The Radiosensitizing Effect of Zinc Oxide Nanoparticles in Sub-Cytotoxic Dosing Is Associated with Oxidative Stress In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ZnO-NP Suspension
2.2. Characterization of ZnO Nanoparticles
2.3. Cell Culture
2.4. MTT Cytotoxicity Assay
2.5. Cell Cycle Analysis
2.6. Irradiation
2.7. Clonogenic Survival Assay
2.8. FPG-Modified Single-Cell Microgel Electrophoresis (Comet) Assay
2.9. Trypan Blue Exclusion Test
2.10. Statistical Analysis
3. Results
3.1. ZnO-NP Characterization
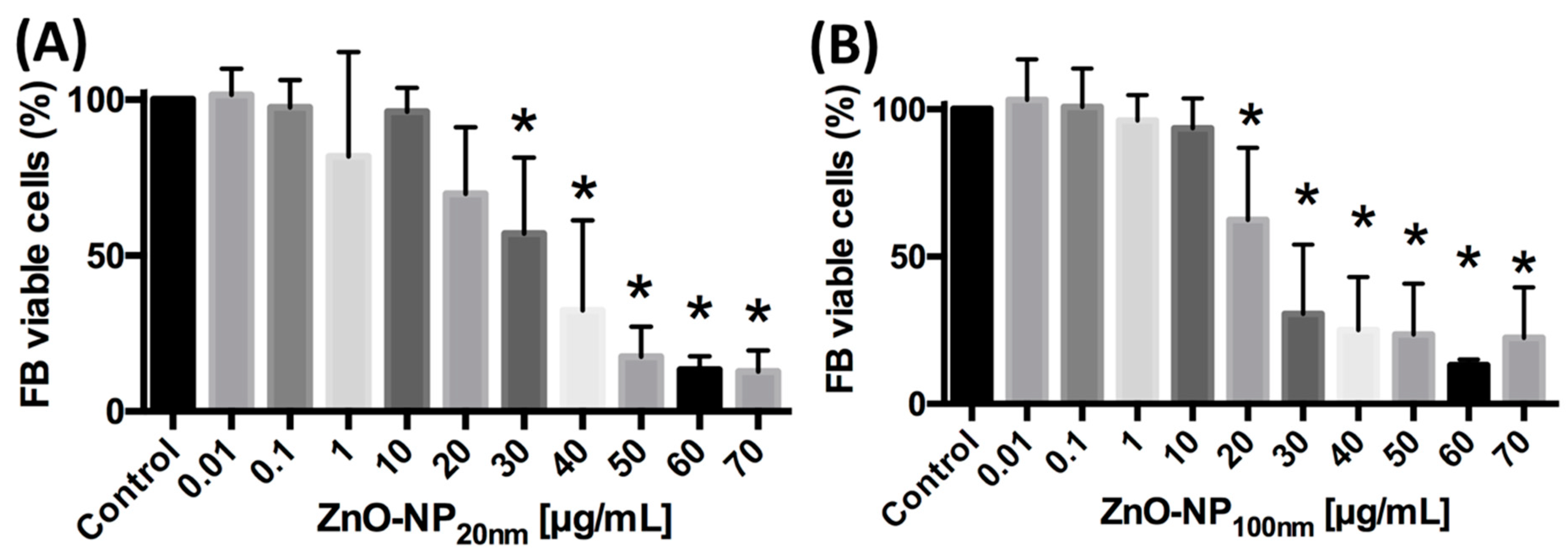
3.2. ZnO-NP-Mediated Cytotoxicity
3.2.1. ZnO-NP-Mediated Cytotoxicity in FaDu
3.2.2. ZnO-NP-Mediated Cytotoxicity in FB
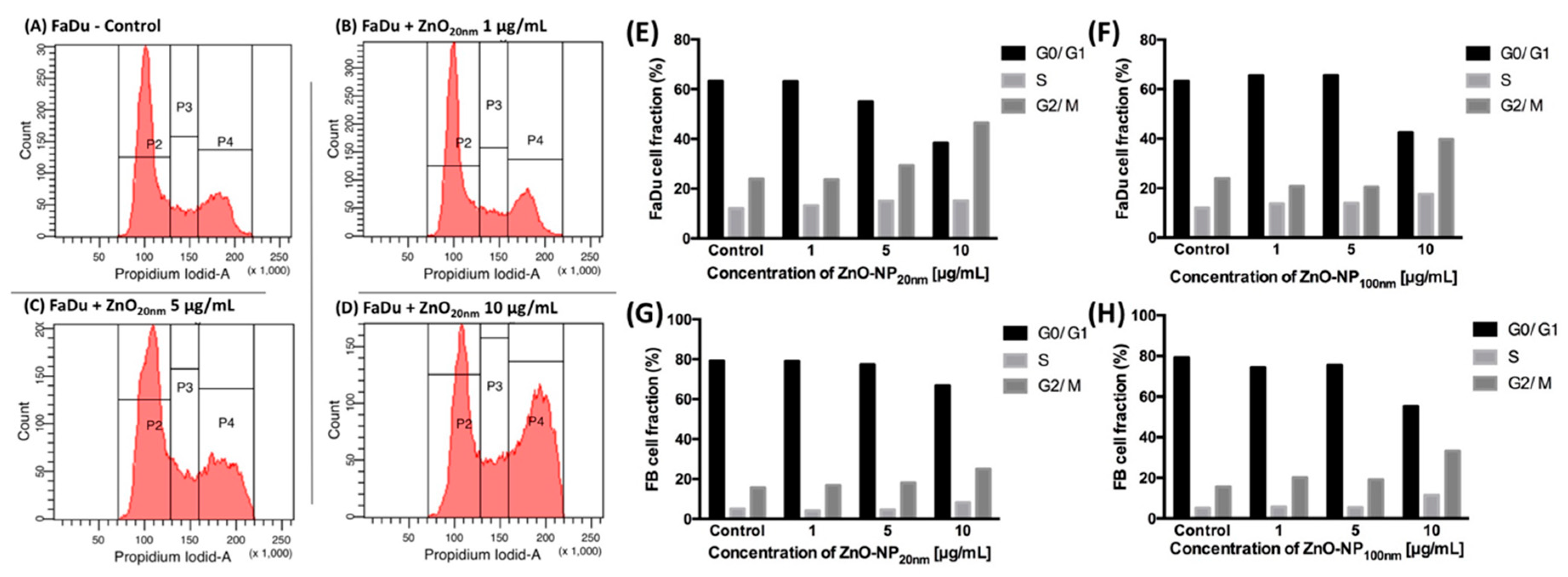
3.3. Influence of ZnO-NP on the Cell Cycle Distribution in FaDu and FB
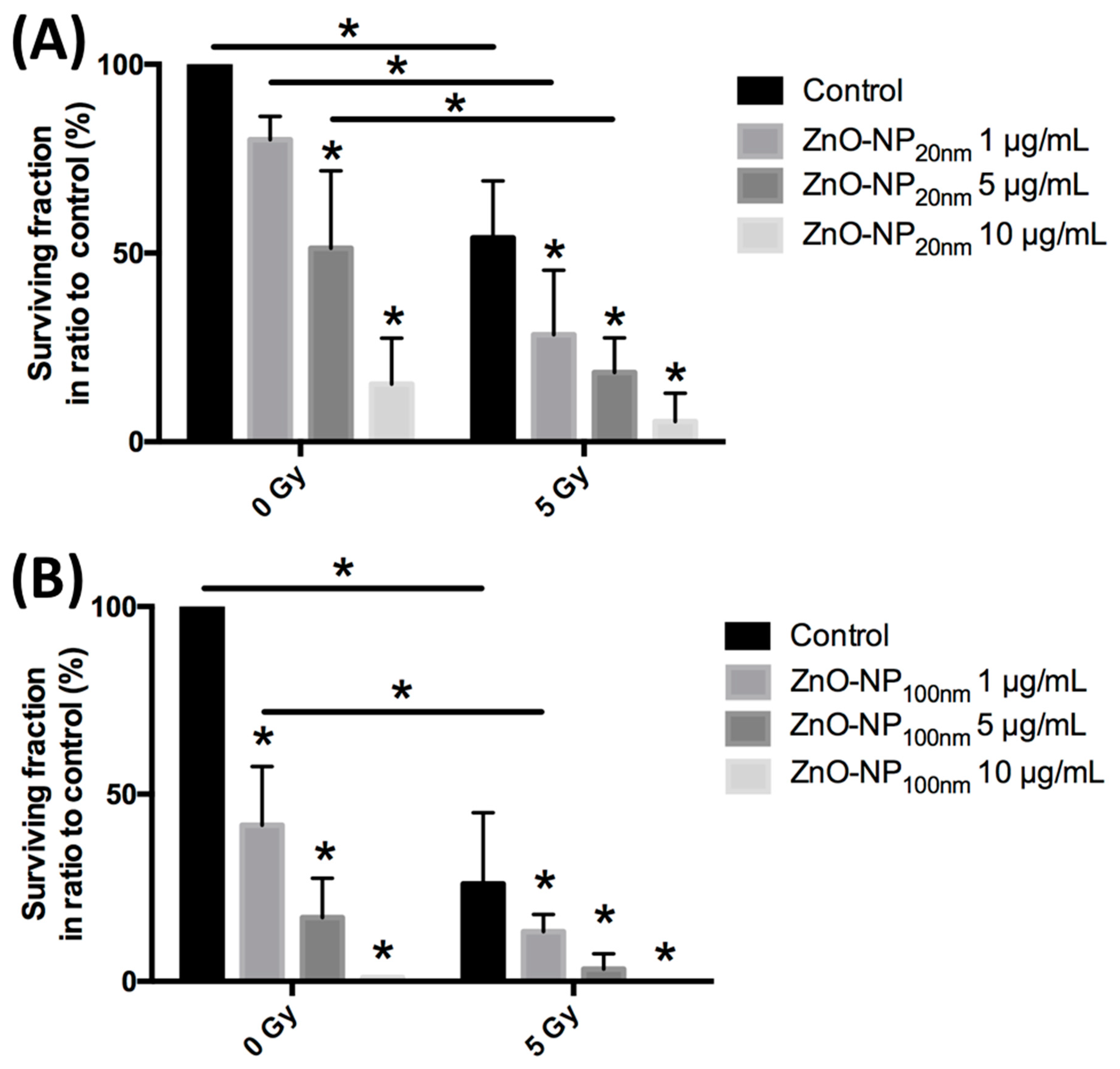
3.4. Colonial Cell Survival in Relation to ZnO-NP Concentration and Irradiation
3.5. Cytotoxicity Assessment by the Trypan Blue Exclusion Test
3.6. DNA Damage and Changes of Oxidative Stress after Exposure to ZnO-NP and Irradiation
3.6.1. DNA Damage Immediately after Exposure to ZnO-NP and Irradiation
3.6.2. Changes of Oxidative Stress Immediately after Exposure to ZnO-NP and Irradiation
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Kong, W.; Peng, Y.; Miao, Q.; Mackillop, W.J. Temporal trends in the incidence and survival of cancers of the upper aerodigestive tract in Ontario and the United States. Int. J. Cancer 2009, 125, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.; Nuyts, S. Can Intensity-Modulated-Radiotherapy Reduce Toxicity in Head and Neck Squamous Cell Carcinoma? Cancers 2017, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, D.G. Nanotechnology and medicine. Expert Opin. Biol. Ther. 2003, 3, 655–663. [Google Scholar] [CrossRef]
- Hanley, C.; Layne, J.; Punnoose, A.; Reddy, K.M.; Coombs, I.; Coombs, A.; Feris, K.; Wingett, D. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology 2008, 19, 295103. [Google Scholar] [CrossRef]
- DeLong, R.K.; Cheng, Y.H.; Pearson, P.; Lin, Z.; Coffee, C.; Mathew, E.N.; Hoffman, A.; Wouda, R.M.; Higginbotham, M.L. Translating Nanomedicine to Comparative Oncology-the Case for Combining Zinc Oxide Nanomaterials with Nucleic Acid Therapeutic and Protein Delivery for Treating Metastatic Cancer. J. Pharmacol. Exp. Ther. 2019, 370, 671–681. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Zinc oxide nanoparticles induce photocatalytic cell death in human head and neck squamous cell carcinoma cell lines in vitro. Int. J. Oncol. 2010, 37, 1583–1590. [Google Scholar]
- Youssef, Z.; Vanderesse, R.; Colombeau, L.; Baros, F.; Roques-Carmes, T.; Frochot, C.; Wahab, H.; Toufaily, J.; Hamieh, T.; Acherar, S.; et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zou, X.; Chen, W. A new X-ray activated nanoparticle photosensitizer for cancer treatment. J. Biomed. Nanotechnol. 2014, 10, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.R.; Ordaz, J.; Lo, C.L.; Damayanti, N.P.; Zhou, F.; Irudayaraj, J. From the Cover: Zinc oxide Nanoparticles-Induced Reactive Oxygen Species Promotes Multimodal Cyto- and Epigenetic Toxicity. Toxicol. Sci. 2017, 156, 261–274. [Google Scholar] [PubMed]
- Kocbek, P.; Teskac, K.; Kreft, M.E.; Kristl, J. Toxicological aspects of long-term treatment of keratinocytes with ZnO and TiO2 nanoparticles. Small 2010, 6, 1908–1917. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Kim, H.; Kim, Y.; Yi, J.; Choi, K.; Park, K. Inflammatory responses may be induced by a single intratracheal instillation of iron nanoparticles in mice. Toxicology 2010, 275, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bihari, P.; Vippola, M.; Schultes, S.; Praetner, M.; Khandoga, A.G.; Reichel, C.A.; Coester, C.; Tuomi, T.; Rehberg, M.; Krombach, F. Optimized dispersion of nanoparticles for biological in vitro and in vivo studies. Part. Fibre Toxicol. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Moratin, H.; Scherzad, A.; Gehrke, T.; Ickrath, P.; Radeloff, K.; Kleinsasser, N.; Hackenberg, S. Toxicological characterization of ZnO nanoparticles in malignant and non-malignant cells. Environ. Mol. Mutagen. 2018, 59, 247–259. [Google Scholar] [CrossRef]
- Radeloff, K.; Radeloff, A.; Tirado, M.R.; Scherzad, A.; Hagen, R.; Kleinsasser, N.H.; Hackenberg, S. Long-Term Impact of Zinc Oxide Nanoparticles on Differentiation and Cytokine Secretion of Human Adipose-Derived Stromal Cells. Materials 2019, 12, 1832. [Google Scholar] [CrossRef]
- Rangan, S.R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer 1972, 29, 117–121. [Google Scholar] [CrossRef]
- Vangipuram, M.; Ting, D.; Kim, S.; Diaz, R.; Schule, B. Skin punch biopsy explant culture for derivation of primary human fibroblasts. J. Vis. Exp. 2013, 7, e3779. [Google Scholar] [CrossRef]
- Gehrke, T.; Scherzad, A.; Hackenberg, S.; Schendzielorz, P.; Hagen, R.; Kleinsasser, N. Differences in tumor stroma derived from irradiated versus non-irradiated fibroblasts in a co-culture model with head and neck squamous cell carcinoma. Oncol. Lett. 2016, 12, 3549–3554. [Google Scholar] [CrossRef][Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Collins, A.R.; Duthie, S.J.; Dobson, V.L. Direct enzymic detection of endogenous oxidative base damage in human lymphocyte DNA. Carcinogenesis 1993, 14, 1733–1735. [Google Scholar] [CrossRef]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Scherzad, A.; Hackenberg, S.; Schramm, C.; Froelich, K.; Ginzkey, C.; Hagen, R.; Kleinsasser, N. Geno- and cytotoxicity of salinomycin in human nasal mucosa and peripheral blood lymphocytes. Toxicol. Vitr. 2015, 29, 813–818. [Google Scholar] [CrossRef]
- Olive, P.L.; Durand, R.E.; Le Riche, J.; Olivotto, I.A.; Jackson, S.M. Gel electrophoresis of individual cells to quantify hypoxic fraction in human breast cancers. Cancer Res. 1993, 53, 733–736. [Google Scholar]
- Bisht, G.; Rayamajhi, S. ZnO Nanoparticles: A Promising Anticancer Agent. Nanobiomedicine (Rij) 2016, 3, 9. [Google Scholar] [CrossRef]
- Ostrovsky, Stella; Kazimirsky, Gila; Gedanken, Aharo; Brodie, Chaya Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009, 2, 882–890. [CrossRef]
- He, T.; Peterson, T.E.; Holmuhamedov, E.L.; Terzic, A.; Caplice, N.M.; Oberley, L.W.; Katusic, Z.S. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2021–2027. [Google Scholar] [CrossRef]
- Hackenberg, S.; Zimmermann, F.Z.; Scherzed, A.; Friehs, G.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Repetitive exposure to zinc oxide nanoparticles induces dna damage in human nasal mucosa mini organ cultures. Environ. Mol. Mutagen. 2011, 52, 582–589. [Google Scholar] [CrossRef]
- Hackenberg, S.; Scherzed, A.; Technau, A.; Kessler, M.; Froelich, K.; Ginzkey, C.; Koehler, C.; Burghartz, M.; Hagen, R.; Kleinsasser, N. Cytotoxic, genotoxic and pro-inflammatory effects of zinc oxide nanoparticles in human nasal mucosa cells in vitro. Toxicol. Vitr. 2011, 25, 657–663. [Google Scholar] [CrossRef]
- Heim, J.; Felder, E.; Tahir, M.N.; Kaltbeitzel, A.; Heinrich, U.R.; Brochhausen, C.; Mailander, V.; Tremel, W.; Brieger, J. Genotoxic effects of zinc oxide nanoparticles. Nanoscale 2015, 7, 8931–8938. [Google Scholar] [CrossRef]
- Sadjadpour, S.; Safarian, S.; Zargar, S.J.; Sheibani, N. Antiproliferative effects of ZnO, ZnO-MTCP, and ZnO-CuMTCP nanoparticles with safe intensity UV and X-ray irradiation. Biotechnol. Appl. Biochem. 2016, 63, 113–124. [Google Scholar] [CrossRef]
- Zangeneh, M.; Nedaei, H.A.; Mozdarani, H.; Mahmoudzadeh, A.; Salimi, M. Enhanced cytotoxic and genotoxic effects of gadolinium-doped ZnO nanoparticles on irradiated lung cancer cells at megavoltage radiation energies. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109739. [Google Scholar] [CrossRef]
- Generalov, R.; Kuan, W.B.; Chen, W.; Kristensen, S.; Juzenas, P. Radiosensitizing effect of zinc oxide and silica nanocomposites on cancer cells. Colloids Surf. B Biointerfaces 2015, 129, 79–86. [Google Scholar] [CrossRef]
- Yaghini, E.; Pirker, K.F.; Kay, C.W.; Seifalian, A.M.; MacRobert, A.J. Quantification of reactive oxygen species generation by photoexcitation of PEGylated quantum dots. Small 2014, 10, 5106–5115. [Google Scholar] [CrossRef]
- Ghaemi, B.; Mashinchian, O.; Mousavi, T.; Karimi, R.; Kharrazi, S.; Amani, A. Harnessing the Cancer Radiation Therapy by Lanthanide-Doped Zinc Oxide Based Theranostic Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 3123–3134. [Google Scholar] [CrossRef]
- Karlsson, H.L.; Gustafsson, J.; Cronholm, P.; Moller, L. Size-dependent toxicity of metal oxide particles--a comparison between nano- and micrometer size. Toxicol. Lett. 2009, 188, 112–118. [Google Scholar] [CrossRef]
- Golbamaki, N.; Rasulev, B.; Cassano, A.; Marchese Robinson, R.L.; Benfenati, E.; Leszczynski, J.; Cronin, M.T. Genotoxicity of metal oxide nanomaterials: Review of recent data and discussion of possible mechanisms. Nanoscale 2015, 7, 2154–2198. [Google Scholar] [CrossRef]
- Warheit, D.B.; Webb, T.R.; Sayes, C.M.; Colvin, V.L.; Reed, K.L. Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: Toxicity is not dependent upon particle size and surface area. Toxicol. Sci. 2006, 91, 227–236. [Google Scholar] [CrossRef]
- Swartz, J.E.; Pothen, A.J.; Stegeman, I.; Willems, S.M.; Grolman, W. Clinical implications of hypoxia biomarker expression in head and neck squamous cell carcinoma: A systematic review. Cancer Med. 2015, 4, 1101–1116. [Google Scholar] [CrossRef]
- Gammon, L.; Mackenzie, I.C. Roles of hypoxia, stem cells and epithelial-mesenchymal transition in the spread and treatment resistance of head and neck cancer. J. Oral Pathol. Med. 2016, 45, 77–82. [Google Scholar] [CrossRef]
- Gao, F.; Ma, N.; Zhou, H.; Wang, Q.; Zhang, H.; Wang, P.; Hou, H.; Wen, H.; Li, L. Zinc oxide nanoparticles-induced epigenetic change and G2/M arrest are associated with apoptosis in human epidermal keratinocytes. Int. J. Nanomed. 2016, 1, 3859–3874. [Google Scholar]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meyer, T.J.; Scherzad, A.; Moratin, H.; Gehrke, T.E.; Killisperger, J.; Hagen, R.; Wohlleben, G.; Polat, B.; Dembski, S.; Kleinsasser, N.; et al. The Radiosensitizing Effect of Zinc Oxide Nanoparticles in Sub-Cytotoxic Dosing Is Associated with Oxidative Stress In Vitro. Materials 2019, 12, 4062. https://doi.org/10.3390/ma12244062
Meyer TJ, Scherzad A, Moratin H, Gehrke TE, Killisperger J, Hagen R, Wohlleben G, Polat B, Dembski S, Kleinsasser N, et al. The Radiosensitizing Effect of Zinc Oxide Nanoparticles in Sub-Cytotoxic Dosing Is Associated with Oxidative Stress In Vitro. Materials. 2019; 12(24):4062. https://doi.org/10.3390/ma12244062
Chicago/Turabian StyleMeyer, Till Jasper, Agmal Scherzad, Helena Moratin, Thomas Eckert Gehrke, Julian Killisperger, Rudolf Hagen, Gisela Wohlleben, Bülent Polat, Sofia Dembski, Norbert Kleinsasser, and et al. 2019. "The Radiosensitizing Effect of Zinc Oxide Nanoparticles in Sub-Cytotoxic Dosing Is Associated with Oxidative Stress In Vitro" Materials 12, no. 24: 4062. https://doi.org/10.3390/ma12244062
APA StyleMeyer, T. J., Scherzad, A., Moratin, H., Gehrke, T. E., Killisperger, J., Hagen, R., Wohlleben, G., Polat, B., Dembski, S., Kleinsasser, N., & Hackenberg, S. (2019). The Radiosensitizing Effect of Zinc Oxide Nanoparticles in Sub-Cytotoxic Dosing Is Associated with Oxidative Stress In Vitro. Materials, 12(24), 4062. https://doi.org/10.3390/ma12244062






