Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification
- Two-stage modification of bentonite with cationic and nonionic surfactants. First, the modification of bentonite by exchanging naturally occurring cations at the mineral ion exchange positions for organic cations of HDTMA was conducted. The samples were prepared by mixing 100 g of natural bentonite with 2000 g of the HDTMA solution at a concentration of 48 mmol (1.0 CEC). After 8 h of stirring at 60 °C, the samples were centrifuged for 10 min at 14,000 rpm, washed thrice with distilled water and once with hot ethanol, and then dried at 40 °C for 24 h. This procedure has been well documented and proven to be highly effective [36]. Then, the prepared material was modified using a nonionic surfactant (TX100). The samples were stirred with 2000 g of TX100 solution at a concentration range of 2–120 mmol/100 g at 40 °C. After 24 h of stirring, the samples were centrifuged for 10 min at 14,000 rpm and dried at 40 °C for 24 h.
- Single-stage modification of bentonite by exclusively using a nonionic surfactant with different initial molar concentrations was conducted. The samples were stirred with 2000 g of TX100 solution at concentrations of 2–120 mmol/100 g at 40 °C. After 24 h of stirring, the samples were centrifuged for 10 min at 14,000 rpm and dried at 40 °C for 24 h.
- Natural bentonite: BENT
- Bentonite modified with HDTMA only: HD-B
- Bentonite modified with HDTMA and TX100 (2 mmol of TX100/100 g): HD-TX-2
- Bentonite modified with HDTMA and TX100 (48 mmol of TX100/100 g): HD-TX-48
- Bentonite modified with HDTMA and TX100 (120 mmol of TX100/100 g): HD-TX-120
- Bentonite modified with TX100 alone (2 mmol of TX100/100 g): TX-2
- Bentonite modified with TX100 alone (48 mmol of TX100/100 g): TX-48
- Bentonite modified with TX100 alone (120 mmol of TX100/100 g): TX-120
2.3. Methods
3. Results and Discussion
3.1. Modification
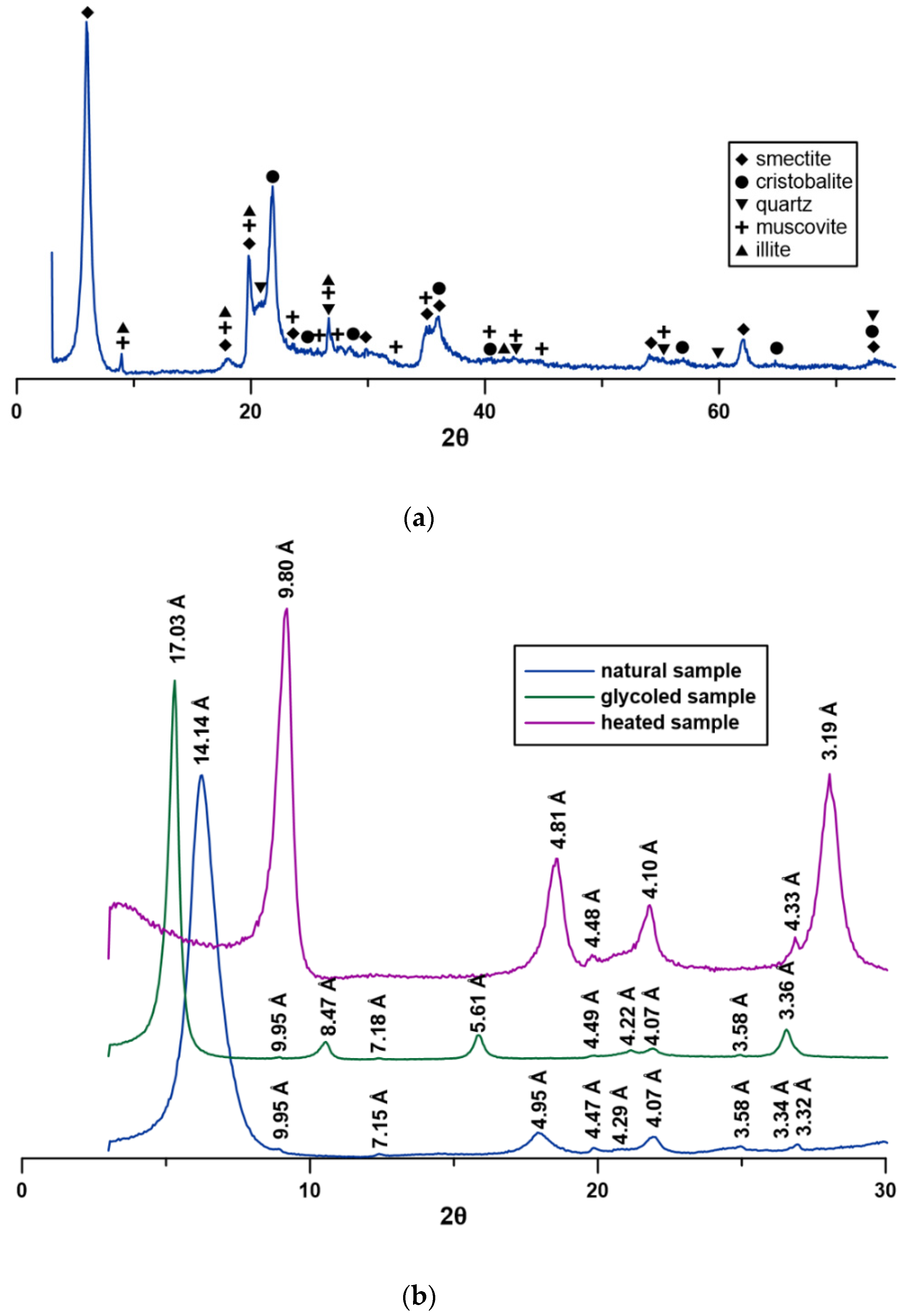
3.2. XRD
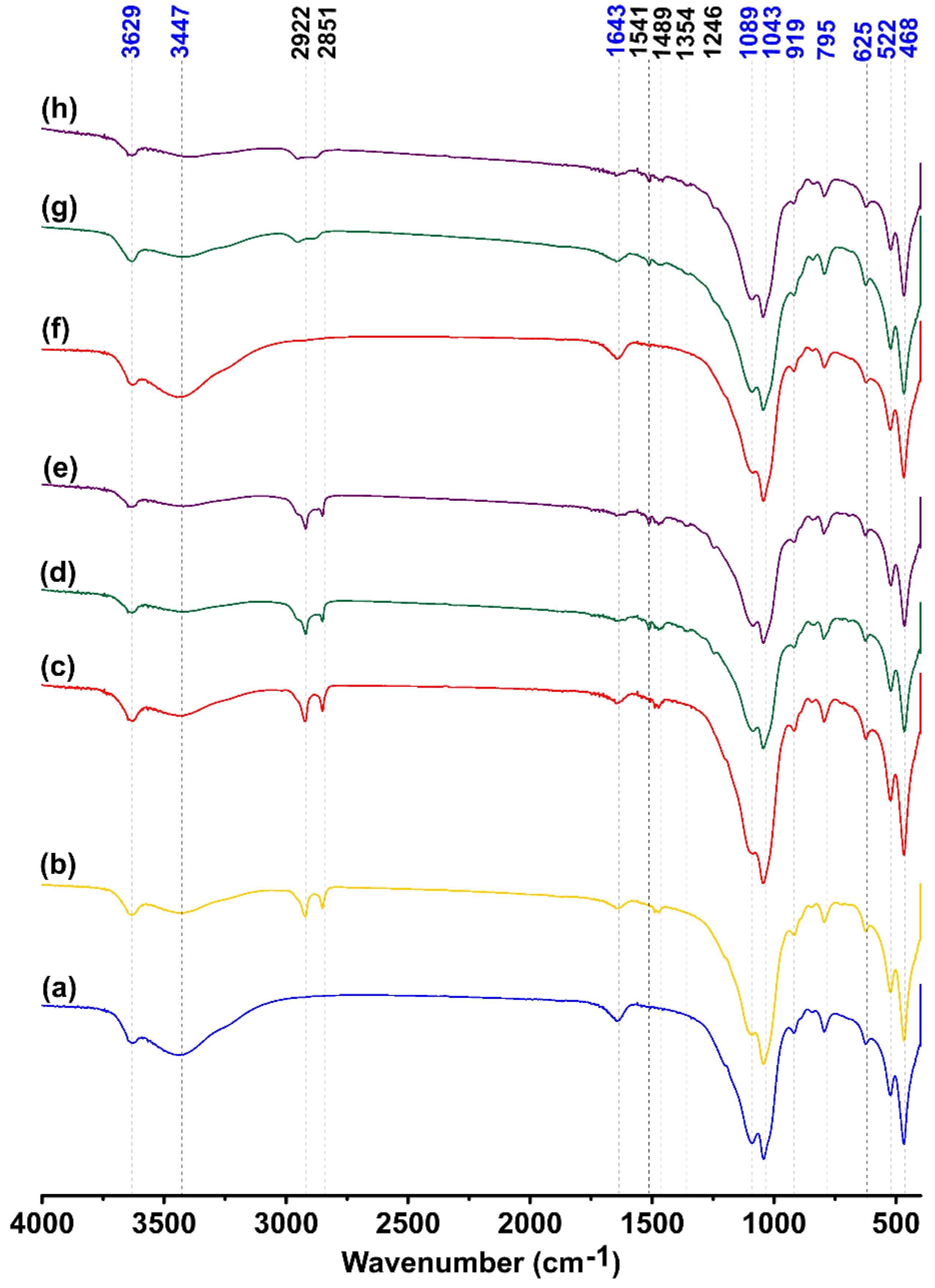
3.3. FTIR
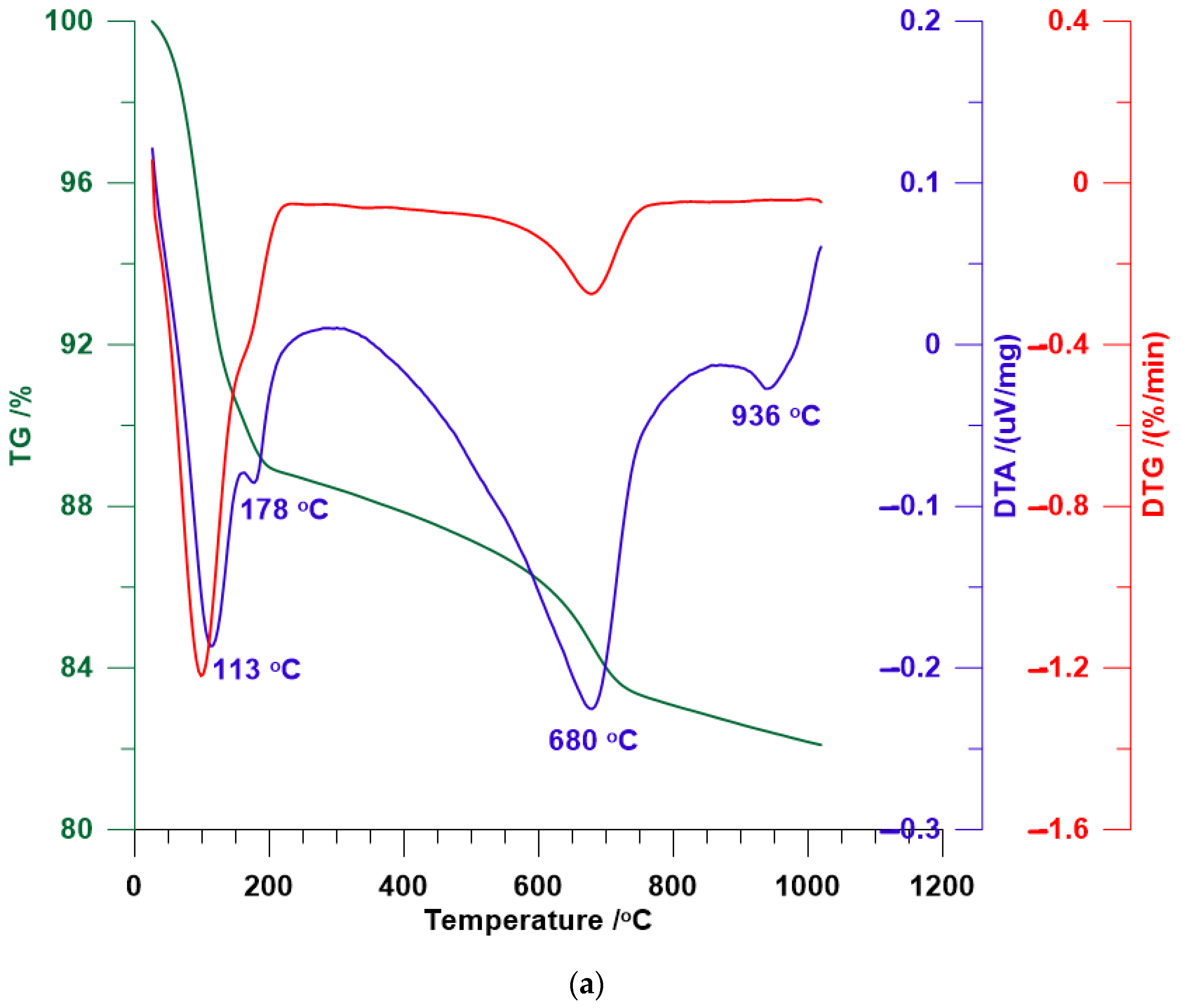
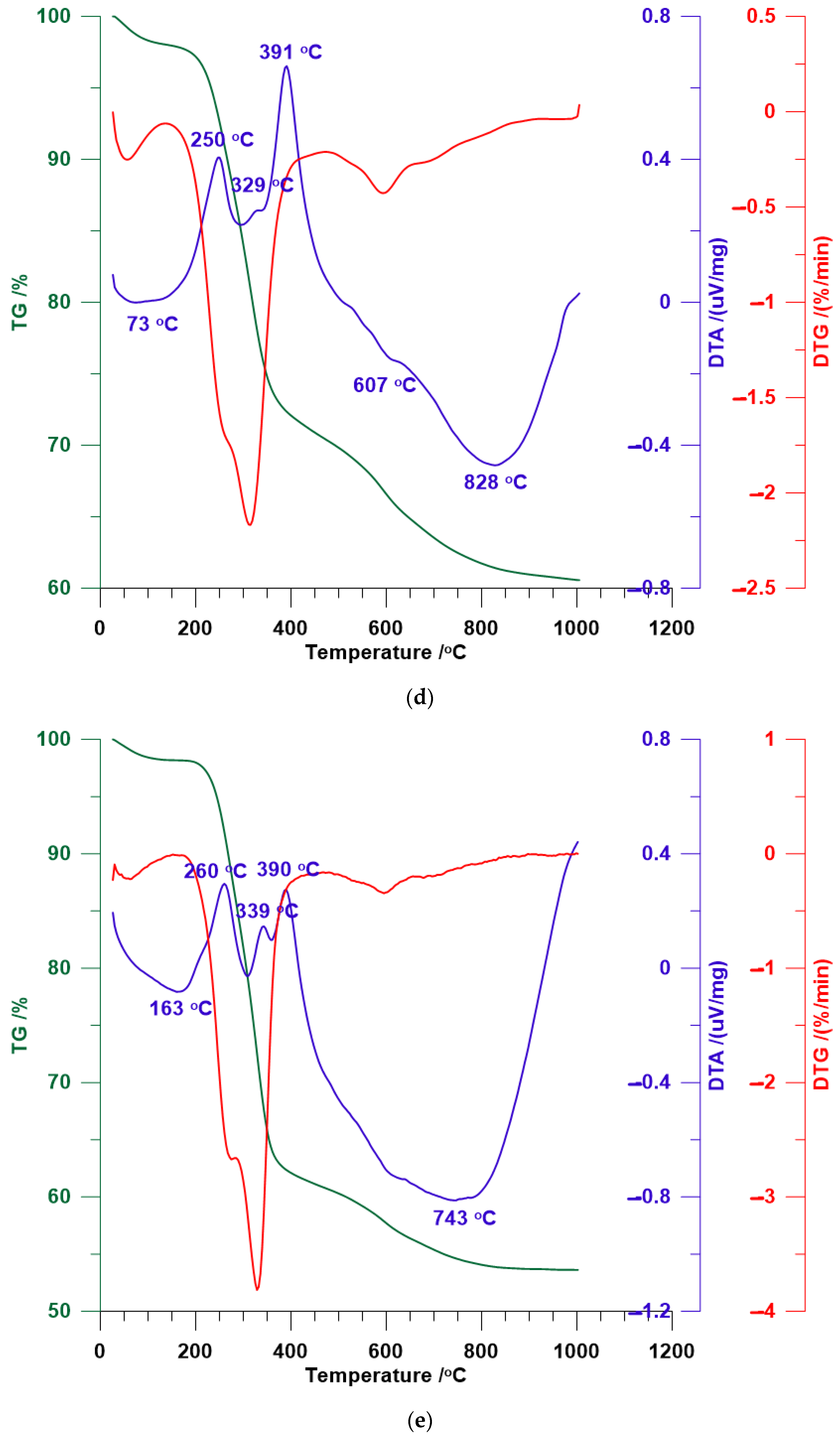
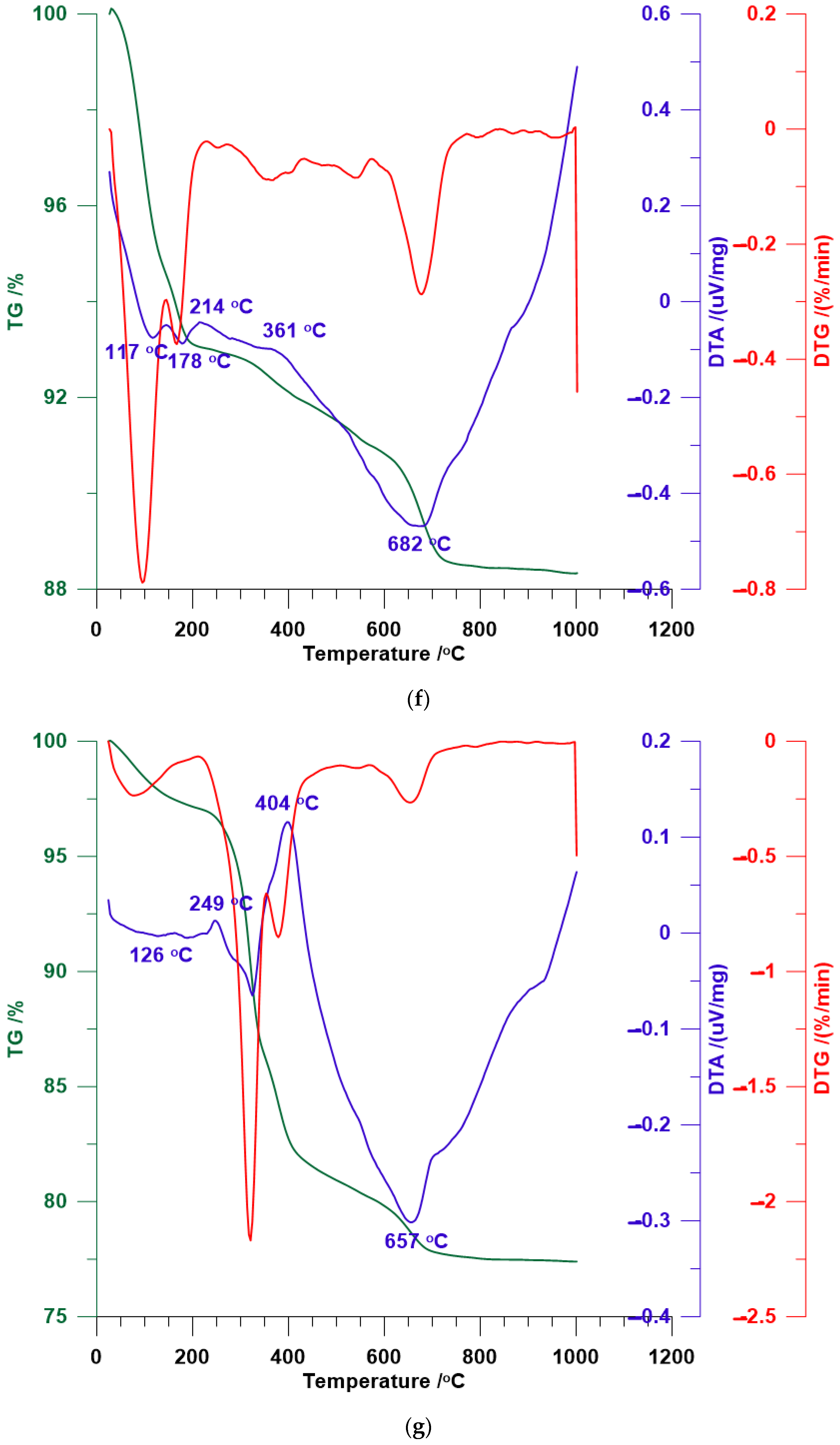
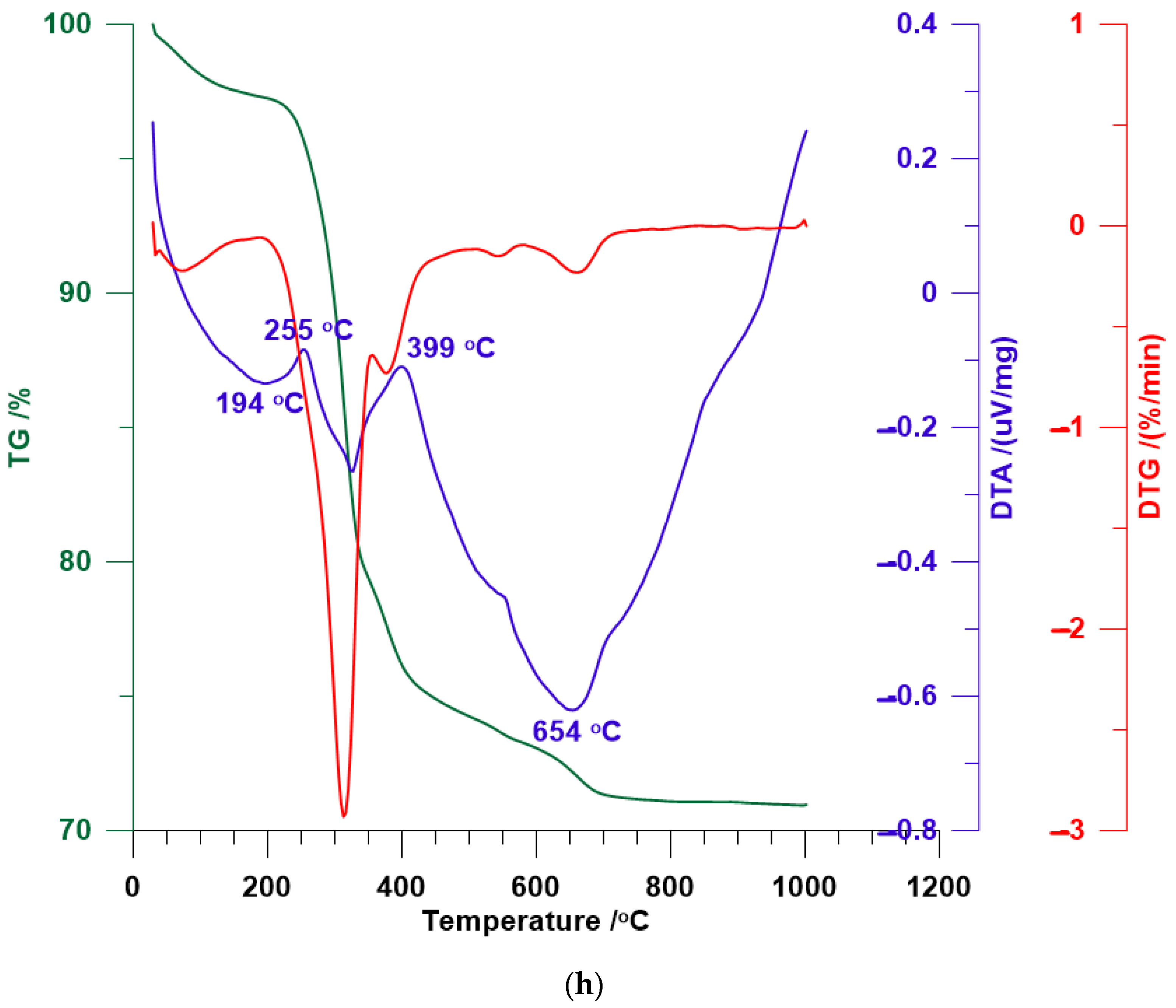
3.4. TG/DTA
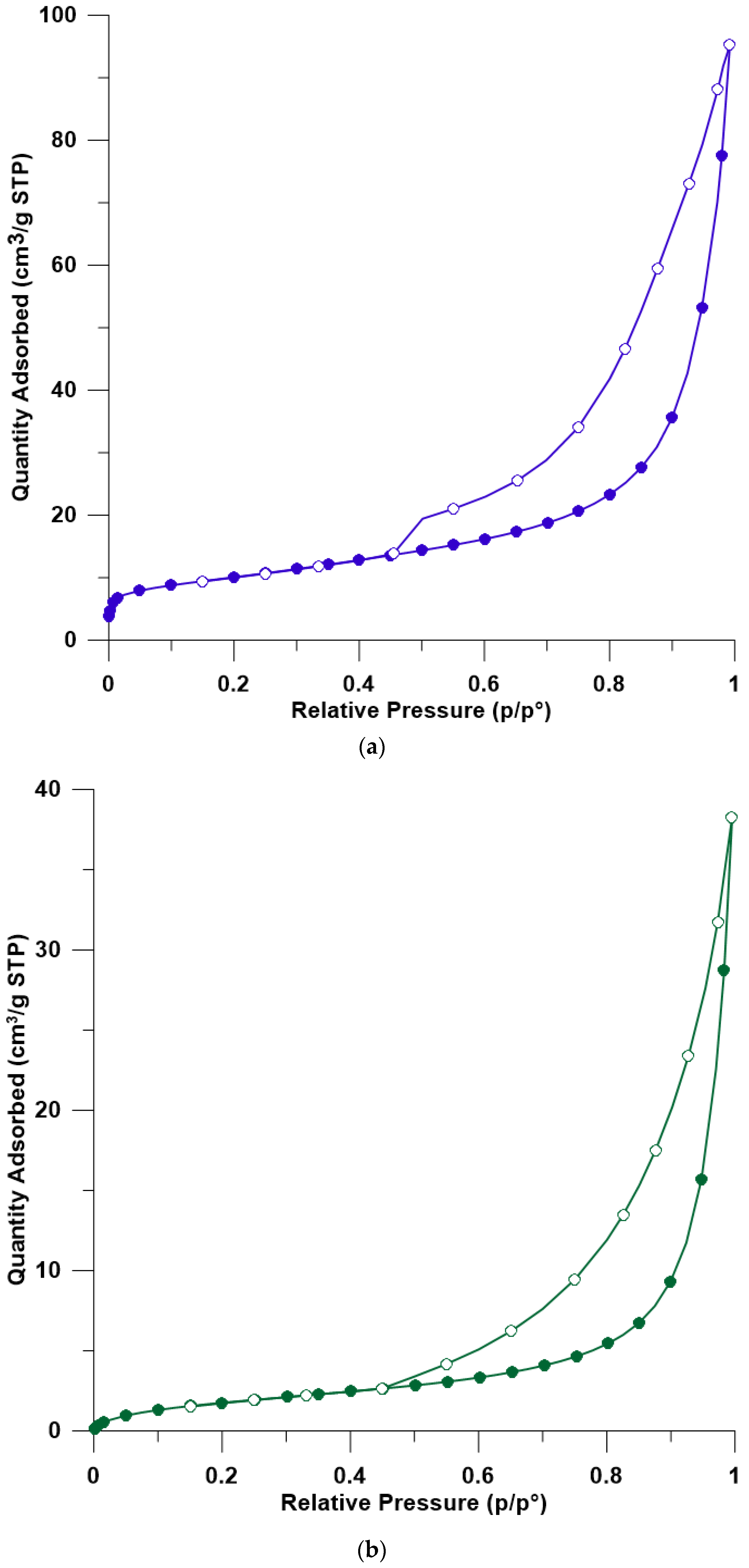
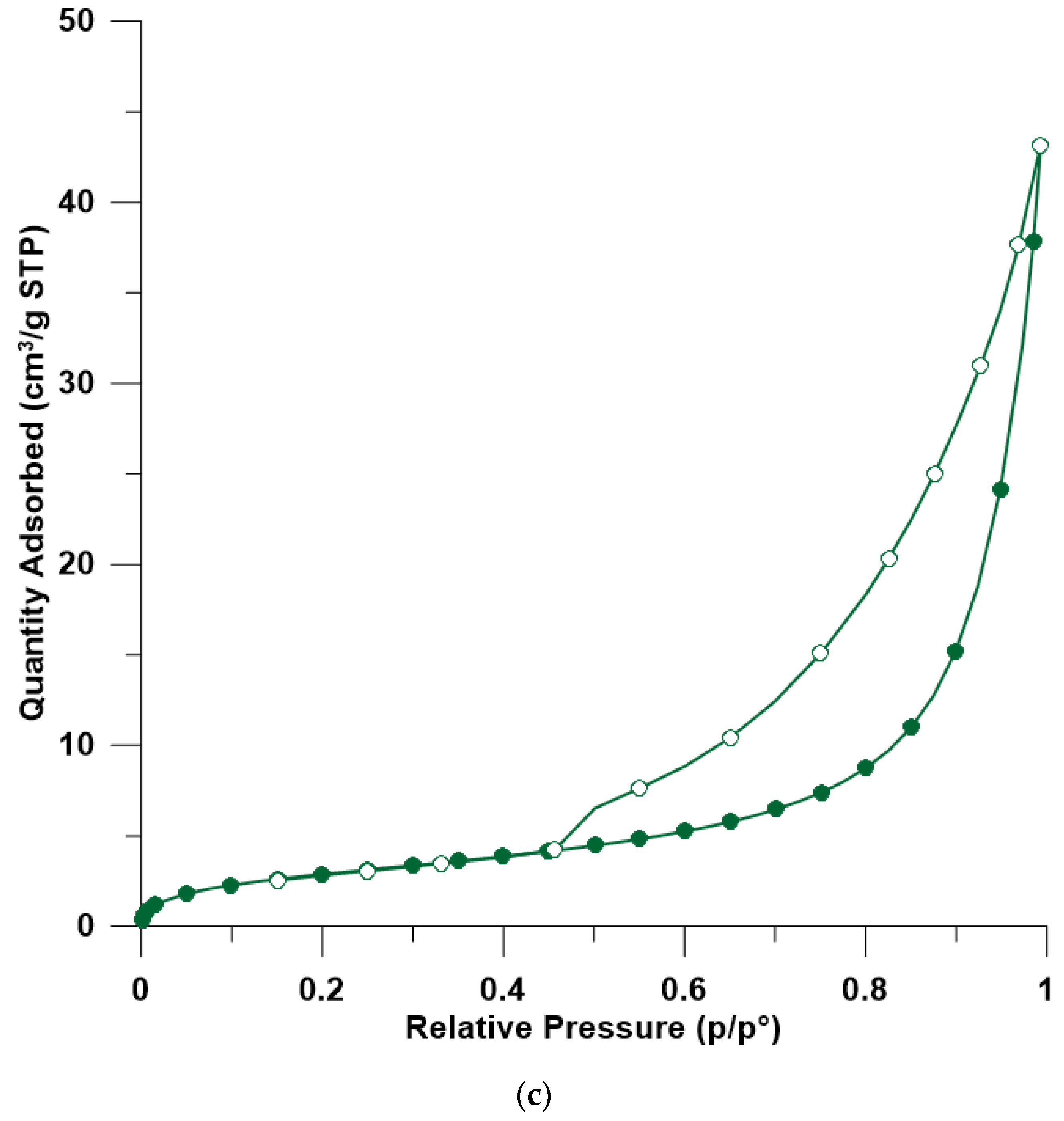
3.5. BET
3.6. SEM
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouellet-Plamondon, C.M.; Stasiak, J.; Ai-Tabbaa, A. The effect of cationic, non-ionic and amphiphilic surfactants on the intercalation of bentonite. Colloids Surf. A Physicochem. Eng. Asp. 2014, 444, 330–337. [Google Scholar] [CrossRef]
- Zhou, W.J.; Zhu, L.Z. Distribution of polycyclic aromatic hydrocarbons in soil-water system containing a nonionic surfactant. Chemosphere 2005, 60, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Zhu, W.; Xu, H.Q.; Fan, X.H.; Wu, S.L.; Shi, J.Y.; Song, J. A new method for determination of heavy metal adsorption parameters in compacted clay by batch tests. Ecotoxicol. Environ. Saf. 2019, 181, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, R.; Manjaiah, K.M.; Datta, S.C.; Sarkar, B. Comparison of properties and aquatic arsenic removal potentials of organically modified smectite adsorbents. J. Hazard. Mater. 2019, 377, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.A.; Zaini, M.A.A.; Surajudeen, A.; Aliyu, E.U.; Omeiza, A.U. Surface modification of low-cost bentonite adsorbents—A review. Part Sci. Technol. 2019, 37, 534–545. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.H.; Juang, R.S. Heavy metal removal from water by sorption using surfactant-modified montmorillonite. J. Hazard. Mater. 2002, 92, 315–326. [Google Scholar] [CrossRef]
- Bajda, T.; Klapyta, Z. Adsorption of chromate from aqueous solutions by HDTMA-modified clinoptilolite, glauconite and montmorillonite. Appl. Clay Sci. 2013, 86, 169–173. [Google Scholar] [CrossRef]
- Iwai, T.; Hashimoto, Y. Adsorption of tungstate (WO4) on birnessite, ferrihydrite, gibbsite, goethite and montmorillonite as affected by pH and competitive phosphate (PO4) and molybdate (MoO4) oxyanions. Appl. Clay Sci. 2017, 143, 372–377. [Google Scholar] [CrossRef]
- Li, Z.H.; Bowman, R.S. Sorption of chromate and PCE by surfactant-modified clay minerals. Environ. Eng. Sci. 1998, 15, 237–245. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhao, Y.; Zhu, Y.; Wu, H.Y.; Wang, H.T.; Lu, W.J. Adsorption of mixed cationic-nonionic surfactant and its effect on bentonite structure. J. Environ. Sci. 2012, 24, 1525–1532. [Google Scholar] [CrossRef]
- Toledo-Jaldin, H.P.; Blanco-Flores, A.; Sanchez-Mendieta, V.; Martin-Hernandez, O. Influence of the chain length of surfactant in the modification of zeolites and clays. Removal of atrazine from water solutions. Environ. Technol. 2018, 39, 2679–2690. [Google Scholar] [CrossRef] [PubMed]
- Senturk, H.B.; Ozdes, D.; Gundogdu, A.; Duran, C.; Soylak, M. Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: Equilibrium, kinetic and thermodynamic study. J. Hazard. Mater. 2009, 172, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Tuchowska, M.; Muir, B.; Kowalik, M.; Socha, R.P.; Bajda, T. Sorption of molybdates and tungstates on functionalized montmorillonites: Structural and textural features. Materials 2019, 12, 21. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G.; Vayer, M. Cation and anion exchange. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G.G.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, pp. 979–1001. [Google Scholar]
- Lee, S.; Chen, Y.H.; Wang, J.Y. Isothermal sheet formability of magnesium alloy AZ31 and AZ61. J. Mater. Process. Technol. 2002, 124, 19–24. [Google Scholar] [CrossRef]
- He, H.P.; Frost, R.L.; Bostrom, T.; Yuan, P.; Duong, L.; Yang, D.; Yunfel, X.F.; Kloprogge, J.T. Changes in the morphology of organoclays with HDTMA(+) surfactant loading. Appl. Clay Sci. 2006, 31, 262–271. [Google Scholar] [CrossRef]
- Ouellet-Plamondon, C.; Lynch, R.J.; Al-Tabbaa, A. Comparison between granular pillared, organo- and inorgano-organo-bentonites for hydrocarbon and metal ion adsorption. Appl. Clay Sci. 2012, 67–68, 91–98. [Google Scholar] [CrossRef]
- Chang, Y.; Lv, X.Q.; Zha, F.; Wang, Y.G.; Lei, Z.Q. Sorption of p-nitrophenol by anion-cation modified palygorskite. J. Hazard. Mater. 2009, 168, 826–831. [Google Scholar] [CrossRef]
- He, H.P.; Zhou, Q.; Frost, R.L.; Wood, B.J.; Duong, L.V.; Kloprogge, J.T. A X-ray photoelectron spectroscopy study of HDTMAB distribution within organoclays. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 66, 1180–1188. [Google Scholar] [CrossRef]
- Rodriguez-Cruz, M.S.; Sanchez-Martin, M.J.; Andrades, M.S.; Sanchez-Camazano, M. Modification of clay barriers with a cationic surfactant to improve the retention of pesticides in soils. J. Hazard. Mater. 2007, 139, 363–372. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, J.X.; Zhu, R.L.; Ge, F.; Yuan, P.; He, H.P. Enhancing the sorption capacity of CTMA-bentonite by simultaneous intercalation of cationic polyacrylamide. J. Hazard. Mater. 2010, 178, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Reza, E.M.; Pérez Bueno, J.J.; Arreola, F.E.; Aviles-Arellano, L.; Pérez-Robles, J.F.; Nava Mendoza, N.; Hurtado-Macias, A. Organobentonites with crystalline layer separation used for adsorption in water treatment. In Handbook of Research on Diverse Applications of Nanotechnology in Biomedicine, Chemistry, and Engineering; Soni, S., Salhotra, A., Suar, M., Eds.; Engineering Science Reference: Hershey, PA, USA, 2014. [Google Scholar]
- Lee, S.M.; Tiwari, D. Organo and inorgano-organo-modified clays in the remediation of aqueous solutions: An overview. Appl. Clay Sci. 2012, 59–60, 84–102. [Google Scholar] [CrossRef]
- Celis, R.; Hermosin, M.C.; Cornejo, J. Heavy metal adsorption by functionalized clays. Environ. Sci. Technol. 2000, 34, 4593–4599. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M.; Rytwo, G. Hybrid materials based on clays for environmental and biomedical applications. J. Mater. Chem. 2010, 20, 9306–9321. [Google Scholar] [CrossRef]
- Shen, Y.H. Preparations of organobentonite using nonionic surfactants. Chemosphere 2001, 44, 989–995. [Google Scholar] [CrossRef]
- Cross, J. Nonionic Surfactants: Chemical Analysis; Marcel Dekker Inc.: New York, NY, USA, 1987. [Google Scholar]
- Landoll, L.M. Non-ionic polymer surfactants. J. Polym. Sci. Part A Polym. Chem. 1982, 20, 443–455. [Google Scholar] [CrossRef]
- Reeve, P.J.; Fallowfield, H.J. The toxicity of cationic surfactant HDTMA-Br, desorbed from surfactant modified zeolite, towards faecal indicator and environmental microorganisms. J. Hazard. Mater. 2017, 339, 208–215. [Google Scholar] [CrossRef]
- Wolowiec, M.; Muir, B.; Zieba, K.; Bajda, T.; Kowalik, M.; Franus, W. Experimental Study on the Removal of VOCs and PAHs by Zeolites and Surfactant-Modified Zeolites. Energy Fuels 2017, 31, 8803–8812. [Google Scholar] [CrossRef]
- Thanos, A.G.; Sotiropoulos, A.; Malamis, S.; Katsou, E.; Pavlatou, E.A.; Haralambous, K.J. Regeneration of HDTMA-modified minerals after sorption with chromate anions. Desalin. Water Treat. 2016, 57, 27869–27878. [Google Scholar] [CrossRef]
- Krajnak, A.; Viglasova, E.; Galambos, M.; Krivosudsky, L. Kinetics, thermodynamics and isotherm parameters of uranium(VI) adsorption on natural and HDTMA-intercalated bentonite and zeolite. Desalin. Water Treat. 2018, 127, 272–281. [Google Scholar] [CrossRef]
- Gammoudi, S.; Frini-Srasra, N.; Srasra, E. Influence of exchangeable cation of smectite on HDTMA adsorption: Equilibrium, kinetic and thermodynamic studies. Appl. Clay Sci. 2012, 69, 99–107. [Google Scholar] [CrossRef]
- Dultz, S.; An, J.H.; Riebe, B. Organic cation exchanged montmorillonite and vermiculite as adsorbents for Cr(VI): Effect of layer charge on adsorption properties. Appl. Clay Sci. 2012, 67–68, 125–133. [Google Scholar] [CrossRef]
- Bajda, T.; Szala, B.; Solecka, U. Removal of lead and phosphate ions from aqueous solutions by organo-smectite. Environ. Technol. 2015, 36, 2872–2883. [Google Scholar] [CrossRef] [PubMed]
- Muir, B.; Andrunik, D.; Hyla, J.; Bajda, T. The removal of molybdates and tungstates from aqueous solution by organo-smectites. Appl. Clay Sci. 2017, 136, 8–17. [Google Scholar] [CrossRef]
- Bajda, T.; Szala, B. Removal of vanadium(VI) from aqueous solution by HDTMA-modified clinoptilolite. In Proceedings of the International Conference on Applied Mineralogy & Advanced Materials, AMAM 2015, Taranto, Italy, 7–12 June 2015. [Google Scholar]
- Krishna, B.S.; Murty, D.S.R.; Prakash, B.S.J. Surfactant-modified clay as adsorbent for chromate. Appl. Clay Sci. 2001, 20, 65–71. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Taghizadeh, A.; Taghizadeh, M.; Azimi, M. Surface modified montmorillonite with cationic surfactants: Preparation, characterization, and dye adsorption from aqueous solution. J. Environ. Chem. Eng. 2019, 7, 11. [Google Scholar] [CrossRef]
- Guegan, R. Organoclay applications and limits in the environment. Comptes Rendus Chim. 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; de Oliveira, V.R.L.; dos Santos, F.K.G.; Neto, E.L.D.; Leite, R.H.D.; Aroucha, E.M.M.; Silva, K.N.D. Influence of the ionic and nonionic surfactants mixture in the structure and properties of the modified bentonite clay. J. Mol. Liq. 2018, 272, 990–998. [Google Scholar] [CrossRef]
- Tuchowska, M.; Bajda, T. Bentonite modified with ionic-nonionic surfactants as a sorbent of pesticides. In Proceedings of the 19th International Conference of Young Geologists, Herľany, Slovakia, 5–7 April 2018; pp. 25–26. [Google Scholar]
- Zhuang, G.Z.; Zhang, Z.P.; Wu, H.; Zhang, H.X.; Zhang, X.M.; Liao, L.B. Influence of the nonionic surfactants’ nature on the structures and properties of organo-montmorillonites. Colloids Surf. A Physicochem. Eng. Asp. 2017, 518, 116–123. [Google Scholar] [CrossRef]
- Zhuang, G.Z.; Zhang, Z.P.; Fu, M.; Ye, X.; Liao, L.B. Comparative study on the use of cationic-nonionic-organo-montmorillonite in oil-based drilling fluids. Appl. Clay Sci. 2015, 116, 257–262. [Google Scholar] [CrossRef]
- Zhuang, G.Z.; Zhang, Z.P.; Guo, J.S.; Liao, L.B.; Zhao, J.L. A new ball milling method to produce organo-montmorillonite from anionic and nonionic surfactants. Appl. Clay Sci. 2015, 104, 18–26. [Google Scholar] [CrossRef]
- Samiey, B.; Golestan, S. Adsorption of Triton X-100 on silica gel: Effects of temperature and alcohols. Cent. Eur. J. Chem. 2010, 8, 361–369. [Google Scholar] [CrossRef]
- Huang, J.; Cao, J.; Tu, N.; Dong, H.P.; Li, J.F.; Shou, J.X.; Li, Y.M. Effect of surfactants on the removal of nitrobenzene by Fe-bearing montmorillonite/Fe(II). J. Colloid Interface Sci. 2019, 533, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Breen, C.; Thompson, G.; Webb, M. Preparation, thermal stability and decomposition routes of clay/Triton-X100 composites. J. Mater. Chem. 1999, 9, 3159–3165. [Google Scholar] [CrossRef]
- Shariatmadari, H.; Mermut, A.R.; Benke, M.B. Sorption of selected cationic and neutral organic molecules on palygorskite and sepiolite. Clays Clay Miner. 1999, 47, 44–53. [Google Scholar] [CrossRef]
- Barhoumi, M.; Said, H. Adsorption of alkylphenols onto green clay surface in the presence of nonionic surfactant. Rev. Roum. Chim. 2017, 62, 735–739. [Google Scholar]
- Zhang, W.; Zhang, F.W.; Han, Z.T.; Lu, X.L.; Lin, D.H.; Werner, D. Effect of clay minerals on transport of surfactants dispersed multi-walled carbon nanotubes in porous media. Acta Geol. Sin. Engl. Ed. 2017, 91, 135–144. [Google Scholar] [CrossRef]
- Qiao, L.; Easteal, A.J. Mass transport in Triton X series nonionic surfactant solutions: A new approach to solute-solvent interactions. Colloid Polym. Sci. 1996, 274, 974–980. [Google Scholar] [CrossRef]
- Garewal, H.S. Procedure for the estimation of microgram quantities of Triton X-100. Anal. Biochem. 1973, 54, 319–324. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances II. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Xu, S.H.; Boyd, S.A. Cationic surfactant adsorption by swelling and nonswelling layer silicates. Langmuir 1995, 11, 2508–2514. [Google Scholar] [CrossRef]
- Zhang, L.; Somasundaran, P. Adsorption of mixtures of nonionic sugar-based surfactants with other surfactants at solid/liquid interfaces I. Adsorption of n-dodecyl-beta-D-maltoside with anionic sodium dodecyl sulfate on alumina. J. Colloid Interface Sci. 2006, 302, 20–24. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1997; p. 400. [Google Scholar]
- Xi, Y.F.; Ding, Z.; He, H.P.; Frost, R.L. Structure of organoclays—An X-ray diffraction and thermogravimetric analysis study. J. Colloid Interface Sci. 2004, 277, 116–120. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, L.B.; Morales, A.R.; Diaz, F.R.V. Organoclays: Properties, preparation and applications. Appl. Clay Sci. 2008, 42, 8–24. [Google Scholar] [CrossRef]
- Zhu, J.X.; He, H.P.; Guo, J.G.; Yang, D.; Xie, X.D. Arrangement models of alkylammonium cations in the interlayer of HDTMA(+) pillared montmorillonites. Chin. Sci. Bull. 2003, 48, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.M.; Deng, Y.X.; Zhu, Q.; Du, G.X.; Zhou, F.S. Characterization of cationic-nonionic surfactants modified montmorillonite and its application for the Removal of p-nitrophenol. Sci. Adv. Mater. 2013, 5, 1041–1051. [Google Scholar] [CrossRef]
- Navratilova, Z.; Wojtowicz, P.; Vaculikova, L.; Sugarkova, V. Sorption of alkylammonium cations on montmorillonite. Acta Geodyn. Geomater. 2007, 4, 59–65. [Google Scholar]
- Zhang, Y.X.; Long, Y.Y.; Zhang, Y.C.; Zhu, Y.; Wang, H.T.; Wu, H.Y.; Lu, W.J. Effect of a mixed anionic-nonionic surfactant adsorption on bentonite structure and on distribution of pentachlorophenol. Appl. Clay Sci. 2012, 69, 93–98. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications, Analytical Techniques in the Sciences; John Wiley & Sons Ltd.: London, UK, 2005. [Google Scholar]
- Del Hoyo, C.; Dorado, C.; Rodriguez-Cruz, M.S.; Sanchez-Martin, M.J. Physico-chemical study of selected surfactant-clay mineral systems. J. Therm. Anal. Calorim. 2008, 94, 227–234. [Google Scholar] [CrossRef]
- Hubetska, T.S.; Krivtsov, I.; Kobylinska, N.G.; Menendez, J.R.G. Hydrophobically functionalized magnetic nanocomposite as a new adsorbent for preconcentration of organochlorine pesticides in water solution. IEEE Magn. Lett. 2018, 9, 5. [Google Scholar] [CrossRef]
- Madejova, J.; Sekerakova, L.; Bizovska, V.; Slany, M.; Jankovic, L. Near-infrared spectroscopy as an effective tool for monitoring the conformation of alkylammonium surfactants in montmorillonite interlayers. Vib. Spectrosc. 2016, 84, 44–52. [Google Scholar] [CrossRef]
- Mozgawa, W.; Krol, M.; Bajda, T. IR spectra in the studies of anion sorption on natural sorbents. J. Mol. Struct. 2011, 993, 109–114. [Google Scholar] [CrossRef]
- Földvári, M. Handbook of Thermogravimetric System of Minerals and its Use in Geological Practice; Fancsik, T., Ed.; Geological Institute of Hungary: Budapest, Hungary, 2011; Volume 213. [Google Scholar]
- He, H.P.; Ding, Z.; Zhu, J.X.; Yuan, P.; Xi, Y.F.; Yang, D.; Frost, R.L. Thermal characterization of surfactant-modified montmorillonites. Clays Clay Miner. 2005, 53, 287–293. [Google Scholar] [CrossRef]
- Zhou, Q.; Frost, R.L.; He, H.P.; Xi, Y.F. Changes in the surfaces of adsorbed para-nitrophenol on HDTMA organoclay—The XRD and TG study. J. Colloid Interface Sci. 2007, 307, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Apreutesei, R.E.; Catrinescu, C.; Teodosiu, C. Surfactant-modified natural zeolites for environmental applications in water purification. Environ. Eng. Manag. J. 2008, 7, 149–161. [Google Scholar]
- Tuchowska, M.; Wolowiec, M.; Solinska, A.; Koscielniak, A.; Bajda, T. Organo-modified vermiculite: Preparation, characterization, and sorption of arsenic compounds. Minerals 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas solid systems with special reference to the determination of surface-area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhu, L.Z.; Zhu, R.L.; Tian, S.L.; Li, J.W. Surface microtopography of surfactant modified montmorillonite. Appl. Clay Sci. 2009, 45, 70–75. [Google Scholar] [CrossRef]


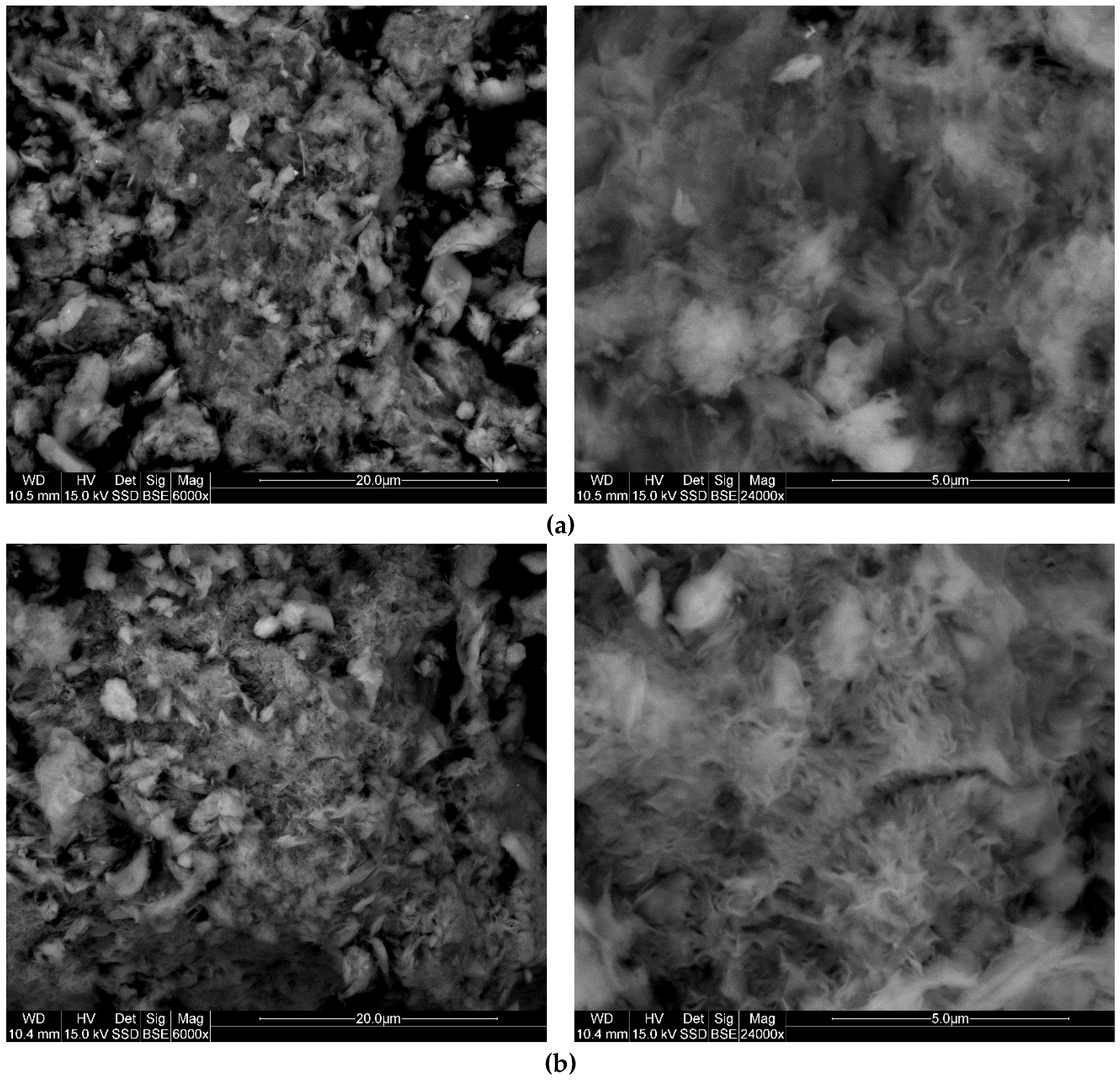
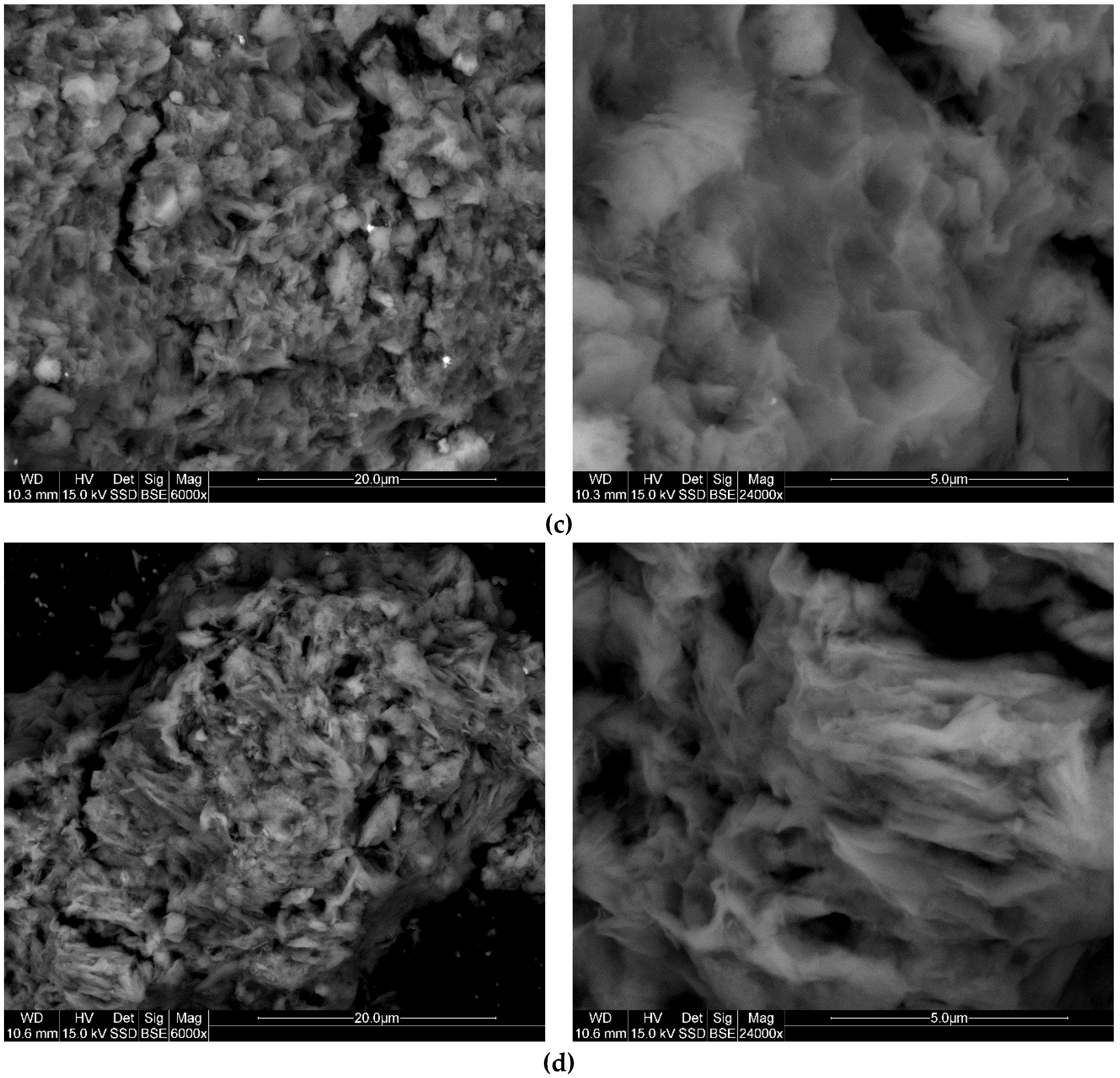
| Initial Concentration of TX100 (mmol/100 g) | Amount of TX100 Adsorbed (mmol/100 g) | |
|---|---|---|
| Bentonite with HDTMA Pre-Adsorbed | Natural Bentonite | |
| 2 | 1.96 | 1.74 |
| 6 | 5.08 | 4.66 |
| 12 | 11.94 | 11.08 |
| 24 | 22.30 | 23.72 |
| 48 | 43.24 | 32.68 |
| 60 | 59.30 | 29.24 |
| 80 | 73.70 | 31.62 |
| 120 | 108.92 | 34.50 |
| 160 | 124.72 | 39.96 |
| Sample | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Volume of Micropores (cm3/g) | Volume of Mesopores (cm3/g) | Volume of Macropores (cm3/g) |
|---|---|---|---|---|---|
| BENT | 35.7 | 0.143 | 0.015 | 0.098 | 0.03 |
| HD-TX-48 | 7.1 | 0.053 | 0.002 | 0.032 | 0.019 |
| TX-48 | 11.0 | 0.063 | 0.004 | 0.047 | 0.012 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrunik, M.; Bajda, T. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials 2019, 12, 3772. https://doi.org/10.3390/ma12223772
Andrunik M, Bajda T. Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials. 2019; 12(22):3772. https://doi.org/10.3390/ma12223772
Chicago/Turabian StyleAndrunik, Magdalena, and Tomasz Bajda. 2019. "Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features" Materials 12, no. 22: 3772. https://doi.org/10.3390/ma12223772
APA StyleAndrunik, M., & Bajda, T. (2019). Modification of Bentonite with Cationic and Nonionic Surfactants: Structural and Textural Features. Materials, 12(22), 3772. https://doi.org/10.3390/ma12223772






