Soil Recycling Geopolymers Fabricated from High Power Ultrasound Treated Soil Slurry in the Presence of Ammonia
Abstract
:1. Introduction
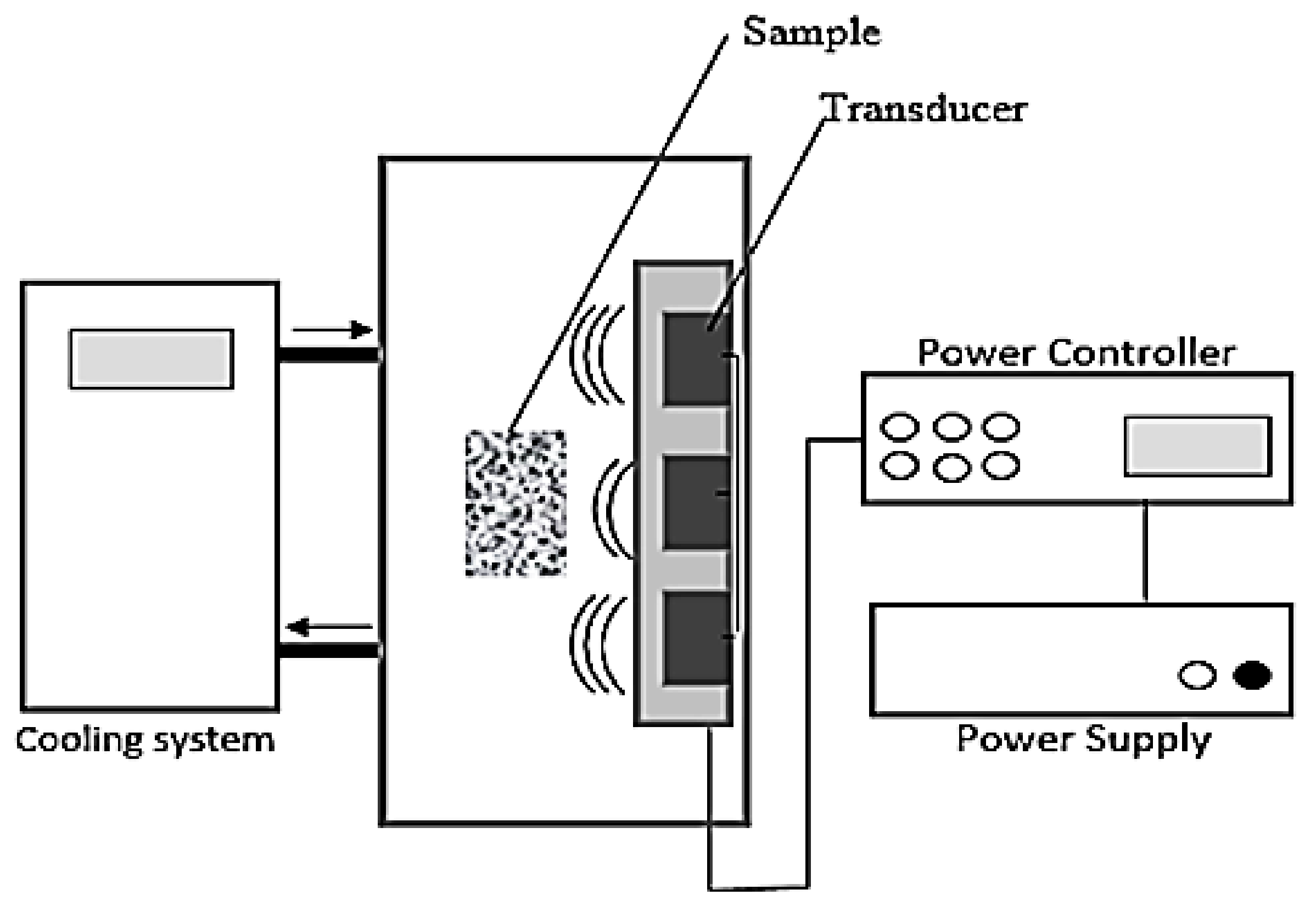
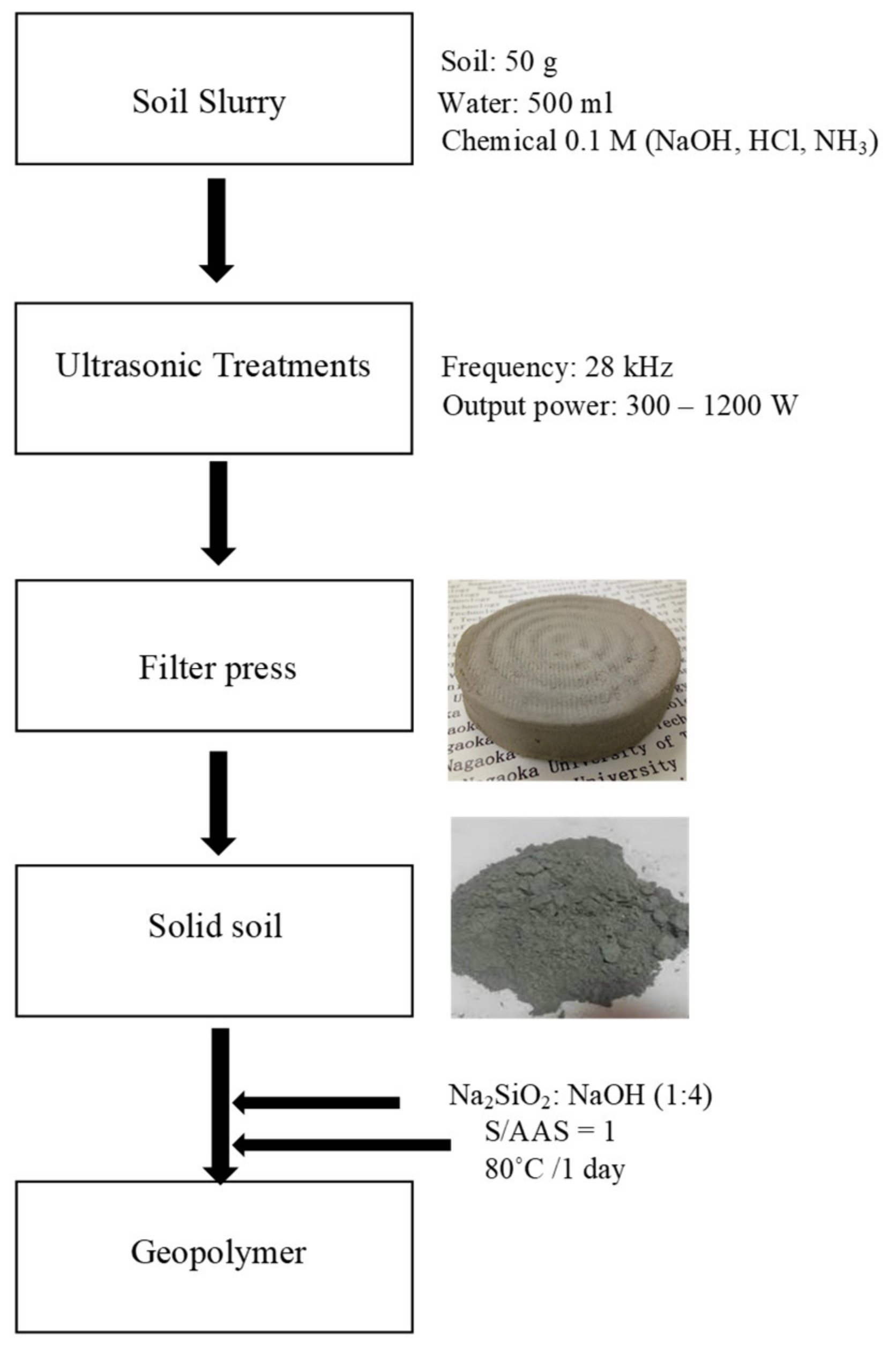
2. Materials and Methods
3. Results and Discussion
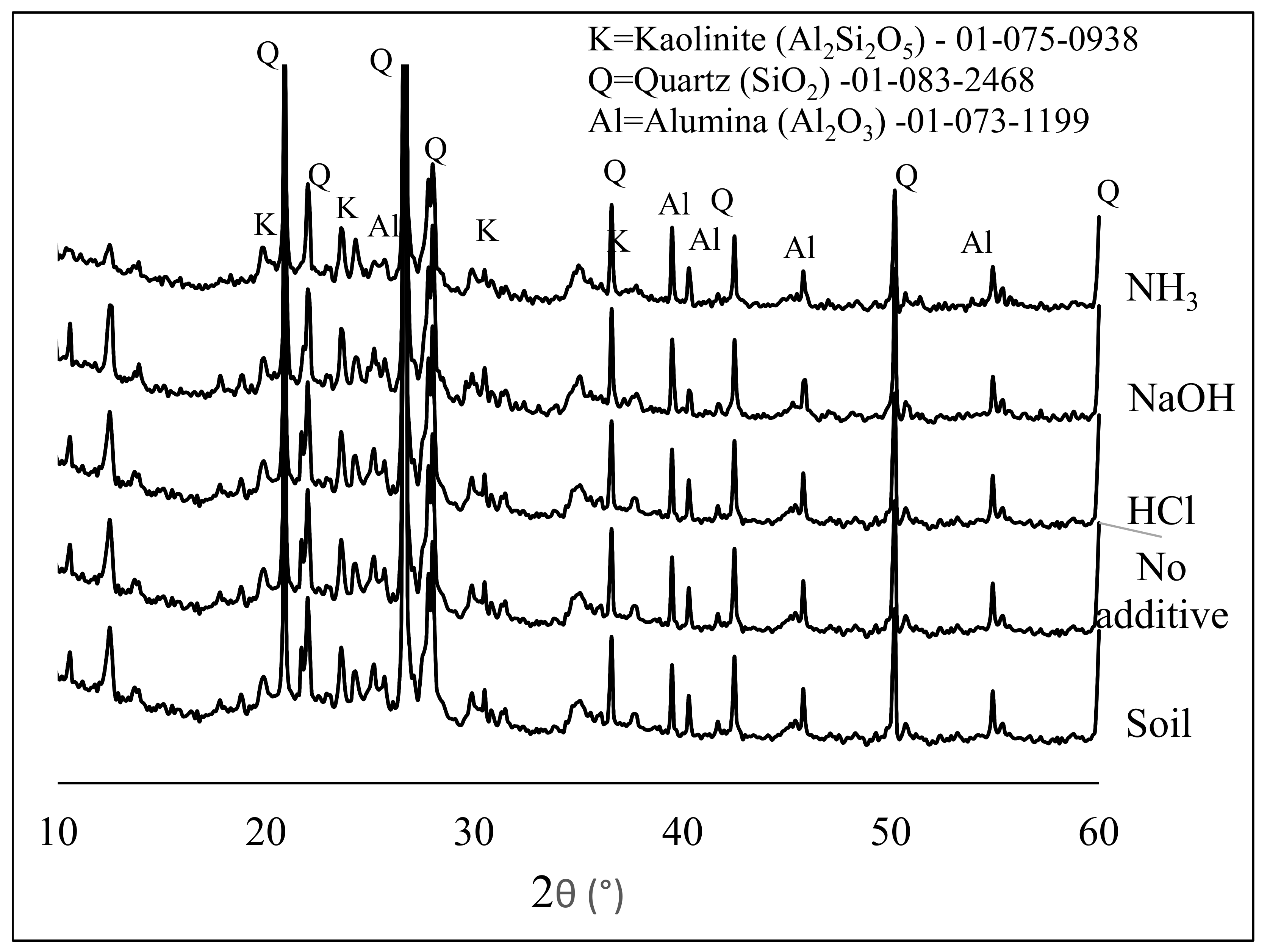
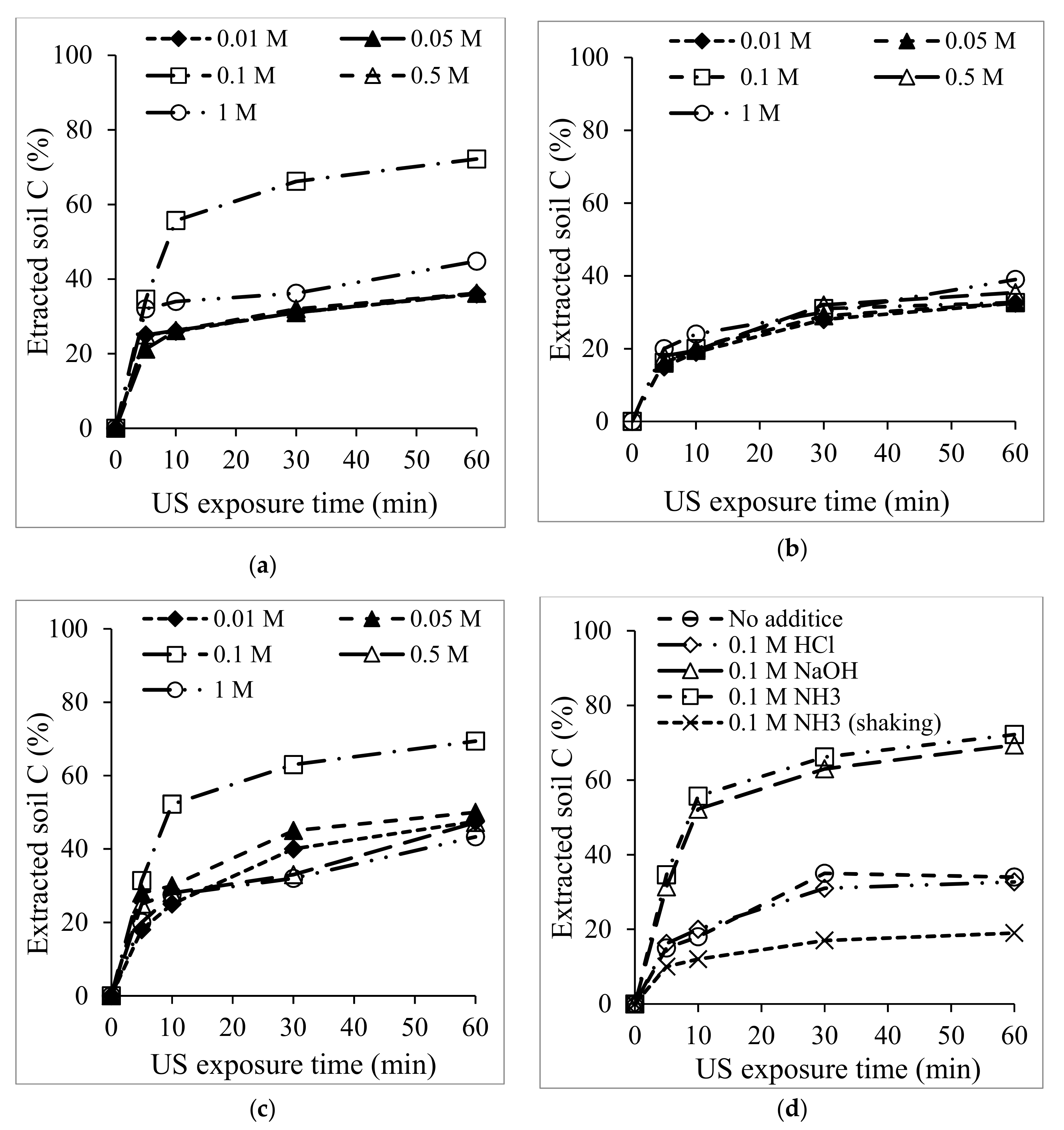
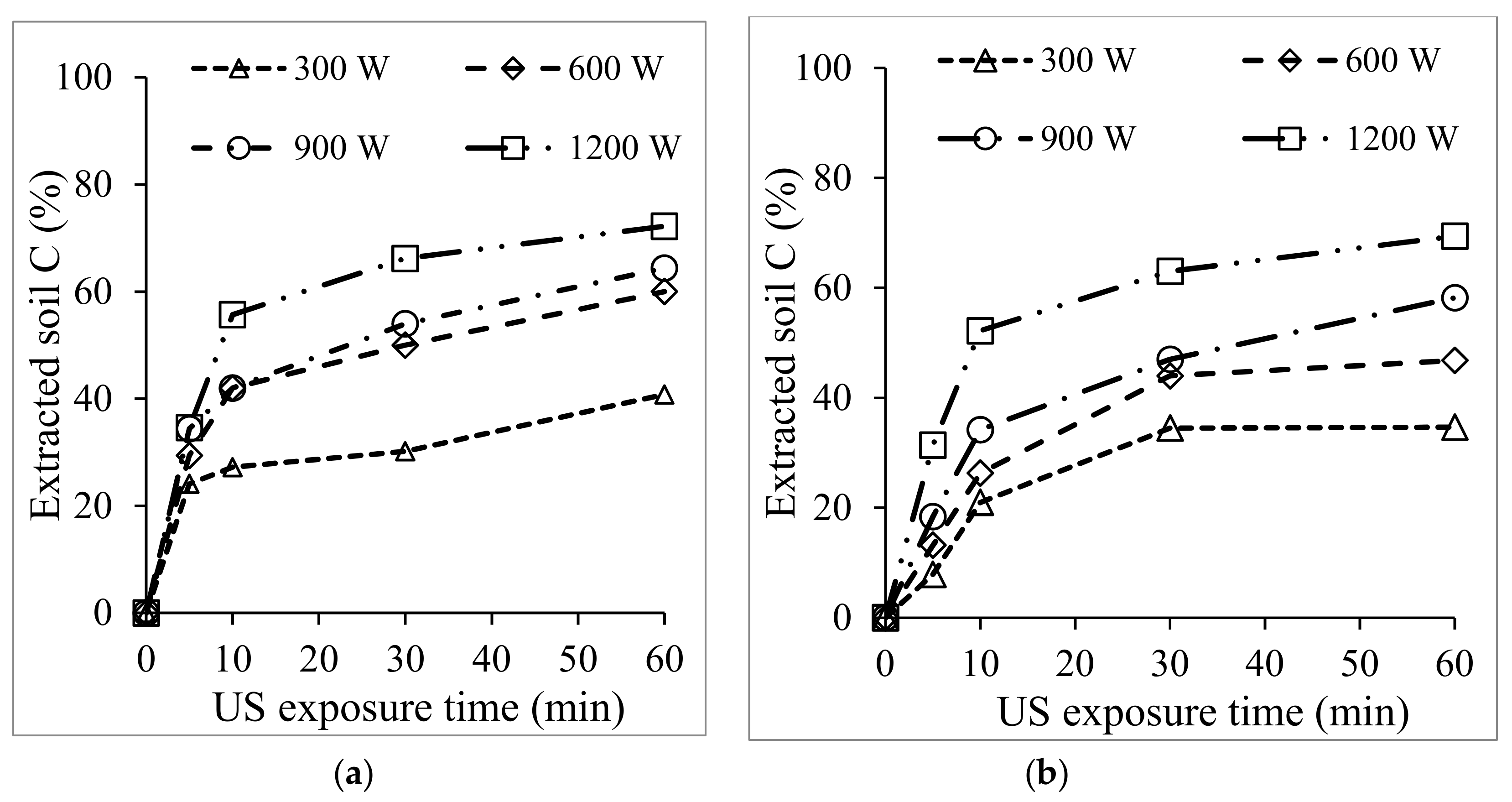
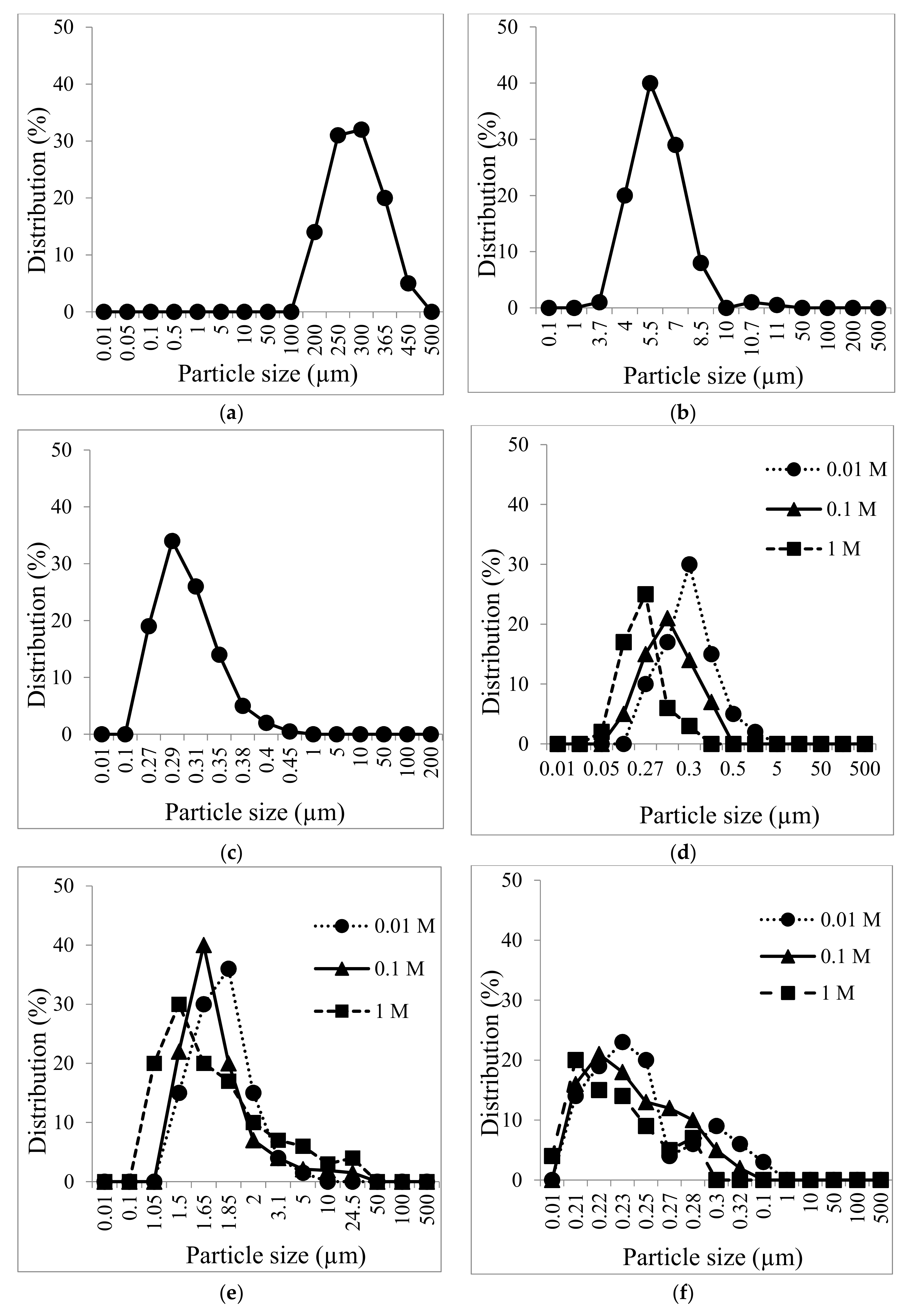
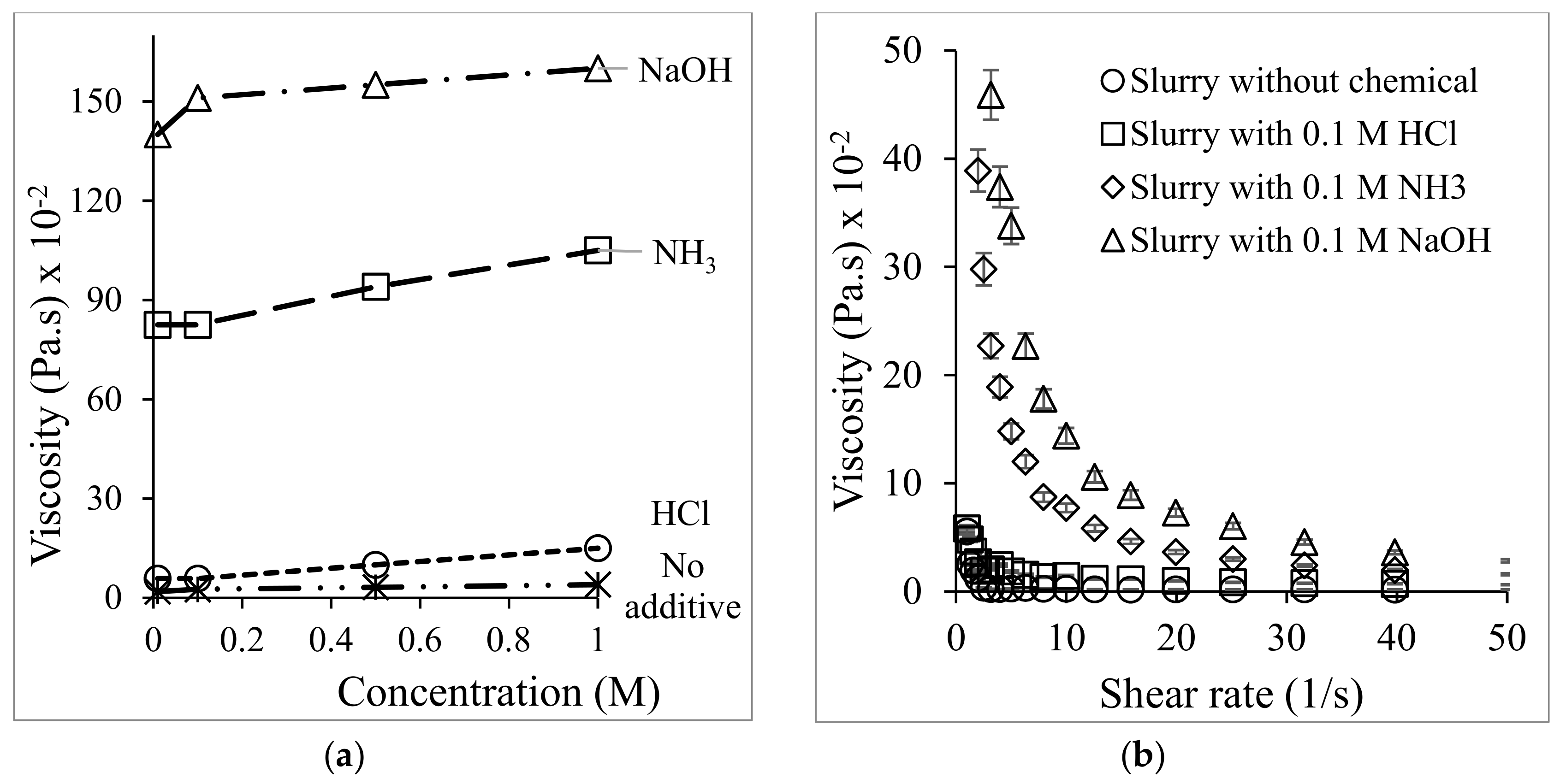
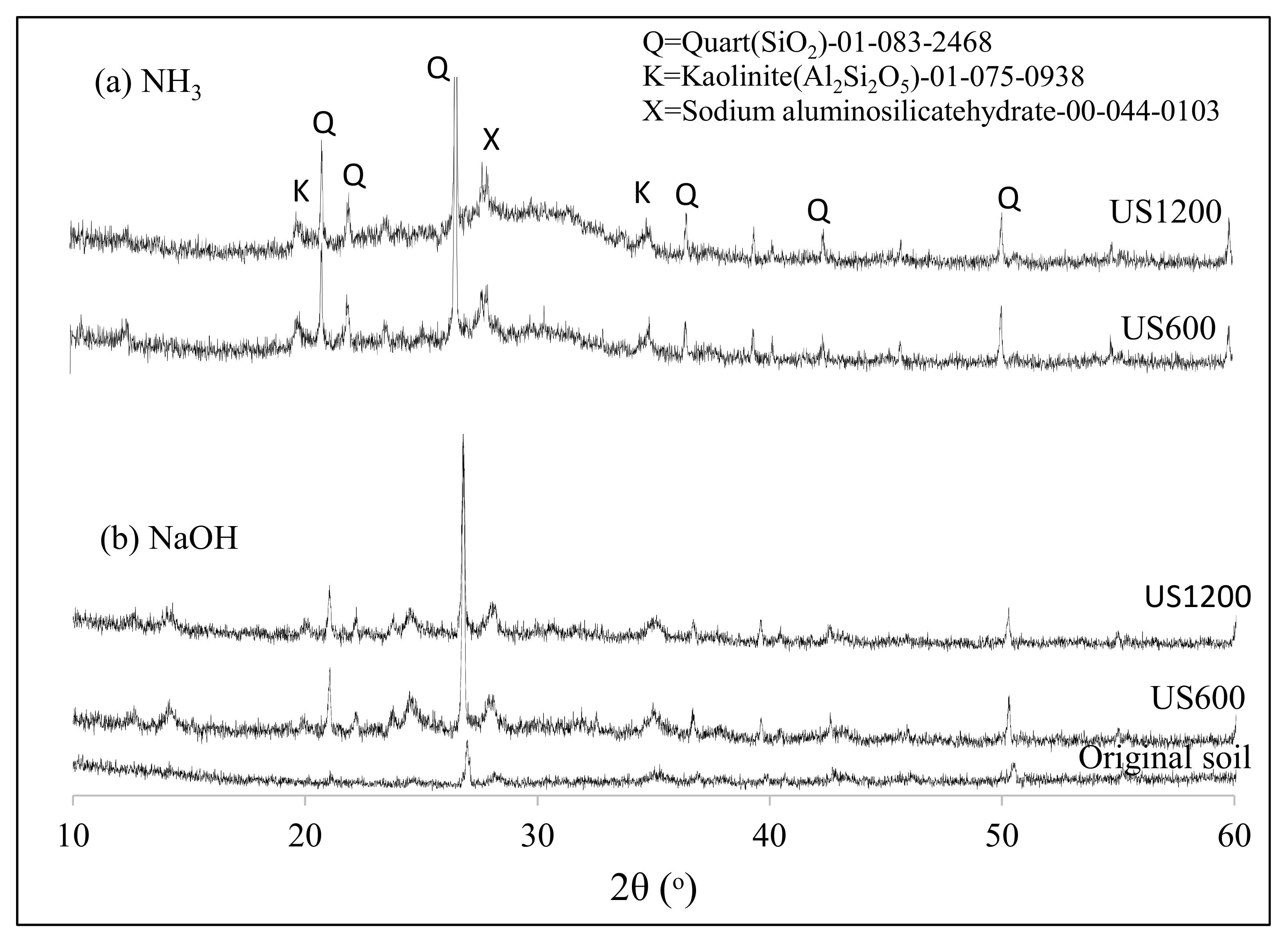
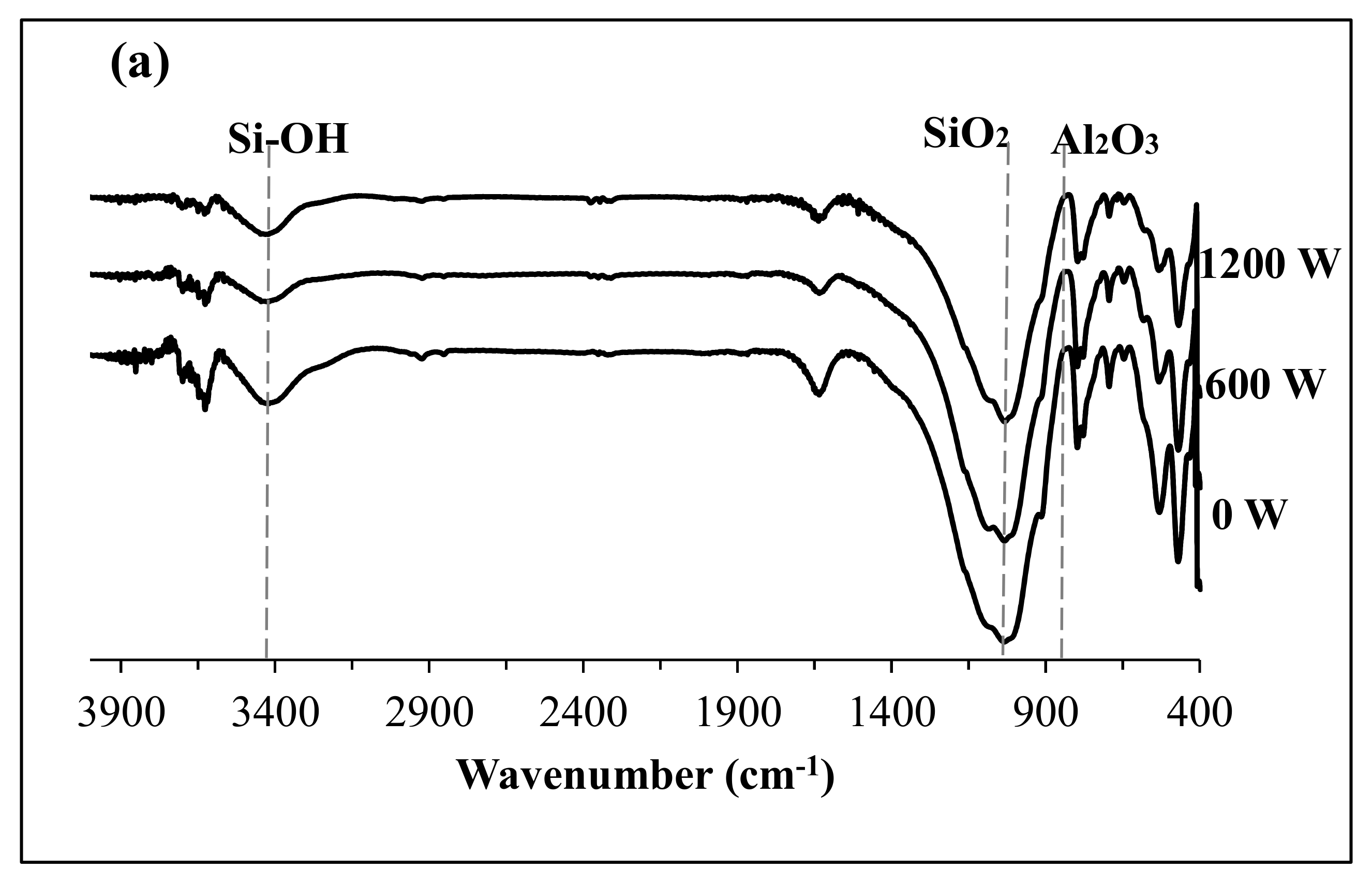
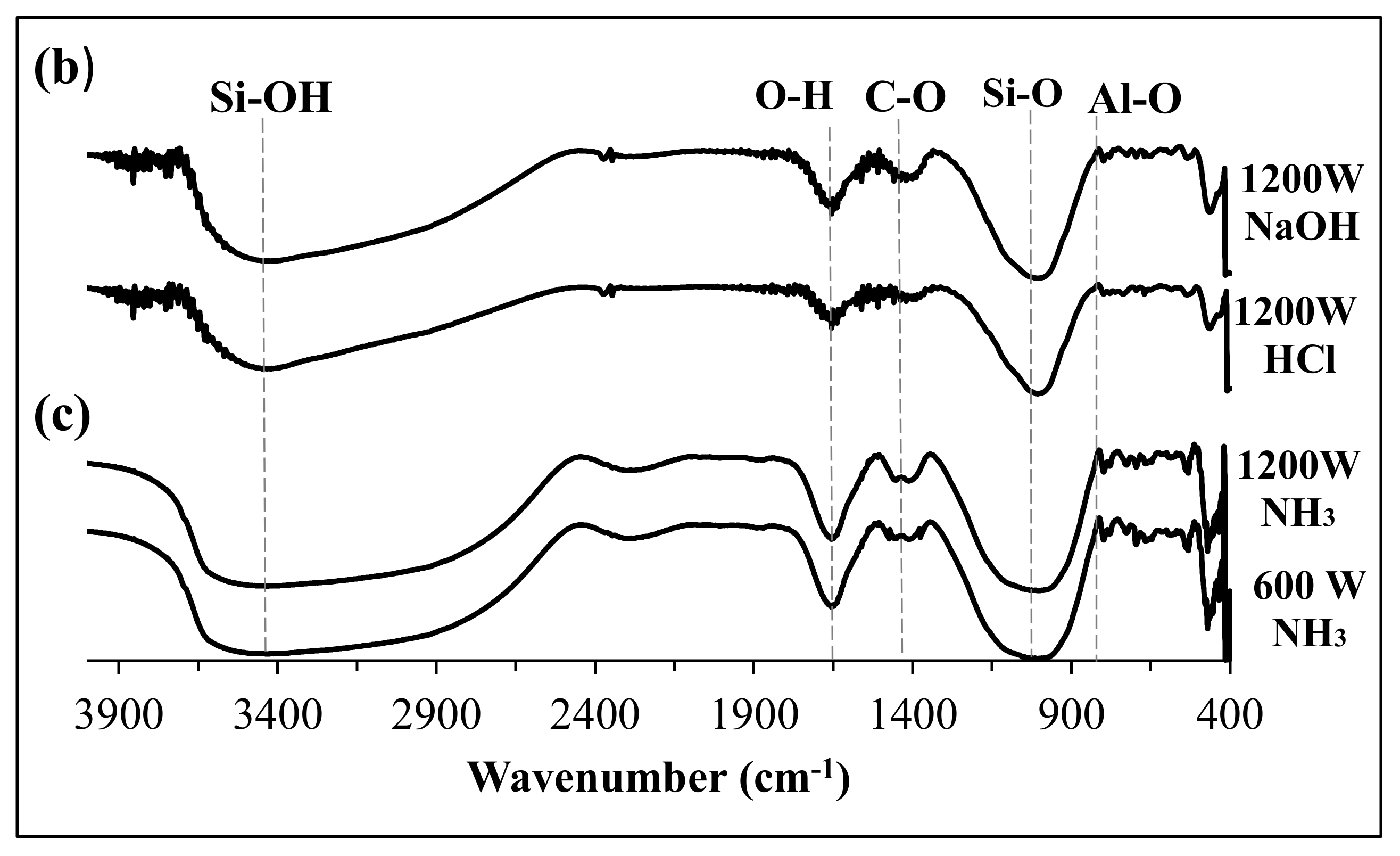
3.1. US Soil Washing in the Presence of Acid or Base
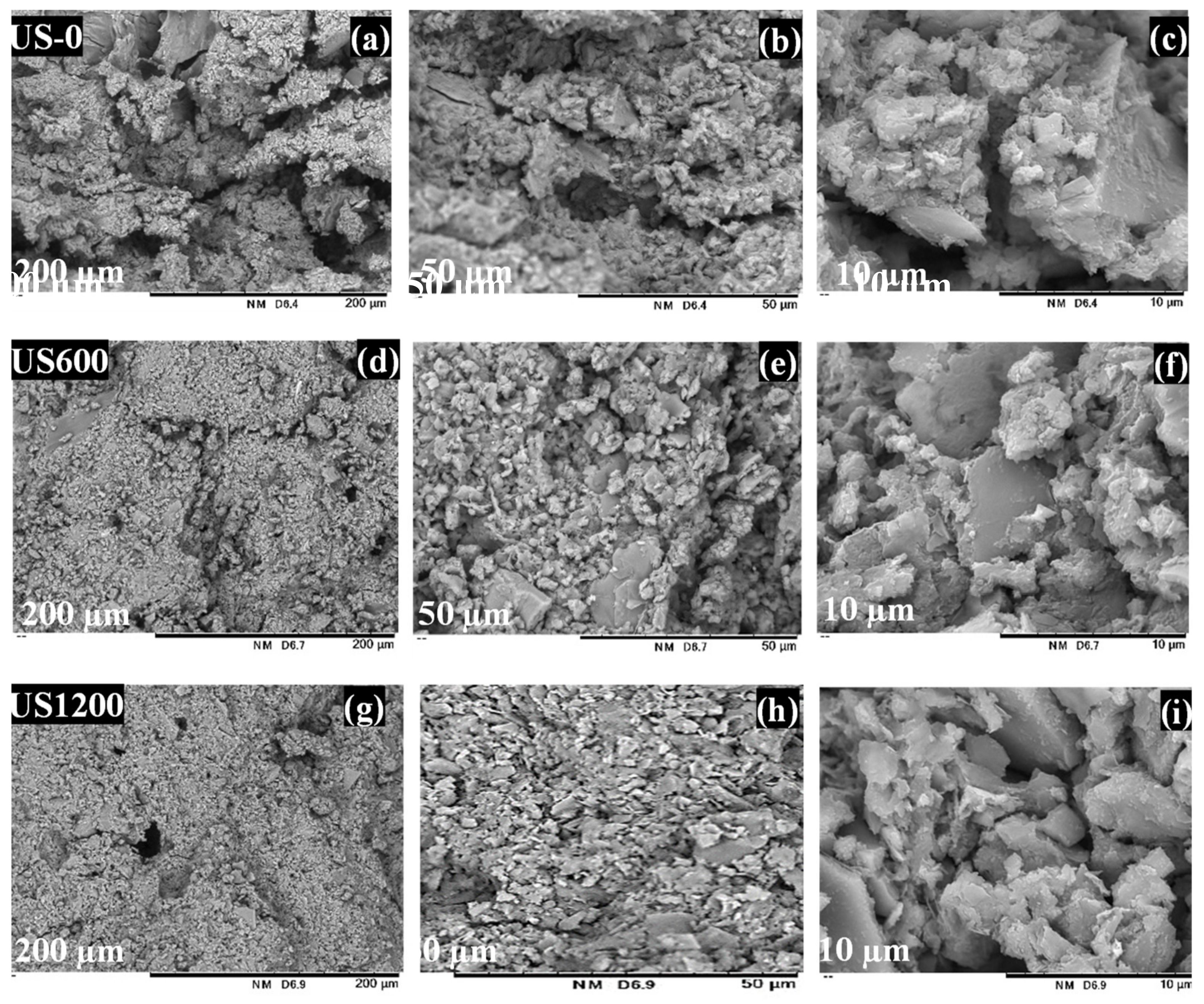
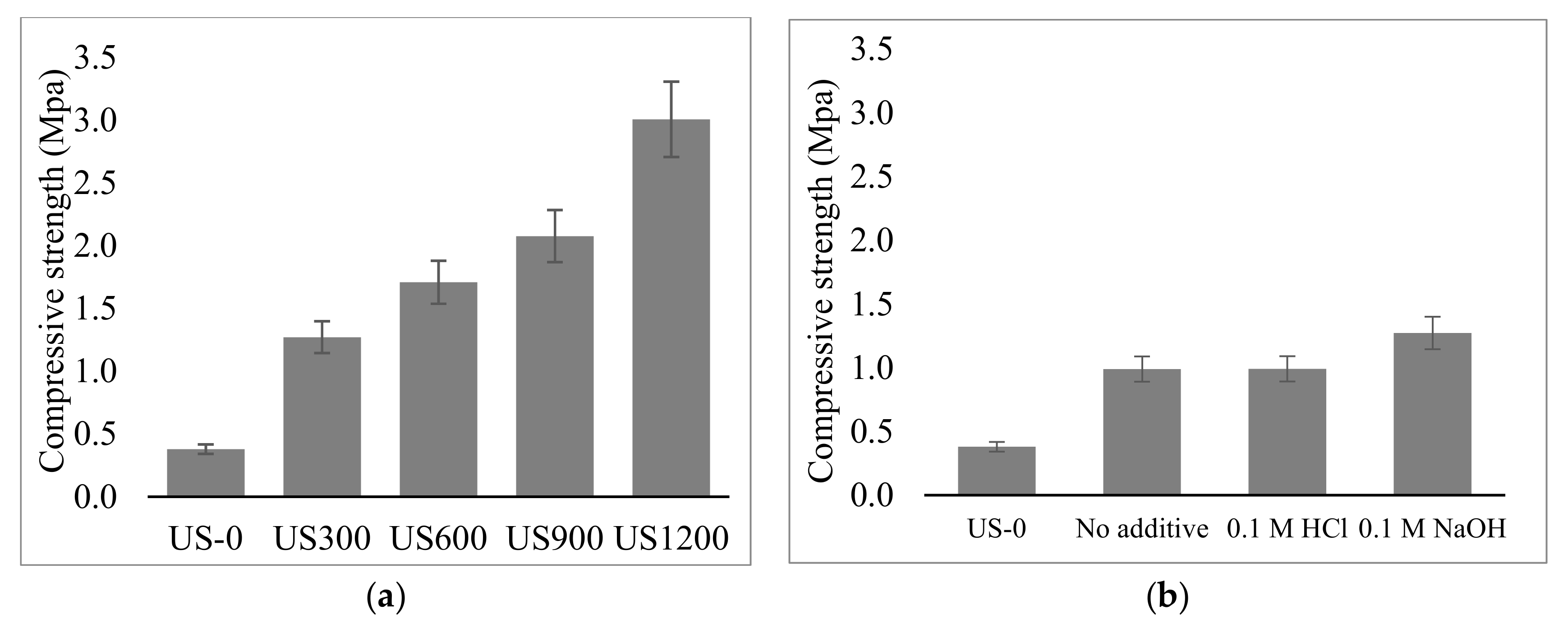
3.2. Fabrication and Properties of Soil Geopolymers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Institut Geopolymere: Saint-Quentin, France, 2015; pp. 4–5. [Google Scholar]
- Huang, T.Y.; Chuieh, P.T. Life cycle assessment of reusing fly ash from municipal waste incineration. Procedia Eng. 2015, 11, 984–991. [Google Scholar] [CrossRef]
- Nakshatra, B.S. Fly ash based geopolymer binder: A construction material. Mineral 2018, 8, 299. [Google Scholar]
- Johansson, N.; Krook, J.; Eklund, M.; Berglund, B. An Integrated Review of Concepts and Initiatives for Mining the technosphere towards a new taxonomy. J. Clean. Prod. 2013, 55, 35–44. [Google Scholar] [CrossRef]
- Residential and Civil Construction Alliance of Ontario (RCCAO) Best Management Practices for Handling Excess Construction Soils in Ontario. Available online: http://www.rccao.com/news/files/RCCAO_NOV2012.pdf (accessed on 10 July 2019).
- Wilpiszeski, R.L.; Aufrecht, J.A.; Retterer, S.T.; Sullivan, M.B.; Graham, D.E.; Pierce, E.M.; Zablocki, O.D.; Palumbo, A.V.; Elias, D.A. Soil Aggregate Microbial Communities: Towards Understanding Microbiome Interactions at Biologically Relevant Scales. Appl. Environ. Microbiol. 2019, 85, e00324-19. [Google Scholar] [CrossRef] [PubMed]
- DeGomez, T.; Kolb, P.; Kleinman, S. Basic Soil Components. 2019. Available online: https://climate-woodlands.extension.org/basic-soil-components/ (accessed on 19 November 2019).
- Mohammadi, K.; Heidari, G.; Khalesro, S.; Sohrabi, Y. Soil management, microorganisms and organic matter interactions: A review. Afr. J. Biotechnol. 2011, 10, 19840–19849. [Google Scholar]
- Robertson, G.P.; Paul, E.A.; Harwood, R.R. Greenhouse gases in intensive agriculture: Contributions of individual gases to the radiative forcing of the atmosphere. Science 2000, 289, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, M.U.F. The temperature dependence of soil organic matter 587 decomposition and the effect of global warming on soil organic carbon storage. Soil Biol. Biochem. 1995, 27, 753–760. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Linn, D.M.; Doran, J.W. Effect of water-filled pore-space on carbon-dioxide and nitrous-oxide production in tilled and non-tilled soils. Soil Sci. Soc. Am. J. 1984, 48, 1267–1272. [Google Scholar] [CrossRef]
- Skopp, J.; Jawson, M.D.; Doran, J.W. Steady-state aerobic microbial activity as a function of soil-water content. Soil Sci. Soc. Am. J. 1990, 54, 1619–1625. [Google Scholar] [CrossRef]
- Barnwell Low Level Radioactive Waste Disposal Facility. Available online: http://www.atlanticcompact.org/pdf_file/29f3737663937c53aebb326b1a87c628.ACC_Conceptual_Design_Report_071004.pdf (accessed on 10 July 2019).
- Gunasekara, M.P.C.M.; Law, D.W.; Setunge, S. Effect of composition of fly ash on compressive strength of fly ash based geopolymer morta. ACMSM 2014, 23, 113–118. [Google Scholar]
- Kaiser, M.; Berhe, A.A.; Sommer, M.; Kleber, M. Application of ultrasound to disperse soil aggregates of high mechanical stability. J. Plant Nutr. Soil Sci. 2012, 4, 521–526. [Google Scholar] [CrossRef]
- Jackson, M.L.; Helman, N.N. X-ray Diffraction Procedure for Positive Differentiation of Montmorillonite from Hydrous Mica. Soil Sci. Soc. Am. Proc. 1942, 7, 194–200. [Google Scholar]
- Griffiths, R.A. Soil-washing technology and practice. J. Hazard. Mater. 1995, 40, 175–189. [Google Scholar] [CrossRef]
- Dermont, G.; Bergeron, M.; Mercier, G. Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater. 2008, 152, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.W.I.; Rumpel, C.; Kögel-Knabner, I. Evaluation of an ultrasonic dispersion procedure to isolate primary organo-mineral complexes from soils. Eur. J. Soil Sci. 1996, 50, 87–94. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Rumpel, C.; Kögel-Knabner, I. Particle size fractionation of soil containing coal and combusted particle. Eur. J. Soil Sci. 1996, 50, 515–522. [Google Scholar] [CrossRef]
- Levesque, M.; Schnitzer, M. Effect of NaOH concentration on the extraction of organic matter and of major inorganic constituents from a soil. Can. J. Soil Sci. 1996, 46, 7–12. [Google Scholar] [CrossRef]
- Kulhawy, F.H.; Jie-Ru, C. Identification and description of soils containing very coarse fractions. J. Geotech. Geoenviron. Eng. 2009, 135, 635–646. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Kachanoski, R.G.; Voroney, R.P. Ultrasonic dispersion of aggregates: Distribution of organic matter in size fractions. Can. J. Soil Sci. 1998, 68, 395–403. [Google Scholar]
- Raine, S.R.; So, H.B. Ultrasonic dispersion of soil water: The effect of suspension properties on energy dissipation and soil dispersion. Aust. J. Soil Res. 1994, 32, 1157–1174. [Google Scholar] [CrossRef]
- Roscoe, R.; Buurman, P.; Velthorst, E.J. Disruption of soil aggregates by varied amount of ultrasound energy in fractionation of organic matter of a clay latosol: Carbon, nitrogen and delta 13C distribution in particle size fractions. Eur. J. Soil Sci. 2000, 51, 445–454. [Google Scholar] [CrossRef]
- Bray, R.H. The significance of particle-size within the clay fraction. J. Am. Ceramic Soc. 1937, 20, 257–261. [Google Scholar] [CrossRef]
- Feng, D.; Aldrich, C. Sonochemical treatment of simulated soil contaminated with diesel. Adv. Environ. Res. 2000, 4, 103–112. [Google Scholar] [CrossRef]
- Freeman, H.M.; Harris, E.F. Hazardous Waste Remediation: Innovative Treatment Technologies; Tehnomic Publishing Co. Inc.: Lancaster, UK, 1995; pp. 103–112. [Google Scholar]
- Torres, R.A.; Pétrier, C.; Combet, E.; Carrier, M.; Pulgarin, C. Ultrasonic cavitation applied to the treatment of bisphenol A. Effect of sonochemical parameters and analysis of BPA by-products. Ultrason. Sonochem. 2008, 15, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Masuda, Y.; Okada, F.; Sakurai, A.; Ichiro, T.; Sakakibara, M. Degradation of bisphenol-A using sonochemical reactions. Water Res. 2008, 42, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.M.; Chen, F.; Hatton, T.A.; Grieser, F.; Ashokkumar, M. Novel one-pot synthesis of magnetite latex nanoparticles by ultrasound irradiation. Langmuir 2009, 29, 2593–2595. [Google Scholar] [CrossRef] [PubMed]
- Teo, B.M.; Prescott, S.W.; Ashokkumar, M.; Grieser, F. Ultrasound initiated miniemulsion polymerization of methacrylate monomers. Ultrason. Sonochem. 2008, 15, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Farmer, A.D.; Collings, A.F.; Jameson, G.J. The application of power ultrasound to the durface cleaning of silica and heavy mineral sands. Ultrason. Sonochem. 2000, 7, 243–247. [Google Scholar] [CrossRef]
- Balachandran, S.; Kentish, S.E.; Mawson, R.; Ashokkumar, M. Ultrasonics enhancement of the supercritical extraction from ginger. Ultrason. Sonochem. 2006, 13, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Rovnavik, P. Effect of curing temperature on the development of hard structure of metakaoline based geopolymer. Contr. Build. Mater. 2012, 24, 1176–1183. [Google Scholar] [CrossRef]
- Singhi, B.; Laskar, A.I.; Ahmed, M.A. Investigation on soil-geopolymer with slag, fly ash and their blending. Arab. J. Sci. Eng. 2016, 41, 393–400. [Google Scholar] [CrossRef]
- Rao, M.A. Flow and Functional Models for Rheological Properties of Fluid Foods, Rheology of Fluid, Semisolid, and Solid Foods; Food Engineering Series; Science and Business Media: New York, NY, USA, 2014; pp. 21–67. [Google Scholar]
- Panda, B.; Unluer, C.; Tan, M.J. Extrusion and rheology characterization of geopolymer nanocomposites used in 3D printing. Compos. Part B Eng. 2019, 176, 107290. [Google Scholar] [CrossRef]
- Shimna, P.; Prasad Reddy, H.; Jayakumar, K.V. Swell Potential Studies on Soils Contaminated with NaOH Solutions. In Proceedings of the Indian Geotechnical Conference IGC, Kakinada, India, 18–20 December 2014; pp. 1362–1369. [Google Scholar]
- Mitchell, J.K. Fundamentals of Soil Behaviour, 2nd ed.; John Wiley and Sons Inc.: New York, NY, USA, 1993. [Google Scholar]
- Ramakrishnegowda, C.; Yaji, R.; Shivashankar, R.; Sivapullaiah, P.V. Geotechnical properties of shedi soil affected by alkali contamination. Int. J. Environ. Protection 2011, 1, 45–52. [Google Scholar]
- Isabella, C.; Lukey, G.C.; Xu, H.; van Deventer, J.S. The Effect of Aggregate Particle Size on Formation of Geopolymeric Gel. Adv. Mater. Constr. Bridges Build. Struct. III Art. 2003, 9, 1–10. [Google Scholar]
- Raphaëlle, P. Formulation and Durability of Metakaolin-Based Geopolymers. Ph.D. Thesis, Université de Toulouse, Université Toulouse III-Paul Sabatier, Toulouse, France, 2015. [Google Scholar]
- Heah, C.Y.; Kamarudin, H.; Mustafa Al Bakri, A.M.; Bnhussain, M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Adam, A.; Horianto, A. The effect of temperature and duration of curing on the strength of fly ash based geopolymer mortar. Procedia Eng. 2014, 95, 410–414. [Google Scholar] [CrossRef]
- Zuhua, Z.; Xiao, Y.; Yue, C. Role of water in the synthesis of calcined kaolin-based geopolymer. Appl. Clay Sci. 2009, 43, 218–223. [Google Scholar] [CrossRef]
| HCl | NH3 | NaOH | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| US Treatment | Soil | No additive | 0.01 M | 0.1 M | 1 M | 0.01 M | 0.1 M | 1 M | 0.01 M | 0.1 M | 1 M |
| C | 3.6 | 2.4 | 2.4 | 2.4 | 2.1 | 2.3 | 1.0 | 1.9 | 1.9 | 1.1 | 2.0 |
| Na2O | 1.3 | 1.4 | 2.0 | 1.84 | 2.2 | 2.1 | 2.1 | 2.0 | 2.2 | 2.8 | 5.0 |
| SiO2 | 50.4 | 55.3 | 63.6 | 62.8 | 63.9 | 64.8 | 65.2 | 63.5 | 64.2 | 63.3 | 62.6 |
| Al2O3 | 18.8 | 17.1 | 15.8 | 15.4 | 14.7 | 15.7 | 16.2 | 16 | 16 | 16.1 | 15.3 |
| Fe2O3 | 4.1 | 4.0 | 3.9 | 3.2 | 2.9 | 3.9 | 4.1 | 4.2 | 4.1 | 3.9 | 3.4 |
| Mass % | |||
|---|---|---|---|
| No Additive | NH3 | NaOH | |
| CO2 | 21.5 | 11.6 | 12.4 |
| Na2O | 20.9 | 17.1 | 18 |
| Al2O3 | 11 | 13.9 | 12.7 |
| SiO2 | 40.4 | 48.9 | 48.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwedi-Nsah, L.-M.; Watanabe, Y.; Kobayashi, T. Soil Recycling Geopolymers Fabricated from High Power Ultrasound Treated Soil Slurry in the Presence of Ammonia. Materials 2019, 12, 3804. https://doi.org/10.3390/ma12223804
Kwedi-Nsah L-M, Watanabe Y, Kobayashi T. Soil Recycling Geopolymers Fabricated from High Power Ultrasound Treated Soil Slurry in the Presence of Ammonia. Materials. 2019; 12(22):3804. https://doi.org/10.3390/ma12223804
Chicago/Turabian StyleKwedi-Nsah, Louis-Marly, Yuta Watanabe, and Takaomi Kobayashi. 2019. "Soil Recycling Geopolymers Fabricated from High Power Ultrasound Treated Soil Slurry in the Presence of Ammonia" Materials 12, no. 22: 3804. https://doi.org/10.3390/ma12223804
APA StyleKwedi-Nsah, L.-M., Watanabe, Y., & Kobayashi, T. (2019). Soil Recycling Geopolymers Fabricated from High Power Ultrasound Treated Soil Slurry in the Presence of Ammonia. Materials, 12(22), 3804. https://doi.org/10.3390/ma12223804




