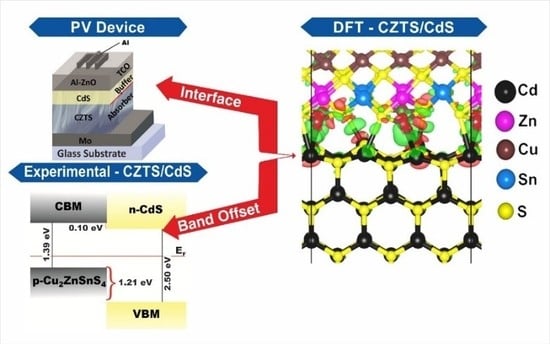
Interface Structure and Band Alignment of CZTS/CdS Heterojunction: An Experimental and First-Principles DFT Investigation
Abstract
1. Introduction
2. Experimental and DFT Calculations Details
2.1. Experimental Details
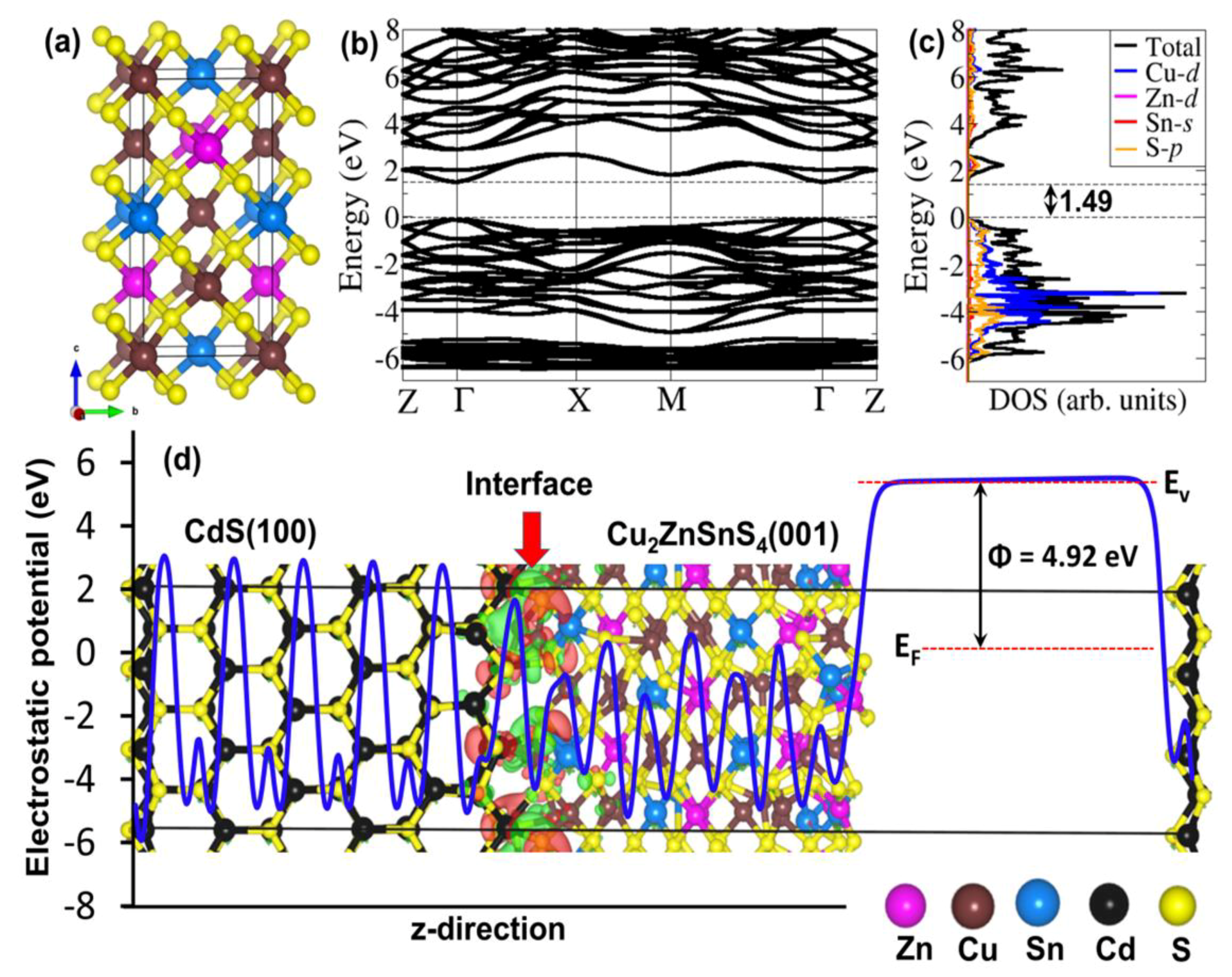
2.2. Computational Details
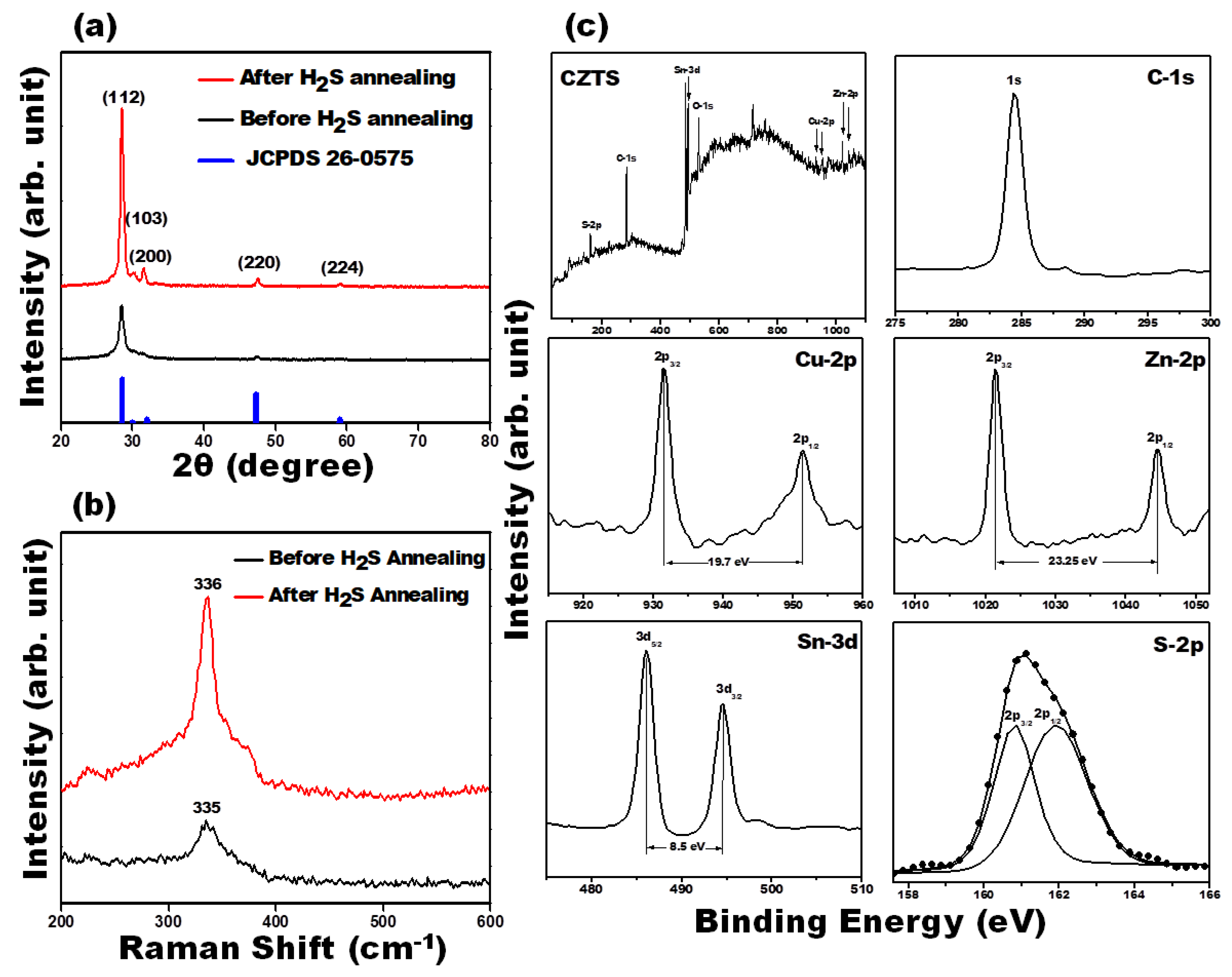
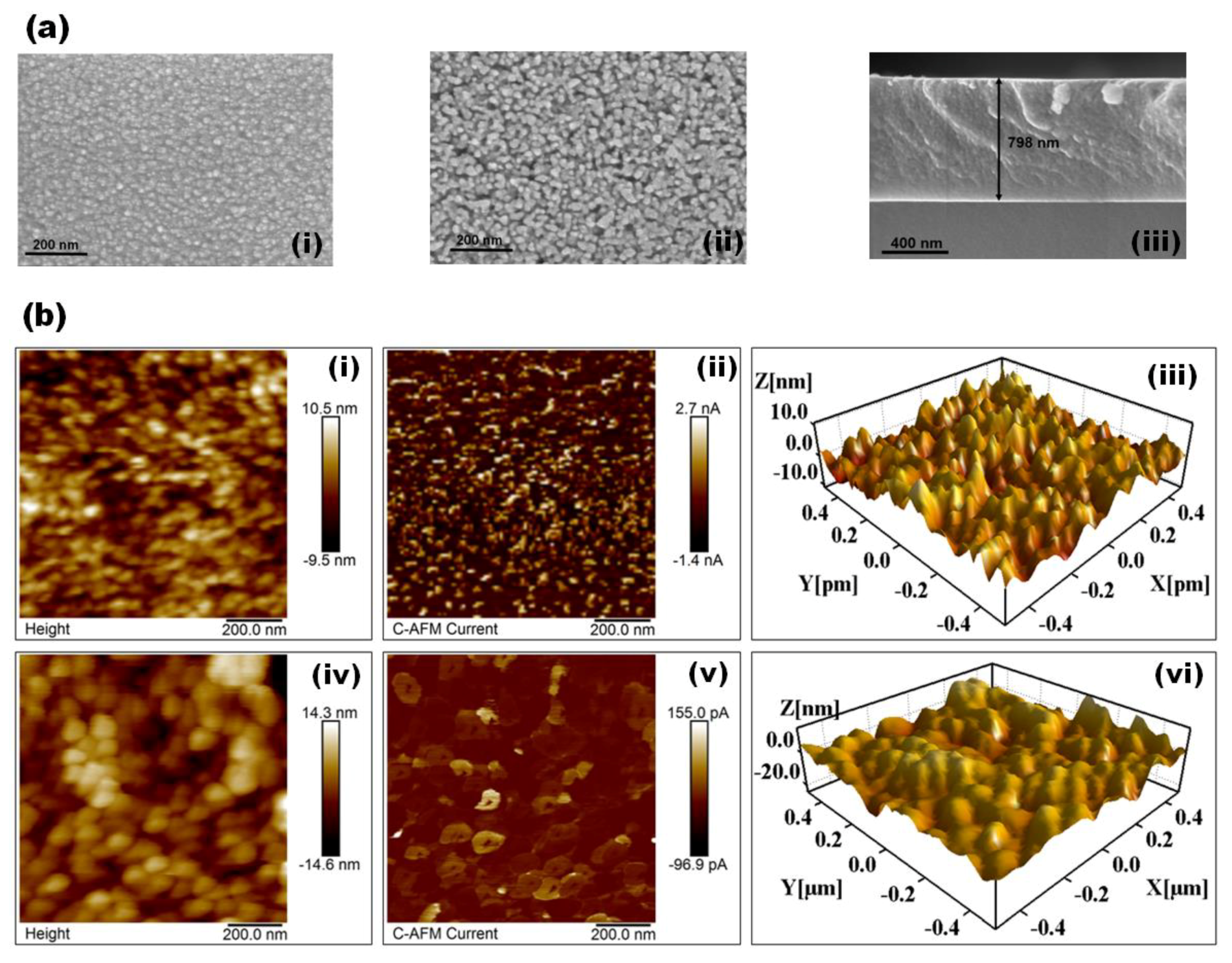
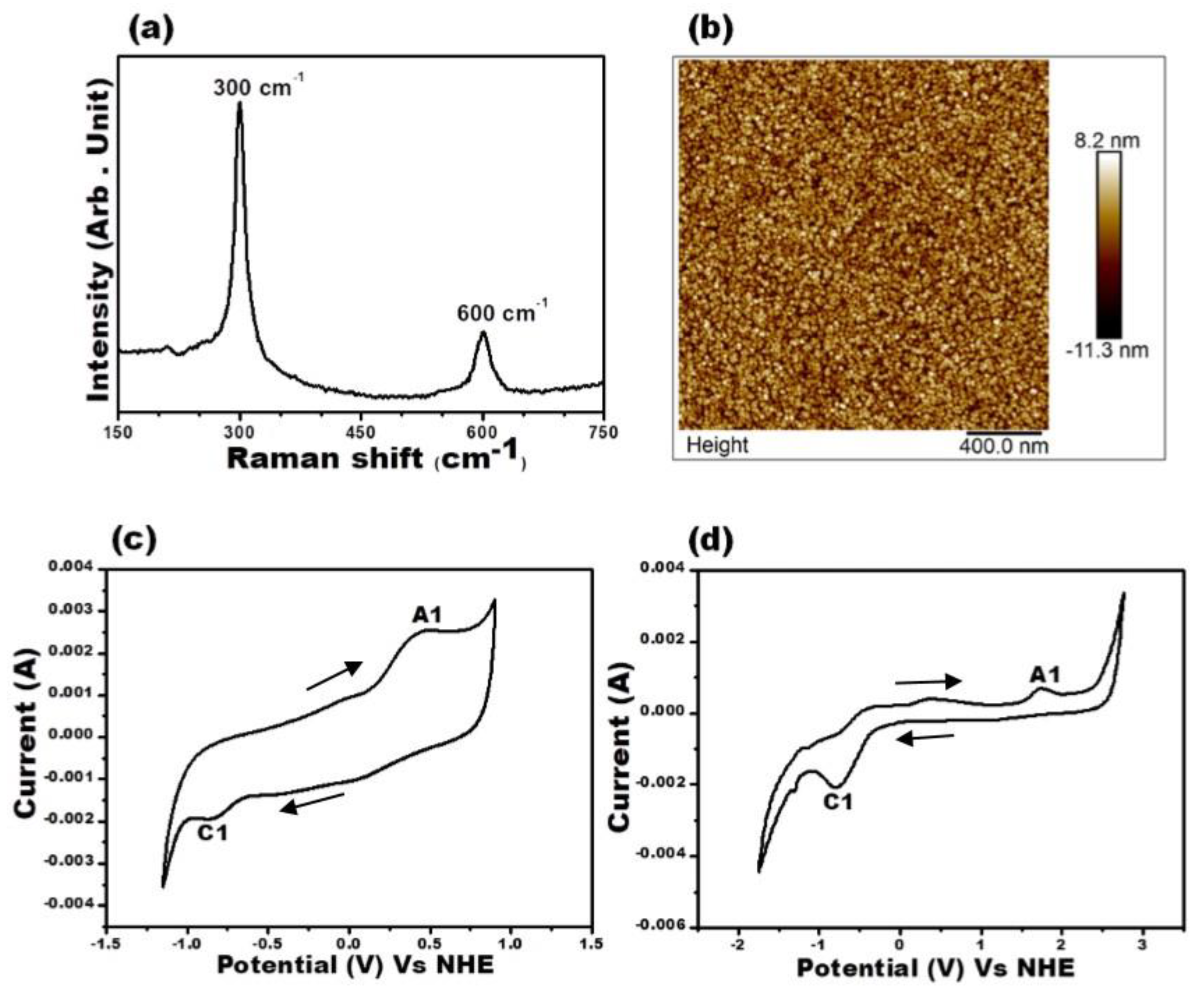
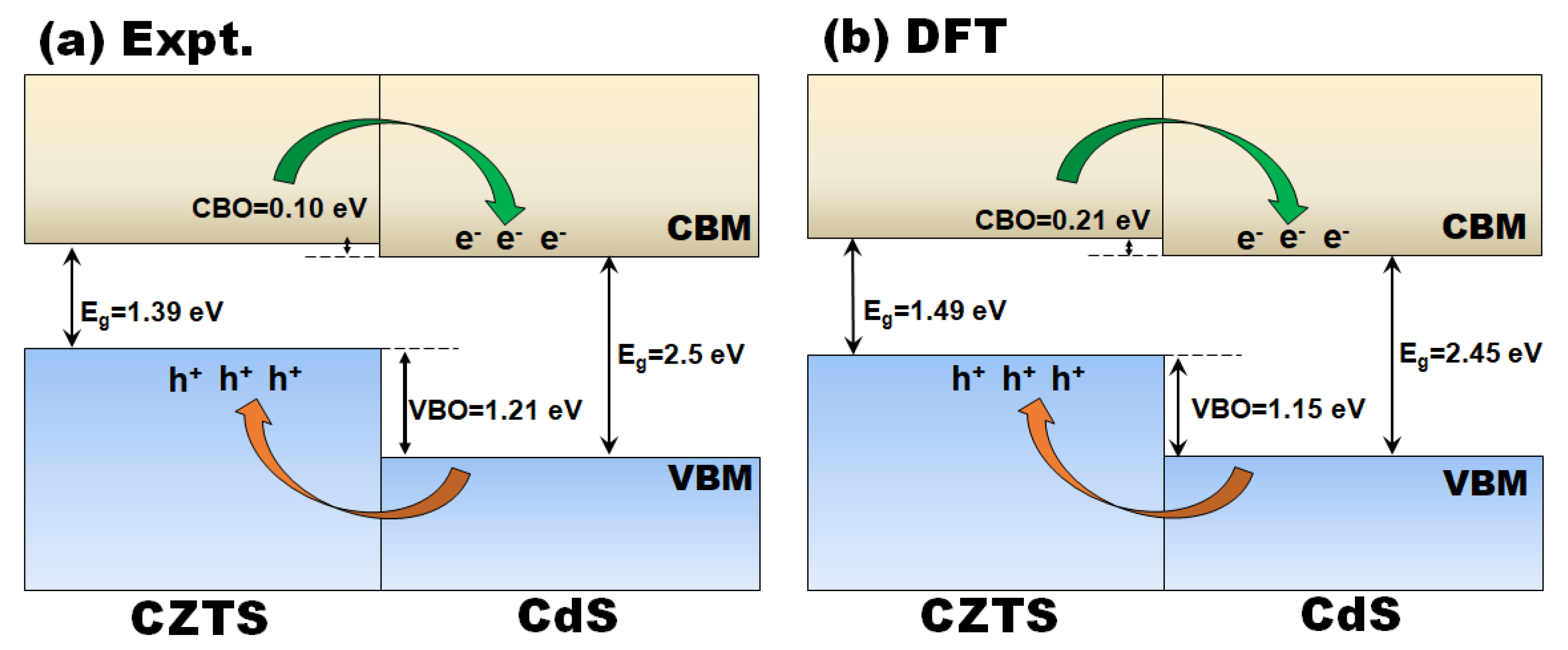
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shockley, W.; Queisser, H.J. Detailed balance limit of efficiency of p-n junction solar cells. J. Appl. Phys. 1961, 32, 510. [Google Scholar] [CrossRef]
- Kush, P.; Deka, S. Multifunctional copper-based quaternary chalcogenide semiconductors toward state-of-the-art energy applications. ChemNanoMat 2018, 4, 1–31. [Google Scholar] [CrossRef]
- Steinhagen, C.; Panthani, M.G.; Akhavan, V.; Goodfellow, B.; Koo, B.; Korgel, B.A. Synthesis of Cu2ZnSnS4 nanocrystals for use in low-cost photovoltaics. J. Am. Chem. Soc. 2009, 131, 12554–12555. [Google Scholar] [CrossRef] [PubMed]
- Embden, J.V.; Chesman, A.S.R.; Gaspera, E.D.; Duffy, N.W.; Watkins, S.E.; Jasieniak, J.J. Cu2ZnSnS4xSe4(1−x) solar cells from polar nanocrystal inks. J. Am. Chem. Soc 2014, 136, 5237–5240. [Google Scholar] [CrossRef] [PubMed]
- Wojtyła, S.; Baran, T. Copper zinc oxide heterostructure nanoflowers for hydrogen evolution. Int. J. Hydrogen Energy 2019, 50, 27343–27353. [Google Scholar]
- Kar, M.K.A.; Fazaeli, R.; Manteghi, F.; Ghahari, M. Structural, Optical, and Isothermic Studies of CuFe2O4 and Zn-Doped CuFe2O4 Nanoferrite as a Magnetic Catalyst for Photocatalytic Degradation of Direct Red 264 Under Visible Light Irradiation. Environ. Prog. Sustain. Energy 2019, 38, 13109. [Google Scholar] [CrossRef]
- Kush, P.; Deori, K.; Kumar, A.; Deka, S. Efficient hydrogen/oxygen evolution and photocatalytic dye degradation and reduction of aqueous Cr(vi) by surfactant free hydrophilic Cu2ZnSnS4 nanoparticles. J. Mater. Chem. A 2015, 3, 8098–8106. [Google Scholar] [CrossRef]
- Digraskar, R.V.; Sapner, V.S.; Narwade, S.S.; Mali, S.M.; Ghuleb, A.V.; Sathe, B.R. Enhanced electrocatalytic hydrogen generation from water via cobalt-doped Cu2ZnSnS4 nanoparticles. RSC Adv. 2018, 8, 20341. [Google Scholar] [CrossRef]
- Green, M.A.; Emery, K.; Hishikawa, Y.; Warta, W.; Dunlop, E.D. Characterizing electrical output of bifacial photovoltaic modules by altering reflective materials. Prog. Photovolt. Res. Appl. 2015, 23, 1–9. [Google Scholar] [CrossRef]
- Lee, Y.S.; Gershon, T.; Gunawan, O.; Todorov, T.K.; Gokmen, T.; Virgus, Y.; Guha, S. Cu2ZnSnSe4 thin-film solar cells by thermal co-evaporation with 11.6% efficiency and improved minority carrier diffusion length. Adv. Energy Mater. 2014, 5, 140372. [Google Scholar]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device characteristics of CZTSSe thin film solar cells with 12.6% efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Shin, B.; Gunawan, O.; Zhu, Y.; Bojarczuk, N.A.; Chey, S.J.; Guha, S. Thin film solar cell with 8.4% power conversion efciency using an earth-abundant Cu2ZnSnS4 absorber. Progress. Photovolt. Res. Appl 2013, 21, 72–76. [Google Scholar] [CrossRef]
- Tajima, S.; Umehara, M.; Hasegawa, M.; Mise, T.; Itoh, T. Cu2ZnSnS4 photovoltaic cell with improved efciency fabricated by high-temperature annealing afer CdS bufer-layer deposition. Prog. Photovolt. Res. Appl. 2017, 25, 14–22. [Google Scholar] [CrossRef]
- Shin, D.; Saparov, B.; Mitzi, D.B. Defect engineering in multinary earth-abundant chalcogenide photovoltaic materials. Adv. Energy Mater. 2017, 7, 1602366. [Google Scholar] [CrossRef]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Zhai, X.; Jia, H.; Zhang, Y.; Lei, Y.; Wei, J.; Gao, Y.; Chu, J.; He, W.; Yin, J.; Zheng, Z. In situ fabrication of Cu2ZnSnS4 nanoflake thin films on both rigid and flexible substrates. CrystEngComm 2014, 16, 6244–6249. [Google Scholar] [CrossRef]
- Scragg, J.J.; Kubart, T.; Watjen, J.T.; Ericson, T.; Linnarsson, M.K.; Bjorkman, C.P. Effects of back contact instability on Cu2ZnSnS4 devices and processes. Chem. Mater 2013, 25, 3162–3171. [Google Scholar] [CrossRef]
- Siebentritt, S. Why are kesterite solar cells not 20% efficient? Thin Solid Films 2013, 535, 1–4. [Google Scholar] [CrossRef]
- Crovetto, A.; Hansen, O. What is the band alignment of Cu2ZnSn(S,Se)4 solar cells? Sol. Energy Mater. Sol. Cells 2017, 169, 177–194. [Google Scholar] [CrossRef]
- Kumar, M.; Dubey, A.; Adhikari, N.; Venkatesan, S.; Qiao, Q. Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS-Se solar cells. Energy Environ. Sci. 2015, 8, 3134–3159. [Google Scholar] [CrossRef]
- Jadhav, Y.A.; Thakur, P.R.; Haram, S.K. Voltammetry investigation on copper zinc tin sulphide/selenide (CZTSxSe1−x) alloy nanocrystals: Estimation of composition dependent band edge parameters. Sol. Energy Mater. Sol. Cells 2016, 155, 273–279. [Google Scholar] [CrossRef]
- Haram, S.K.; Quinn, B.M.; Bard, A.J. Electrochemistry of CdS nanoparticles: A correlation between optical and electrochemical band gaps. J. Am. Chem. Soc. 2001, 123, 8860–8861. [Google Scholar]
- Kresse, G.; Furthmiiller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the exchange screening parameter on the performance of screened hybrid functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef]
- Surgina, G.D.; Nevolin, V.N.; Sipaylo, I.P.; Teterin, P.E.; Medvedeva, S.S.; Lebedinsky, Y.Y.; Zenkevich, A.V. Effect of annealing on structural and optical properties of Cu2ZnSnS4 thin films grown by pulsed laser deposition. Thin Solid Films 2015, 594, 74–79. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S. Elements of X–Ray Diffraction; Prentice-Hall: Upper Saddle River, NJ, USA, 2001; Volume 3. [Google Scholar]
- Zhenghua, S.; Kaiwen, S.; Zili, H.; Hongtao, C.; Fangyang, L.; Yanqing, L.; Jie, L.; Xiaojing, H.; Yexiang, L.; Green, M.A. Fabrication of Cu2ZnSnS4 solar cells with 5.1% efficiency via thermal decomposition and reaction using a non-toxic sol–gel route. J. Mater. Chem. A 2014, 2, 500–509. [Google Scholar]
- Li, M.; Zhou, W.H.; Guo, J.; Zhou, Y.L.; Hou, Z.L.; Jiao, J.; Zhou, Z.J.; Du, Z.L.; Wu, S.X. Synthesis of pure metastable wurtzite CZTS nanocrystals by facile one-pot method. J. Phys. Chem. C 2012, 116, 26507–26516. [Google Scholar] [CrossRef]
- Singh, A.; Geaney, H.; Laffir, F.; Ryan, K.M. Colloidal synthesis of wurtzite Cu2ZnSnS4 nanorods and their perpendicular assembly. J. Am. Chem. Soc. 2012, 134, 2910–2913. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Wang, H.; Xie, M.; Zong, K.; Zheng, H.; Shu, Y.; Liu, J.; Yan, H.; Zhua, M.; et al. Novel non-hydrazine solution processing of earth abundant Cu2ZnSn(S,Se)4 absorbers for thin-film solar cells. J. Mater. Chem. A 2013, 1, 6880–6887. [Google Scholar] [CrossRef]
- Yin, X.; Tang, C.; Chen, M.; Adams, S.; Wang, H.; Gong, H. Hierarchical porous Cu2ZnSnS4 films for high-capacity reversible lithium storage applications. J. Mater. Chem. A 2013, 1, 7927–7932. [Google Scholar] [CrossRef]
- Rondiya, S.; Wadnerkar, N.; Jadhav, Y.; Jadkar, S.; Haram, S.; Kabir, M. Structural, electronic, and optical properties of Cu2NiSnS4: A combined experimental and theoretical study toward photovoltaic applications. Chem. Mater. 2017, 29, 3133–3142. [Google Scholar] [CrossRef]
- Persson, C. Electronic and optical properties of Cu2ZnSnS4 and Cu2ZnSnSe4. J. Appl. Phys. 2010, 107, 053710. [Google Scholar] [CrossRef]
- Chen, S.; Gong, X.G.; Walsh, A.; Wei, S.-H. Crystal and electronic band structure of Cu2ZnSnX4 (X=S/Se) photovoltaic absorbers: First-principles insights. Appl. Phys. Lett. 2009, 94, 041903. [Google Scholar] [CrossRef]
- Burton, L.A.; Kumagai, Y.; Walsh, A.; Oba, F. DFT investigation into the underperformance of sulfide materials in photovoltaic applications. J. Mater. Chem. A 2017, 5, 9132–9140. [Google Scholar] [CrossRef]
- Seboui, Z.; Gassoumi, A.; Cuminal, Y.; Turki, N.K. The post-growth effect on the properties of Cu2ZnSnS4 thin films. J. Renew. Sustain. Energy 2015, 7, 011203. [Google Scholar] [CrossRef]
- Ericson, T.; Scragg, J.J.; Kubart, T.; Törndahl, T.; Platzer-Björkman, C. Annealing behavior of reactively sputtered precursor films for Cu2ZnSnS4 solar cells. Thin Solid Films 2013, 535, 22–26. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rondiya, S.; Jadhav, Y.; Nasane, M.; Jadkar, S.; Dzade, N.Y. Interface Structure and Band Alignment of CZTS/CdS Heterojunction: An Experimental and First-Principles DFT Investigation. Materials 2019, 12, 4040. https://doi.org/10.3390/ma12244040
Rondiya S, Jadhav Y, Nasane M, Jadkar S, Dzade NY. Interface Structure and Band Alignment of CZTS/CdS Heterojunction: An Experimental and First-Principles DFT Investigation. Materials. 2019; 12(24):4040. https://doi.org/10.3390/ma12244040
Chicago/Turabian StyleRondiya, Sachin, Yogesh Jadhav, Mamta Nasane, Sandesh Jadkar, and Nelson Y. Dzade. 2019. "Interface Structure and Band Alignment of CZTS/CdS Heterojunction: An Experimental and First-Principles DFT Investigation" Materials 12, no. 24: 4040. https://doi.org/10.3390/ma12244040
APA StyleRondiya, S., Jadhav, Y., Nasane, M., Jadkar, S., & Dzade, N. Y. (2019). Interface Structure and Band Alignment of CZTS/CdS Heterojunction: An Experimental and First-Principles DFT Investigation. Materials, 12(24), 4040. https://doi.org/10.3390/ma12244040