General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Electrospun Matrices
2.2. Analysis of Matrix Surface Structure and Properties
2.3. Treatment of Matrices with Glutaraldehyde
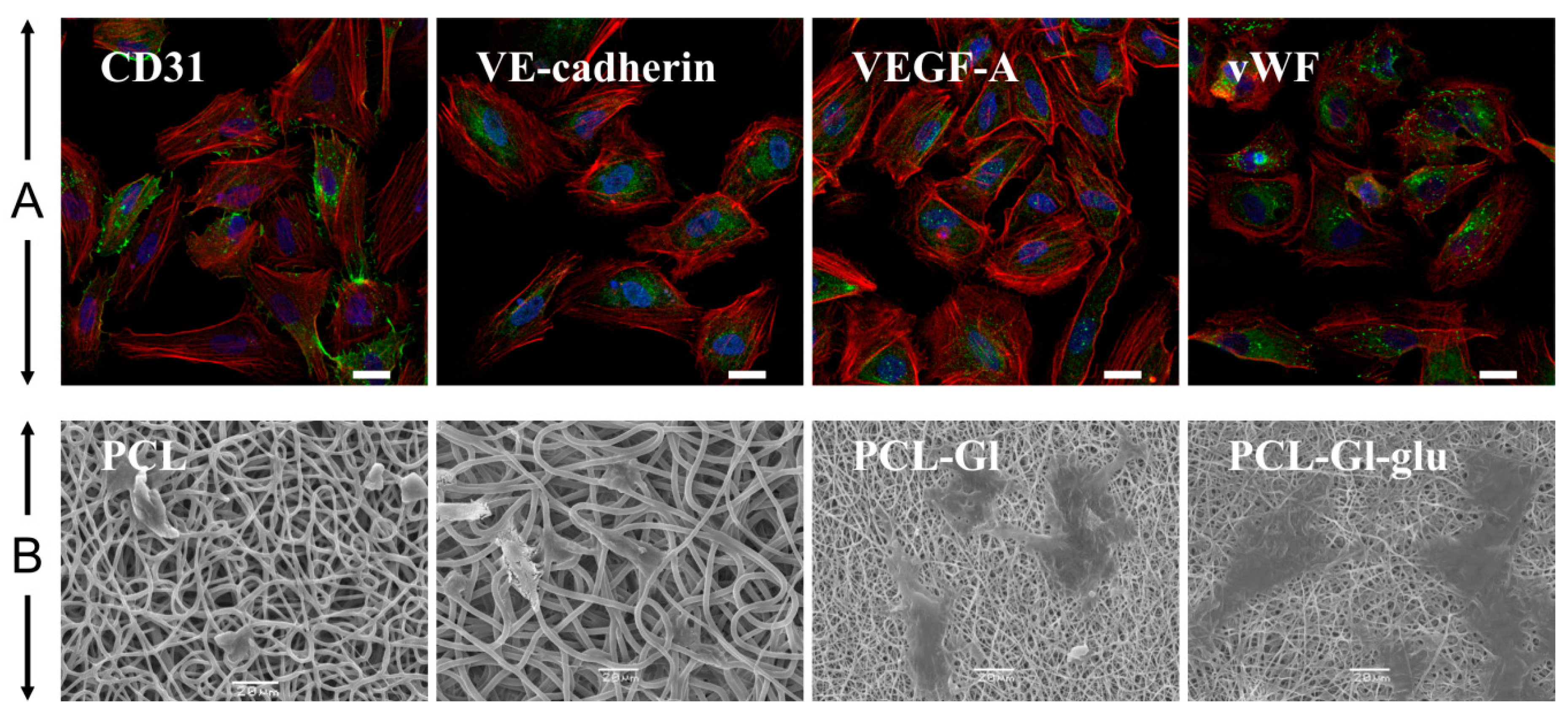
2.4. Isolation, Cultivation and Characterization of Human Umbilical Vein Endothelial Cells
2.5. Assessment of Adhesion and Viability of Endothelial Cells on the Matrix Surface
2.6. Cultivation of HUVEC for Gene Expression Profiling and RNA Isolation
2.7. RNA Sequencing and Data Analysis
2.8. Statistical Data Analysis
3. Results and Discussion
3.1. Characterization of 3D Matrices
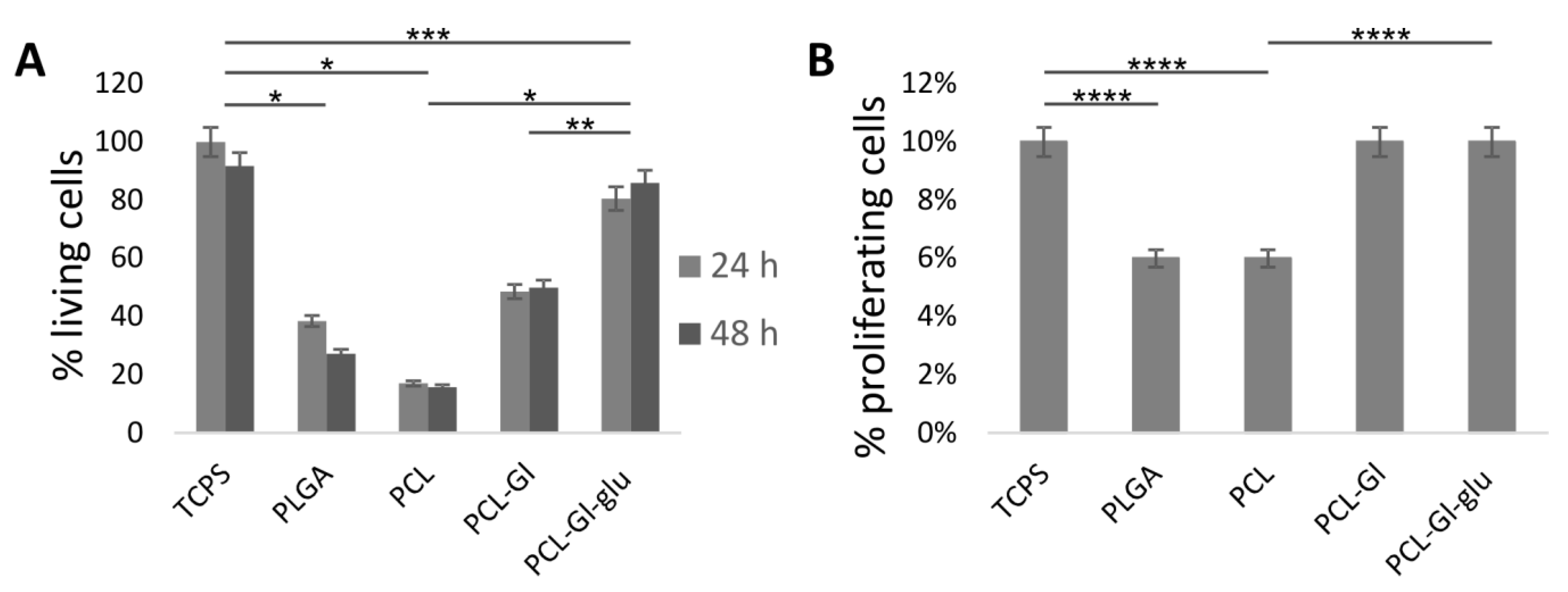
3.2. Cultivation of HUVEC on 3D Matrices
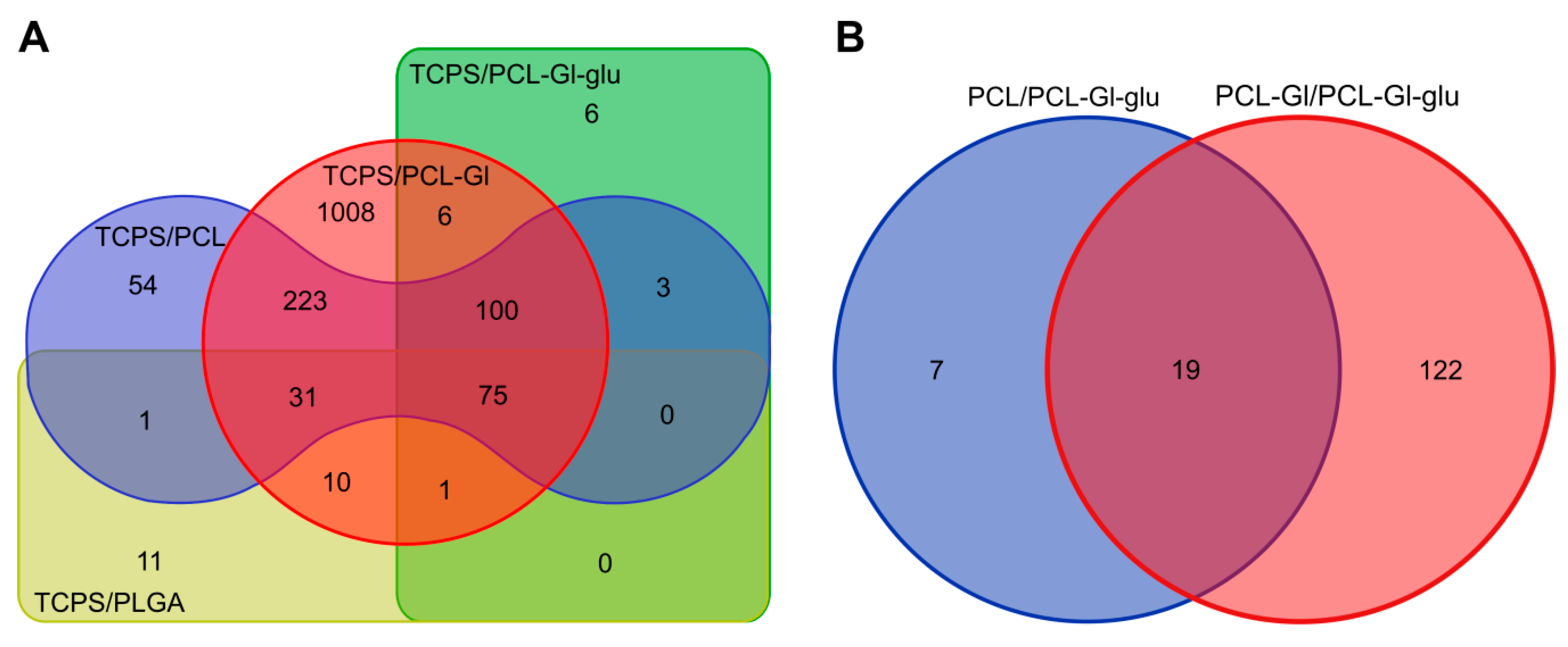
3.3. Transcriptome Profiling
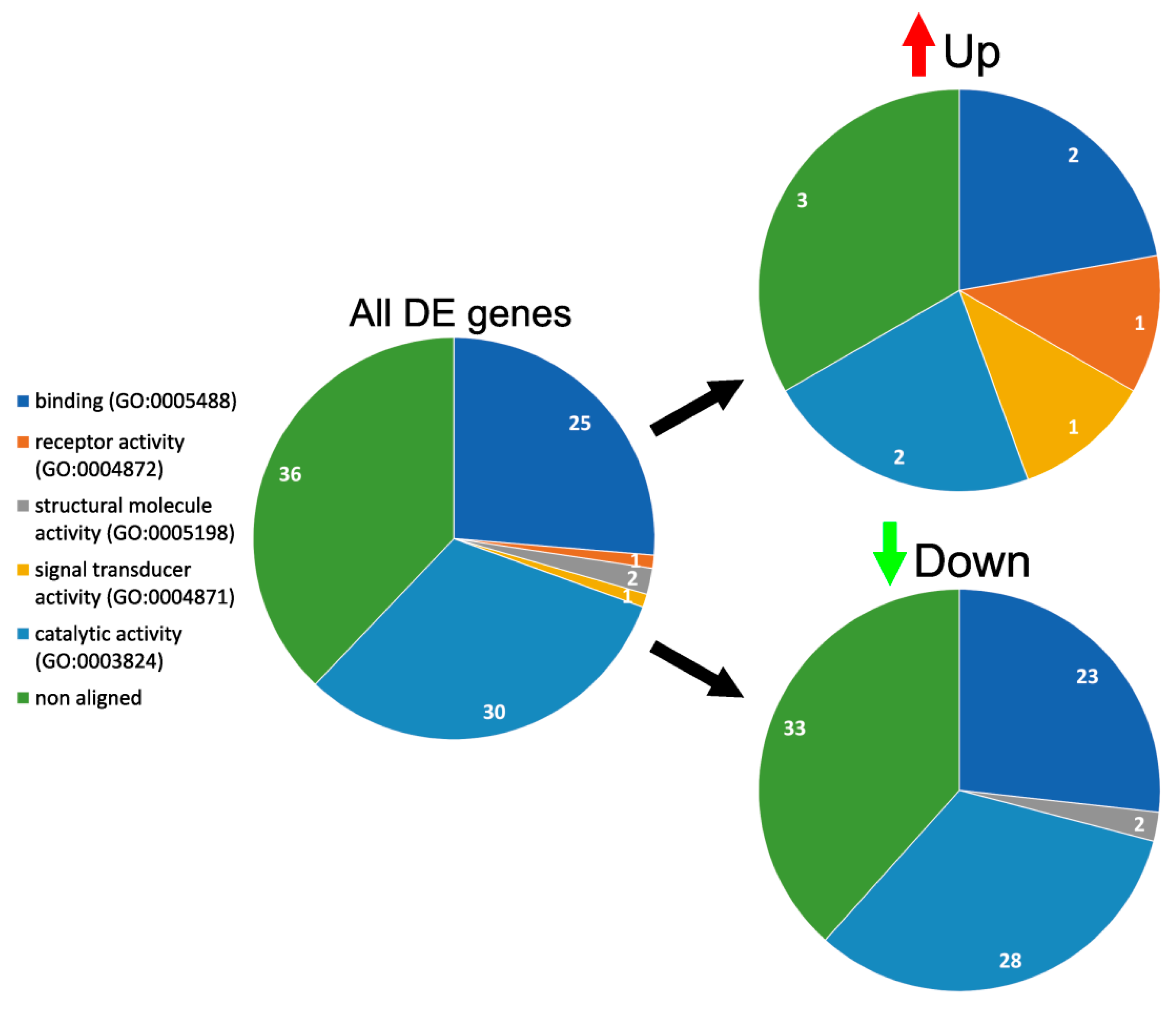
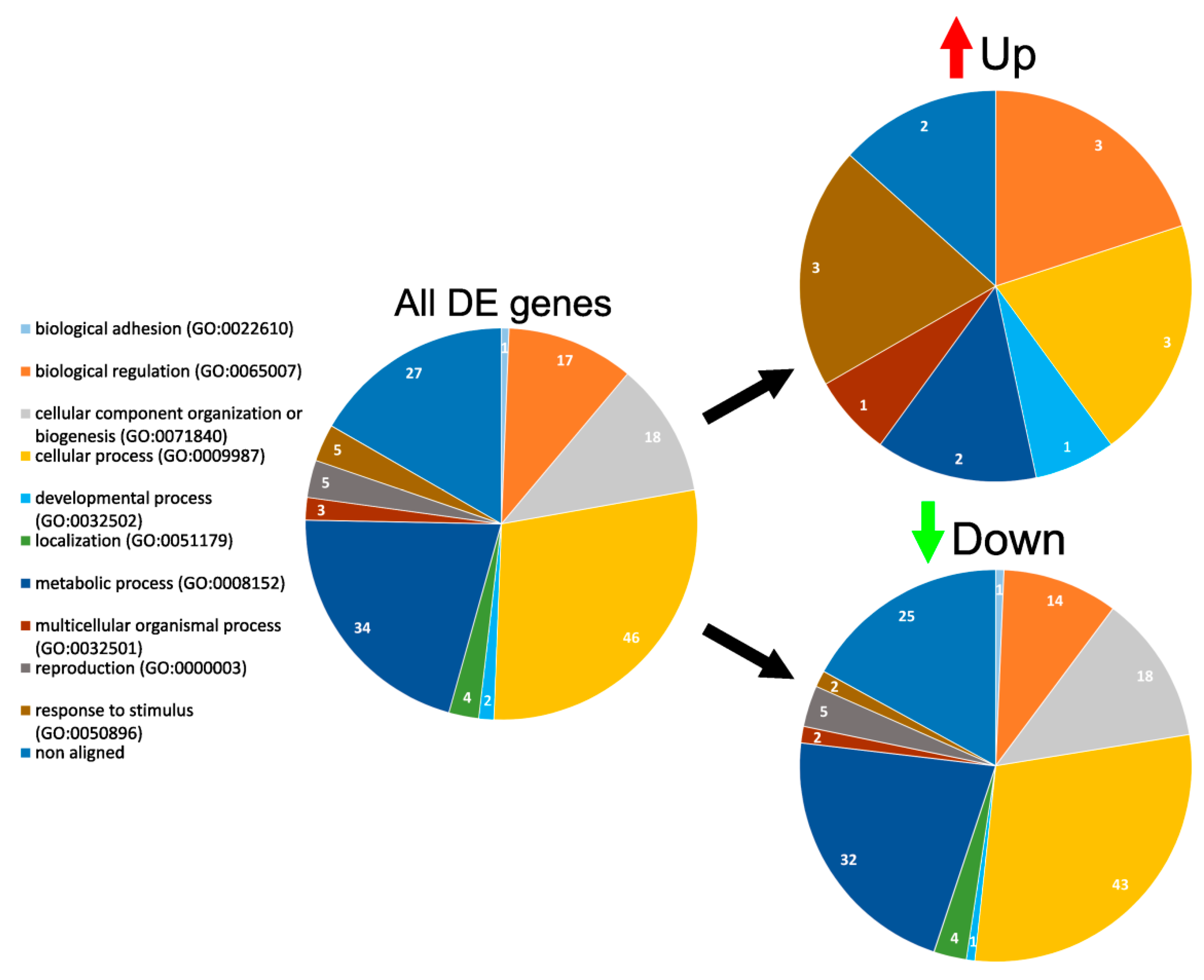
3.4. GO Enrichment and Functional Analysis of DE Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, T.; Liu, S.; Zhang, K.; Chen, J.; Huang, N. Endothelialization of implanted cardiovascular biomaterial surfaces: The development from in vitro to in vivo. J. Biomed. Mater. Res. Part A 2014, 102, 3754–3772. [Google Scholar] [CrossRef] [PubMed]
- Seifalian, A.M.; Tiwari, A.; Hamilton, G.; Salacinski, J.H. Improving the Clinical Patency of Prosthetic Vascular and Coronary Bypass Grafts: The Role of Seeding and Tissue Engineering. Artif. Organs 2002, 26, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Aubin, H.; Mas-Moruno, C.; Iijima, M.; Schutterle, N.; Steinbrink, M.; Assmann, A.; Gil, F.J.; Lichtenberg, A.; Pegueroles, M.; Akhyari, P. Customized Interface Biofunctionalization of Decellularized Extracellular Matrix: Toward Enhanced Endothelialization. Tissue Eng. Part C Methods 2016, 22, 496–508. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Henry, J.J.D. Nonthrombogenic Approaches to Cardiovascular Bioengineering. Annu. Rev. Biomed. Eng. 2011, 13, 451–475. [Google Scholar] [CrossRef] [PubMed]
- Natorska, J.; Undas, A. Blood coagulation and fibrinolysis in aortic valve stenosis: Links with inflammation and calcification. Thromb. Haemost. 2015, 114, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Effects of shear stress on endothelial cells: Go with the flow. Acta Physiol. 2017, 219, 382–408. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Osborne, L.D.; Guilluy, C.; Chen, Z.; O’Brien, E.T.; Reader, J.S.; Burridge, K.; Superfine, R.; Tzima, E. Haemodynamic and extracellular matrix cues regulate the mechanical phenotype and stiffness of aortic endothelial cells. Nat. Commun. 2014, 5, 3984. [Google Scholar] [CrossRef]
- Xu, C.; Yang, F.; Wang, S.; Ramakrishna, S. In vitro study of human vascular endothelial cell function on materials with various surface roughness. J. Biomed. Mater. Res. 2004, 71, 154–161. [Google Scholar] [CrossRef]
- Montoya, Y.; Valencia, R.A.; Ortiz, I.C.; Hoyos, L.M.; Bustamante, J. In Vitro Study of Proliferation and Cellularisation on Electrospun Membranes for Vascular Prosthesis. In VII Latin American Congress on Biomedical Engineering CLAIB 2016, Bucaramanga, Santander, Colombia, October 26th–28th, 2016; Springer: Singapore, 2017; pp. 589–592. [Google Scholar] [CrossRef]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft—Past, Present, and Future, Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Manea, L.R.; Hristian, L.; Leon, A.L.; Popa, A. Recent Advances of Basic Materials to Obtain Electrospun Polymeric Nanofibers for Medical Applications. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; p. 032006. [Google Scholar] [CrossRef]
- Stepanova, A.O.; Chernonosova, V.S.; Popova, I.V.; Karpenko, A.A.; Pokushalov, E.A.; Karaskov, A.M.; Laktionov, P.P.; Vlassov, V.V. Method for Producing Small-Diameter Low-Porosity Vascular Prostheses (Versions). RU2572333C1, 10 January 2016. [Google Scholar]
- Henry, J.D.; Yu, J.; Wang, A.; Lee, R.; Fang, J.; Li, S. Engineering the mechanical and biological properties of nanofibrous vascular grafts for in situ vascular tissue engineering. Biofabrication 2017, 9, 035007. [Google Scholar] [CrossRef]
- Catto, V.; Fare, S.; Cattaneo, I.; Figliuzzi, M.; Alessandrino, A.; Freddi, G.; Remuzzi, A.; Tanzi, M.C. Small diameter electrospun silk fibroin vascular grafts: Mechanical properties, in vitro biodegradability, and in vivo biocompatibility. Mater. Sci. Eng. C 2015, 54, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Nicast. Nano-fibrous Medical Devices. Available online: http://nicast.com/ (accessed on 5 December 2019).
- D’Amore, A.; Luketich, S.K.; Raffa, G.M.; Olia, S.; Menallo, G.; Mazzola, A.; D’Accardi, F.; Grunberg, T.; Gu, X.; Pilato, M.; et al. Heart valve scaffold fabrication: Bioinspired control of macro-scale morphology, mechanics and micro-structure. Biomaterials 2018, 150, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Lei, D.; Li, S.; Huang, P.; Qi, Q.; Sun, Y.; Zhang, Y.; Wang, Z.; You, Z.; Ye, X.; et al. Hybrid small-Diameter vascular grafts: Anti-Expansion effect of electrospun poly ε-Caprolactone on heparin-Coated decellularized matrices. Biomaterials 2016, 76, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, Y.; Wang, J.; Yang, X.; Wu, Y.; Wang, K.; Gao, X.; Li, D.; Li, Y.; Zheng, X.L.; et al. The effect of thick fibers and large pores of electrospun poly(ε-Caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials 2014, 35, 5700–5710. [Google Scholar] [CrossRef] [PubMed]
- Fu, W.; Liu, Z.; Feng, B.; Hu, R.; He, X.; Wang, H.; Yin, M.; Huang, H.; Zhang, H.; Wang, W. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int. J. Nanomed. 2014, 9, 2335. [Google Scholar] [CrossRef]
- Chernonosova, V.S.; Kvon, R.I.; Stepanova, A.O.; Larichev, Y.V.; Karpenko, A.A.; Chelobanov, B.P.; Kiseleva, E.V.; Laktionov, P.P. Human serum albumin in electrospun PCL fibers: Structure, release, and exposure on fiber surface. Polym. Adv. Technol. 2017, 28, 819–827. [Google Scholar] [CrossRef]
- Olmer, R.; Engels, L.; Usman, A.; Menke, S.; Malik, M.N.H.; Pessler, F.; Göhring, G.; Bornhorst, D.; Bolten, S.; Abdelilah-Seyfried, S.; et al. Differentiation of Human Pluripotent Stem Cells into Functional Endothelial Cells in Scalable Suspension Culture. Stem Cell Rep. 2018, 10, 1657–1672. [Google Scholar] [CrossRef]
- Hauser, S.; Jung, F.; Pietzsch, J. Human Endothelial Cell Models in Biomaterial Research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef]
- Munoz-Pinto, D.J.; Erndt-Marino, J.D.; Becerra-Bayona, S.M.; Guiza-Arguello, V.R.; Samavedi, S.; Malmut, S.; Reichert, W.M.; Russell, B.; Höök, M.; Hahn, M.S. Evaluation of late outgrowth endothelial progenitor cell and umbilical vein endothelial cell responses to thromboresistant collagen-mimetic hydrogels. J. Biomed. Mater. Res. Part A 2017, 105, 1712–1724. [Google Scholar] [CrossRef]
- Kang, T.Y.; Lee, J.H.; Kim, B.J.; Kang, J.A.; Hong, J.M.; Kim, B.S.; Cha, H.J.; Rhie, J.W.; Cho, D.W. In vivo endothelization of tubular vascular grafts through in situ recruitment of endothelial and endothelial progenitor cells by RGD-fused mussel adhesive proteins. Biofabrication 2015, 7, 015007. [Google Scholar] [CrossRef]
- Ritarwan, K.; Lelo, A.; Pane, Y.S.; Nerdy, N. Increasing Atherosclerosis in Streptozotocin-Induced Diabetes into Four Groups of Mice, Open Access Maced. J. Med. Sci. 2018, 6, 287–292. [Google Scholar] [CrossRef]
- Bouis, D.; Hospers, G.A.P.; Meijer, C.; Molema, G.; Mulder, N.H. Endothelium in vitro: A review of human vascular endothelial cell lines for blood vessel-Related research. Angiogenesis 2001, 4, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Chennazhy, K.P.; Krishnan, L.K. Effect of passage number and matrix characteristics on differentiation of endothelial cells cultured for tissue engineering. Biomaterials 2005, 26, 5658–5667. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Florczyk, S.J.; Leung, M.C.; Wang, K.; Wu, J.D.; Silber, J.R.; Ellenbogen, R.G.; Lee, J.S.H.; Zhang, M. Proliferation and enrichment of CD133+ glioblastoma cancer stem cells on 3D chitosan-Alginate scaffolds. Biomaterials 2014, 35, 9137–9143. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M.; Hashemian, S.J.; Milan, P.B.; Hamzehlou, S.; Soleimani, M.; Joghataei, M.T.; Gholipourmalekabadi, M.; Korourian, A.; Mousavizadeh, K.; et al. Osteogenic potential of stem cells-Seeded bioactive nanocomposite scaffolds: A comparative study between human mesenchymal stem cells derived from bone, umbilical cord Wharton’s jelly, and adipose tissue. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 61–72. [Google Scholar] [CrossRef]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.E.; Wallace, G.G.; Chung, J.; Quigley, A.; Choong, P.F.M.; Myers, D.E. Chondrogenesis of Infrapatellar Fat Pad Derived Adipose Stem Cells in 3D Printed Chitosan Scaffold. PLoS ONE 2014, 9, e99410. [Google Scholar] [CrossRef]
- Pekar-Zlotin, M.; Hirsch, F.R.; Soussan-Gutman, L.; Ilouze, M.; Dvir, A.; Boyle, T.; Wynes, M.; Miller, V.A.; Lipson, D.; Palmer, G.A.; et al. Fluorescence In Situ Hybridization, Immunohistochemistry, and Next-Generation Sequencing for Detection of EML4-ALK Rearrangement in Lung Cancer. Oncologist 2015, 20, 316–322. [Google Scholar] [CrossRef]
- Khodakov, D.; Wang, C.; Zhang, D.Y. Diagnostics based on nucleic acid sequence variant profiling: PCR, hybridization, and NGS approaches. Adv. Drug Deliv. Rev. 2016, 105, 3–19. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, Y.T.; Li, F.; Wu, K.; Hou, S.; Yu, J.; Shen, Q.; Wu, D.; Song, M.; OuYang, W.H.; et al. High-Purity Prostate Circulating Tumor Cell Isolation by a Polymer Nanofiber-Embedded Microchip for Whole Exome Sequencing. Adv. Mater. 2013, 25, 2897–2902. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, L.; Guo, X.; Wang, H.; Chen, W.; Wu, G.; Gu, B.; Miao, W.; Kong, J.; Jin, X.; et al. Comparative Analysis of microRNA Expression Profiles of Exosomes Derived from Normal and Hypoxic Preconditioning Human Neural Stem Cells by Next Generation Sequencing. J. Biomed. Nanotechnol. 2018, 14, 1075–1089. [Google Scholar] [CrossRef]
- Eissa, A.M.; Barros, F.S.V.; Vrljicak, P.; Brosens, J.J.; Cameron, N.R. Enhanced Differentiation Potential of Primary Human Endometrial Cells Cultured on 3D Scaffolds. Biomacromolecules 2018, 19, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
- Hrdlickova, R.; Toloue, M.; Tian, B. RNA-Seq methods for transcriptome analysis. Wiley Interdiscip. Rev. RNA 2017, 8, e1364. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, E.A.; Nachman, R.L.; Becker, C.G.; Miinick, C.R. Culture of Human Endothelial Cells Derived from Umbilical Veins. J. Clin. Investig. 1973, 52, 2745–2756. [Google Scholar] [CrossRef] [PubMed]
- Mal’shakova, V.S.; Laktionov, P.P.; Pyshnyi, D.V.; Vlasov, V.V. Intracellular localization of natural and modified oligonucleotides in primary human endothelial cells. Bull. Exp. Biol. Med. 2007, 143, 204–206. [Google Scholar] [CrossRef]
- Hannon, G.J. FASTX-Toolkit. 2010. Available online: http://hannonlab.cshl.edu/fastx_toolkit (accessed on 5 December 2019).
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. Featurecounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Stepanova, A.O.; Chernonosova, V.S.; Kvon, R.I.; Laktionov, P.P. Gelatin and Heparin Exposure on the Surface of Electrospun PCL Fibers. In Proceedings of the TERMIS-EU 2016 Conference, Uppsala, Sweden, 15 February 2016; p. 56. [Google Scholar]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Ko, Y.G.; Park, J.H.; Lee, J.B.; Oh, H.H.; Park, W.H.; Cho, D.; Kwon, O.H. Growth behavior of endothelial cells according to electrospun poly(D,L-Lactic-Co-Glycolic acid) fiber diameter as a tissue engineering scaffold. Tissue Eng. Regen. Med. 2016, 13, 343–351. [Google Scholar] [CrossRef]
- Whited, B.M.; Rylander, M.N. The influence of electrospun scaffold topography on endothelial cell morphology, alignment, and adhesion in response to fluid flow. Biotechnol. Bioeng. 2014, 111, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Ramos, T.; Wieringa, P.; Van Blitterswijk, C.; De Boer, J.; Moroni, L. Geometric constraints of endothelial cell migration on electrospun fibres. Sci. Rep. 2018, 8, 6386. [Google Scholar] [CrossRef] [PubMed]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(D,L-Lactic-Co-Glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef] [PubMed]
- Lutter, C.; Nothhaft, M.; Rzany, A.; Garlichs, C.D.; Cicha, I. Effect of specific surface microstructures on substrate endothelialisation and thrombogenicity: Importance for stent design. Clin. Hemorheol. Microcirc. 2015, 59, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi-Mobarakeh, L.; Prabhakaran, M.P.; Morshed, M.; Nasr-Esfahani, M.H.; Ramakrishna, S. Electrospun poly(ɛ-Caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 2008, 29, 4532–4539. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. 2005, 72, 156–165. [Google Scholar] [CrossRef]
- Jiang, Y.C.; Jiang, L.; Huang, A.; Wang, X.F.; Li, Q.; Turng, L.S. Electrospun polycaprolactone/gelatin composites with enhanced cell–Matrix interactions as blood vessel endothelial layer scaffolds. Mater. Sci. Eng. C. 2017, 71, 901–908. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Kyriakides, T.R. Cell Interactions with Polymers. In Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2014; pp. 385–406. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Alves, N.M.; Mano, J.F. Cell interactions with superhydrophilic and superhydrophobic surfaces. J. Adhes. Sci. Technol. 2014, 28, 843–863. [Google Scholar] [CrossRef]
- Mohan, T.; Niegelhell, K.; Nagaraj, C.; Reishofer, D.; Spirk, S.; Olschewski, A.; Kleinschek, K.S.; Kargl, R. Interaction of Tissue Engineering Substrates with Serum Proteins and Its Influence on Human Primary Endothelial Cells. Biomacromolecules 2017, 18, 413–421. [Google Scholar] [CrossRef]
- Stepanova, A.O.; Korobeinikov, M.V.; Yunoshev, A.S.; Laktionov, P.P. Effect of Electron-Beam Irradiation on Electrospinning Produced Scaffolds. In 2015 International Conference on Biomedical Engineering and Computational Technologies; IEEE: Piscataway, NJ, USA, 2015; pp. 39–42. [Google Scholar] [CrossRef]
- Hiep, N.T.; Lee, B.T. Electro-Spinning of PLGA/PCL blends for tissue engineering and their biocompatibility. J. Mater. Sci. Mater. Med. 2010, 21, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Baker, S.C.; Rohman, G.; Southgate, J.; Cameron, N.R. The relationship between the mechanical properties and cell behaviour on PLGA and PCL scaffolds for bladder tissue engineering. Biomaterials 2009, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Heng, B.C.; Bezerra, P.P.; Preiser, P.R.; Law, S.K.A.; Xia, Y.; Boey, F.; Venkatraman, S.S. Effect of cell-Seeding density on the proliferation and gene expression profile of human umbilical vein endothelial cells within ex vivo culture. Cytotherapy 2011, 13, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, Z.; Zhong, J.; Luo, D.; Zhou, J.; Yang, J.; Xiao, L.; Shu, D.; Tan, H. Comparative transcriptome analysis between an evolved abscisic acid-Overproducing mutant Botrytis cinerea TBC-A and its ancestral strain Botrytis cinerea TBC-6. Sci. Rep. 2016, 6, 37487. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, V.; Head, S.R.; Ordoukhanian, P.; Mercola, M.; Subramaniam, S. Technical Variations in Low-Input RNA-Seq Methodologies. Sci. Rep. 2015, 4, 3678. [Google Scholar] [CrossRef]
- Lother, A.; Bergemann, S.; Deng, L.; Moser, M.; Bode, C.; Hein, L. Cardiac Endothelial Cell Transcriptome. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 566–574. [Google Scholar] [CrossRef]
- Seo, H.J.; Yu, S.M.; Lee, S.H.; Choi, J.B.; Park, J.C.; Kim, J.K. Effect of PLGA Nano-Fiber/Film Composite on HUVECs for Vascular Graft Scaffold. In 13th International Conference on Biomedical Engineering; Springer: Berlin, Heidelberg, Germany, 2009; pp. 2147–2150. [Google Scholar] [CrossRef]
- Chi, J.T.; Chang, H.Y.; Haraldsen, G.; Jahnsen, F.L.; Troyanskaya, O.G.; Chang, D.S.; Wang, Z.; Rockson, S.G.; van de Rijn, M.; Botstein, D.; et al. Endothelial cell diversity revealed by global expression profiling. Proc. Natl. Acad. Sci. USA 2003, 100, 10623–10628. [Google Scholar] [CrossRef]
- Gozzelino, R.; Jeney, V.; Soares, M.P. Mechanisms of Cell Protection by Heme Oxygenase-1. Ann. Rev. Pharmacol. Toxicol. 2010, 50, 323–354. [Google Scholar] [CrossRef]
- He, C.; Zhao, C.; Kumar, A.; Lee, C.; Chen, M.; Huang, L.; Wang, J.; Ren, X.; Jiang, Y.; Chen, W.; et al. Vasoprotective effect of PDGF-CC mediated by HMOX1 rescues retinal degeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 14806–14811. [Google Scholar] [CrossRef]
- Kinderlerer, A.R.; Gregoire, I.P.; Hamdulay, S.S.; Ali, F.; Steinberg, R.; Silva, G.; Ali, N.; Wang, B.; Haskard, D.O.; Soares, M.P.; et al. Heme oxygenase-1 expression enhances vascular endothelial resistance to complement-Mediated injury through induction of decay-Accelerating factor: A role for increased bilirubin and ferritin. Blood 2008, 113, 1598–1607. [Google Scholar] [CrossRef]
- Brouard, S.; Otterbein, L.E.; Anrather, J.; Tobiasch, E.; Bach, F.H.; Choi, A.M.K.; Soares, M.P. Carbon Monoxide Generated by Heme Oxygenase 1 Suppresses Endothelial Cell Apoptosis. J. Exp. Med. 2000, 192, 1015–1026. [Google Scholar] [CrossRef]
- Seldon, M.P.; Silva, G.; Pejanovic, N.; Larsen, R.; Gregoire, I.P.; Filipe, J.; Anrather, J.; Soares, M.P. Heme Oxygenase-1 Inhibits the Expression of Adhesion Molecules Associated with Endothelial Cell Activation via Inhibition of NF-B RelA Phosphorylation at Serine 276. J. Immunol. 2007, 179, 7840–7851. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Wagener, F.A.D.T.G.; Immenschuh, S. The macrophage heme-Heme oxygenase-1 system and its role in inflammation. Biochem. Pharmacol. 2018, 153, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Au, C.G.; Cooper, S.T.; Lo, H.P.; Compton, A.G.; Yang, N.; Wintour, E.M.; North, K.N.; Winlaw, D.S. Expression of aquaporin 1 in human cardiac and skeletal muscle. J. Mol. Cell. Cardiol. 2004, 36, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y. Different expressions of AQP1, AQP4, eNOS, and VEGF proteins in ischemic versus non-Ischemic cerebropathy in rats: Potential roles of AQP1 and eNOS in hydrocephalic and vasogenic edema formation. Anat. Cell Biol. 2011, 44, 295. [Google Scholar] [CrossRef]
- Bondy, C.; Chin, E.; Smith, B.L.; Preston, G.M.; Agre, P. Developmental gene expression and tissue distribution of the CHIP28 water-Channel protein. Proc. Natl. Acad. Sci. USA 1993, 90, 4500–4504. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Bliksøen, M.; Hillestad, V.; Amin, M.; Czibik, G.; Valen, G.; Vaage, J.; Amiry-Moghaddam, M.; Stensløkken, K.O. Aquaporin-1 in cardiac endothelial cells is downregulated in ischemia, hypoxia and cardioplegia. J. Mol. Cell. Cardiol. 2013, 56, 22–33. [Google Scholar] [CrossRef]
- Kaneko, K.; Yagui, K.; Tanaka, A.; Yoshihara, K.; Ishikawa, K.; Takahashi, K.; Bujo, H.; Sakurai, K.; Saito, Y. Aquaporin 1 is required for hypoxia-Inducible angiogenesis in human retinal vascular endothelial cells. Microvasc. Res. 2008, 75, 297–301. [Google Scholar] [CrossRef]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflug. Arch. Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Sastry, K.S.; Chouchane, A.I.; Wang, E.; Kulik, G.; Marincola, F.M.; Chouchane, L. Cytoprotective effect of neuropeptides on cancer stem cells: Vasoactive intestinal peptide-Induced antiapoptotic signaling. Cell Death Dis. 2017, 8, e2844. [Google Scholar] [CrossRef]
- RefSeq Gene: TSPAN7, NC_000023.11. 2019. Available online: https://www.ncbi.nlm.nih.gov/gene/7102 (accessed on 20 February 2019).
- Vitturi, D.A.; Chen, C.S.; Woodcock, S.R.; Salvatore, S.R.; Bonacci, G.; Koenitzer, J.R.; Stewart, N.A.; Wakabayashi, N.; Kensler, T.W.; Freeman, B.A.; et al. Modulation of Nitro-fatty Acid Signaling PROSTAGLANDIN REDUCTASE-1 IS A NITROALKENE REDUCTASE. J. Biol. Chem. 2013, 288, 25626–25637. [Google Scholar] [CrossRef]
- Chou, W.L.; Chuang, L.M.; Chou, C.C.; Wang, A.H.J.; Lawson, J.A.; FitzGerald, G.A.; Chang, Z.F. Identification of a Novel Prostaglandin Reductase Reveals the Involvement of Prostaglandin E 2 Catabolism in Regulation of Peroxisome Proliferator-Activated Receptor γ Activation. J. Biol. Chem. 2007, 282, 18162–18172. [Google Scholar] [CrossRef] [PubMed]
- Samaras, S.E.; Chen, B.; Koch, S.R.; Sawyer, D.B.; Lim, C.C.; Davidson, J.M. 26S Proteasome regulation of Ankrd1/CARP in adult rat ventricular myocytes and human microvascular endothelial cells. Biochem. Biophys. Res. Commun. 2012, 425, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Brigstock, D.R. Regulation of angiogenesis and endothelial cell function by connective tissue growth factor (CTGF) and cysteine-Rich 61 (CYR61). Angiogenesis 2002, 5, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, G.S.; Schaeffer, D.F.; Cho, C.K.J.; Musrap, N.; Saraon, P.; Batruch, I.; Grin, A.; Mitrovic, B.; Kirsch, R.; Riddell, R.H.; et al. Collective migration of cancer-Associated fibroblasts is enhanced by overexpression of tight junction-Associated proteins claudin-11 and occluding. Mol. Oncol. 2014, 8, 178–195. [Google Scholar] [CrossRef] [PubMed]
- Findley, M.K.; Koval, M. Regulation and roles for claudin-Family tight junction proteins. Iubmb Life. 2009, 61, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, L.; Liu, A. Dickkopf-1: Current knowledge and related diseases. Life Sci. 2018, 209, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.J. Neuregulin and the ErbB signalling cascade in cardiovascular control. J. Hypertens. 2014, 32, 735–737. [Google Scholar] [CrossRef] [PubMed]
- Britsch, S. The neuregulin-I/ErbB signaling system in development and disease. Adv. Anat. Embryol. Cell Biol. 2007, 190, 1–65. [Google Scholar]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Kobayashi, T.; Vischer, U.M.; Rosnoblet, C.; Lebrand, C.; Lindsay, M.; Parton, R.G.; Kruithof, E.K.O.; Gruenberg, J. The Tetraspanin CD63/lamp3 Cycles between Endocytic and Secretory Compartments in Human Endothelial Cells. Mol. Biol. Cell. 2000, 11, 1829–1843. [Google Scholar] [CrossRef]
- Fisher, A.B.; Chien, S.; Barakat, A.I.; Nerem, R.M. Endothelial cellular response to altered shear stress. Am. J. Physiol. Cell. Mol. Physiol. 2001, 281, L529–L533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.J.; Haga, J.H.; Chien, S. Molecular basis of the effects of shear stress on vascular endothelial cells. J. Biomech. 2005, 38, 1949–1971. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Goldschmidt-Clermont, P.; Wille, J.; Yin, F.C. Specificity of endothelial cell reorientation in response to cyclic mechanical stretching. J. Biomech. 2001, 34, 1563–1572. [Google Scholar] [CrossRef]
- Brown, T.D. Techniques for mechanical stimulation of cells in vitro: A review. J. Biomech. 2000, 33, 3–14. [Google Scholar] [CrossRef]
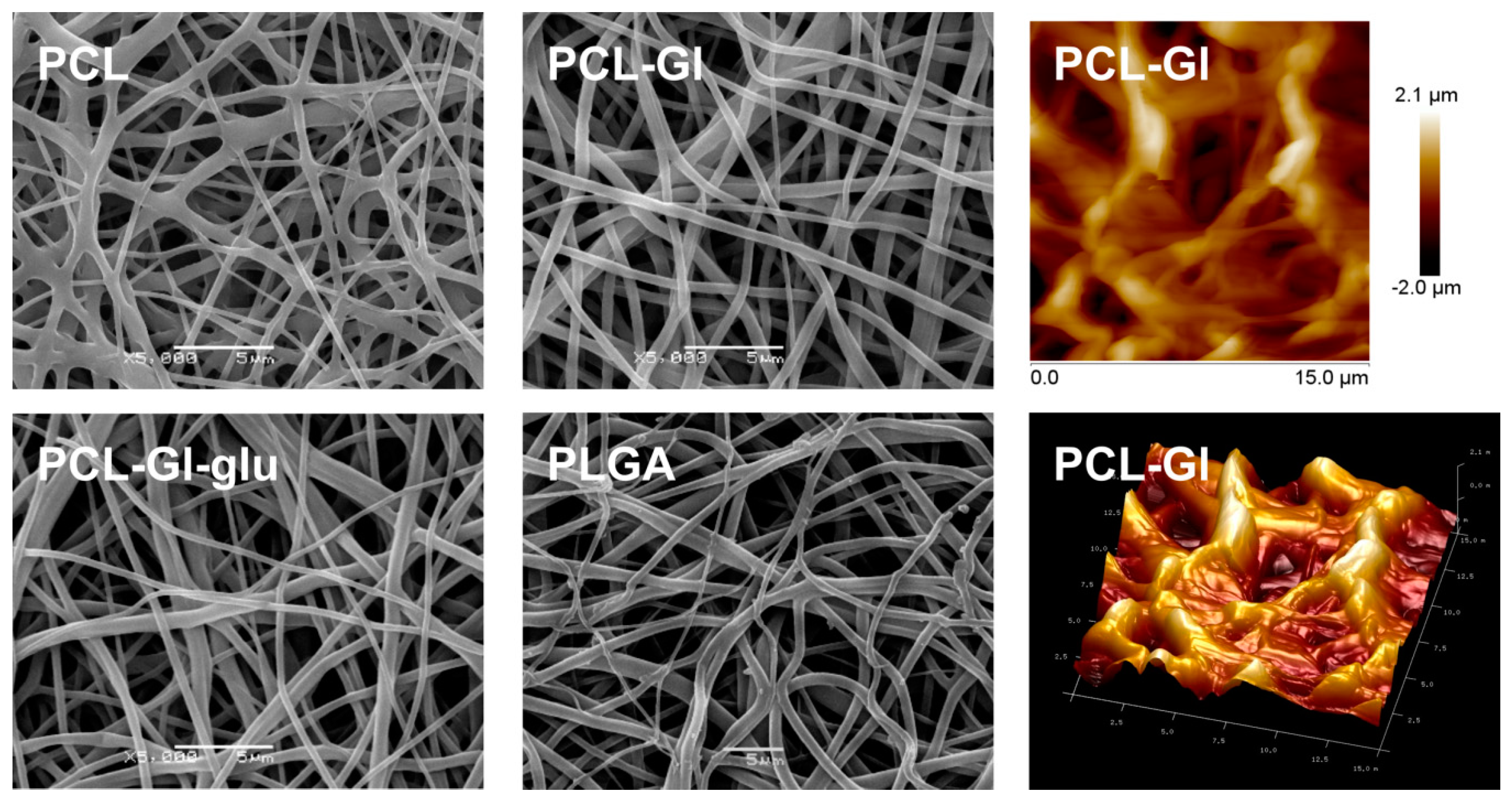
| Blend Composition | Electrospinning Parameters | Characteristics of Matrices | ||||
|---|---|---|---|---|---|---|
| Voltage, kV | Solution Supply Rate, mL/h | Capillary-collector Distance, cm | Fiber Diameter, µm | Pore Size, µm | Contact Angle, ° | |
| PCL | 23.5 | 1.5 | 20 | 1.05 ± 0.12 1 | 6.6 ± 0.22 1 | 127.5 ± 1.29 2 |
| PCL-Gl | 24.5 | 1.3 | 20 | 0.89 ± 0.09 | 7.0 ± 0.57 | 85.66 ± 2.9 |
| PLGA | 20.0 | 1.5 | 20 | 1.12 ± 0.13 | 10.0 ± 0.32 | 113.5 ± 1.3 |
| No. | Control | Comparison | DE Genes | |||||
|---|---|---|---|---|---|---|---|---|
| Total | by RPKM1 | Common | Common, by RPKM 1 | |||||
| 1 | TCPS | PCL | 487 | 152/167/140/24/4 | 100 | 75 | 42/41/15/0/2 | 46/19/8/2/0 |
| 2 | PCL-Gl | 1454 | 268/493/478/176/39 | 45/37/15/1/2 | 47/18/8/2/0 | |||
| 3 | PCL-Gl-glu | 191 | 47/106/31/5/2 | 27/54/17/0/2 | 17/48/7/3/0 | |||
| 4 | PLGA | 129 | 58/35/29/6/1 | 46/19/9/1/0 | ||||
| 5 | PCL-Gl-glu | PCL | 26 | 5/6/10/4/1 | 19 | 4/6/5/2/1 | ||
| 6 | PCL-Gl | 141 | 37/37/44/21/2 | 4/6/5/2/1 | ||||
| 7 | PCL | PLGA | 0 | - | - | - | ||
| 8 | PCL-Gl | 0 | - | - | ||||
| No. | Control | Comparison | DE Genes, Up/Down | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Common | by FC | ||||||||
| 1–2 | 2–3 | 3–4 | 4–5 | >5 | ||||||
| 1 | TCPS | PCL | 125/362 | 100 | 75 | 112/219 [7/35] 1 (5/3) 2 | 12/86 [3/42] (0/29) | 1/43 [0/11)] (1/29) | 0/13 [0/2] (0/8) | 0/1 [0/0] (0/0) |
| 2 | PCL-Gl | 657/797 | 635/602 [7/31] (1/1) | 20/105 [3/32] (4/18) | 2/58 [0/22] (1/30) | 0/23 [0/5] (0/16) | 0/9 [0/0] (0/4) | |||
| 3 | PCL-GL-glu | 22/169 | 21/159 [10/88] (5/62) | 1/10 [0/2] (1/7) | 0/0 [0/0] (0/0) | 0/0 [0/0] (0/0) | 0/0 [0/0] (0/0) | |||
| 4 | PLGA | 30/99 | 18/17 (3/6) | 11/61 (3/49) | 0/20 (0/14) | 1/1 (0/0) | 0/0 (0/0) | |||
| 5 | PCL-Gl-glu | PCl | 13/13 | 19 | 13/13 (7/12) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | |
| 6 | PCL-Gl | 47/96 | 46/93 (7/11) | 1/1 (1/0) | 0/0 (0/0) | 0/0 (0/0) | 0/0 (0/0) | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, A.O.; Laktionov, P.P.; Cherepanova, A.V.; Chernonosova, V.S.; Shevelev, G.Y.; Zaporozhchenko, I.A.; Karaskov, A.M.; Laktionov, P.P. General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials. Materials 2019, 12, 4082. https://doi.org/10.3390/ma12244082
Stepanova AO, Laktionov PP, Cherepanova AV, Chernonosova VS, Shevelev GY, Zaporozhchenko IA, Karaskov AM, Laktionov PP. General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials. Materials. 2019; 12(24):4082. https://doi.org/10.3390/ma12244082
Chicago/Turabian StyleStepanova, Alena O., Petr P. Laktionov, Anna V. Cherepanova, Vera S. Chernonosova, Georgiy Yu. Shevelev, Ivan A. Zaporozhchenko, Alexander M. Karaskov, and Pavel P. Laktionov. 2019. "General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials" Materials 12, no. 24: 4082. https://doi.org/10.3390/ma12244082
APA StyleStepanova, A. O., Laktionov, P. P., Cherepanova, A. V., Chernonosova, V. S., Shevelev, G. Y., Zaporozhchenko, I. A., Karaskov, A. M., & Laktionov, P. P. (2019). General Study and Gene Expression Profiling of Endotheliocytes Cultivated on Electrospun Materials. Materials, 12(24), 4082. https://doi.org/10.3390/ma12244082





