Open-Cell Aluminum Foams by the Sponge Replication Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Characterisation
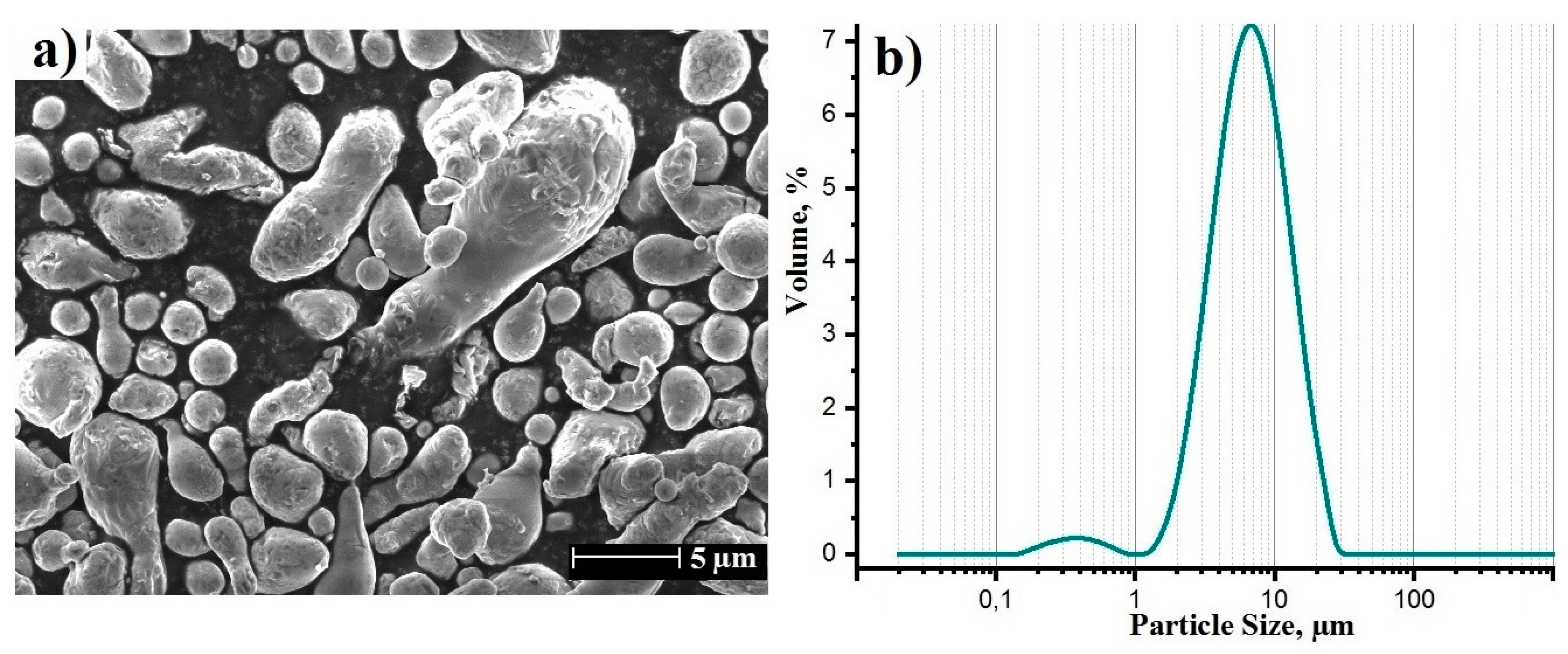
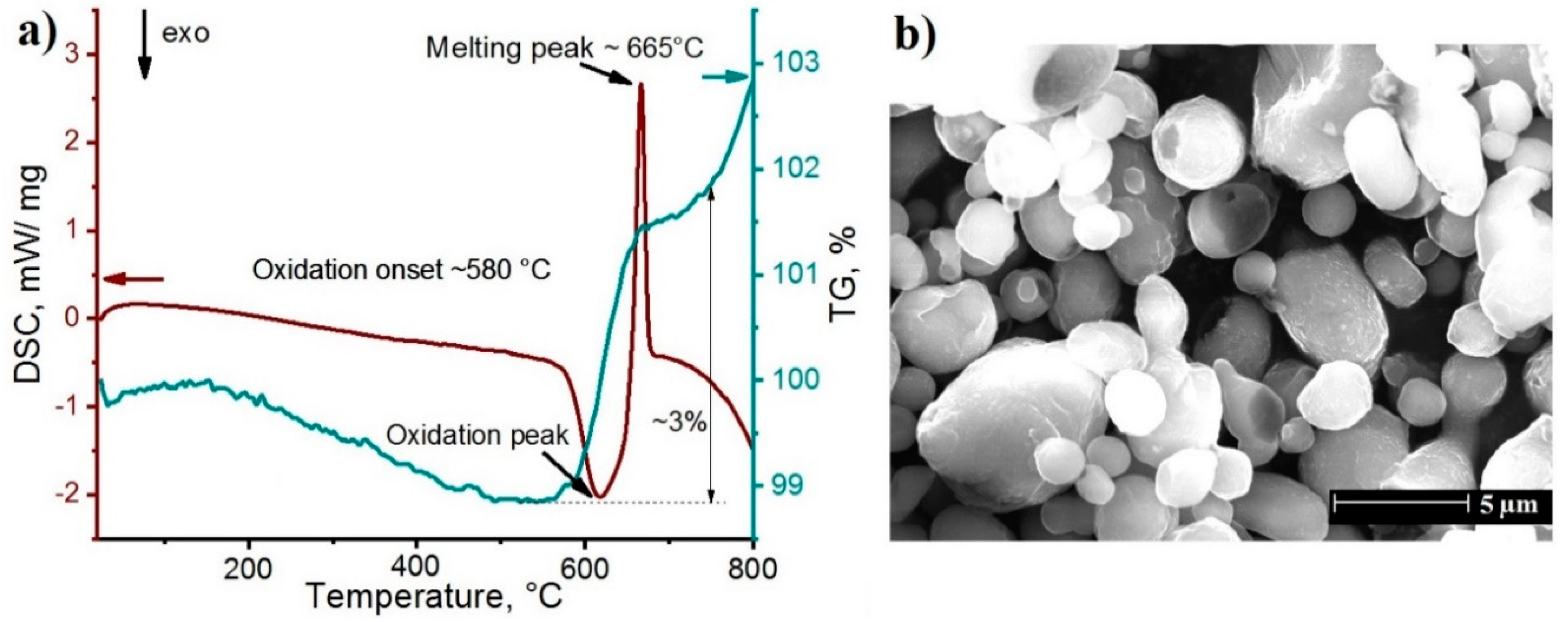
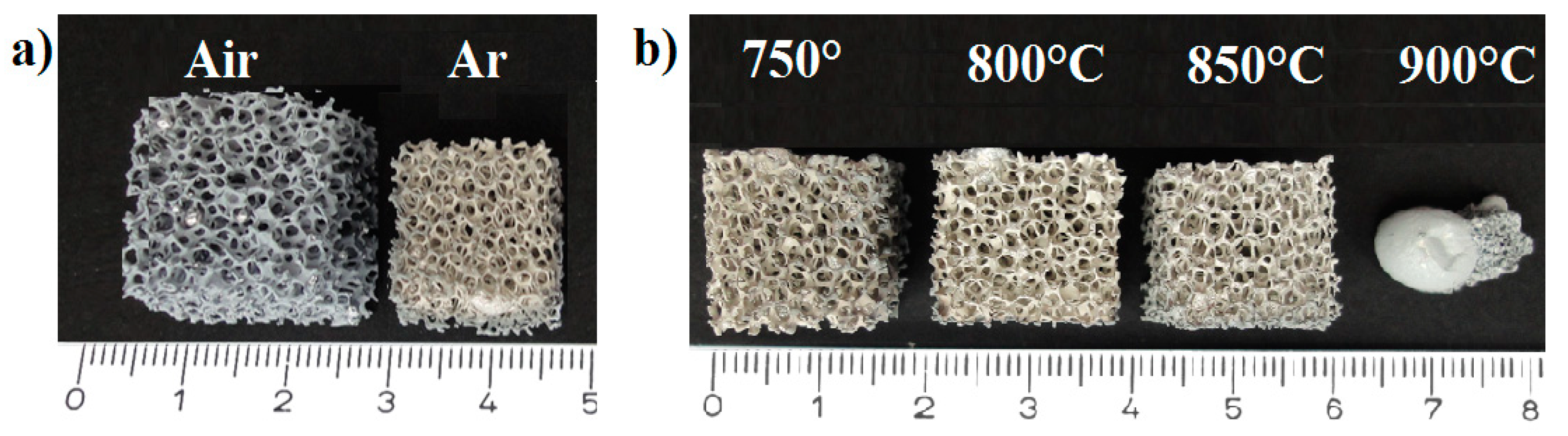
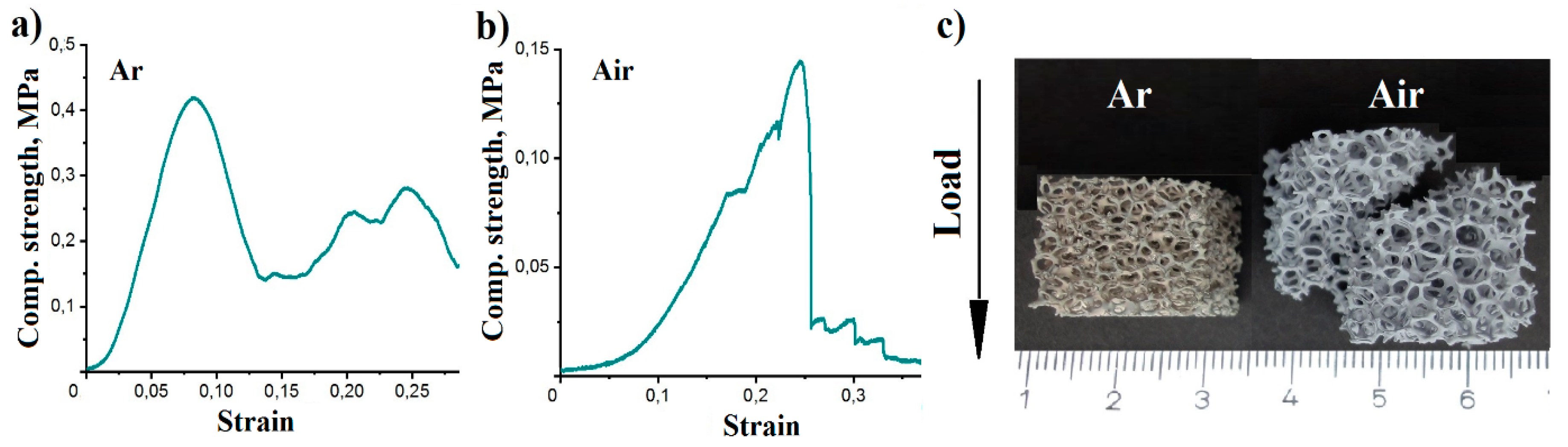
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, S.; Bhatnagar, N. A survey of fabrication and application of metallic foams (1925–2017). J. Porous Mater. 2018, 25, 537–554. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, characterisation and application of cellular metals and metal foams. Prog. Mater. Sci. 2001, 46, 559–563. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.-W. A Review on Manufacturing and Application of Open-cell Metal Foam. Procedia Mater. Sci. 2014, 4, 305–309. [Google Scholar] [CrossRef]
- Bortolozzi, J.P.; Banús, E.D.; Milt, V.G.; Gutierrez, L.B.; Ulla, M.A. The significance of passivation treatments on AISI 314 foam pieces to be used as substrates for catalytic applications. Appl. Surf. Sci. 2010, 257, 495–502. [Google Scholar] [CrossRef]
- Gancarczyk, A.; Sindera, K.; Iwaniszyn, M.; Piątek, M.; Macek, W.; Jodłowski, P.J.; Wroński, S.; Sitarz, M.; Łojewska, J.; Kołodziej, A. Metal Foams as Novel Catalyst Support in Environmental Processes. Catalysts 2019, 9, 587. [Google Scholar] [CrossRef]
- Li, C.-L.; Wang, H.; Zhou, X.; JieLi, H.-z.L. Debinding of stainless steel foamprecursor with 3-D open-cell network structure. Trans. Nonfer. Met. Soc. 2010, 20, 2340–2344. [Google Scholar] [CrossRef]
- Gupta, N.; Rohatgi, P.K. Metal Matrix Syntactic Foams: Processing, Microstructure, Properties and Applications; DEStech Publications Inc.: Lancaster, PA, USA, 2014. [Google Scholar]
- Szlancsik, A.; Katona, B.; Károly, D.; Orbulov, I.N. Notch (In)Sensitivity of Aluminum Matrix Syntactic Foams. Materials 2019, 12, 574. [Google Scholar] [CrossRef]
- Cybulski, A.; Moulijn, J.A. Monoliths in Heterogeneous Catalysis. Catal. Rev. 1994, 36, 179–270. [Google Scholar] [CrossRef]
- Hassani, A.; Habibolahzadeh, A.; Bafti, H. Production of graded aluminum foams via powder space holder technique. Mater. Des. 2012, 40, 510–515. [Google Scholar] [CrossRef]
- Parvanian, A.M.; Panjepour, M. Mechanical behavior improvement of open-pore copper foams synthesized through space holder technique. Mater. Des. 2013, 49, 834–841. [Google Scholar] [CrossRef]
- Choe, H. Synthesis, structure, and mechanical properties of Ni–Al and Ni–Cr–Al superalloy foams. Acta Mater. 2004, 52, 1283–1295. [Google Scholar] [CrossRef]
- Mutlu, I.; Oktay, E. Mechanical properties of sinter-hardened Cr–Si–Ni–Mo based steel foam. Mater. Des. 2013, 44, 274–282. [Google Scholar] [CrossRef]
- García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef]
- Quadbeck, P.; Kümmel, K.; Hauser, R.; Standke, G.; Adler, J.; Stephani, G. Open Cell Metal Foams–Application-oriented Structure and Material Selection. In Proceedings of the CELLMAT 2010, Dresden, Germany, 27–29 October 2010. [Google Scholar]
- Soni, B.; Biswas, S. Development of Al Foams by a Low-cost Salt Replication Method for Industrial Applications. Mat. Today Proc. 2015, 2, 1886–1891. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Egan, D.R.; Ponce de León, C.; Wood, R.J.K.; Jones, R.L.; Stokes, K.R.; Walsh, F.C. Developments in electrode materials and electrolytes for aluminium–air batteries. J. Power Sources 2013, 236, 293–310. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Quadbeck, P.; Kümmel, K.; Hauser, R.; Standke, G.; Adler, J.; Stephani, G.; Kieback, B. Structural and Material Design of Open-Cell Powder Metallurgical Foams. Adv. Eng. Mater. 2011, 13, 1024–1030. [Google Scholar] [CrossRef]
- Banhart, J. Aluminum Foams: On the Road to Real Applications. MRS Bull. 2003, 28, 290–295. [Google Scholar] [CrossRef]
- Zaragoza, G.; Goodall, R. Metal Foams with Graded Pore Size for Heat Transfer Applications. Adv. Eng. Mater. 2013, 15, 123–128. [Google Scholar] [CrossRef]
- Yang, X.; Hu, Q.; Du, J.; Song, H.; Zou, T.; Sha, J.; He, C.; Zhao, N. Compression fatigue properties of open-cell aluminum foams fabricated by space-holder method. Int. J. Fatigue 2019, 121, 272–280. [Google Scholar] [CrossRef]
- Salvo, L.; Martin, G.; Suard, M.; Marmottant, A.; Dendievel, R.; Blandin, J.-J. Processing and structures of solids foams. C. R. Phys. 2014, 15, 662–673. [Google Scholar] [CrossRef]
- Davies, G.J.; Zhen, S. Metallic foams: Their production, properties and applications. J. Mater. Sci. 1983, 18, 1899–1911. [Google Scholar] [CrossRef]
- Kennedy, A. Porous Metals and Metal Foams Made from Powders. In Powder Metallurgy; Kondoh, K., Ed.; IntechOpen: London, UK, 2012; pp. 31–46. [Google Scholar]
- Karl, S.; Somers, A.V. Method of Making Porous Ceramic Articles. U.S. Patent 3090094 A, 21 May 1963. [Google Scholar]
- Jamaludin, A.R.; Kasim, S.R.; Ismail, A.K.; Abdullah, M.Z.; Ahmad, Z.A. The effect of sago as binder in the fabrication of alumina foam through the polymeric sponge replication technique. J. Eur. Ceram. Soc. 2015, 35, 1905–1914. [Google Scholar] [CrossRef]
- Dietrich, B.; Schell, G.; Bucharsky, E.C.; Oberacker, R.; Hoffmann, M.J.; Schabel, W.; Kind, M.; Martin, H. Determination of the thermal properties of ceramic sponges. Int. J. Heat Mass Transfer 2010, 53, 198–205. [Google Scholar] [CrossRef]
- Bakan, H.I.; Korkmaz, K. Synthesis and properties of metal matrix composite foams based on austenitic stainless steels–titanium carbonitrides. Mater. Des. 2015, 83, 154–158. [Google Scholar] [CrossRef]
- Choudhary, A.; Pratihar, S.K.; Agrawal, A.K.; Behera, S.K. Macroporous SiOC Ceramics with Dense Struts by Positive Sponge Replication Technique. Adv. Eng. Mater. 2018, 20, 1700586. [Google Scholar] [CrossRef]
- Kwon, H.; Park, D.H.; Park, Y.; Silvain, J.F.; Kawasaki, A.; Park, Y. Spark plasma sintering behavior of pure aluminum depending on various sintering temperatures. Metals Mater. Int. 2010, 16, 71–75. [Google Scholar] [CrossRef]
- Trunov, M.A.; Umbrajkar, M.S.; Schoenitz, M.; Mang, J.T.; Dreizin, E.L. Oxidation and melting of aluminum nanopowders. J. Phys. Chem. B 2006, 110, 13094–13099. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, L.; Su, Y.; Tan, J.; Bao, L.; Lu, Y.; Wang, J.; Chen, R.; Chen, S.; Wu, F. An interfacial framework for breaking through the Li-ion transport barrier of Li-rich layered cathode materials. J. Mater. Chem. A 2017, 5, 24292–24298. [Google Scholar] [CrossRef]
- Manonukul, A.; Srikudvien, P.; Tange, M.; Puncreobutr, C. Geometry anisotropy and mechanical property isotropy in titanium foam fabricated by replica impregnation method. Mater. Sci. Eng. A 2016, 655, 388–395. [Google Scholar] [CrossRef]
- Li, J.P.; Li, S.H.; van Blitterswijk, C.A.; de Groot, K. A novel porous Ti6Al4V: Characterization and cell attachment. J. Biomed. Mater. Res. A 2005, 73, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Zaman, E.; Keleş, Ö. Open Cell Aluminum Foams Produced by Polymer Impregnation Method. Acta Phys. Pol. A 2014, 125, 445–448. [Google Scholar] [CrossRef]
- Coelho, A.A. Topas Academic V5; Coelho Software: Brisbane, Australia, 2012. [Google Scholar]
- Henon, J.; Alzina, A.; Absi, J.; Smith, D.S.; Rossignol, S. Potassium geopolymer foams made with silica fume pore forming agent for thermal insulation. J. Porous Mater. 2013, 20, 37–46. [Google Scholar] [CrossRef]
- Ross, R.B. Metallic Materials Specification Handbook, 4th ed.; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Hochleistungskeramik; Monolithische Keramik; Allgemeine und strukturelle Eigenschaften; Teil 2: Bestimmung von Dichte und Porosität; DIN EN 623–2:1993–11; Beuth Verlag: Berlin, Germany, 1993. (In German)
- Betke, U.; Lieb, A.; Scheffler, F.; Scheffler, M. Manufacturing of Reticulated Open-Cellular Aluminum Nitride Ceramic Foams from Aqueous AlN Suspensions. Adv. Eng. Mater. 2017, 19, 1600660. [Google Scholar] [CrossRef]
- Log, T.; Gustafsson, S.E. Transient plane source (TPS) technique for measuring thermal transport properties of building materials. Fire Mater. 1995, 19, 43–49. [Google Scholar] [CrossRef]
- He, Y. Rapid thermal conductivity measurement with a hot disk sensor. Thermochim. Acta 2005, 436, 122–129. [Google Scholar] [CrossRef]
- Sercombe, T.B.; Schaffer, G.B. Rapid manufacturing of aluminum components. Science 2003, 301, 1225–1227. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Patnaik, P. Handbook of Inorganic Chemicals; McGraw-Hill: New York, NY, USA, 2002. [Google Scholar]
- Harper, C.A. Handbook of Ceramics, Glasses and Diamond; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Barg, S.; Soltmann, C.; Schwab, A.; Koch, D.; Schwieger, W.; Grathwohl, G. Novel open cell aluminum foams and their use as reactive support for zeolite crystallization. J. Porous Mater. 2011, 18, 89–98. [Google Scholar] [CrossRef]
- Hasani, S.; Panjepour, M.; Shamanian, M. Oxidation and Kinetic Analysis of Pure Aluminum Powder under Nonisothermal Condition. J. Aquac. Res. Dev. 2012, 01. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, H.; Jiao, Q.J. Oxidation mechanism of micron-sized aluminum particles in Al-CO 2 gradually heating system. IOP Conf. Ser. Mater. Sci. Eng. 2017, 248, 12002. [Google Scholar] [CrossRef]
- Körner, C.; Arnold, M.; Singer, R.F. Metal foam stabilization by oxide network particles. Mater. Sci. Eng. A 2005, 396, 28–40. [Google Scholar] [CrossRef]
- Ashby, M.F. The properties of foams and lattices. Philos. Trans. A Math. Phys. Eng. Sci. 2006, 364, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Eucken, A. Die Wärmeleitfähigkeit feuerfester Stoffe. Ihre Berechnung aus der Wärmeleitfähigkeit der Bestandteile; VDI-Verlag: Berlin, Germany, 1932. [Google Scholar]
- Lemmon, E.W.; Jacobsen, R.T. Viscosity and Thermal Conductivity Equations for Nitrogen, Oxygen, Argon, and Air. Int. J. Thermophys. 2004, 25, 21–67. [Google Scholar] [CrossRef]
- Touloukian, Y.S.; Powell, R.W.; Ho, C.Y.; Klemens, P.G. Thermophysical Properties of Matter—The TPRC Data Series; IFI/Plenum: New York, NY, USA, 1973. [Google Scholar]
- McNeil, L.E.; Grimsditch, M.; French, R.H. Vibrational spectroscopy of aluminum nitride. J. Am. Ceram. Soc. 1993, 76, 1132–1136. [Google Scholar] [CrossRef]
- Slack, G.A.; Tanzilli, R.A.; Pohl, R.O.; Vandersande, J.W. The intrinsic thermal conductivity of AIN. J. Phys. Chem. Solids 1987, 48, 641–647. [Google Scholar] [CrossRef]
- Smith, D.S.; Fayette, S.; Grandjean, S.; Martin, C.; Telle, R.; Tonnessen, T. Thermal resistance of grain boundaries in alumina ceramics and refractories. J. Am. Ceram. Soc. 2003, 86, 105–111. [Google Scholar] [CrossRef]


| Binder Burning | PU Burning | Thermal Processing | ||||
|---|---|---|---|---|---|---|
| T, °C | Time, h | T, °C | Time, h | T, °C | Atmosphere | Time, h |
| 250 | 3 | 500 | 3 | 750 | Air | 3 |
| 750 | Ar | |||||
| 800 | ||||||
| 850 | ||||||
| 900 | ||||||
| Sample | α-Al2O3, wt.% | γ-Al2O3, wt.% |
|---|---|---|
| Al powder | - | - |
| Air 750 °C | 20.2 | 2.4 |
| Ar 750 °C | - | 4.6 |
| Ar 800 °C | - | 3.8 |
| Ar 850 °C | 1.5 | 4.3 |
| Ar 900 °C (shrink part of the foam) | 28.8 | 7.4 |
| Sample | Linear Shrinkage, % | Total Porosity a, % | Cell Porosity (Pcell) b, % | Total Strut Porosity c, % | Strut Porosity (Ps) d, % | Hollow Strut Porosity e, % |
|---|---|---|---|---|---|---|
| Air 750°C | 0 | 90.4 | 78.6 | 58.5 | 51.6 | 6.9 |
| Ar 750 °C | 21 | 90.7 | 85.9 | 39.7 | 29.8 | 9.9 |
| Ar 800 °C | 21 | 90.2 | 86.6 | 35.6 | 25.0 | 10.6 |
| Ar 850 °C | 23 | 90.9 | 86.5 | 40.0 | 29.5 | 10.5 |
| Sample | Therm. Cond. of Foam λf, W·m−1K−1 | Therm. Cond. of Porous Strut Material λs, W·m−1K−1 | Bulk Therm. Cond. of Bulk Material λb, W·m−1 K−1 |
|---|---|---|---|
| Air 750 °C | 0.45 ± 0.05 | 6.0 | 17.1 |
| Ar 750 °C | 2.32 ± 0.14 | 38.4 | 66.8 |
| Ar 800 °C | 2.98 ± 0.17 | 66.0 | 103.0 |
| Sample | Comp. Strength, MPa | Weibull Parameter m | Total Strut Porosity, % | Aluminum Oxides, wt.% |
|---|---|---|---|---|
| Air 750 °C | 0.133 ± 0.023 | 7.2 | 58.5 | 22.6 |
| Ar 750 °C | 0.339 ± 0.078 | 3.2 | 39.7 | 4.6 |
| Ar 800 °C | 0.304 ± 0.051 | 6.56 | 36.5 | 3.8 |
| Ar 850 °C | 0.224 ± 0.030 | 40.0 | 5.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutygina, A.; Betke, U.; Scheffler, M. Open-Cell Aluminum Foams by the Sponge Replication Technique. Materials 2019, 12, 3840. https://doi.org/10.3390/ma12233840
Sutygina A, Betke U, Scheffler M. Open-Cell Aluminum Foams by the Sponge Replication Technique. Materials. 2019; 12(23):3840. https://doi.org/10.3390/ma12233840
Chicago/Turabian StyleSutygina, Alina, Ulf Betke, and Michael Scheffler. 2019. "Open-Cell Aluminum Foams by the Sponge Replication Technique" Materials 12, no. 23: 3840. https://doi.org/10.3390/ma12233840
APA StyleSutygina, A., Betke, U., & Scheffler, M. (2019). Open-Cell Aluminum Foams by the Sponge Replication Technique. Materials, 12(23), 3840. https://doi.org/10.3390/ma12233840







