Synthesis and Mechanical Characterization of a Ti(C,N)/Mo–Co–Ni/CaF2@Al2O3 Self-Lubricating Cermet
Abstract
:1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Testing and Characterization
3. Results and Discussion
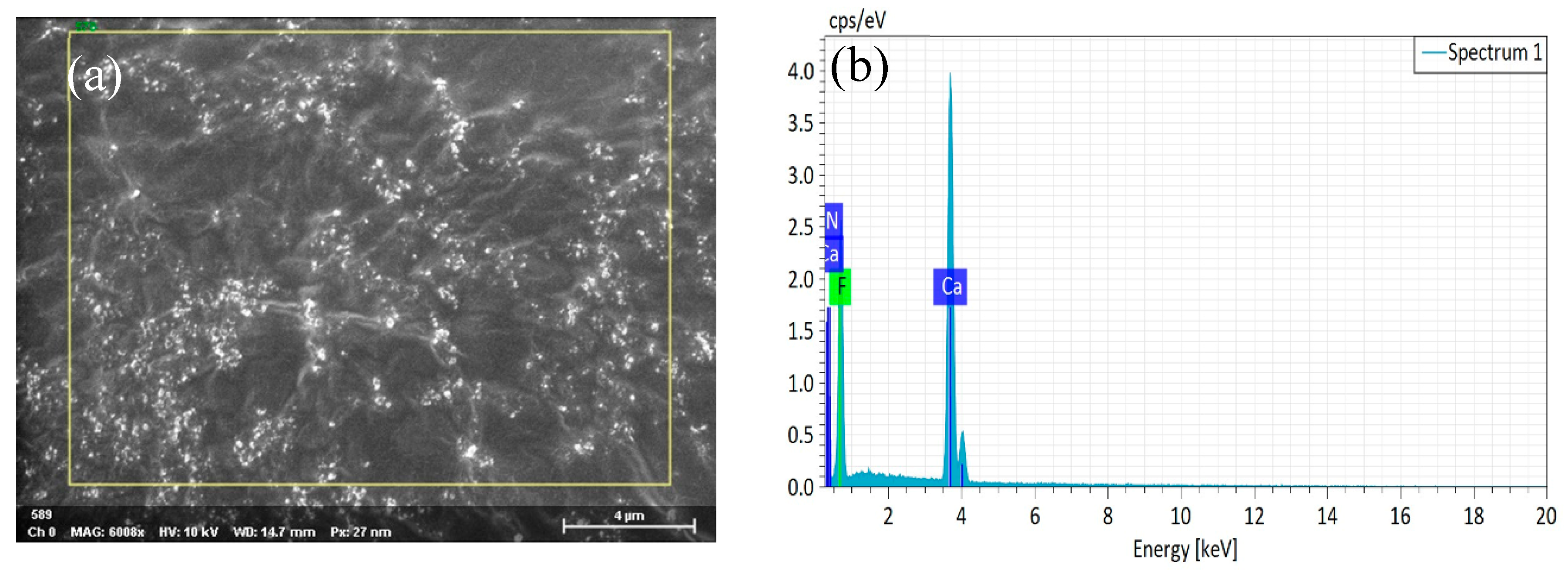
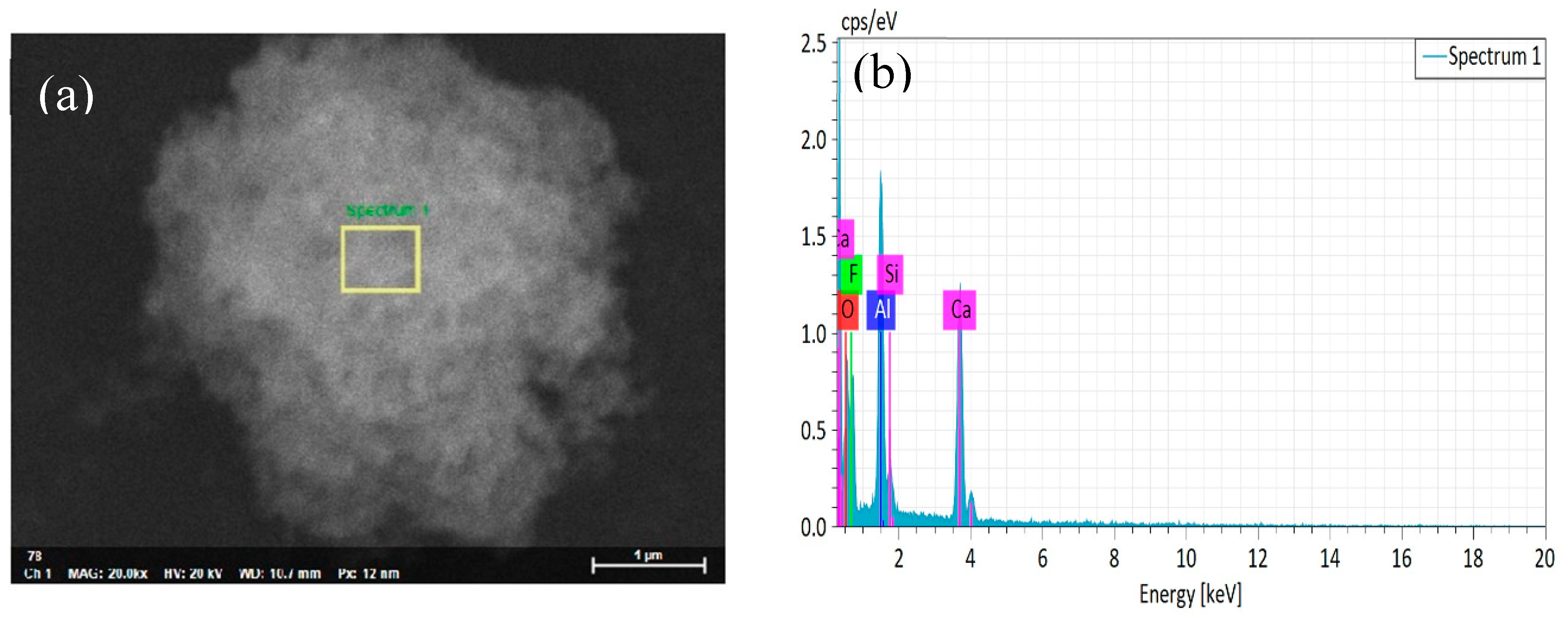
3.1. Phase Analysis of the Coated Powder
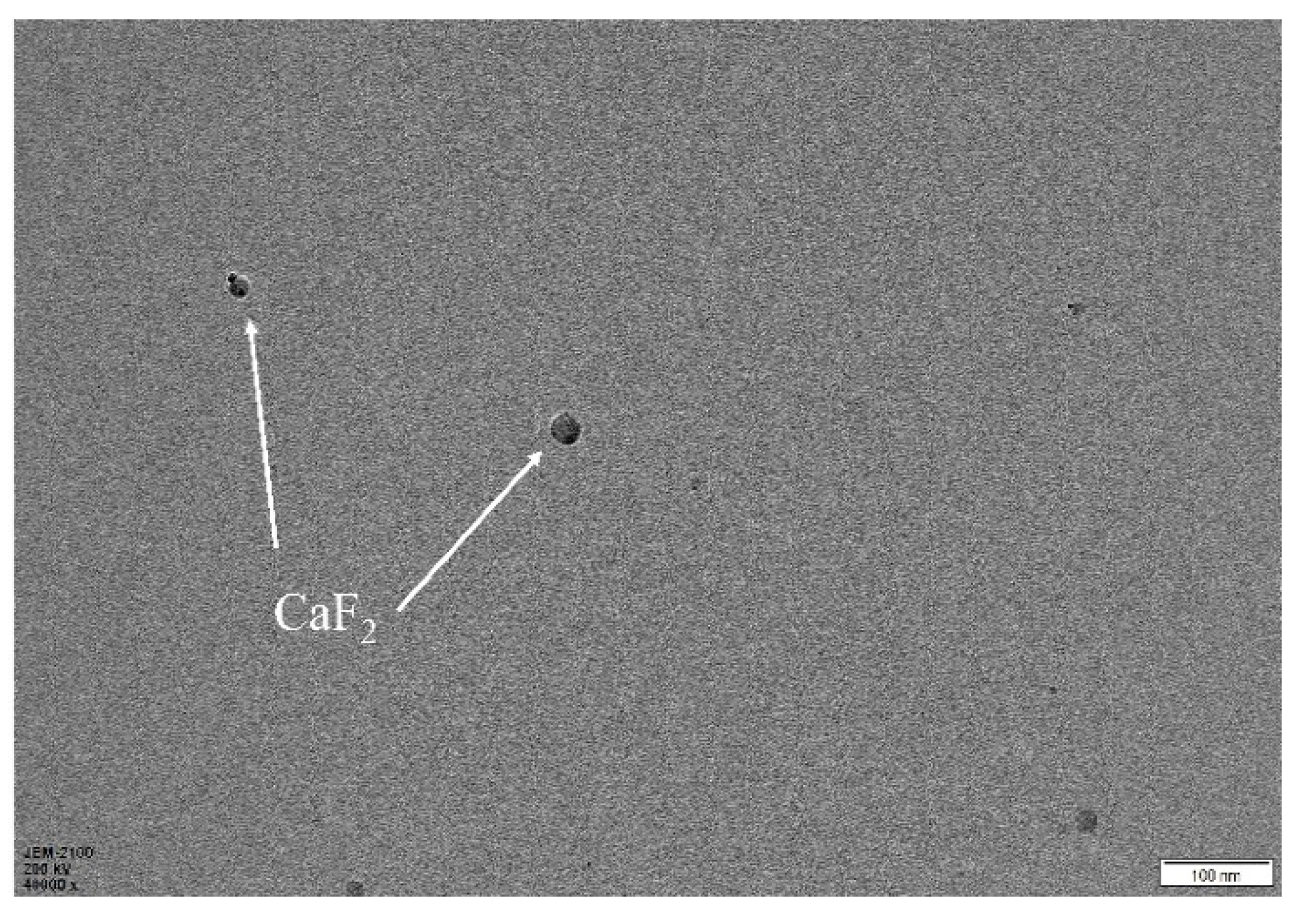
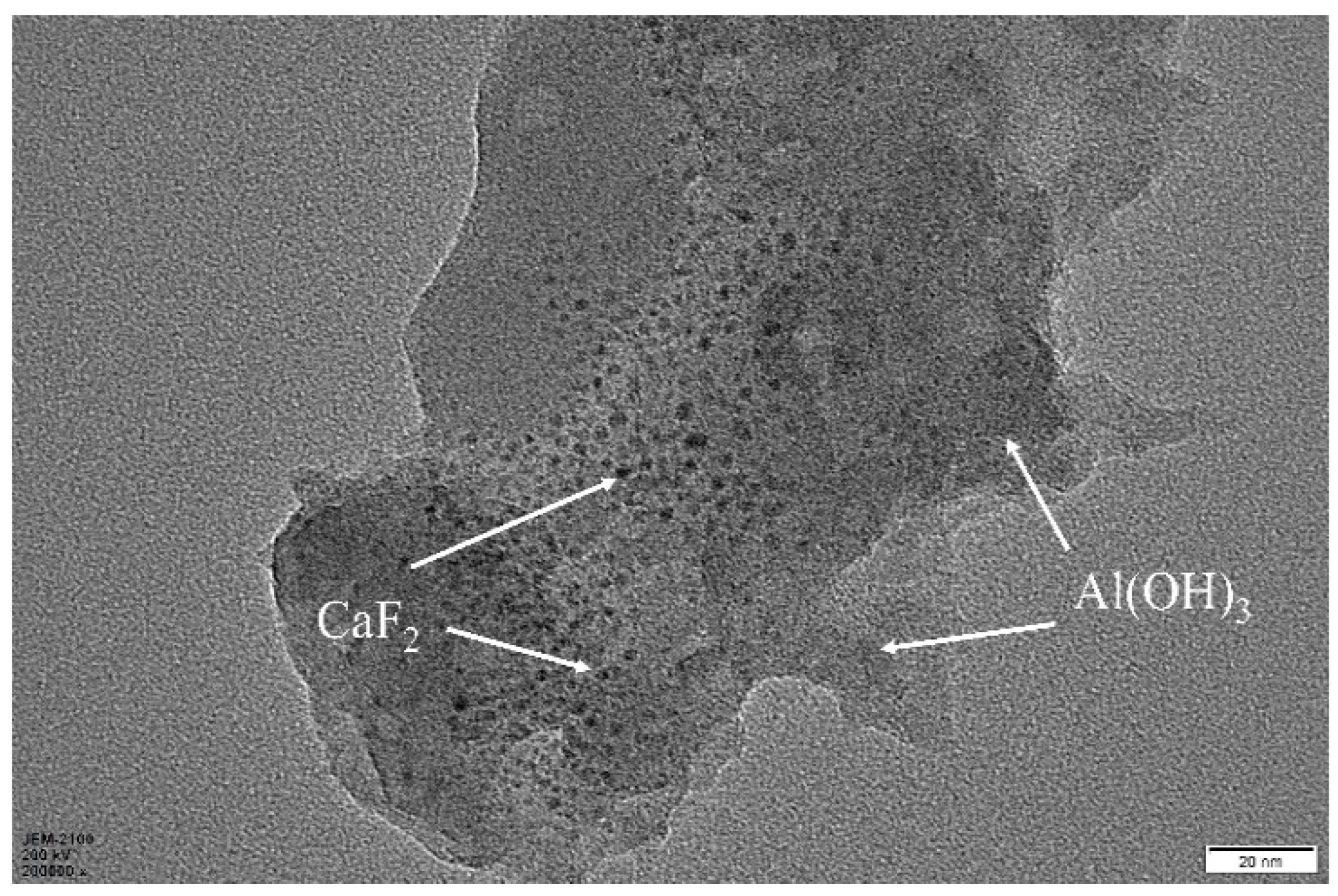
3.2. Metal Ceramic Material Microstructure
3.3. Mechanical Properties of Cermet Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Liu, N.; Liu, A.; Zhang, H. Effects of NiTi additions on the microstructure and mechanical properties of Ti(C,N)-based cermets. Int. J. Refract. Met. Hard Mater. 2013, 40, 43–50. [Google Scholar] [CrossRef]
- Manoj Kumar, B.V.; Balasubramaniam, R.; Basu, B. Electrochemical behavior of TiCN–Ni-based cermets. J. Am. Ceram. Soc. 2007, 90, 205–210. [Google Scholar]
- Wang, Y.; Zou, B.; Huang, C. Tool wear mechanisms and micro-channels quality in micro-machining of Ti-6Al-4V alloy using the Ti(C7N3)-based cermet micro-mills. Tribol. Int. 2019, 134, 60–76. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, B.; Huang, C. Feasibility study of the Ti (C7N3)-based cermet micro-mill based on dynamic fatigue behavior and modeling of the contact stress distribution on the round cutting edge. Int. J. Mech. Sci. 2019, 155, 143–158. [Google Scholar] [CrossRef]
- Hao, Y.; Zou, B.; Wang, J. Effects of sintering temperature and nano Ti(C,N) on the microstructure and mechanical properties of Ti(C,N) cermets cutting tool materials with low Ni-Co. Mater. Sci. Eng. 2017, 705, 98–104. [Google Scholar]
- Xiang, J.; Xie, Z.; Huang, Y. Synthesis of Ti(C,N) ultrafine powders by carbothermal reduction of TiO2 derived from sol-gel process. J. Eur. Ceram. Soc. 2000, 20, 933–938. [Google Scholar] [CrossRef]
- Monteverde, F.; Medri, V.; Bellosi, A. Microstructure of hot-pressed Ti(C,N)-based cermet. J. Eur. Ceram. Soc. 2002, 22, 2587–2593. [Google Scholar] [CrossRef]
- Qu, J.; Xiong, W.; Ye, D. Effect of WC content on the microstructure and mechanical properties of Ti(C0.5N0.5)–WC–Mo–Ni cermets. Inter. J. Refract. Metal. Mater. 2010, 28, 243–249. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, X.Q.; Zhang, Y.H. The effect of Co particle structures on the mechanical properties and microstructure of TiCN-based cermets. Mater. Sci. Eng. A 2016, 675, 164–170. [Google Scholar] [CrossRef]
- Wu, P.; Liu, S.C.; Jiang, X.R. Effect of the Ratio of Co to Ni+Co on the Microstructures and Mechanical Properties of Ti(C,N)-Based Cermets. Key Eng. Mater. 2017, 726, 292–296. [Google Scholar] [CrossRef]
- Xu, Q.; Xing, A.; Zhao, J. Effects of metal binder on the microstructure and mechanical properties of Ti(C,N)-based cermets. J. Alloy Comp. 2015, 644, 663–672. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, X.; Xiu, Z. Influences of sintering temperature on chemical reaction and microstructure in Al2O3-Ti(C0.7N0.3)-cBN composite. J. Nanoelect. Optoelec. 2017, 12, 701–705. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X. Fabrication of a novel Al2O3-Ti(C0.7N0.3)-cBN composite with excellent performance in the turning of difficult-to-machine stellite alloys. Ceram. Int. 2018, 44, 12815–12824. [Google Scholar] [CrossRef]
- Guo, G.; Yu, J.; Luo, Z.; Qian, Z.; Tu, M. Effect of rutile titanium dioxide nano-particles and hindered amine light stabilizer on the ageing resistant properties of abs. Acta Polyme. Sinica. 2008, 8, 733–739. [Google Scholar] [CrossRef]
- Kong, L.; Zhu, S.; Bi, Q. Friction and wear behavior of self-lubricating ZrO2(Y2O3)-CaF2-Mo-graphite composite from 20 °C to 1000 °C. Ceram. Int. 2014, 40, 10787–10792. [Google Scholar] [CrossRef]
- Wu, G.; Xu, C.; Xiao, G. Self-lubricating ceramic cutting tool material with the addition of nickel coated CaF2 solid lubricant powders. Int. J. Refract. Met. Hard Mater. 2016, 56, 51–58. [Google Scholar] [CrossRef]
- Wang, H.M.; Yu, Y.L.; Li, S.Q. Microstructure and tribological properties of laser clad CaF2/Al2O3 self-lubrication wear-resistant ceramic matrix composite coatings. Appl. Laser. 2002, 47, 57–61. [Google Scholar] [CrossRef]
- Ni, J.X.; Chen, L.J.; Zhao, K.M. Preparation of gel-silica/ammonium polyphosphate core-shell flame retardant and properties of polyurethane composites. Polym. Adv. Technol. 2011, 22, 1824–1831. [Google Scholar] [CrossRef]
- Han, M.; Yin, X.; Cheng, L. Effect of core-shell microspheres as pore-forming agent on the properties of porous alumina ceramics. Mater. Design 2017, 113, 384–390. [Google Scholar] [CrossRef]
- Zhao, B.; Shao, G.; Fan, B. Fabrication and enhanced microwave absorption properties of Al2O3 nanoflake-coated Ni core–shell composite microspheres. Rsc Adv. 2014, 4, 57424–57429. [Google Scholar] [CrossRef]
- Han, C.; Jing, M.X.; Shen, X.Q. Fabrication and properties of core–shell structural nano-TiO2@Fe magnetic photocatalyst for removal of phenol waste water. Monatshefte fuer Chemie/Chem. Mon. 2016, 148, 1–6. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, F.; Dong, Q. Core-shell expandable graphite@aluminum hydroxide as a flame-retardant for rigid polyurethane foams. Polym. Degrad. Stab. 2017, 146, 267–276. [Google Scholar] [CrossRef]
- Tejamaya, M.; Römer, I.; Merrifield, R.C. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar]
- Yi, M.; Xu, C.; Chen, Z. Effect of nanosized CaF2 on mechanical properties of selflubricating ceramic material. J. Chin. Ceram. Soc. 2014, 42, 1127–1133. [Google Scholar]
- Li, M.; Xu, C.H.; Chen, Z.Q. Preparation and characterization of Al(OH)3 coated CaF2 composite powder. Adv. Mater. Res. 2014, 5, 1597–1601. [Google Scholar]
- Chen, Z.; Ji, L.; Guo, N. Mechanical properties and microstructure of Al2O3/TiC based self-lubricating ceramic tool with CaF2@Al(HO)3. Inter. J. Refract. Metals Hard Mater. 2018, 75, 50–55. [Google Scholar] [CrossRef]
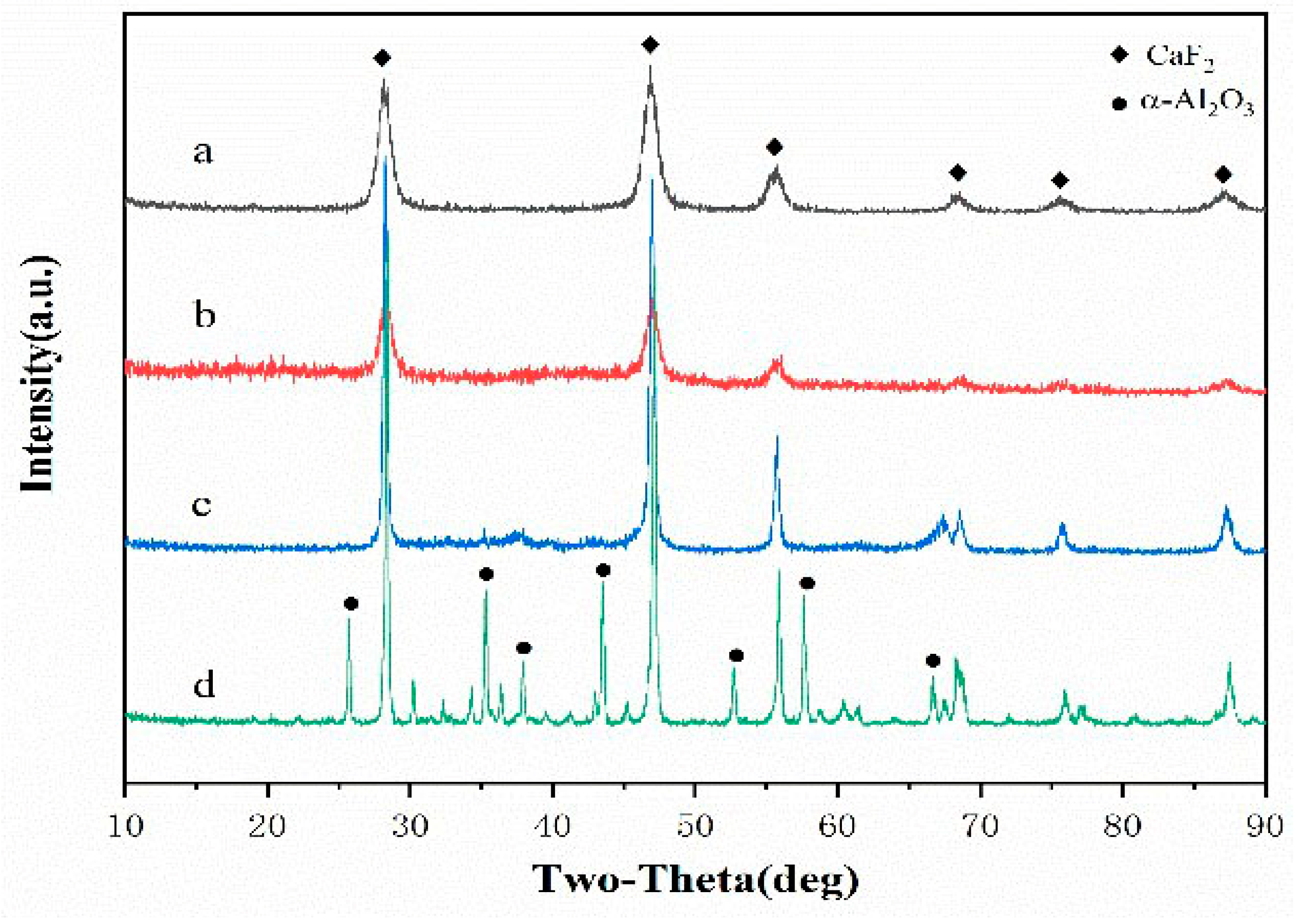


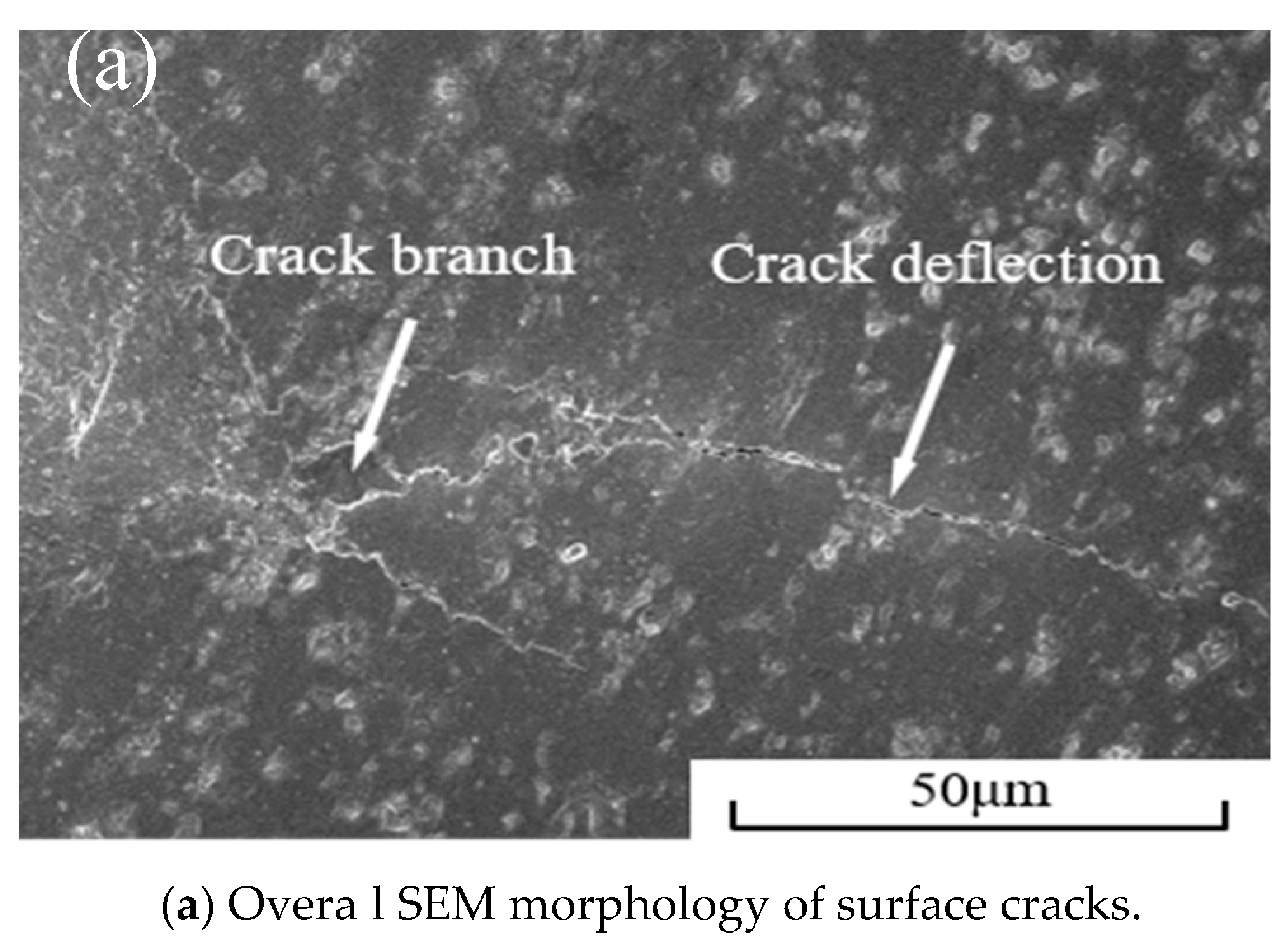
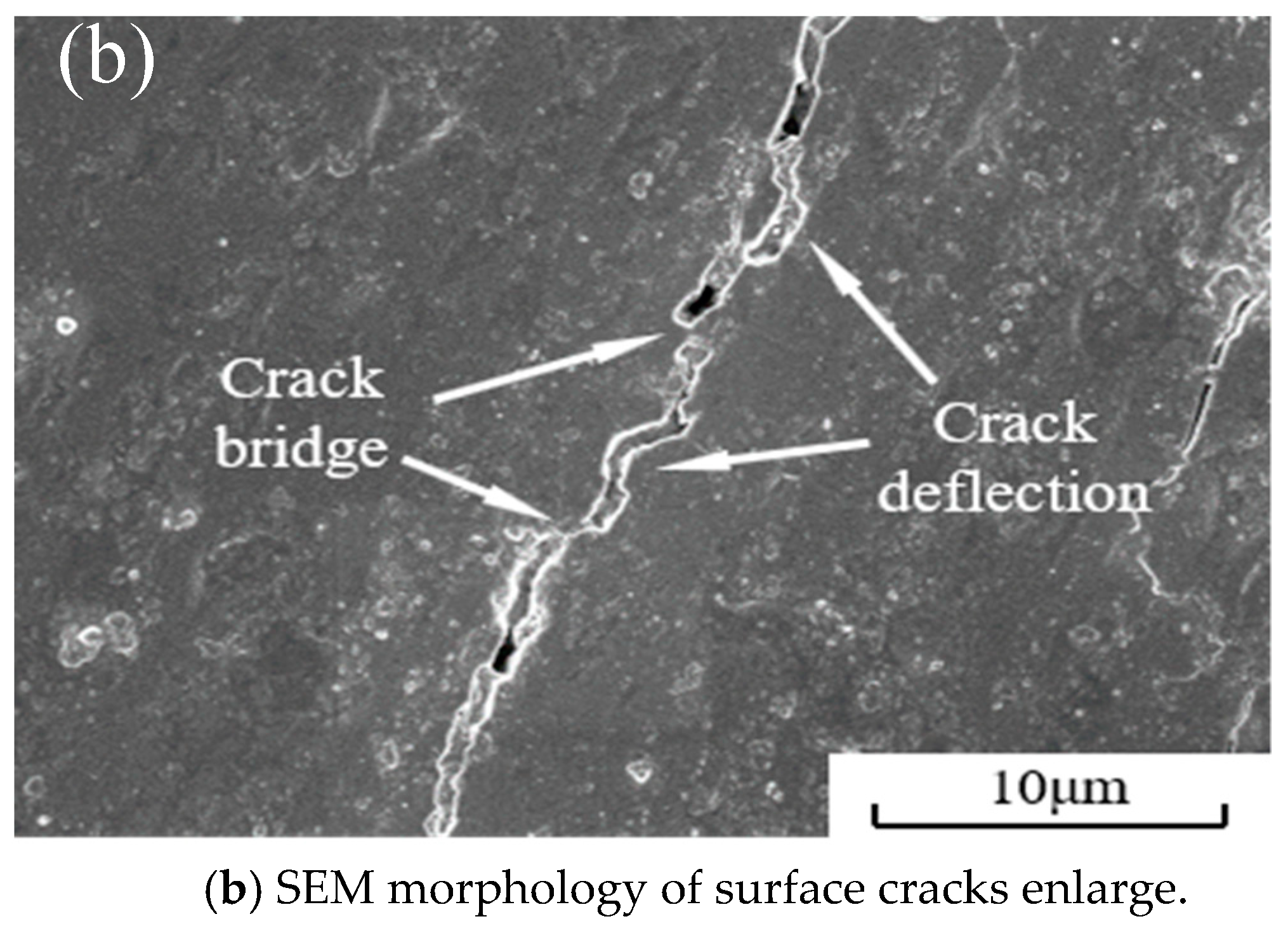
| Component | Flexure Strength /MPa | Fracture Toughness /MPa·m1/2 | Hardness /GPa | Relative Density /% |
|---|---|---|---|---|
| Ti(C,N)/Mo–Co–Ni | 1055 ± 27 | 7.54 ± 0.14 | 19.26 ± 0.22 | 98.87 ± 0.032 |
| Ti(C,N)/Mo–Co–Ni/CaF2/Al2O3 | 1016 ± 20 | 8.11 ± 0.12 | 19.25 ± 0.13 | 98.89 ± 0.033 |
| Ti(C,N)/Mo–Co–Ni/10 vol%CaF2@Al2O3 | 997 ± 21 | 9.94 ± 0.23 | 23.93 ± 0.21 | 99.43 ± 0.021 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yi, M.; Wei, G.; Xu, C. Synthesis and Mechanical Characterization of a Ti(C,N)/Mo–Co–Ni/CaF2@Al2O3 Self-Lubricating Cermet. Materials 2019, 12, 3981. https://doi.org/10.3390/ma12233981
Li C, Yi M, Wei G, Xu C. Synthesis and Mechanical Characterization of a Ti(C,N)/Mo–Co–Ni/CaF2@Al2O3 Self-Lubricating Cermet. Materials. 2019; 12(23):3981. https://doi.org/10.3390/ma12233981
Chicago/Turabian StyleLi, Chuanhao, Mingdong Yi, Gaofeng Wei, and Chonghai Xu. 2019. "Synthesis and Mechanical Characterization of a Ti(C,N)/Mo–Co–Ni/CaF2@Al2O3 Self-Lubricating Cermet" Materials 12, no. 23: 3981. https://doi.org/10.3390/ma12233981
APA StyleLi, C., Yi, M., Wei, G., & Xu, C. (2019). Synthesis and Mechanical Characterization of a Ti(C,N)/Mo–Co–Ni/CaF2@Al2O3 Self-Lubricating Cermet. Materials, 12(23), 3981. https://doi.org/10.3390/ma12233981





