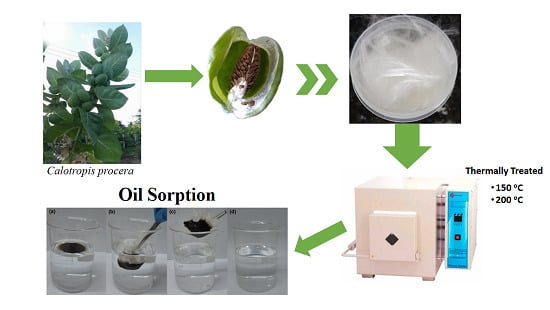
Evaluation of Thermally Treated Calotropis Procera Fiber for the Removal of Crude Oil on the Water Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Fiber Thermal Treatment
2.3. Fiber Characterization
2.4. Selectivity Assay
2.5. Sorption Assay
3. Results
3.1. Characterization of the Calotropis Procera Fibers
3.1.1. Thermogravimetric Analysis—TG
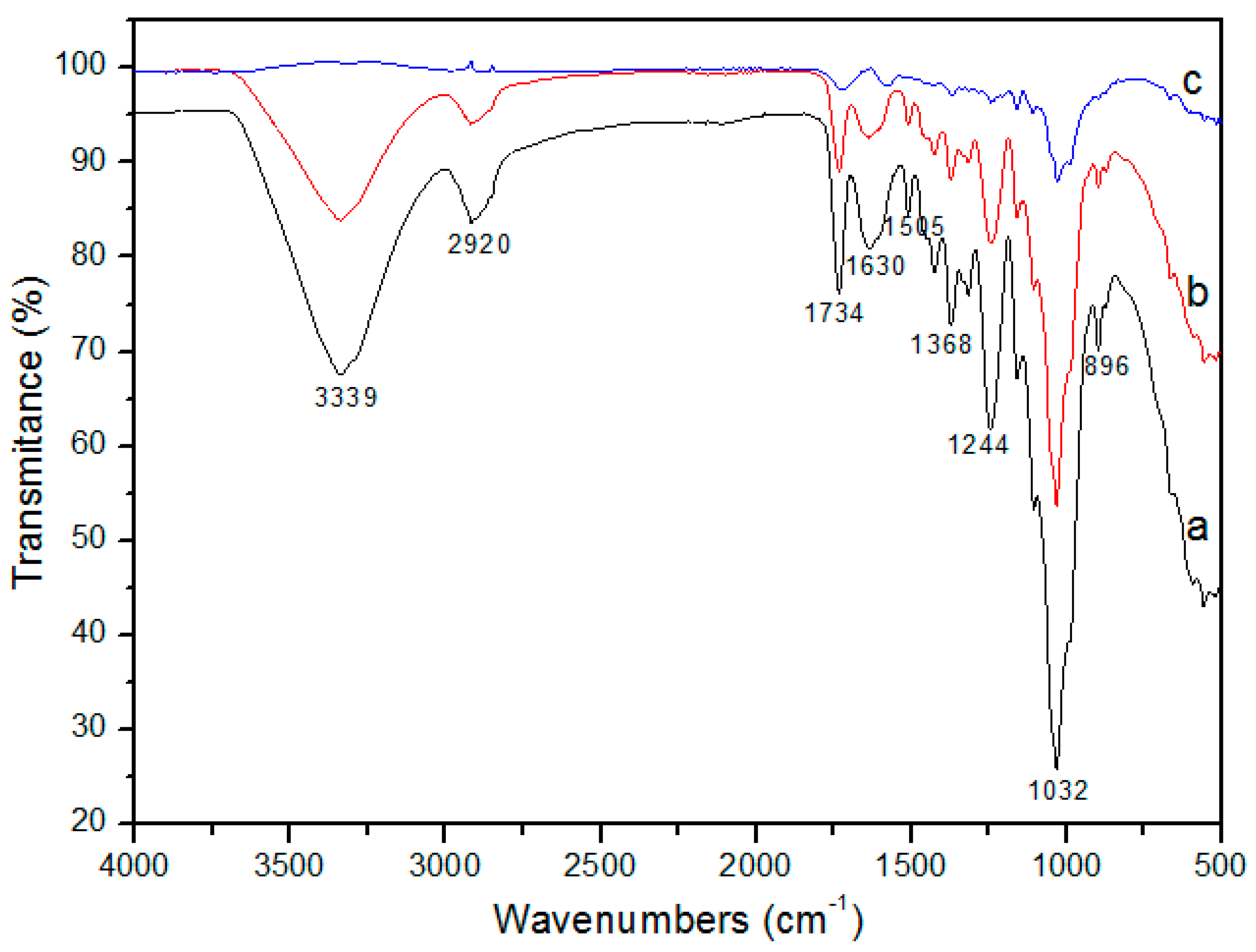
3.1.2. FTIR Analysis
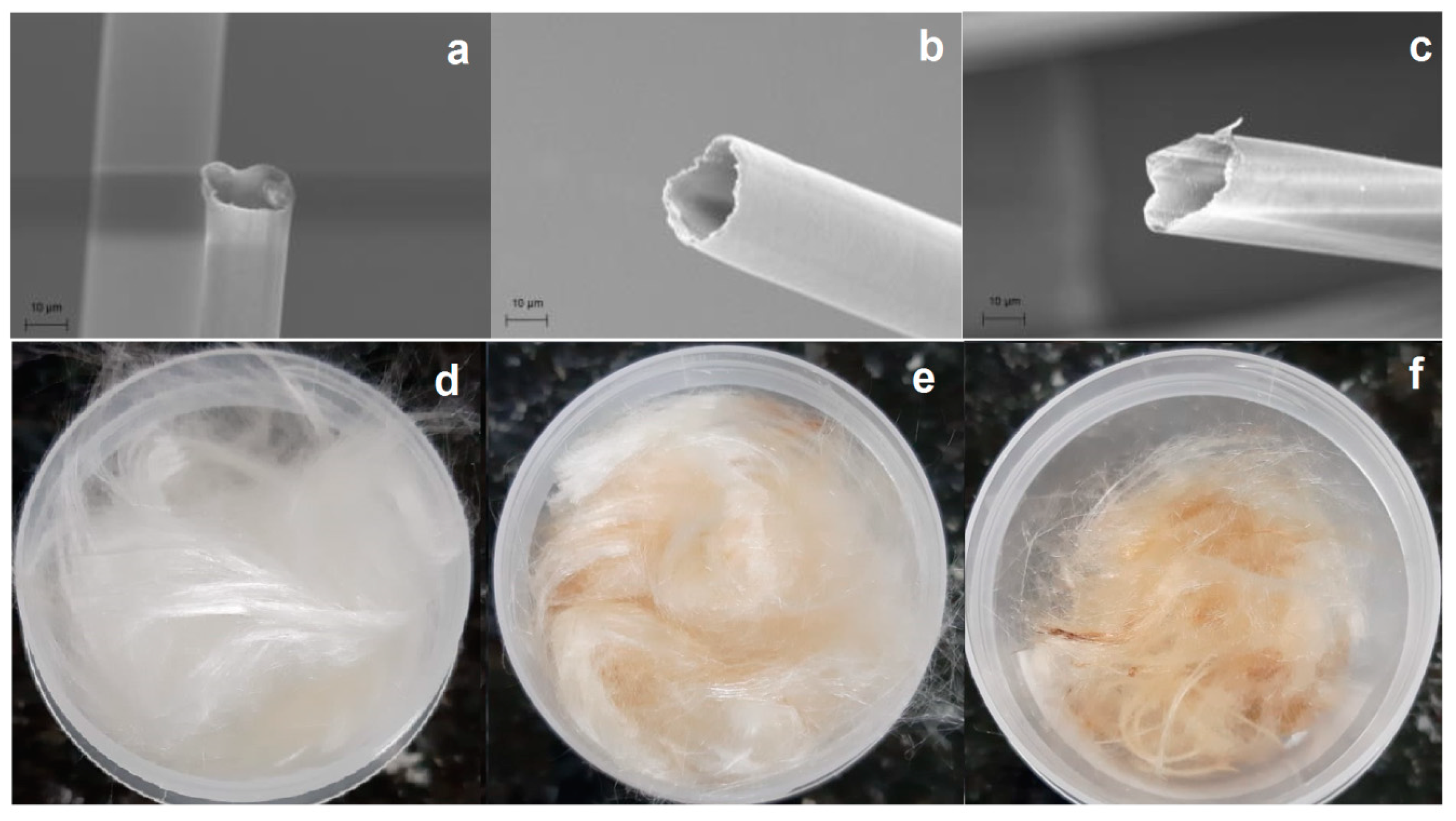
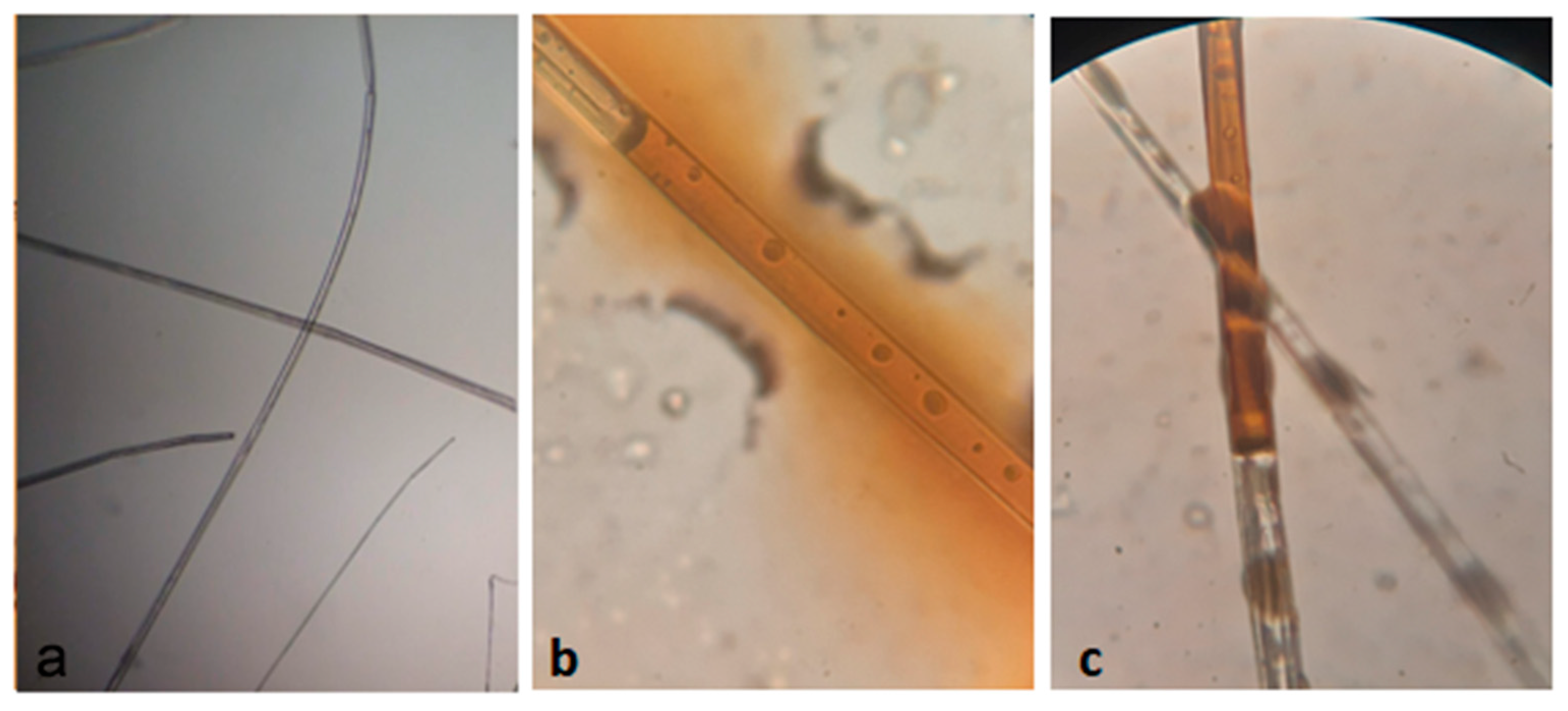
3.1.3. Morphological Analysis—SEM-FEG
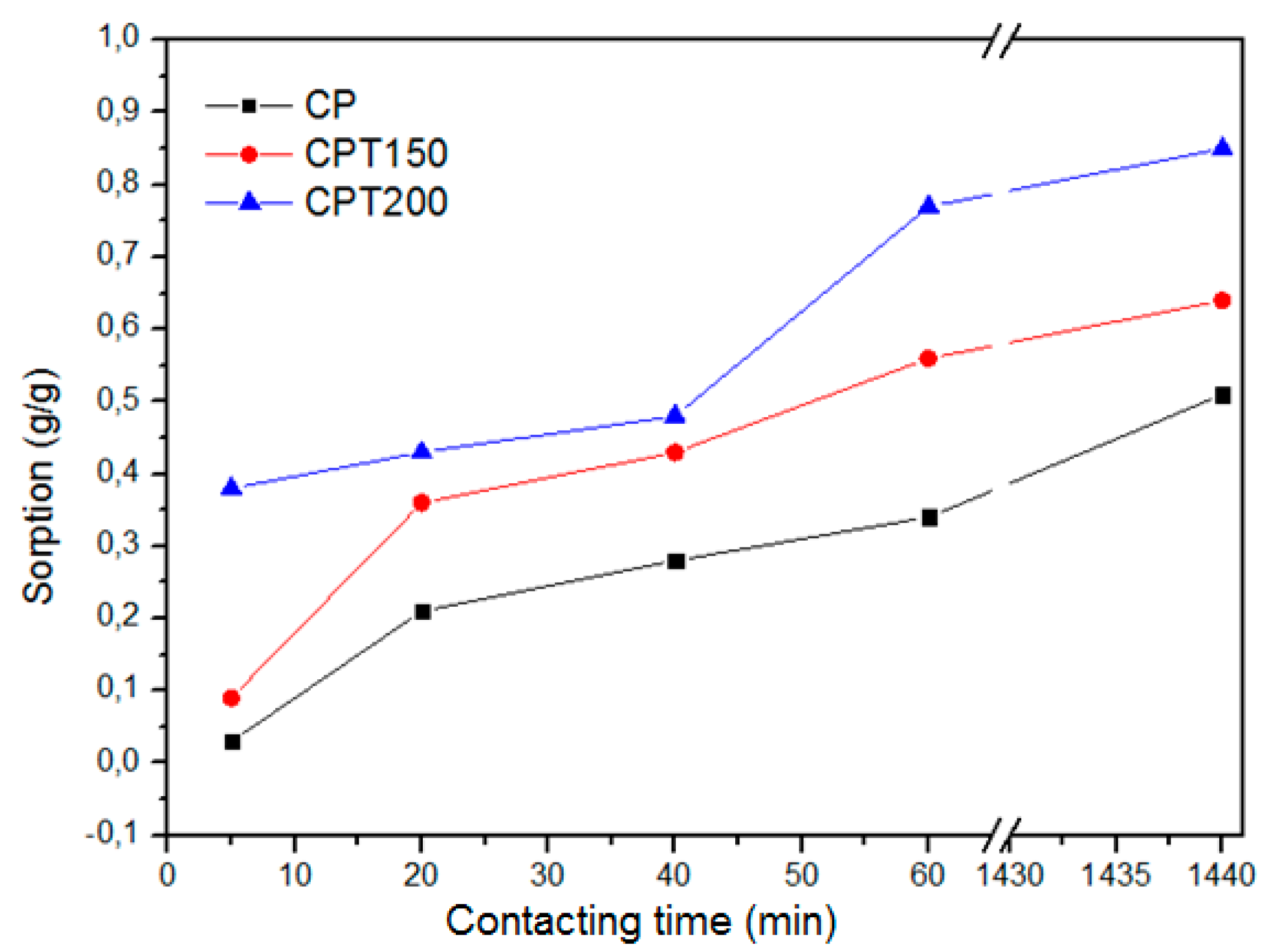
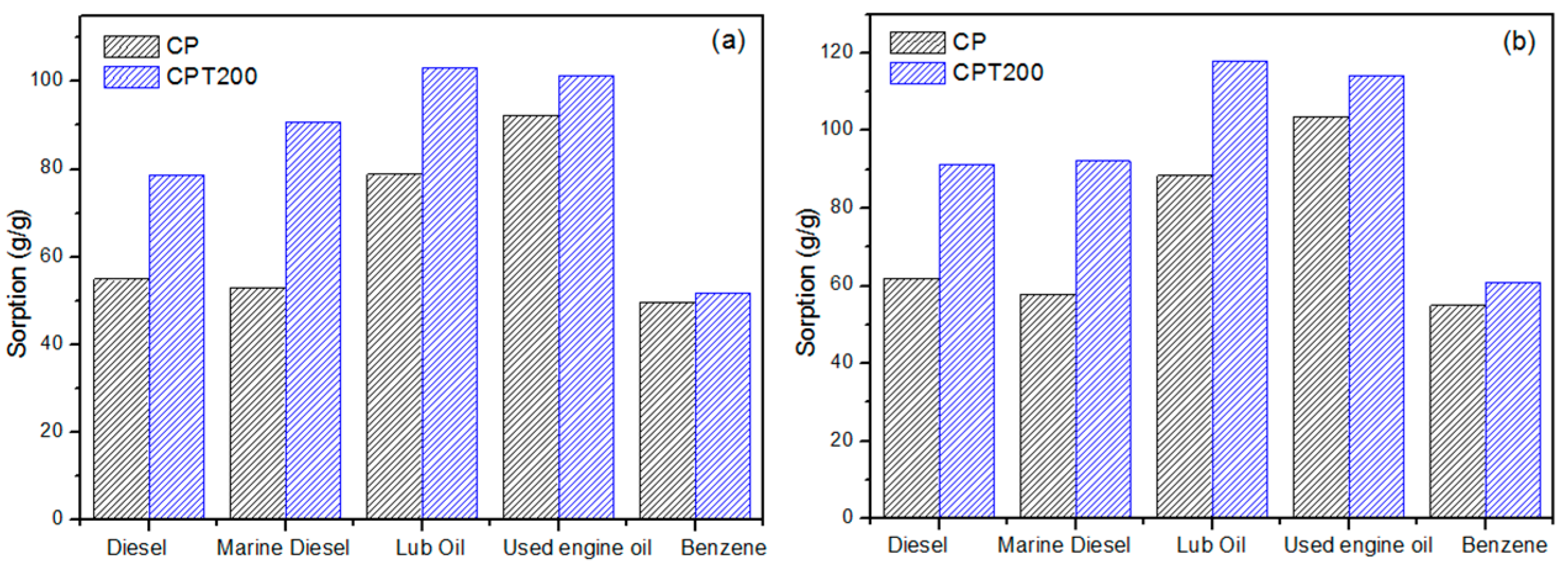
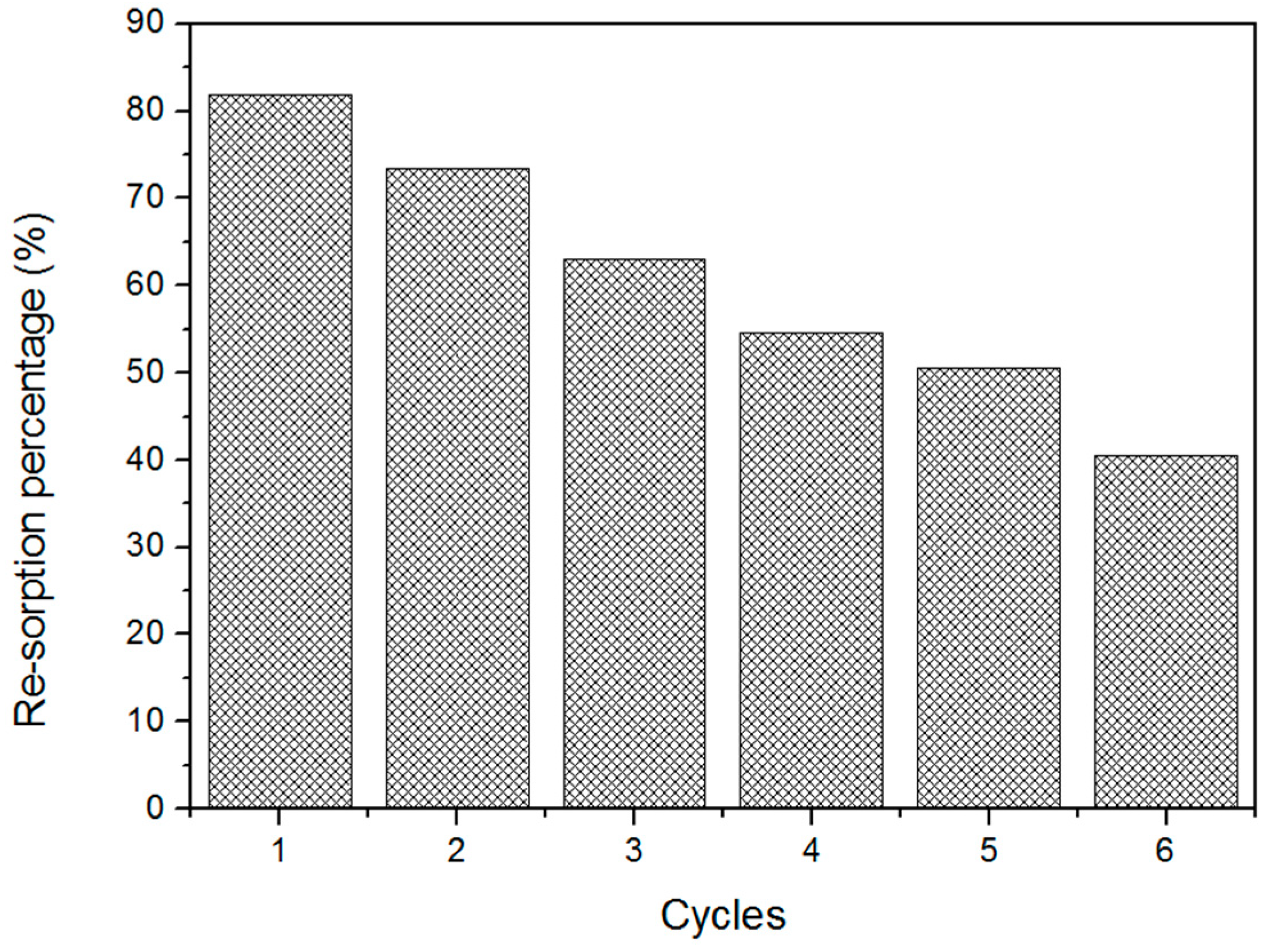
3.2. Sorption Capacity
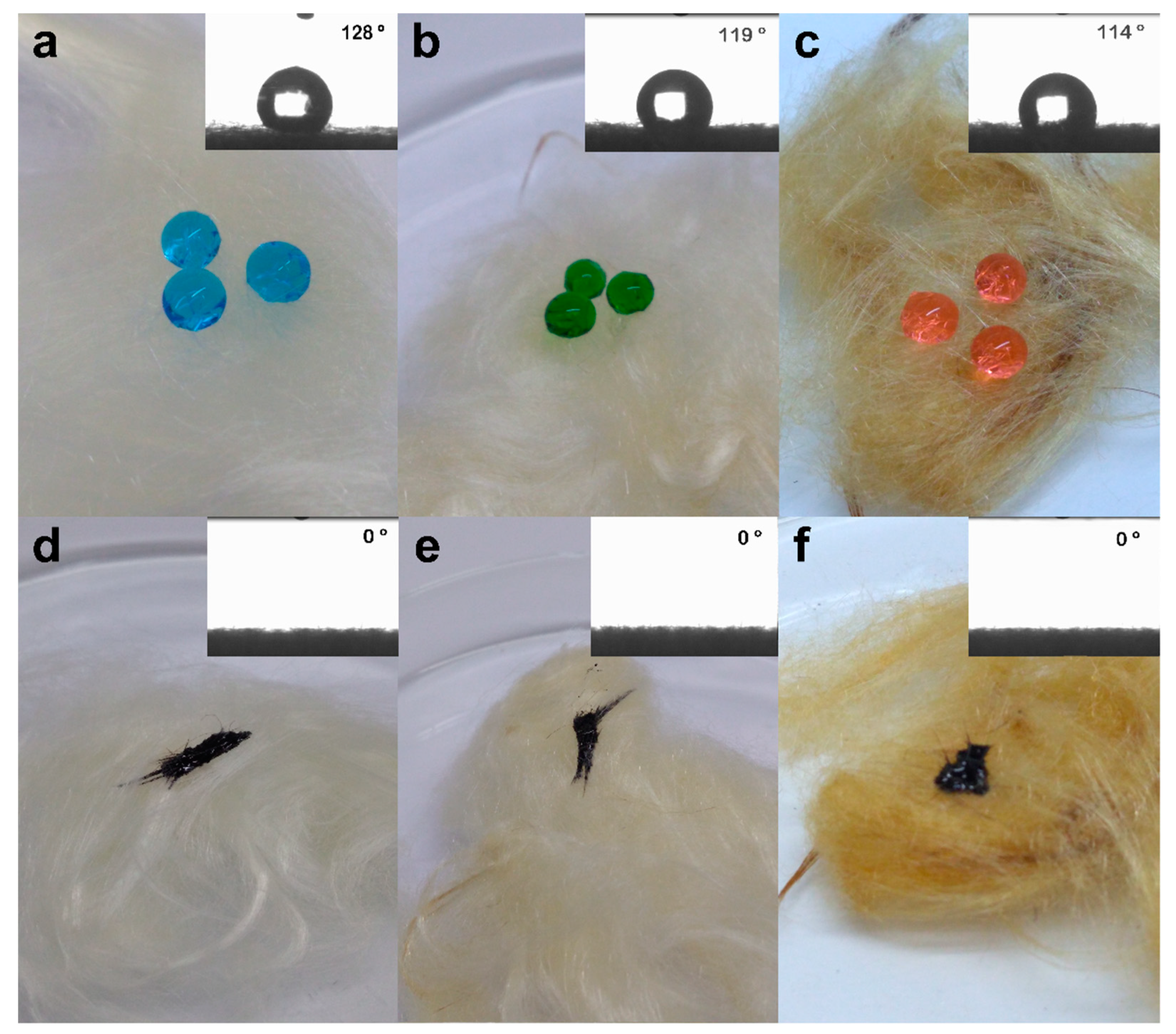
3.3. Wettability and Contact Angle
3.4. Selectivity Test
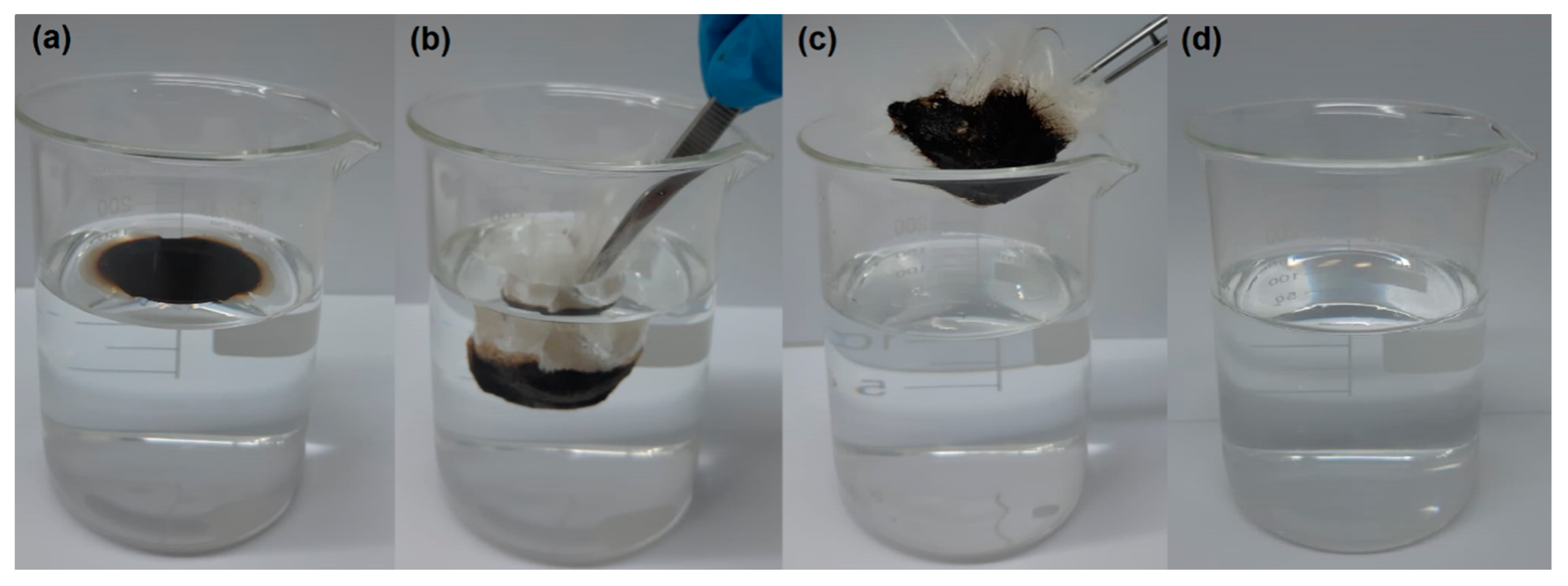
3.5. Oil Fixation to the Sorbent
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paul, J.H.; Hollander, D.; Coble, P.; Daly, K.L.; Murasko, S.; English, D.; Basso, J.; Delaney, J.; McDaniel, L.; Kovach, C.W. Toxicity and mutagenicity of gulf of Mexico waters during and after the deepwater horizon oil spill. Environ. Sci. Technol. 2013, 47, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Nabi, D.; Würz, B.; Wick, L.Y.; Brussaard, C.P.D.; Huisman, J.; van der Meer, J.R.; Reddy, C.M.; Arey, J.S. First day of an oil spill on the open sea: Early mass transfers of hydrocarbons to air and water. Environ. Sci. Technol. 2014, 48, 9400–9411. [Google Scholar] [CrossRef] [PubMed]
- Zengel, S.; Montague, C.L.; Pennings, S.C.; Powers, S.P.; Steinhoff, M.; Fricano, G.; Schlemme, C.; Zhang, M.; Oehrig, J.; Nixon, Z.; et al. Impacts of the deepwater horizon oil spill on salt marsh periwinkles (Littoraria irrorata). Environ. Sci. Technol. 2016, 50, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Kim, J.A.; Park, M.S.; Yim, U.H.; Choi, K. Human health and ecological assessment programs for Hebei Spirit oil spill accident of 2007: Status, lessons, and future challenges. Chemosphere 2017, 173, 180–189. [Google Scholar] [CrossRef]
- Annunciado, T.R.; Sydenstricker, T.H.D.; Amico, S.C. Experimental investigation of various vegetable fibers as sorbent materials for oil spill. Mar. Pollut. Bull. 2005, 50, 1340–1346. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, H.Y.; Zhu, H.W.; Huang, J.; Shi, L.A.; Yu, S.H. Advanced sorbents for oil-spill cleanup: Recent advances and future perspectives. Adv. Mater. 2016, 28, 10459–11049. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y. Oil/water mixtures and emulsions separation of stearic acidfunctionalized sponge fabricated via a facile one-step coating method. Sep. Purif. Technol. 2017, 181, S183–S191. [Google Scholar] [CrossRef]
- Reed, M.; Johansen, O.; Brandvik, P.J.; Daling, P.; Lewis, A.; Fiocco, R.; Mackay, D.; Prentki, R. Oil spill modeling towards the close of the 20th century: Overview of the state of the art. Spill Sci. Technol. Bull. 1999, 1, 3–16. [Google Scholar] [CrossRef]
- Wei, Q.F.; Mather, R.R.; Fotheringham, A.F.; Yang, R.D. Evaluation of nonwoven polypropylene oil sorbents in marine oilspillrecovery. Mar. Pollut. Bull. 2003, 6, 780–783. [Google Scholar] [CrossRef]
- Prince, R.C. Oil spill dispersants: Boon or bane? Environ. Sci. Technol. 2015, 49, 6376–6384. [Google Scholar] [CrossRef]
- Gelderen, L.; Van, L.M.V.; Malmquist, G.J. Vaporization order and burning efficiency of crude oils during in-situ burning on water. Fuel 2017, 191, 528–537. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Enhanced bioremediation of oil spills in the sea. Curr. Opin. Biotechnol. 2014, 27, 191–194. [Google Scholar] [CrossRef]
- Yu, L.; Hao, G.; Xiao, L.; Yin, Q.; Xia, M.; Jiang, W. Robust magnetic polystyrene foam for high efficiency and removal oil from water surface. Sep. Purif. Technol. 2017, 173, 121–128. [Google Scholar] [CrossRef]
- Tu, L.; Duan, W.; Xiao, W.; Fu, C.; Wang, A.; Zheng, Y. Calotropis gigantean fiber derived carbon fiber enables fast and efficient absorption of oils and organic solvents. Sep. Purif. Technol. 2018, 192, 30–35. [Google Scholar] [CrossRef]
- Ma, Q.; Cheng, H.; Fane, A.G.; Wang, R.; Zhang, H. Recent development of advanced materials with special wettability for selective oil/water separation. Small 2016, 12, 2186–2202. [Google Scholar] [CrossRef] [PubMed]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies: A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Saleem, J.; Riaz, M.A.; McKay, G. Oil sorbents from plastic wastes and polymers: A review. J. Hazard. Mater. 2018, 341, 424–437. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, M.; Du, C.; Guo, H.; Bai, H.; Li, L. Poly(dimethylsiloxane) oil absorbent with a three-dimensionally interconnected porous structure and swellable skeleton. ACS Appl. Mater. Interfaces 2013, 5, 10201–10206. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Li, B.; Zhang, J.; Wang, A. Magnetic, durable, and superhydrophobic polyurethane@Fe O @SiO @fluoropolymer sponges for selective oil absorption and oil/water separation. ACS Appl. Mater. Interfaces 2015, 7, 4936–4946. [Google Scholar] [CrossRef]
- Ke, Q.; Jin, Y.; Jiang, P.; Yu, J. Oil/water separation performances of superhydrophobic and superoleophilic sponges. Langmuir 2014, 30, 13137–13142. [Google Scholar] [CrossRef]
- Pan, Y.; Shi, K.; Peng, C.; Wang, W.; Liu, Z.; Ji, X. Evaluation of hydrophobic polyvinylalcohol formaldehyde sponges as absorbents for oil spill. ACS Appl. Mater. Interfaces 2014, 6, 8651–8659. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Alhazzaa, M.I.; Asif, M. Treatment of oily water using hydrophobic nanosilica. Chem. Eng. J. 2011, 167, 99–103. [Google Scholar] [CrossRef]
- Seal, S.; Sakthivel, T.; Reid, D.; Goldstein, I.; Hench, L. Hydrophobic high surfasse area zeolites derived from fly ash for oil spill remediation. Env. Sci. Technol. 2013, 47, 5843–5850. [Google Scholar]
- Bastani, D.; Safekordi, A.; Alihosseini, A.; Taghikhani, V. Study of oil sorption by expanded perlite at 298.15 K. Sep. Purif. Technol. 2006, 52, 295–300. [Google Scholar] [CrossRef]
- Zadaka-Amir, D.; Bleiman, N.; Mishael, Y.G. Sepiolite as an effective natural porous adsorbent for surface oil-spill. Microporous Mesoporous Mater. 2013, 169, 153–159. [Google Scholar] [CrossRef]
- Mysore, D.; Viraragavan, T.; Jin, Y. Treatment of oily waters using vermiculite. Water Res. 2005, 39, 2643–2653. [Google Scholar] [CrossRef]
- Adebajo, M.O.; Frost, R.L. Acetylation of raw cotton for oil spill cleanup application: An FTIR and 13C MAS NMR spectroscopic investigation. Spectrochim. Acta A 2004, 10, 2315–2321. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Kang, Y.; Wang, A. Investigation of oil sorptioncapability of PBMA/SiO2 coated kapok fiber. Chem. Eng. J. 2013, 223, 632–637. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, A. Coated kapok fiber for removal of spilled oil. Mar. Pollut. Bull. 2013, 69, 91–96. [Google Scholar] [CrossRef]
- Lim, T.; Huang, X. Evaluation of kapok (Ceiba pentandra (L.) Gaertn.) as anatural hollow hydrophobic–oleophilic fibrous sorbent for oil spill cleanup. Chemosphere 2007, 66, 955–963. [Google Scholar] [CrossRef]
- Lim, T.; Huang, X. Evaluation of hydrophobicity/oleophilicity of kapok andits performance in oily water filtration: Comparison of raw and solvent-treatedfibers. Ind. Crop. Prod. 2007, 26, 125–134. [Google Scholar] [CrossRef]
- Murti, Y.; Yogi, B.; Pathak, D. Pharmacognostic standardization of leaves of Calotropis Procera (Ait) R. Br. (Asclepiadaceae). Int. J. Ayurveda Res. 2010, 1, 14–17. [Google Scholar] [CrossRef]
- Nascimento, J.H.O.; Coelho, M.P.G.; Silva, A.P.; Silva, K.K.O.S.; dos Santos, A.R.L.; Campos, C.F.; Morais, J.P.S.; Sivam, R.L. Removal of Crude Oil Using a New Natural Fibre—Calotropis procera. In Natural Fibres: Advances in Science and Technology Towards Industrial Applications; Springer Netherlands: Haarlem, The Netherlands, 2016; pp. 113–125. [Google Scholar]
- Razavi, Z.; Mirghaffari, N.; Rezaei, B. Performance Comparison of Raw and Thermal Modified Rice Husk for Decontamination of Oil Polluted Water. Clean—Soil, Air. Water 2015, 43, 182–190. [Google Scholar]
- Anuzyte, E.; Vaisis, V. Natural oil sorbents modification methods for hydrophobicity improvement. Energy Procedia 2018, 147, 295–300. [Google Scholar] [CrossRef]
- Husseien, M.; Amer, A.A.; El-Maghraby, A.; Taha, N.A. Experimental Investigation of Thermal Modification Influence on Sorption Qualities of Barley Straw. J. Appl. Sci. Res. 2008, 4, 652–657. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Riegel, I.; Moura, A.B.D.; Morisso, F.P.; Mello, F.S. Análise termogravimétrica da pirólise da acácia-negra (Acacia mearnsii de Wild.) cultivada no rio grande do sul, Brasil. Revista Árvore 2008, 32, 533–543. [Google Scholar] [CrossRef]
- Yao, F.W.U.; Lei, Y.; Guo, W.; Xu, Y. Thermal decomposition kinetics of natural fibers: Activition energy with dynamic thermogravimetric analysis. Polym. Degrad. Stab. 2008, 93, 90–98. [Google Scholar] [CrossRef]
- Celino, A.; Gonçalves, O.; Jacquemin, F. Qualitative and quantitative assessment of water sorption in natural fibres using ATR-FTIR spectroscopy. Carbohydr. Polym. 2014, 101, 163–170. [Google Scholar] [CrossRef]
- Oun, A.A.; Rhim, J.W. Characterization of nanocelluloses isolated from Ushar (Calotropis procera) seed fiber: Effect of isolation method. Mater. Lett. 2016, 168, 146–150. [Google Scholar] [CrossRef]
- Boni, H.T.; De Oliveira, D.; Ulson De Souza, A.A.; Ulson De Souza, S.M.A.G. Bioadsorption by sugarcane bagasse for the reduction in oil and grease content in aqueous effluent. Int. J. Environ. Sci. Technol. 2016, 13, 1169–1176. [Google Scholar] [CrossRef]
- Likon, M.; Remškar, M.; Ducman, V. Populus seed fibers as a natural source for production of oil super absorbents. J. Environ. Manag. 2013, 114, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Barka, N.; Ouzaouit, K.; Abdennouri, M.; El Makhfouk, M. Dried prickly pear cactus (Opuntia ficus indica) cladodes as a low-cost and eco-friendly biosorbent for dyes removal from aqueous solutions. J. Taiwan Inst. Chem. Eng. 2013, 44, 52–60. [Google Scholar] [CrossRef]
- Mizi, F.; Dasong, D.; Biao, H. Fourier Transform Infrared Spectroscopy for Natural Fibres. In Fourier Transform—Materials Analysis; Salih, S., Ed.; Intech: London, UK, 2012; Volume 83, pp. 46–68. ISBN 978-953-51-0594-7. [Google Scholar]
- Mohebby, B. Application of ATR infrared spectroscopy in wood acetylation. J. Agric. Sci. Technol. 2008, 10, 253–259. [Google Scholar]
- Hergert, H.L. Lignins, Occurrence, Formation, Structure and Reaction; Wiley—Interscience: New York, NY, USA, 1971. [Google Scholar]
- Zheng, Y.; Cao, E.; Zhu, Y.; Wang, A.; Hu, H. Perfluorosilane treated Calotropis gigantea fiber: Instant hydrophobic–oleophilic surface with efficient oil-absorbing performance. Chem. Eng. J. 2016, 295, 477–483. [Google Scholar] [CrossRef]
- Draman, S.F.S.; Daik, R.; Latif, F.A.; El-Sheikh, S.M. Characterization and thermal decomposition kinetics of kapok (Ceiba pentandra L.)- based cellulose. BioResources 2014, 9, 8–23. [Google Scholar] [CrossRef]
- Thilagavathi, G.; Karan, C.P.; Das, D. Oil sorption and retention capacities of thermally-bonded hybrid nonwovens prepared from cotton, kapok, milkweed and polypropylene fibers. J. Environ. Manag. 2018, 219, 340–349. [Google Scholar] [CrossRef]
- Kalia, S.; Kaith, B.S.; Kaur, I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym. Eng. Sci. 2009, 49, 1253–1272. [Google Scholar] [CrossRef]
- Moriwaki, H.; Kitajima, S.; Kurashima, M.; Hagiwara, A.; Haraguchi, K.; Shirai, K.; Kanekatsu, R.; Kiguchi, K. Utilization of silkworm cocoon waste as a sorbent for the removal of oil from water. J. Hazard. Mater. 2009, 165, 266–270. [Google Scholar] [CrossRef]
- Hussein, M.; Amer, A.A.; Sawsan, I.I. Heavy oil spill cleanup using law grade raw cotton fibers: Trial for practical application. J. Pet. Technol. Altern. Fuels 2011, 2, 132–140. [Google Scholar]
- Cojocaru, C.; Macoveanu, M.; Cretescu, I. Peat-based sorbents for the removal of oil spills from water surface: Application of artificial neural network modeling. Colloids Surf. A 2011, 1–3, 675–684. [Google Scholar] [CrossRef]
- Wang, B.; Karthikeyan, R.; Lu, X.Y.; Xuan, J.; Leung, M.K.H. Hollow Carbon Fibers Derived from Natural Cotton as Effective Sorbents for Oil Spill Cleanup. Ind. Eng. Chem. Res. 2013, 52, 18251–18261. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Wu, J.; Yuan, T.; Sun, R. Preparation and Characterization of Lignocellulosic Oil Sorbent by Hydrothermal Treatment of Populus Fiber. Materials 2014, 7, 6733–6747. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, A.; Cojocaru, C.; Cretescu, I.; Pinteala, M.; Timpu, D.; Sacarescu, L.; Harabagiu, V. Performances of clay aerogel polymer composites for oil spill sorption:Experimental design and modeling. Sep. Purif. Technol. 2014, 133, 260–275. [Google Scholar] [CrossRef]
- Feng, J.; Nguyen, S.T.; Fan, Z.; Duong, H.M. Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels. Chem. Eng. J. 2015, 270, 168–175. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhub, Y.Y.; Wangb, A.; Huc, H. Potential of Calotropis gigantea fiber as an absorbent for removal of oil from water. Ind. Crop. Prod. 2016, 83, 387–390. [Google Scholar] [CrossRef]
- Cojocaru, C.; Pricop, L.; Samoila, P.; Rotaru, R.; Harabagiu, V. Surface hydrophobization of polyester fibers with poly(methylhydrodimethyl) siloxane copolymers: Experimental design for testing of modified nonwoven materials as oil spill sorbents. Polym. Test. 2017, 59, 377–389. [Google Scholar] [CrossRef]
- Cojocaru, C.; Dorneanu, P.P.; Airinei, A.; Olaru, N.; Samoila, P.; Rotaru, A. Design and evaluation of electrospun polysulfone fibers and polysulfone/ NiFe2O4 nanostructured composite as sorbents for oil spill cleanup. J. Taiwan Inst. Chem. Eng. 2017, 70, 267–281. [Google Scholar] [CrossRef]
- Balzamo, G.; Singh, N.; Wang, N.; Vladisavljevi’c, G.T.; Bolognesi, G.; Mele, E. 3D Arrays of Super-Hydrophobic Microtubes from Polypore Mushrooms as Naturally-Derived Systems for Oil Absorption. Materials 2019, 12, 132. [Google Scholar] [CrossRef]
- Teas, C.; Kalligeros, S.; Zanikos, F.; Stournas, S.; Lois, E.; Anastopoulos, G. Investigation of the effectiveness of absorbent materials in oil spills clean up. Desalination 2001, 140, 259–264. [Google Scholar] [CrossRef]
- Karan, C.P.; Rengasamy, R.S.; Das, D. Oil spill cleanup by structured fibre assembly. Indian J. Fibre Text. Res. 2011, 36, 190–200. [Google Scholar]


| Materials | Treatment | Sorption Capacity—Oil | Author |
|---|---|---|---|
| Sumauma Fiber | Packed | 36 g/g—Diesel 43 g/g—Hydraulic oil 45 g/g—Motor oil | [30] |
| Barley straw | Pyrolyzed | 5.9–7.6 g/g—Diesel 8.1–9.2 g/g—Heavy oil | [36] |
| Silkworm cocoon | Cocoon residues | 42–52 g/g—Motor oil 37–60 g/g—Vegetable oil | [52] |
| Cotton fiber | Loose fiber Fiber pad shape | 22.5 g/g—Lubricating oil 18.43 g/g—Lubricating oil | [53] |
| Peat | Granular | 9–12 g/g—Diesel | [54] |
| Cotton fiber | Carbonized in N2 atmosphere | 32–77 g/g—Crude oil and solvents | [55] |
| Populus fiber | Acetylation | 21.57 g/g—Corn oil | [56] |
| Clay polymer aerogel | Aerogel | 23.6 g/g—Dodecane | [57] |
| Celulose aerogel | Methyltrimetoxissyan | 40–95 g/g—Oil | [58] |
| Calotropis gigantea fiber | In natura | 22.6–47.6 g/g—Oil and organic solvents | [59] |
| Non-polyester fabrics fiber | (Methylhydro-dimetil) siloxane | 5.52 g/g—Dodecane 10.03 g/g—Motor oil | [60] |
| Nanostructured electrospun fibers | Polissulfona/NiFe2O4 | 9.20 g/g—Dodecane 15.11 g/g—Motor oil | [61] |
| Calotropis gigantea fiber | Carbonized | 80–130 g/g—Oil and organic solvents | [14] |
| Mix of cotton, Sumauma, Asclepias Syriaca, Calotropis procera, Gigantea Polypropylene | Thermal | 40.16 g/g—Heavy oil 23.00 g/g—Diesel | [50] |
| Ganoderma applanatum mushroom | PFOCTS* | 1.8–3.1 g/g—Oil | [62] |
| Calotropis procera | In natura | 74.04 g/g—Petroleum | This research |
| Calotropis procera | Thermal | 124.60 g/g—Petroleum | This research |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobral Hilário, L.; Batista dos Anjos, R.; Borges de Moraes Juviniano, H.; Ribeiro da Silva, D. Evaluation of Thermally Treated Calotropis Procera Fiber for the Removal of Crude Oil on the Water Surface. Materials 2019, 12, 3894. https://doi.org/10.3390/ma12233894
Sobral Hilário L, Batista dos Anjos R, Borges de Moraes Juviniano H, Ribeiro da Silva D. Evaluation of Thermally Treated Calotropis Procera Fiber for the Removal of Crude Oil on the Water Surface. Materials. 2019; 12(23):3894. https://doi.org/10.3390/ma12233894
Chicago/Turabian StyleSobral Hilário, Larissa, Raoni Batista dos Anjos, Henrique Borges de Moraes Juviniano, and Djalma Ribeiro da Silva. 2019. "Evaluation of Thermally Treated Calotropis Procera Fiber for the Removal of Crude Oil on the Water Surface" Materials 12, no. 23: 3894. https://doi.org/10.3390/ma12233894
APA StyleSobral Hilário, L., Batista dos Anjos, R., Borges de Moraes Juviniano, H., & Ribeiro da Silva, D. (2019). Evaluation of Thermally Treated Calotropis Procera Fiber for the Removal of Crude Oil on the Water Surface. Materials, 12(23), 3894. https://doi.org/10.3390/ma12233894