmaceutical oxidation during ozonation of wastewater. Environ. Sci. Technol. 2009, 43, 4858–4863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakretz, A.; Mamane, H.; Cikurel, H.; Avisar, D.; Gelman, E.; Zucker, I. The Role of Soil Aquifer Treatment (SAT) for Effective Removal of Organic Matter, Trace Organic Compounds and Microorganisms from Secondary Effluents Pre-Treated by Ozone. Ozone Sci. Eng. 2017, 39, 385–394. [Google Scholar] [CrossRef]
- Medellin-Castillo, N.A.; Ocampo-Pérez, R.; Leyva-Ramos, R.; Sanchez-Polo, M.; Rivera-Utrilla, J.; Méndez-Díaz, J.D. Removal of diethyl phthalate from water solution by adsorption, photo-oxidation, ozonation and advanced oxidation process (UV/H2O2, O3/H2O2 and O3/activated carbon). Sci. Total Environ. 2013, 442, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Ma, J.; Liu, Z.; Zhao, L. Ozonation kinetics for the degradation of phthalate esters in water and the reduction of toxicity in the process of O3/H2O2. J. Hazard. Mater. 2011, 195, 371–377. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Model Compound | Measured Value |
|---|---|---|
| pH | - | 7.5 |
| Chemical oxygen demand | KHP | 16,400 mg/L |
| Phthalate | Diethyl phthalate | 20 mg/L |
| Chloride | NH4Cl | 1500 mg/L |
| Manganese | MnSO4 | 16 mg/L |
| Zinc | ZnSO4 | 12 mg/L |
| Lead | Pb(NO3)2 | 2 mg/L |
| Chromium | K2Cr2O7 | 1.5 mg/L |
| Copper | CuSO4 | 2.5 mg/L |
| Nickel | NiSO4 | 4.5 mg/L |
| Acetic acid | Organic acid | 7 mg/L |
| Propionic acid | Organic acid | 5 mg/L |
| Pentanoic acid | Organic acid | 1 mg/L |
| Hexanoic acid | Organic acid | 1 mg/L |
| Ozonation Time (min) | pCBA (10 mg/L) | OH Exposure | pCBA (20 mg/L) | OH Exposure |
|---|---|---|---|---|
| 0 | 10 | 0 | 20 | 0 |
| 15 | 1.06 | 4.50 × 10−10 | 1.8 | 4.82 × 10−10 |
| 30 | 1.02 | 4.57 × 10−10 | 1.78 | 4.84 × 10−10 |
| 45 | 0.98 | 4.65 × 10−10 | 1.76 | 4.86 × 10−10 |
| 60 | 0.94 | 4.73 × 10−10 | 1.77 | 4.85 × 10−10 |
| 75 | 0.88 | 4.86 × 10−10 | 1.61 | 5.04 × 10−10 |
| 90 | 0.83 | 4.98 × 10−10 | 1.57 | 5.10 × 10−10 |
| 105 | 0.75 | 5.18 × 10−10 | 1.48 | 5.21 × 10−10 |
| 120 | 0.61 | 5.60 × 10−10 | 1.37 | 5.37 × 10−10 |
| Compound Name | Structure | KO3 (M−1s−1) | KOH (M−1s−1) |
|---|---|---|---|
| Diethyl phthalate | 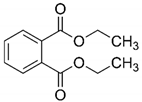 | 0.06–0.1 (Wen et al., 2011) 0.14 | 3–5 × 109 (Haag et al., 2005) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohan, S.; Mamane, H.; Avisar, D.; Gozlan, I.; Kaplan, A.; Dayalan, G. Treatment of Diethyl Phthalate Leached from Plastic Products in Municipal Solid Waste Using an Ozone-Based Advanced Oxidation Process. Materials 2019, 12, 4119. https://doi.org/10.3390/ma12244119
Mohan S, Mamane H, Avisar D, Gozlan I, Kaplan A, Dayalan G. Treatment of Diethyl Phthalate Leached from Plastic Products in Municipal Solid Waste Using an Ozone-Based Advanced Oxidation Process. Materials. 2019; 12(24):4119. https://doi.org/10.3390/ma12244119
Chicago/Turabian StyleMohan, Sankaralingam, Hadas Mamane, Dror Avisar, Igal Gozlan, Aviv Kaplan, and Gokul Dayalan. 2019. "Treatment of Diethyl Phthalate Leached from Plastic Products in Municipal Solid Waste Using an Ozone-Based Advanced Oxidation Process" Materials 12, no. 24: 4119. https://doi.org/10.3390/ma12244119
APA StyleMohan, S., Mamane, H., Avisar, D., Gozlan, I., Kaplan, A., & Dayalan, G. (2019). Treatment of Diethyl Phthalate Leached from Plastic Products in Municipal Solid Waste Using an Ozone-Based Advanced Oxidation Process. Materials, 12(24), 4119. https://doi.org/10.3390/ma12244119






