Magnesium Phosphate Cement as Mineral Bone Adhesive
Abstract
1. Introduction
2. Materials and Methods
2.1. Cement Preparation
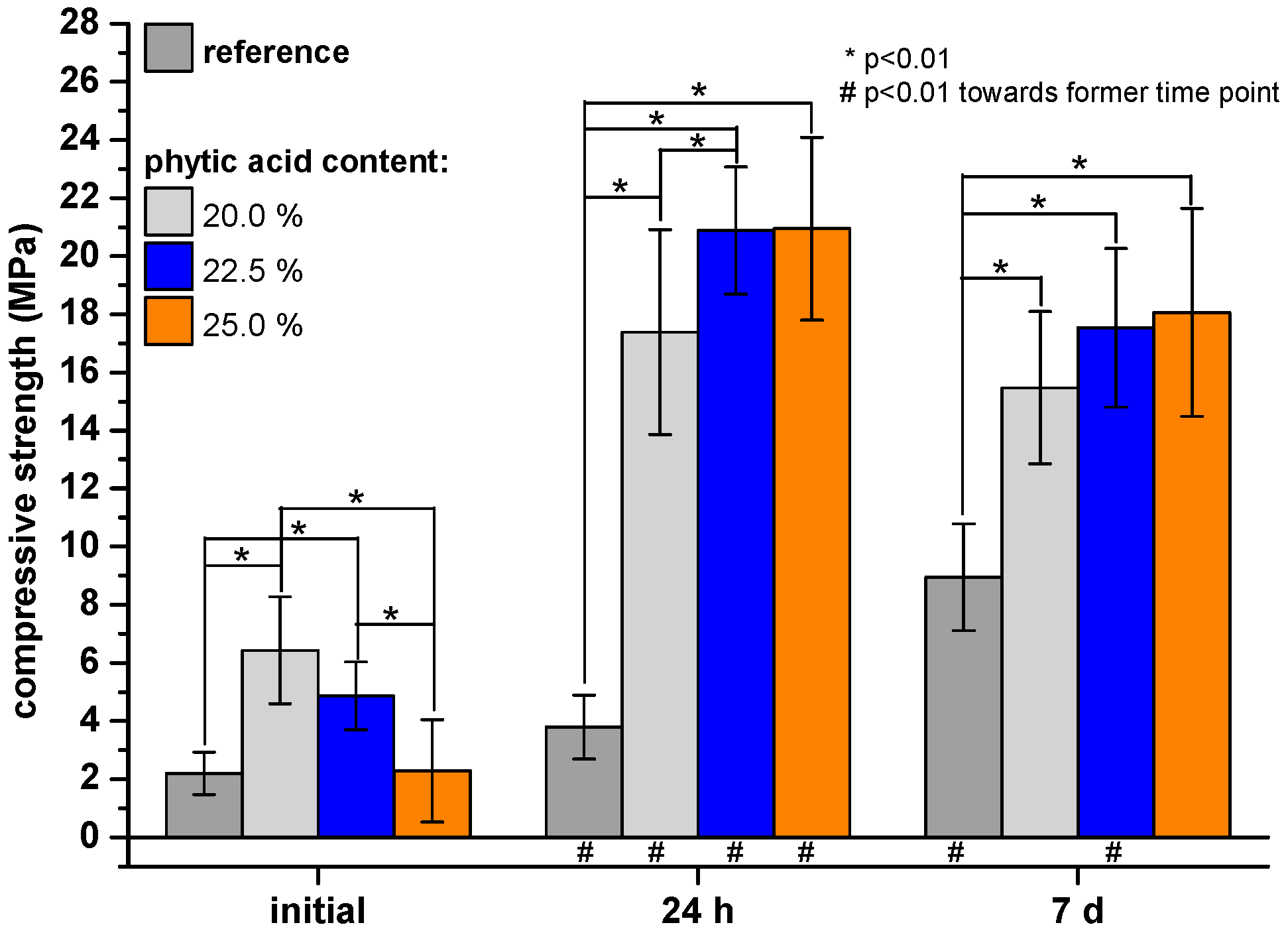
2.2. Compressive Strength Testing
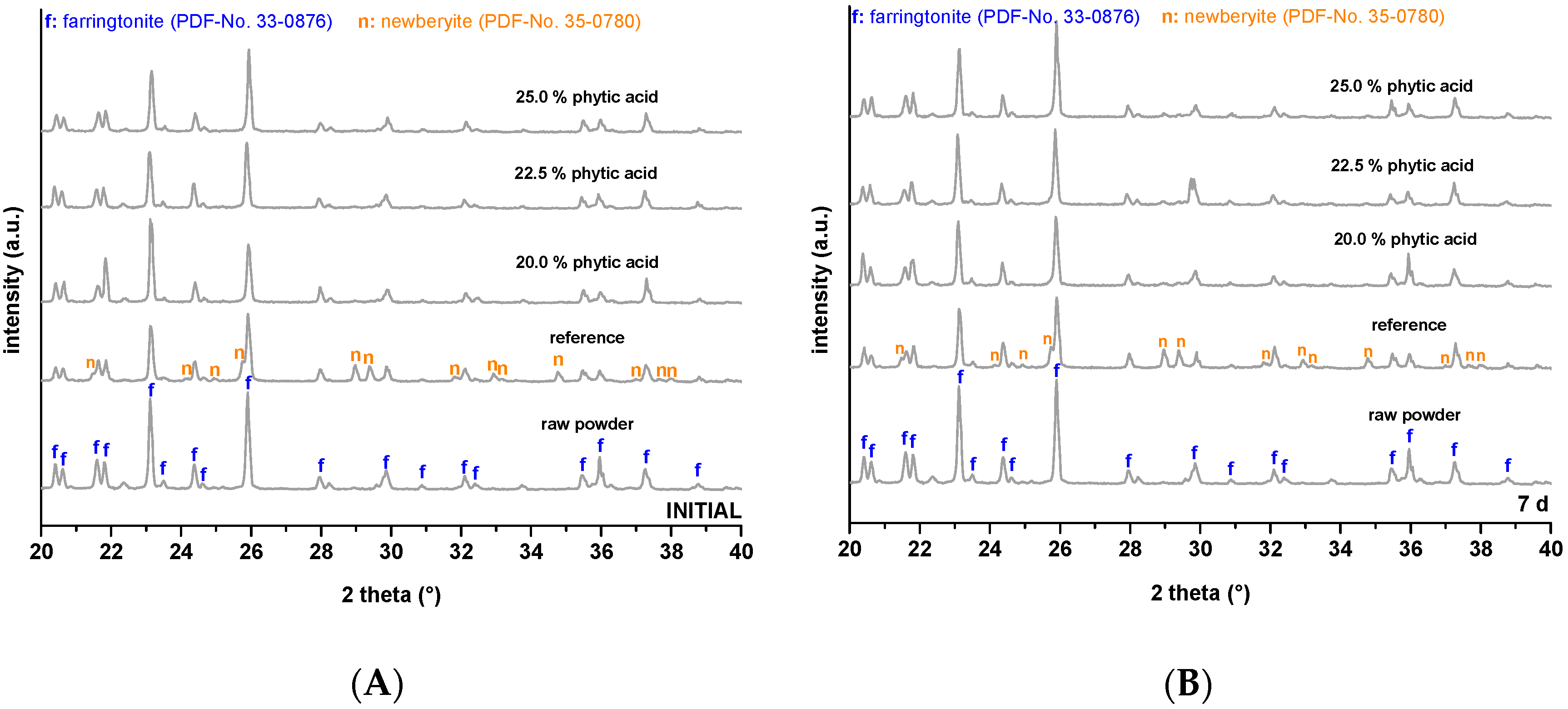
2.3. Phase Composition
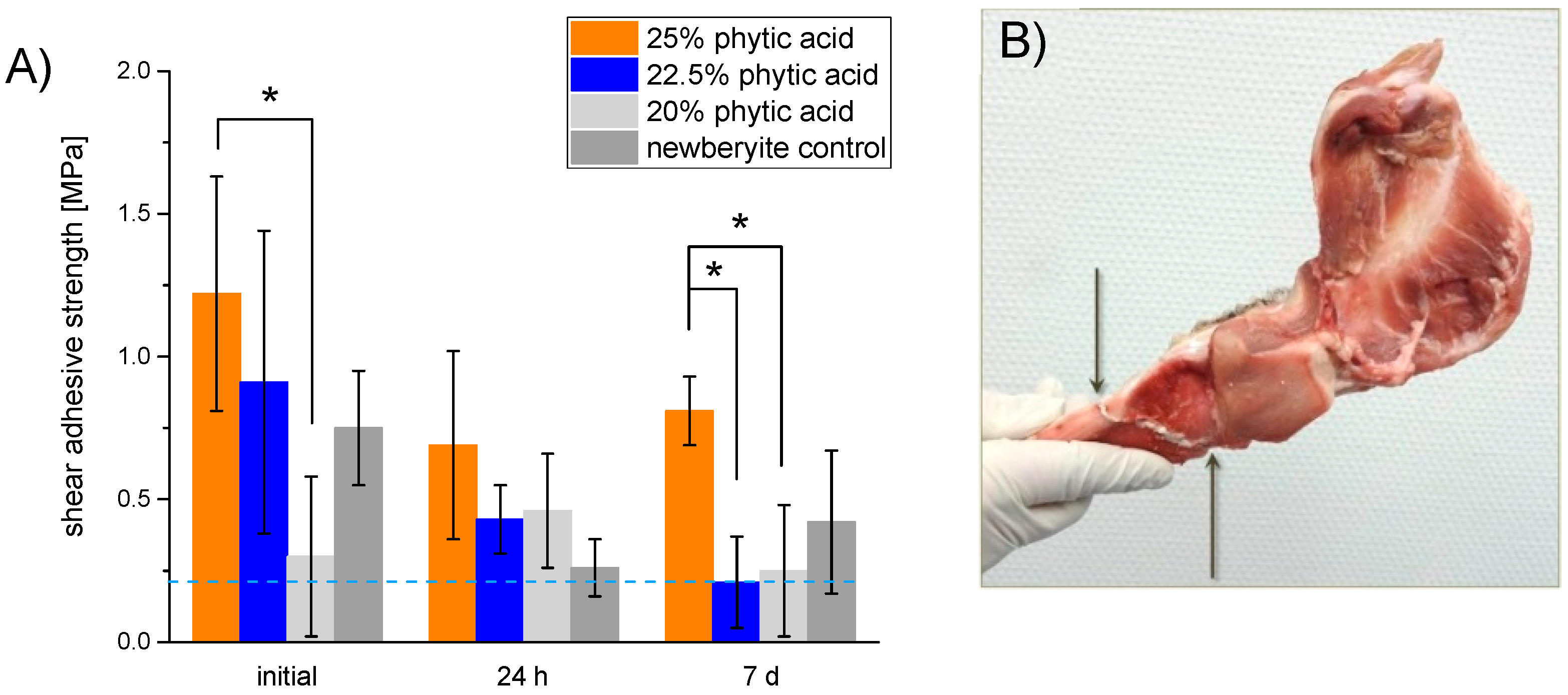
2.4. Adhesion Testing
2.5. Scanning Electron Microscopy and Energy-Dispersive X-ray Spectroscopy
2.6. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schierle, H.P.; Hausamen, J.E. Modern principles in the treatment of craniomaxillofacial fractures. Unfallchirurg 1997, 100, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Endres, K.; Marx, R.; Tinschert, J.; Wirtz, D.C.; Stoll, C.; Riediger, D.; Smeets, R. A new adhesive technique for internal fixation in midfacial surgery. Biomed. Eng. Online 2008, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Esteves, J.C.; Monteiro, J.M.; Aranega, A.M.; Betoni Junior, W.; Sonoda, C.K. Utilization of ethyl cyanoacrylate and 2-Octyl cyanoacrylate adhesives for autogenous bone graft fixation: Histomorphometric study in rats. J. Oral Implantol. 2014, 40, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Akcal, M.A.; Poyanli, O.; Unay, K.; Esenkaya, I.; Gokcen, B.; Firatligil, A.S. Effect of N-butyl cyanoacrylate on fracture healing in segmental rat tibia fracture model. J. Orthop. Surg. Res. 2014, 9, 76. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wistlich, L.; Rucker, A.; Schamel, M.; Kubler, A.C.; Gbureck, U.; Groll, J. A bone glue with sustained adhesion under wet conditions. Adv. Healthc. Mater. 2017, 6, 1600902. [Google Scholar] [CrossRef]
- Smeets, R.; Endres, K.; Stockbrink, G.; Hanken, H.; Hermanns-Sachweh, B.; Marx, R.; Heiland, M.; Blessmann, M.; Wolff, K.-D.; Kolk, A. The innovative application of a novel bone adhesive for facial fracture osteosynthesisin vitro and in vivo results. J. Biomed. Mater. Res. Part A 2013, 101, 2058–2066. [Google Scholar] [CrossRef]
- Zhao, X.; Olsen, I.; Li, H.; Gellynck, K.; Buxton, P.G.; Knowles, J.C.; Salih, V.; Young, A.M. Reactive calcium-phosphate-containing poly(ester-co-ether) methacrylate bone adhesives: Chemical, mechanical and biological considerations. Acta Biomater. 2010, 6, 845–855. [Google Scholar] [CrossRef]
- Song, S.H.; Kyung, H.; Oh, S.-H.; Kang, N. Fixation of fractured anterior wall of maxillary sinus using fibrin glue in a zygomaticomaxillary complex fracture. J. Craniofacial Surg. 2014, 25, 919–921. [Google Scholar] [CrossRef]
- Olofsson, K.; Granskog, V.; Cai, Y.; Hult, A.; Malkoch, M. Activated dopamine derivatives as primers for adhesive-patch fixation of bone fractures. RSC Adv. 2016, 6, 26398–26405. [Google Scholar] [CrossRef]
- Perikamana, S.K.M.; Lee, J.; Lee, Y.B.; Shin, Y.M.; Lee, E.J.; Mikos, A.G.; Shin, H. Materials from mussel-inspired chemistry for cell and tissue engineering applications. Biomacromolecules 2015, 16, 2541–2555. [Google Scholar] [CrossRef]
- Jo, Y.K.; Choi, B.H.; Zhou, C.; Ahn, J.S.; Jun, S.H.; Cha, H.J. Bioengineered mussel glue incorporated with a cell recognition motif as an osteostimulating bone adhesive for titanium implants. J. Mater. Chem. B 2015, 3, 8102–8114. [Google Scholar] [CrossRef]
- Lu, D.D.; Wang, H.S.; Wang, X.Y.; Li, Y.F.; Guo, H.Y.; Sun, S.B.; Zhao, X.L.; Yang, Z.W.; Lei, Z.Q. Biomimetic chitosan-graft-polypeptides for improved adhesion in tissue and metal. Carbohydr. Polym. 2019, 215, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.J.C.; Pinzon, L.M.; Narvaez, D.M.; Paez, C.I.C.; Moreno-Serrano, C.L.; Tabima, D.M.; Salcedo, F.; Briceno, J.C.; Casas-Rodriguez, J.P. Evaluation of a water-resistant and biocompatible adhesive with potential use in bone fractures. J. Adhes. Sci. Technol. 2017, 31, 1480–1495. [Google Scholar] [CrossRef]
- Pinzon, L.M.; Cedano, F.J.; Castro, C.I.; Briceno, J.C.; Casas, J.P.; Tabima, D.M.; Salcedo, F. Formulation and characterization of chitosan-based biocomposites with potential use for bone adhesion. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 697–707. [Google Scholar] [CrossRef]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef]
- Kanter, B.; Geffers, M.; Ignatius, A.; Gbureck, U. Control of in vivo mineral bone cement degradation. Acta Biomater. 2014, 10, 3279–3287. [Google Scholar] [CrossRef]
- Heiss, C.; Kraus, R.; Peters, F.; Henn, W.; Schnabelrauch, M.; Berg, A.; Pautzsch, T.; Weisser, J.; Schnettler, R. Development of a bioresorbable self-hardening bone adhesive based on a composite consisting of polylactide methacrylates and β-tricalcium phosphate. J. Biomed. Mater. Res. B Appl. Biomat. 2009, 90B, 55–66. [Google Scholar] [CrossRef]
- Young, A.M.; Man Ho, S.; Abou Neel, E.A.; Ahmed, I.; Barralet, J.E.; Knowles, J.C.; Nazhat, S.N. Chemical characterization of a degradable polymeric bone adhesive containing hydrolysable fillers and interpretation of anomalous mechanical properties. Acta Biomater. 2009, 5, 2072–2083. [Google Scholar] [CrossRef]
- Gellynck, K.; Abou Neel, E.A.; Li, H.; Mardas, N.; Donos, N.; Buxton, P.; Young, A.M. Cell attachment and response to photocured, degradable bone adhesives containing tricalcium phosphate and purmorphamine. Acta Biomater. 2011, 7, 2672–2677. [Google Scholar] [CrossRef]
- Mestres, G.; Ginebra, M.-P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef] [PubMed]
- Waselau, M.; Samii, V.F.; Weisbrode, S.E.; Litsky, A.S.; Bertone, A.L. Effects of a magnesium adhesive cement on bone stability and healing following a metatarsal osteotomy in horses. Am. J. Vet. Res. 2007, 68, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Christel, T.; Christ, S.; Barralet, J.E.; Groll, J.; Gbureck, U. Chelate bonding mechanism in a novel magnesium phosphate bone cement. J. Am. Ceram. Soc. 2015, 98, 694–697. [Google Scholar] [CrossRef]
- Graf, E.; Eaton, J.W. Antioxidant functions of phytic acid. Free Radic. Biol. Med. 1990, 8, 61–69. [Google Scholar] [CrossRef]
- Ekholm, P.; Virkki, L.; Ylinen, M.; Johansson, L. The effect of phytic acid and some natural chelating agents on the solubility of mineral elements in oat bran. Food Chem. 2003, 80, 165–170. [Google Scholar] [CrossRef]
- Mahanti, H.S.; Barnes, R.M. Determination of major, minor and trace elements in bone by inductively-coupled plasma emission spectrometry. Anal. Chim. Acta 1983, 151, 409–417. [Google Scholar] [CrossRef]
- Weber, S.C.; Chapman, M.W. Adhesives in orthopedic-surgery—A review of the literature and invitro bonding strengths of bone-bonding agents. Clin. Orthop. Relat. Res. 1984, 191, 249–261. [Google Scholar]
- Nabiyouni, M.; Brueckner, T.; Zhou, H.; Gbureck, U.; Bhaduri, S.B. Magnesium-based bioceramics in orthopedic applications. Acta Biomater. 2018, 66, 23–43. [Google Scholar] [CrossRef]
- Barradas, A.M.C.; Yuan, H.; Blitterswijk, C.A.v.; Habibovic, P. Osteoinductive biomaterials: Current knowledge of properties, experimental models and biological mechanisms. Eur. Cells Mater. 2011, 21, 407–429. [Google Scholar] [CrossRef]
- Ooms, E.M.; Wolke, J.G.C.; van de Heuvel, M.T.; Jeschke, B.; Jansen, J.A. Histological evaluation of the bone response to calcium phosphate cement implanted in cortical bone. Biomaterials 2003, 24, 989–1000. [Google Scholar] [CrossRef]
- Grover, L.M.; Gbureck, U.; Farrar, D.; Barralet, J.E. Adhesion of a novel calcium phosphate cement to cortical bone and several common biomaterials. Key Eng. Mater. 2006, 309–311, 849–852. [Google Scholar]
- Dorozhkin, S.V. Bioceramics of calcium orthophosphates. Biomaterials 2010, 31, 1465–1485. [Google Scholar] [CrossRef] [PubMed]
- Gulotta, L.V.; Kovacevic, D.; Ying, L.; Ehteshami, J.R.; Montgomery, S.; Rodeo, S.A. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am. J. Sports Med. 2008, 36, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Galante, J.; Rostoker, W.; Ray, R.D. Physical properties of trabecular bone. Calcif. Tissue Res. 1970, 5, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Reuther, T.; Blank, M.; Reske, I.; Barralet, J.E.; Grover, L.M.; Kübler, A.C.; Gbureck, U. Phase composition, mechanical performance and in vitro biocompatibility of hydraulic setting calcium magnesium phosphate cement. Acta Biomater. 2010, 6, 1529–1535. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakabayashi, N. Adhesive bone cement both to bone and metals: 4-META in MMA initiated with tri-n-butyl borane. J. Biomed. Mater. Res. 1989, 23, 1475–1482. [Google Scholar] [CrossRef]
- Meininger, S.; Blum, C.; Schamel, M.; Barralet, J.E.; Ignatius, A.; Gbureck, U. Phytic acid as alternative setting retarder enhanced biological performance of dicalcium phosphate cement in vitro. Sci. Rep. 2017, 7, 558. [Google Scholar] [CrossRef]

| Ca-to-Mg-Ratio (wt.%/wt.%) | |||
|---|---|---|---|
| Phytic Acid (%) | Time | Cement Residues | Adherend |
| 0 | initial | 0.23 ± 0.02 | 3.0 ± 0.2 |
| 24 h | 0.31 ± 0.03 | 2.7 ± 0.2 | |
| 7 d | 1.6 ± 0.1* | 5.6 ± 0.5 | |
| 20.0 | initial | 0.08 ± 0.01 | 4.9 ± 0.5 |
| 24 h | 0.20 ± 0.02 | 4.7 ± 0.2 | |
| 7 d | 0.42 ± 0.05 | 8.8 ± 0.7 | |
| 22.5 | initial | 0 ± 0 | 6.2 ± 0.5 |
| 24 h | 0.17 ± 0.02 | 8.8 ± 0.9 | |
| 7 d | 0.51 ± 0.04 | 8.3 ± 0.6 | |
| 25.0 | initial | 0.01 ± 0.01 | 7.3 ± 0.8 |
| 24 h | 0.11 ± 0.01 | 3.9 ± 0.3 | |
| 7 d | 0.26 ± 0.02 | 9.9 ± 0.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brückner, T.; Meininger, M.; Groll, J.; Kübler, A.C.; Gbureck, U. Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials 2019, 12, 3819. https://doi.org/10.3390/ma12233819
Brückner T, Meininger M, Groll J, Kübler AC, Gbureck U. Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials. 2019; 12(23):3819. https://doi.org/10.3390/ma12233819
Chicago/Turabian StyleBrückner, Theresa, Markus Meininger, Jürgen Groll, Alexander C. Kübler, and Uwe Gbureck. 2019. "Magnesium Phosphate Cement as Mineral Bone Adhesive" Materials 12, no. 23: 3819. https://doi.org/10.3390/ma12233819
APA StyleBrückner, T., Meininger, M., Groll, J., Kübler, A. C., & Gbureck, U. (2019). Magnesium Phosphate Cement as Mineral Bone Adhesive. Materials, 12(23), 3819. https://doi.org/10.3390/ma12233819






