Photoactive Pore Matrix for In Situ Delivery of a Photosensitizer in Vascular Smooth Muscle Cells Selective PDT
Abstract
1. Introduction
2. Materials and Methods
2.1. Photoactive Matrix Preparation
2.2. Pore Size Distribution Experimental Setup
2.3. Analysis of the Pore Size Distribution
2.4. Light Power and Energy Densities Measurements
2.5. Light Source
2.6. Energy-Dispersive X-Ray Spectroscopy (EDS)
2.7. Atomic Force Microscopy
2.8. Spectrophotometric Measurements
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Cell Culture Conditions and Materials
2.11. Confocal Laser Microscopy
2.12. Biocompatibility and Toxicity Studies
2.13. Intracellular Reactive Oxygen Species Level
3. Results
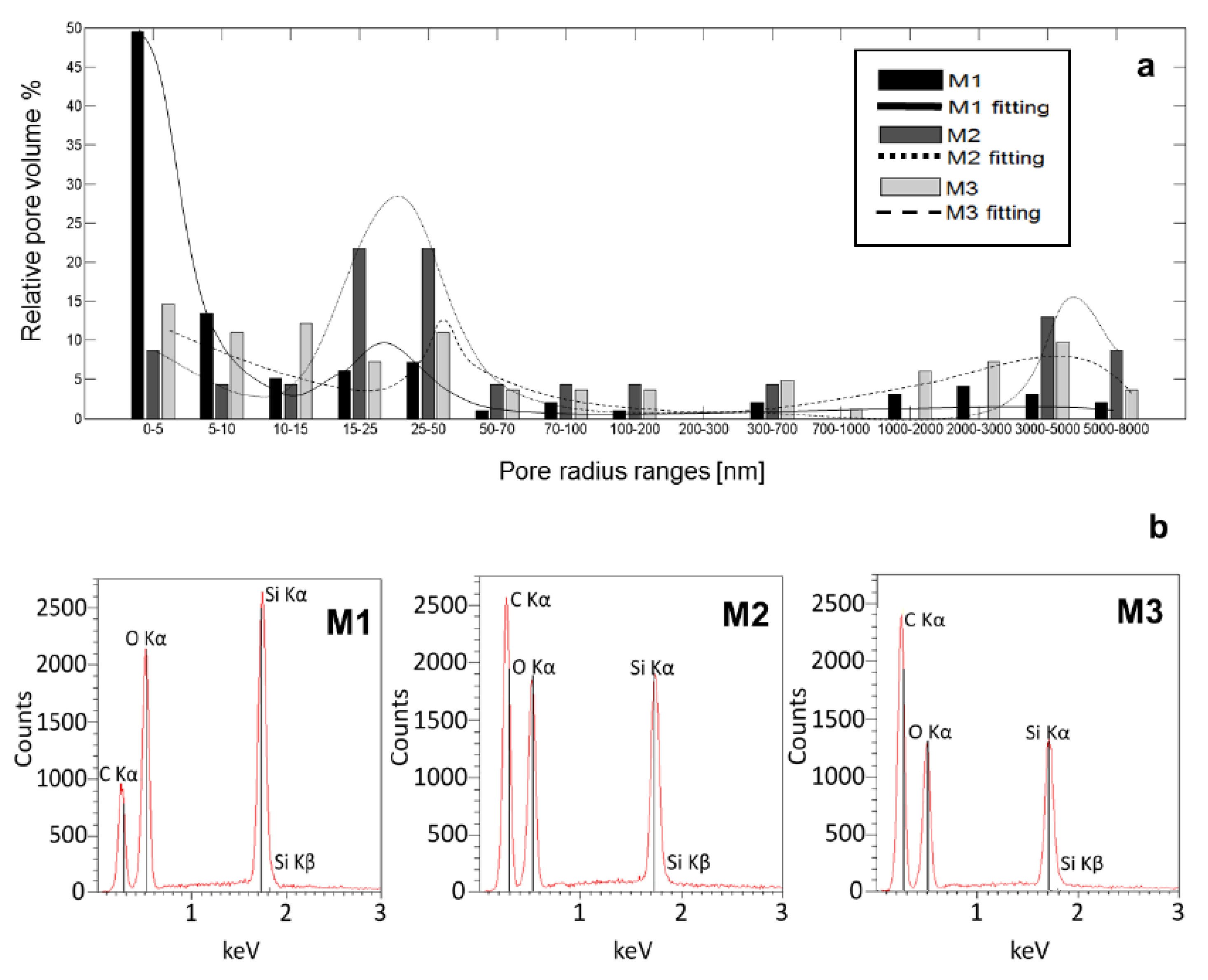
3.1. Analysis of the Pore Size Distribution and Energy Dispersive X-Ray Spectroscopy
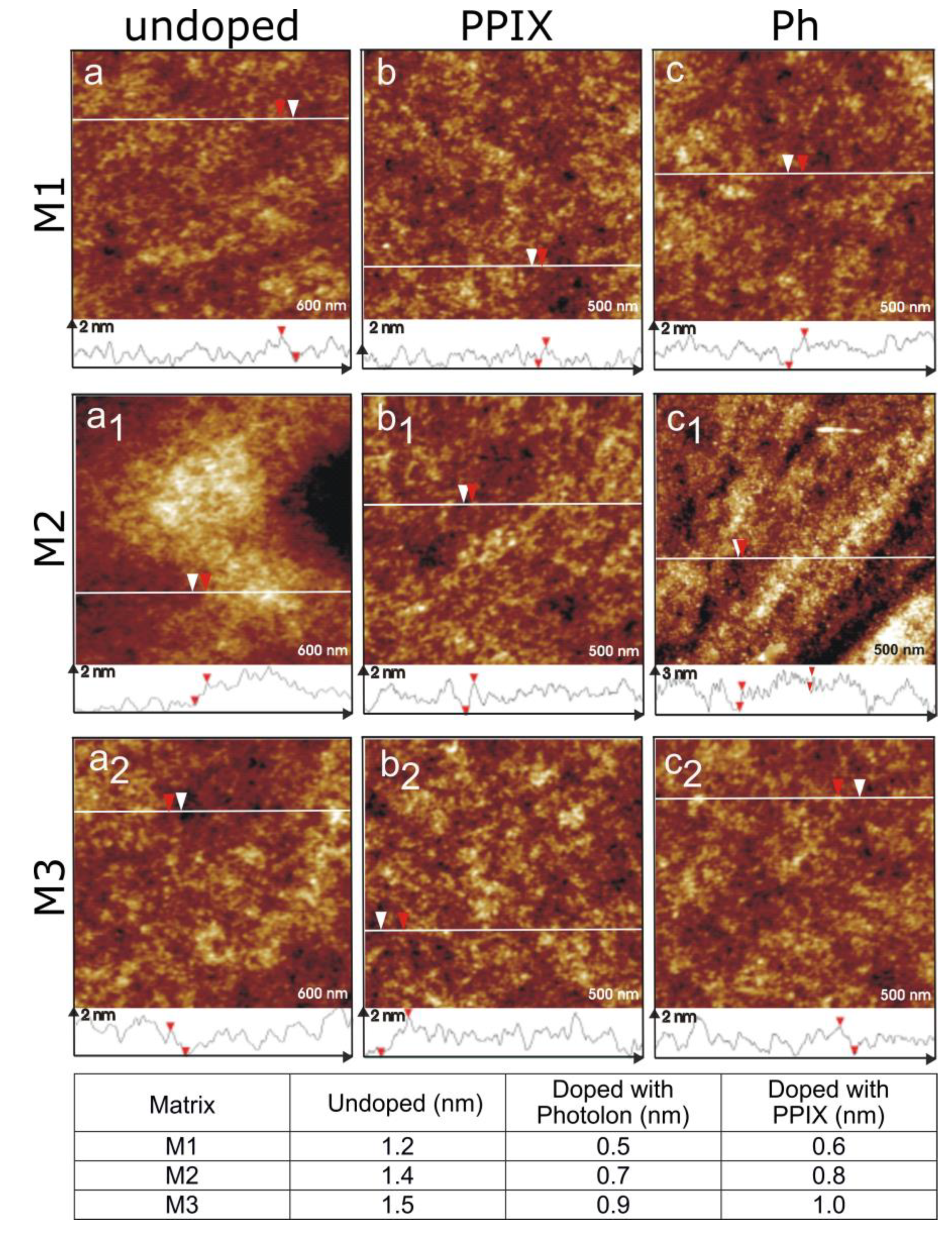
3.2. Atomic Force Microscopy Examination
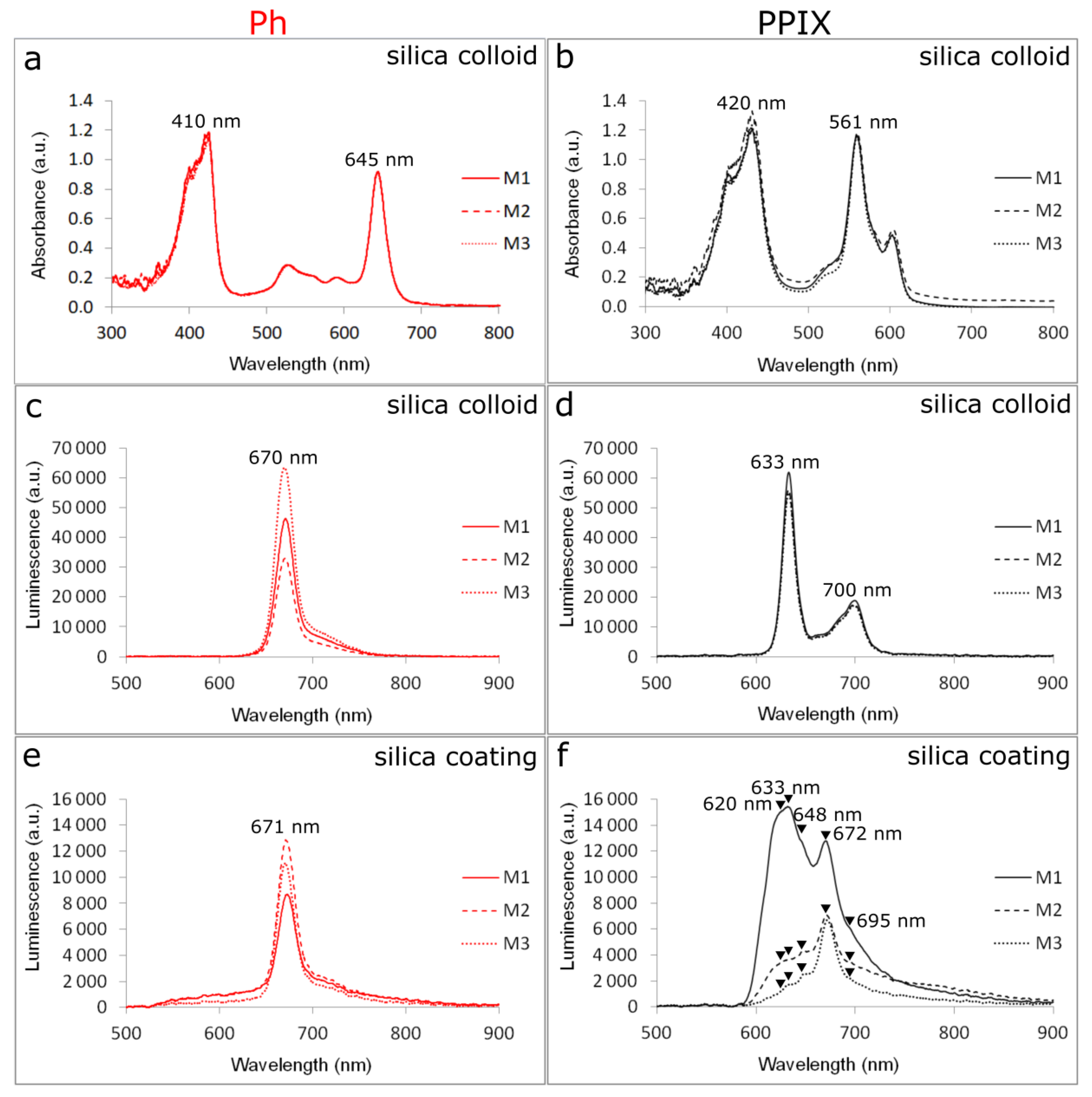
3.3. Spectrophotometric Measurements
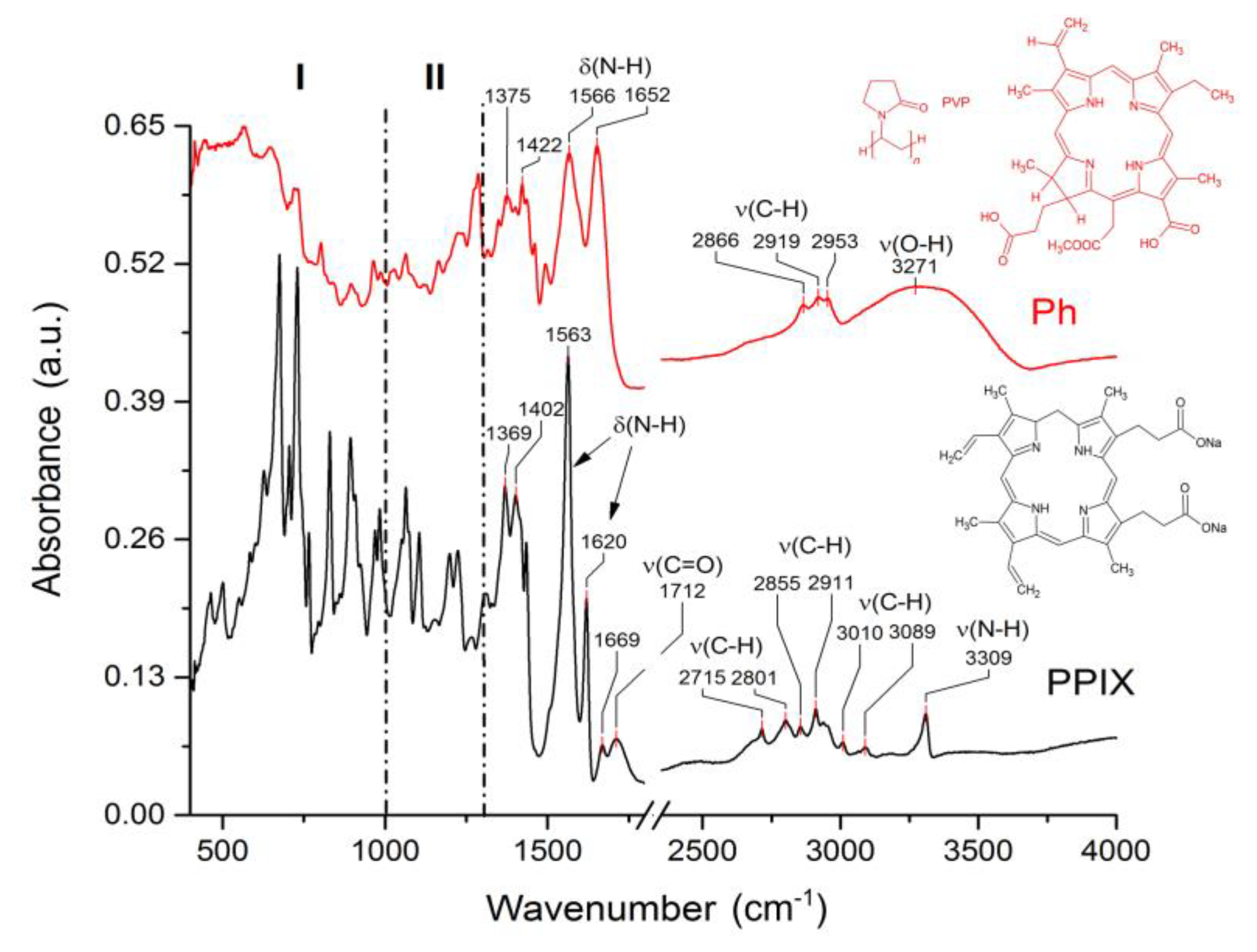
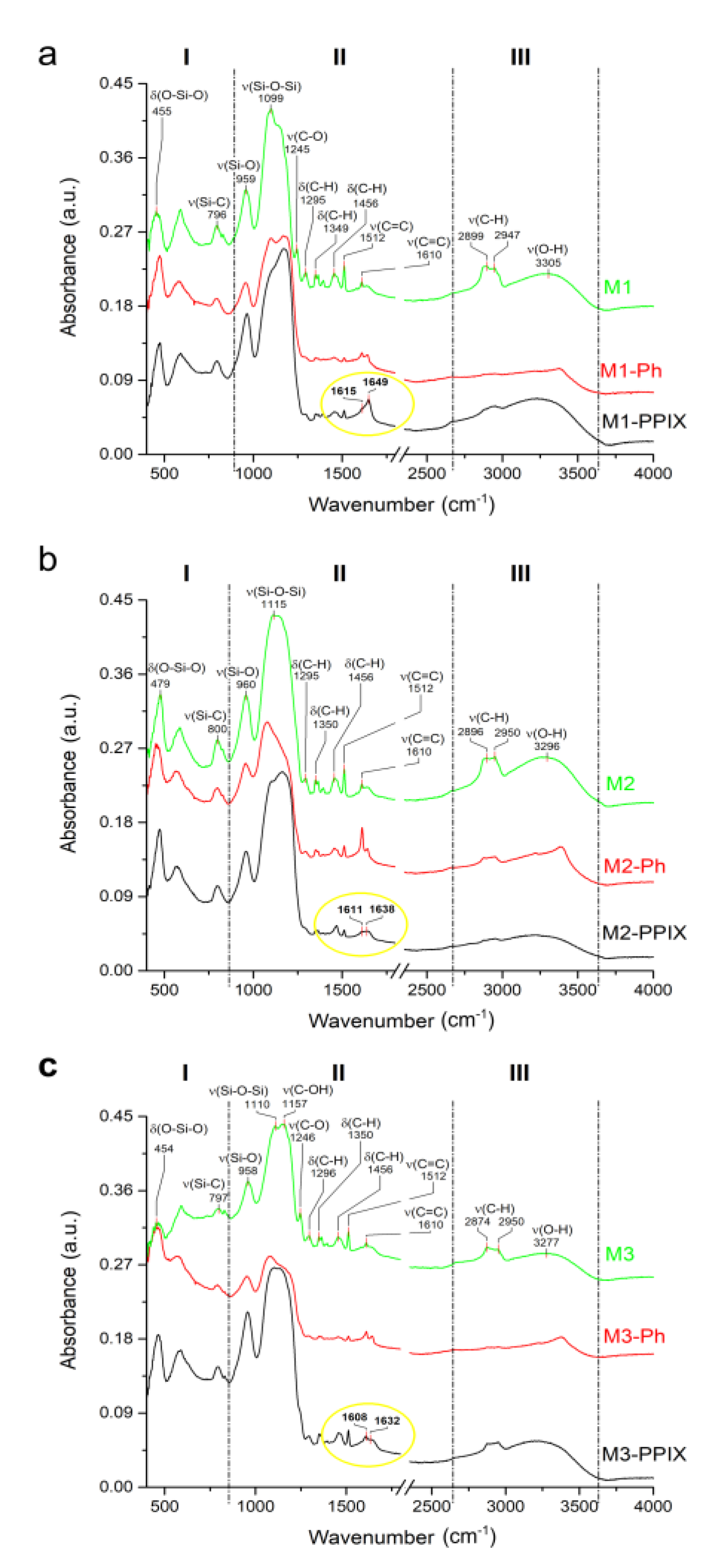
3.4. Spectroscopic Characterization of Functionalized Surfaces
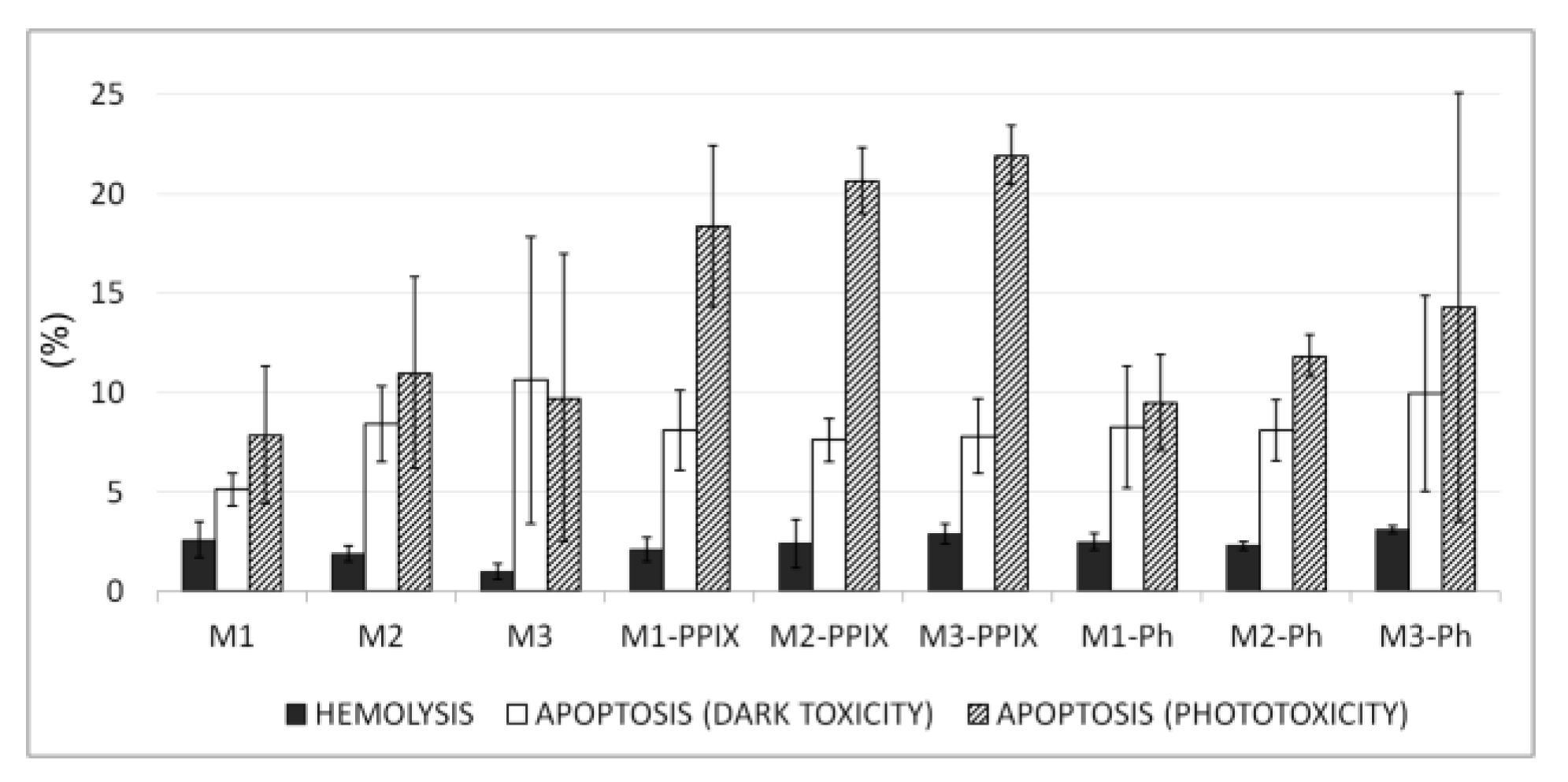
3.5. Blood Biocompatibility and Toxicity Studies
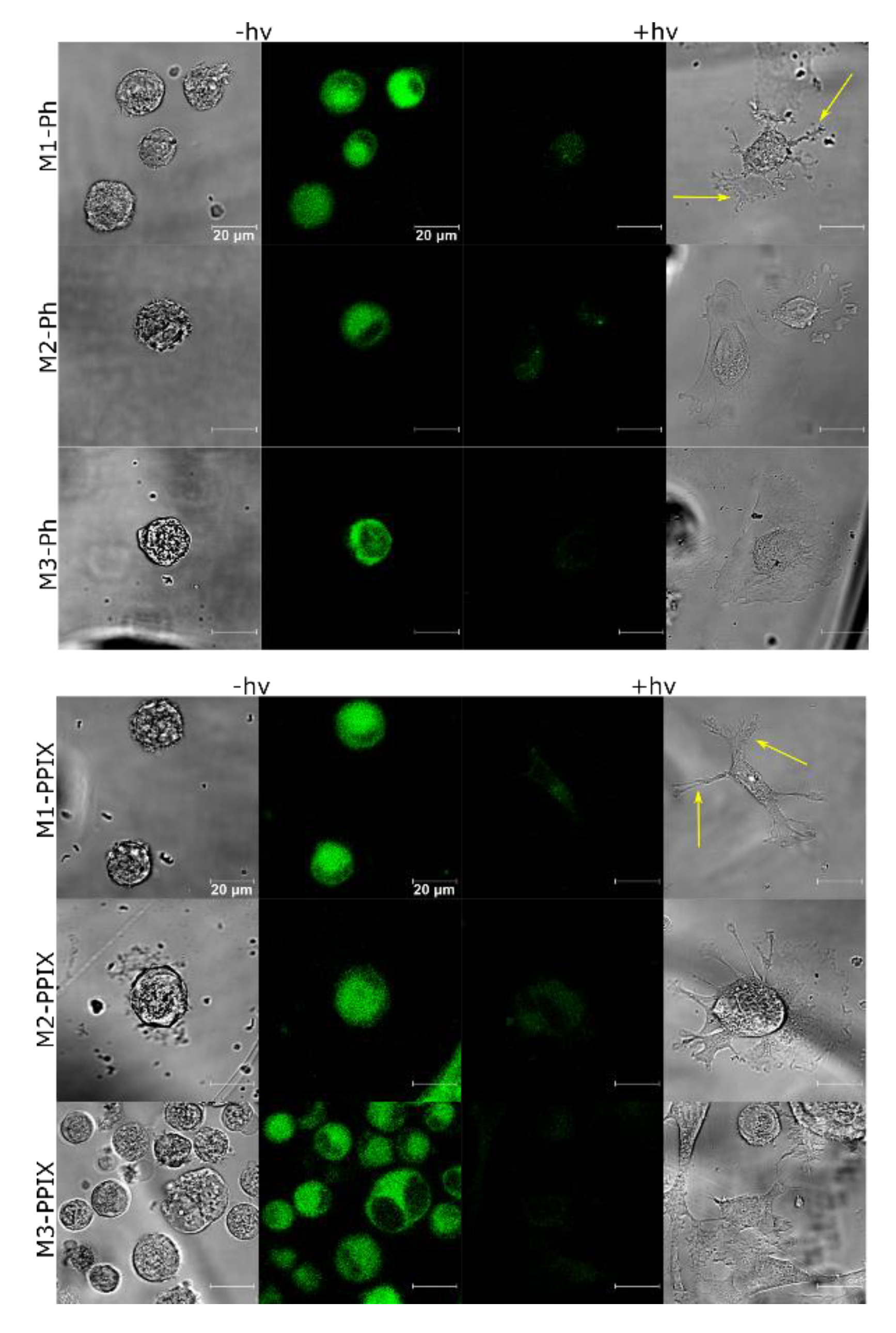
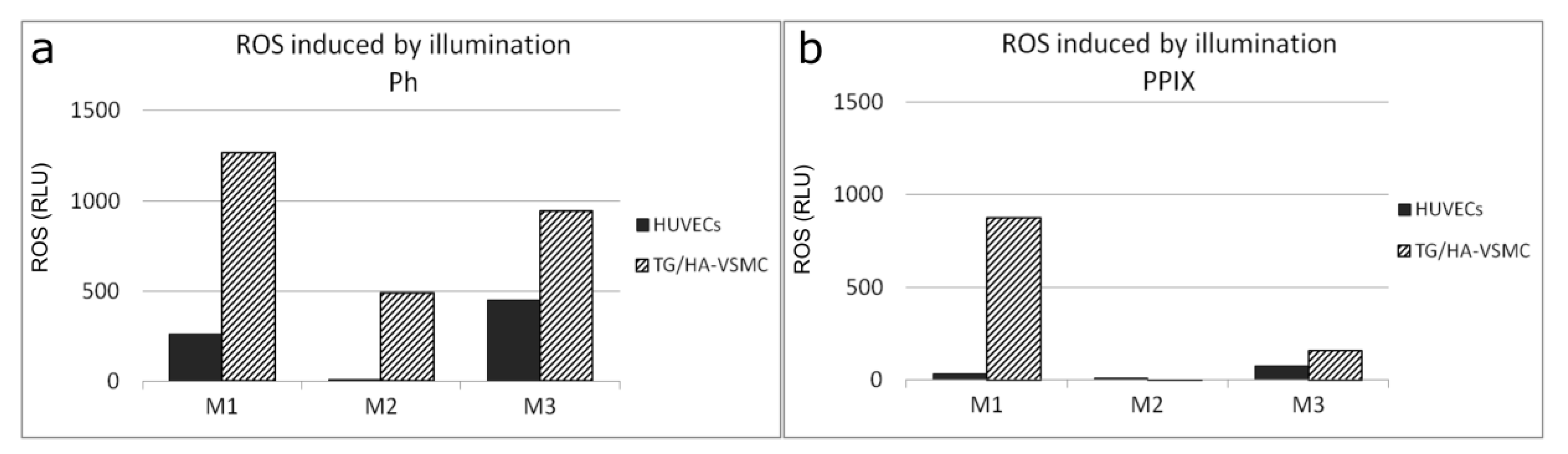
3.6. PS Uptake and ROS Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rui, T.; Xiao-Bing, C.; Jia-Ning, W.; Shi-You, C. CTP synthase 1, a smooth muscle-sensitive therapeutic target for effective vascular repair. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 10. [Google Scholar]
- Wiemer, M.; Stoikovic, S.; Samol, A.; Dimitriadis, Z.; Ruiz-Nodar, J.M.; Birkemeyer, R.; Novo, E.G.; Monsegu, J.; Finet, G.; Tresukosol, D.; et al. Third generation drug eluting stent (DES) with biodegradable polymer in diabetic patients: 5 years follow-up. Cardiovasc. Diabetol. 2017, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Gogas, B.D.; McDaniel, M.; Samady, H.; King, S.B., 3rd. Novel drug-eluting stents for coronary revascularization. Trends Cardiovasc. Med. 2014, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kuchulakanti, P.K.; Chu, W.W.; Torguson, R.; Ohlmann, P.; Rha, S.W.; Clavijo, L.C.; Kim, S.W.; Bui, A.; Gevorkian, N.; Xue, Z.; et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stent. Circulation 2006, 113, 1108–1113. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, R.; Bown, S.; McEwan, J. Photodynamic therapy: Shedding light on restenosis. Heart 2001, 86, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Moussavian, M.; Casterella, P.; Teirstein, P. Restenosis after angioplasty. Curr. Treat. Options Cardiovasc. Med. 2001, 3, 103–113. [Google Scholar] [CrossRef]
- Statius van Eps, R.G.; ChandraSekar, N.R.; Hasan, T.; LaMuraglia, G.M. Importance of the treatment field for the application of vascular photodynamic therapy to inhibit intimal hyperplasia. Photochem. Photobiol. 1998, 67, 337–342. [Google Scholar] [CrossRef]
- Granville, D.J.; Cassidy, B.A.; Ruehlmann, D.O.; Choy, J.C.; Brenner, C.; Kroemer, G.; McManus, B.M.; van Breemen, C.; Margaron, P.; Hunt, D.W.; et al. Mitochondrial release of apoptosis-inducing factor and cytochrome c during smooth muscle cell apoptosis. Am. J. Pathol. 2001, 159, 305–311. [Google Scholar] [CrossRef]
- Chen, Z.; Woodburn, K.W.; Shi, C.; Adelman, D.C.; Rogers, C.; Simon, D.I. Photodynamic therapy with motexafin lutetium induces redox-sensitive apoptosis of vascular cells. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 759–764. [Google Scholar] [CrossRef]
- Wawrzyńska, M.; Kałas, W.; Biały, D.; Zioło, E.; Arkowski, J.; Mazurek, W.; Strządała, L. In vitro photodynamic therapy with chlorin e6 leads to apoptosis of human vascular smooth muscle cells. Arch. Immunol. Ther. Exp. 2010, 58, 67–75. [Google Scholar] [CrossRef]
- Statius van Eps, R.G.; Adili, F.; Watkins, M.T.; Anderson, R.R.; LaMuraglia, G.M. Photodynamic therapy of extracellular matrix stimulates endothelial cell growth by inactivation of matrix-associated transforming growth factor-beta. Lab. Invest. 1997, 76, 257–266. [Google Scholar] [PubMed]
- Magaraggia, M.; Visonà, A.; Furlan, A.; Pagnan, A.; Miotto, G.; Tognon, G.; Jori, G. Inactivation of vascular smooth muscle cells photosensitised by liposome-delivered Zn(II)-phthalocyanine. J. Photochem. Photobiol. B 2006, 82, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nagae, T.; Aizawa, K.; Uchimura, N.; Tani, D.; Abe, M.; Fujishima, K.; Ishimaru, S.; Wilson, S.E. Endovascular photodynamic therapy using mono-l-aspartyl-chlorin e6 to inhibit Intimal hyperplasia in balloon-injured rabbit arteries. Lasers Surg. Med. 2001, 28, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Pai, M.; Jamal, W.; Mosse, A.; Bishop, C.; Bown, S.; McEwan, J. Inhibition of in-stent restenosis in rabbit iliac arteries with photodynamic therapy. Eur. J. Vasc. Endovasc. Surg. 2005, 30, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, M.P.; Buonaccorsi, G.; MacRobert, A.; Bishop, C.C.; Bown, S.G.; McEwan, J.R. Intra-arterial photodynamic therapy using 5-ALA in a swine model. Eur. J. Vasc. Endovasc. Surg. 1998, 16, 284–291. [Google Scholar] [CrossRef]
- Guyon, L.; Farine, M.-O.; Lesage, J.C.; Gevaert, A.M.; Simonin, S.; Schmitt, C.; Collinet, P.; Mordon, S. Photodynamic therapy of ovarian cancer peritoneal metastasis with hexaminolevulinate: A toxicity study. Photodiagnosis Photodyn. Ther. 2014, 11, 265–274. [Google Scholar] [CrossRef]
- Pantelides, M.L.; Moore, J.V.; Blacklock, N.J. A comparison of serum kinetics and tissue distribution of photofrin II following intra-venous and intraperitoneal injection in the mouse. Photochem. Photobiol. 1989, 49, 67–70. [Google Scholar] [CrossRef]
- Henderson, B.W.; Vaughan, L.; Bellnier, D.A.; van Leengoed, H.; Johnson, P.G.; Oseroff, A.R. Photosensitization of murine tumor vasculature and skin by 5-aminolevulinic acid-induced porphyrin. Photochem. Photobiol. 1995, 62, 780–789. [Google Scholar] [CrossRef]
- Eckl, D.B.; Dengler, L.; Nemmert, M.; Eichner, A.; Baumler, W.; Huber, H. A Closer Look at Dark Toxicity of the Photosensitizer TMPyP in Bacteria. Photochem. Photobiol. 2018, 94, 165–172. [Google Scholar] [CrossRef]
- McCarthy, J.R.; Korngold, E.; Weissleder, R.; Jaffer, F.A. A light-activated theranostic nanoagent for targeted macrophage ablation in inflammatory atherosclerosis. Small 2010, 6, 2041–2049. [Google Scholar] [CrossRef]
- Jain, M.; Zellweger, M.; Frobert, A.; Valentin, J.; Bergh, H.V.D.; Wagnières, G.; Cook, S.; Giraud, M.N. Intra-arterial drug and light delivery for photodynamic therapy using Visudyne(R): Implication for atherosclerotic plaque treatment. Front. Physiol. 2016, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, J.; Peng, C.; Li, Z.; Shi, S.; Liang, H.; Tian, Y.; Zhang, Z.; Cao, W. Apoptosis of vascular smooth muscle cells induced by photodynamic therapy with protoporphyrin IX. Biochem. Biophys. Res. Commun. 2010, 391, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Li, Y.; Liang, H.; Cheng, J.; Li, Q.; Sun, X.; Li, Z.; Wang, F.; Guo, Y.; Tian, Z.; et al. Detection and photodynamic therapy of inflamed atherosclerotic plaques in the carotid artery of rabbits. J. Photochem. Photobiol. B 2011, 102, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Pegaz, B.; Debefve, E.; Borle, F.; Ballini, J.P.; Wagnières, G.; Spaniol, S.; Albrecht, V.; Scheglmann, D.; Nifantiev, N.E.; van den Bergh, H.; et al. Preclinical evaluation of a novel water-soluble chlorin E6 derivative (BLC 1010) as photosensitizer for the closure of the neovessels. Photochem. Photobiol. 2005, 81, 1505–1510. [Google Scholar] [CrossRef]
- Nagae, T.; Louie, A.Y.; Aizawa, K.; Ishimaru, S.; Wilson, S.E. Selective targeting and photodynamic destruction of intimal hyperplasia by scavenger-receptor mediated protein-chlorin e6 conjugates. J. Cardiovasc. Surg. 1998, 39, 709–715. [Google Scholar]
- Buzalewicz, I.; Hołowacz, I.; Ulatowska-Jarża, A.; Podbielska, H. Towards dosimetry for photodynamic diagnosis with the low-level dose of photosensitizer. J. Photochem. Photobiol. B 2017, 173, 333–343. [Google Scholar] [CrossRef]
- Kałas, W.; Wysokińska, E.; Przybyło, M.; Langner, M.; Ulatowska-Jarża, A.; Biały, D.; Wawrzyńska, M.; Zioło, E.; Gil, W.; Trzeciak, A.M.; et al. Photoactive liposomal formulation of PVP-conjugated chlorin e6 for photodynamic reduction of atherosclerotic plaque. Int. J. Mol. Sci. 2019, 20, 3852. [Google Scholar] [CrossRef]
- Przybyło, M.; Głogocka, D.; Dobrucki, J.; Frączkowska, K.; Podbielska, H.; Kopaczyńska, M.; Borowik, T.P.; Langner, M. The cellular internalization of liposome encapsulated protoporphyrin IX by HeLa cells. Eur. J. Pharm. Sci. 2016, 85, 39–46. [Google Scholar] [CrossRef]
- Hüsing, N.; Schubert, U. Aerogels—airy materials: Chemistry, structure and properties. Angew. Chem. Int. Ed. 1998, 37, 22–45. [Google Scholar] [CrossRef]
- Producers Internet Site. Available online: http://www.belmedpreparaty.by/product/anot.php?anat_id=234 (accessed on 6 September 2017).
- Optical Fiber System Producers Internet Site. Available online: http://www.cnilaser.com/index.htm (accessed on 6 September 2017).
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013, 73, 50166. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Kelbauskas, L.; Dietel, W. Internalization of aggregated photosensitizers by tumor cells: Subcellular time-resolved fluorescence spectroscopy on derivatives of pyropheophorbide-a ethers and chlorin e6 under femtosecond one- and two-photon excitation. Photochem. Photobiol. 2002, 76, 686–694. [Google Scholar] [CrossRef]
- Leszczak, V.; Popat, K.C. Improved in vitro blood compatibility of polycaprolactonenenowire surfaces. ACS Appl. Mater. Interfaces 2014, 6, 15913–15924. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Li, X.; Xu, Y.; Wu, D.; Sun, Y.; Xu, J.; Deng, F. Bilirubin adsorption on amine/methyl bifunctionalized SBA-15 with platelet morphology. Colloids Surf. B Biointerfaces 2011, 84, 571–578. [Google Scholar] [CrossRef]
- Jovanovic, A.V.; Flint, J.A.; Varshney, M.; Morey, T.E.; Dennis, D.M.; Duran, R.S. Surface modification of silica core-shell nanocapsules: Biomedical implications. Biomacromolecules 2006, 7, 945–949. [Google Scholar] [CrossRef]
- Diociaiuti, M.; Bordi, F.; Gataleta, L.; Baldo, G.; Crateri, P.; Paoletti, L. Morphological and functional alterations of human erythrocytes induced by SiO2 particles: An electron microscopy and dielectric spectroscopy study. Environ. Res. 1999, 80, 197–207. [Google Scholar] [CrossRef]
- Ulatowska-Jarża, A.; Hołowacz, I.; Wysocka, K.; Podbielska, H. Silica-based versus silica-titania sol-gel materials—comparison of the physical properties: Surface tension, gelation time, refractive index and optical transmittance. Opt. Appl. 2009, 39, 211–220. [Google Scholar]
- Lechna, M.; Hołowacz, I.; Ulatowska, A.; Podbielska, H. Optical properties of sol-gel coatings for fiberoptic sensors. Surf. Coat. Technol. 2002, 151–152, 299–302. [Google Scholar] [CrossRef]
- Podbielska, H.; Ulatowska-Jarża, A.; Mueller, G.; Hołowacz, I.; Bauer, J.; Bindig, U. Silica sol-gel matrix doped with Photolon molecules for sensing and medical purposes. Biomol. Eng. 2007, 24, 425–433. [Google Scholar] [CrossRef]
- Podbielska, H.; Bindig, U.; Ulatowska-Jarża, A.; Hołowacz, I.; Müller, G.; Scheller, E.E. Optical properties of sol-gel fiber optic applicators for laser interstitial therapy. Laser Phys. 2006, 16, 816–826. [Google Scholar] [CrossRef]
- Ulatowska-Jarża, A.; Zychowicz, J.; Hołowacz, I.; Bauer, J.; Razik, J.; Wieliczko, A.; Podbielska, H.; Mueller, G.; Stręk, W.; Bindig, U. Antimicrobial PDT with chlorophyll-derived photosensitizer and semiconductor laser. Med. Laser Appl. 2006, 2, 177–183. [Google Scholar] [CrossRef]
- Kopaczyńska, M.; Sobieszczańska, B.; Ulatowska-Jarża, A.; Hołowacz, I.; Buzalewicz, I.; Wasyluk, Ł.; Tofail, S.A.M.; Biały, D.; Wawrzyńska, M.; Podbielska, H. Photoactivated titania-based nanomaterials for potential application as cardiovascular stent coatings. Biocybern. Biomed. Eng. 2014, 34, 189–197. [Google Scholar] [CrossRef]
- Luo, S.; Hao, J.; Gao, Y.; Liu, D.; Cai, Q.; Yang, X. Pore size effect on adsorption and release of metoprolol tartrate in mesoporous silica: Experimental and molecular simulation studies. Mat. Sci. Eng. C 2019, 100, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, S.; Kong, F.; Jiang, T.; Tang, C.; Yin, C. Effects of pore size on in vitro and in vivo anticancer efficacies of mesoporous silica nanoparticles. RSC Adv. 2018, 8, 24633–24640. [Google Scholar] [CrossRef]
- Fateye, B.; Wan, A.; Yang, X.; Myers, K.; Chen, B. Comparison between endothelial and tumor cells in the response to verteporfin-photodynamic therapy and a PI3K pathway inhibitor. Photodiagnosis Photodyn. Ther. 2015, 12, 19–26. [Google Scholar] [CrossRef]
| # C2H5OH (mol) | # TEOS (mol) | # C2H5OH/# TEOS | # Ph (mol) | # PP IX (mol) | |
|---|---|---|---|---|---|
| M1 | 0.288 × 10−3 | 14.38 × 10−6 | 20 | 16.7 × 10−12 | 17.4 × 10−12 |
| M2 | 0.309 × 10−3 | 9.46 × 10−6 | 32 | ||
| M3 | 0.313 × 10−3 | 7.82 × 10−6 | 40 |
| M1 | M2 | M3 | |
|---|---|---|---|
| R2 statistic | 0.9966 | 0.9241 | 0.9366 |
| Adjusted-R2 | 0.9947 | 0.9034 | 0.9121 |
| RMSE | 4.181 | 6.509 | 3.616 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wawrzyńska, M.; Duda, M.; Hołowacz, I.; Kaczorowska, A.; Ulatowska-Jarża, A.; Buzalewicz, I.; Kałas, W.; Wysokińska, E.; Biały, D.; Podbielska, H.; et al. Photoactive Pore Matrix for In Situ Delivery of a Photosensitizer in Vascular Smooth Muscle Cells Selective PDT. Materials 2019, 12, 4110. https://doi.org/10.3390/ma12244110
Wawrzyńska M, Duda M, Hołowacz I, Kaczorowska A, Ulatowska-Jarża A, Buzalewicz I, Kałas W, Wysokińska E, Biały D, Podbielska H, et al. Photoactive Pore Matrix for In Situ Delivery of a Photosensitizer in Vascular Smooth Muscle Cells Selective PDT. Materials. 2019; 12(24):4110. https://doi.org/10.3390/ma12244110
Chicago/Turabian StyleWawrzyńska, Magdalena, Maciej Duda, Iwona Hołowacz, Aleksandra Kaczorowska, Agnieszka Ulatowska-Jarża, Igor Buzalewicz, Wojciech Kałas, Edyta Wysokińska, Dariusz Biały, Halina Podbielska, and et al. 2019. "Photoactive Pore Matrix for In Situ Delivery of a Photosensitizer in Vascular Smooth Muscle Cells Selective PDT" Materials 12, no. 24: 4110. https://doi.org/10.3390/ma12244110
APA StyleWawrzyńska, M., Duda, M., Hołowacz, I., Kaczorowska, A., Ulatowska-Jarża, A., Buzalewicz, I., Kałas, W., Wysokińska, E., Biały, D., Podbielska, H., & Kopaczyńska, M. (2019). Photoactive Pore Matrix for In Situ Delivery of a Photosensitizer in Vascular Smooth Muscle Cells Selective PDT. Materials, 12(24), 4110. https://doi.org/10.3390/ma12244110








