The Effect of Rare Earths Additions on the Microstructure and the Corrosion Behavior of Sn-0.7Cu-0.075Al Solder Alloy
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials and Samples Preparation
2.2. Microstructural Observation
2.3. Electrochemical Corrosion
3. Results and Discussion
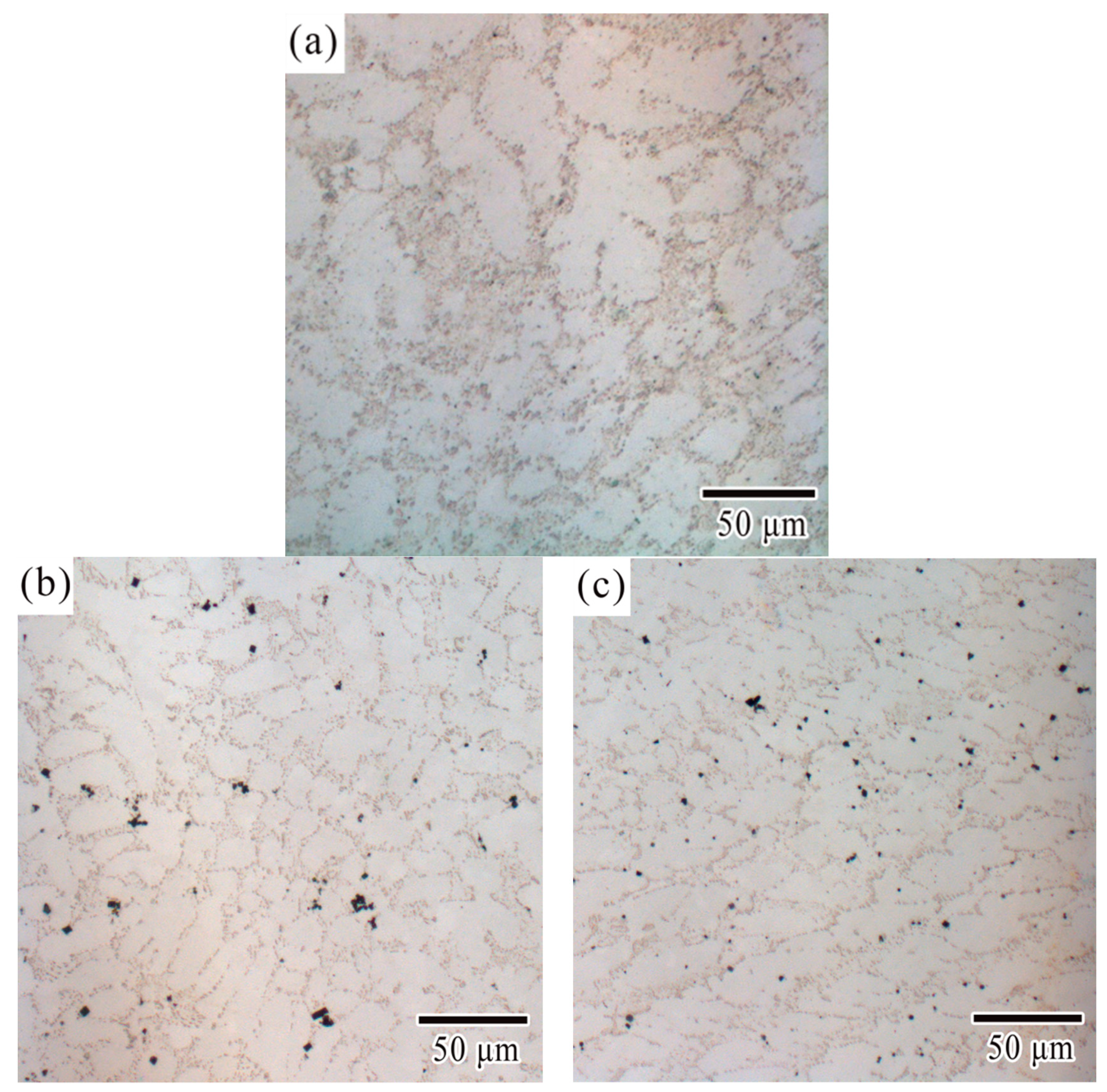
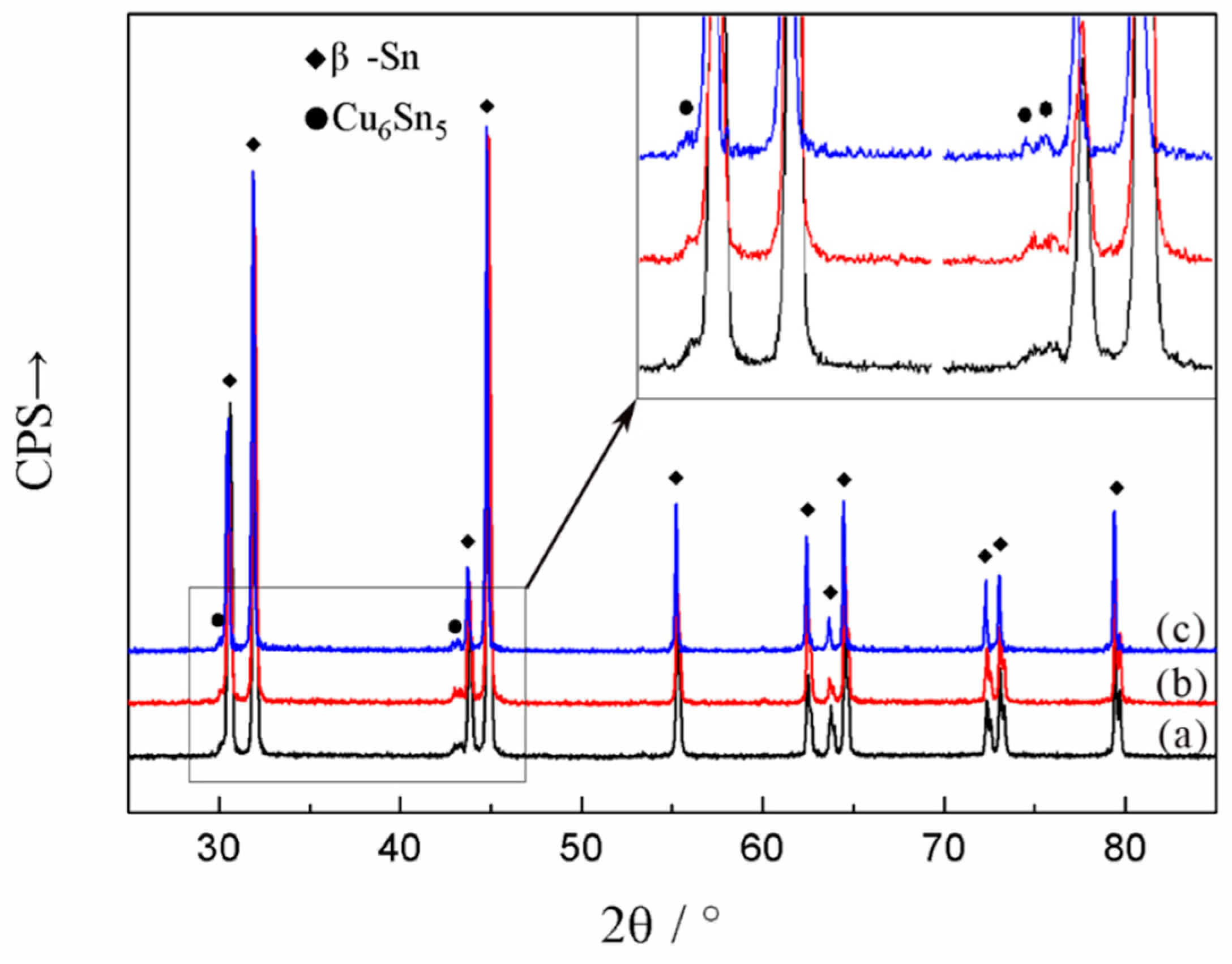
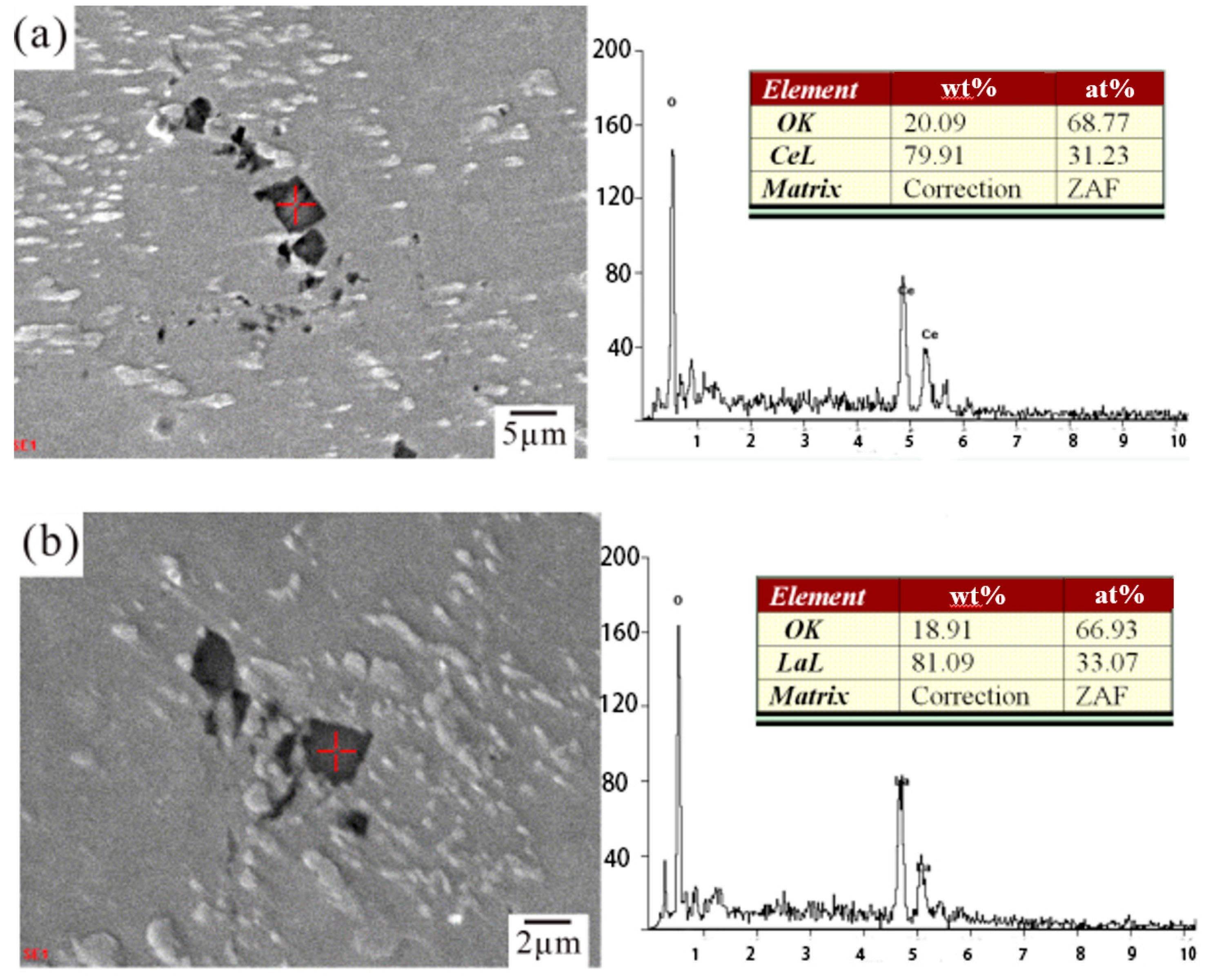
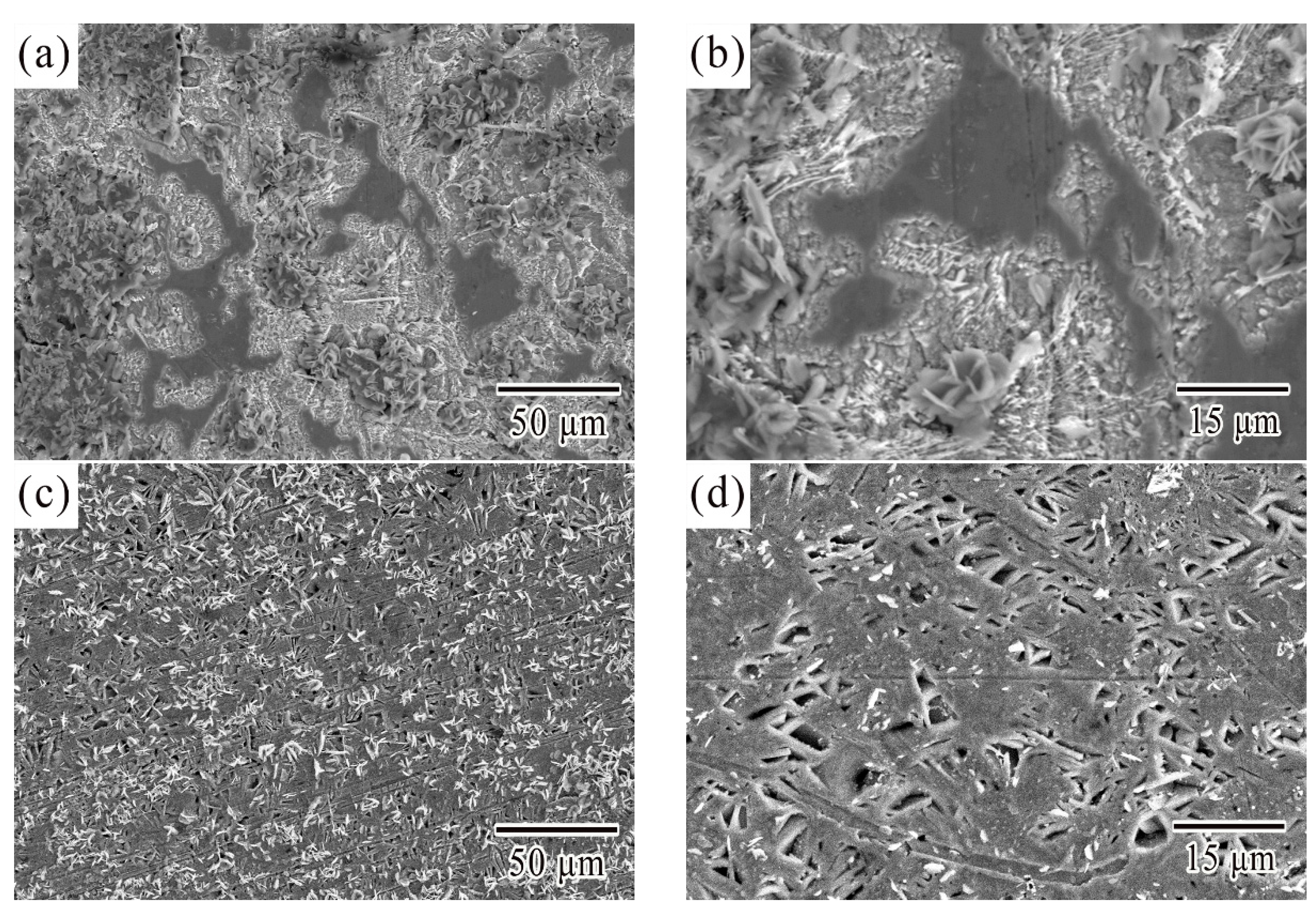
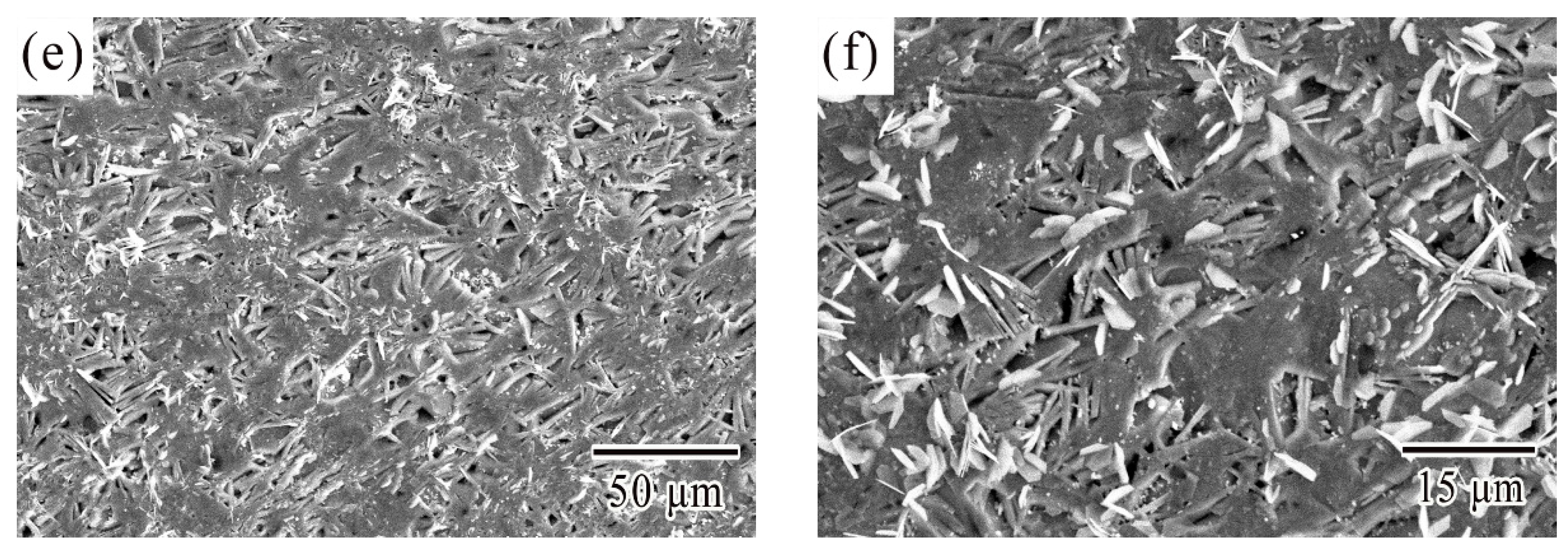
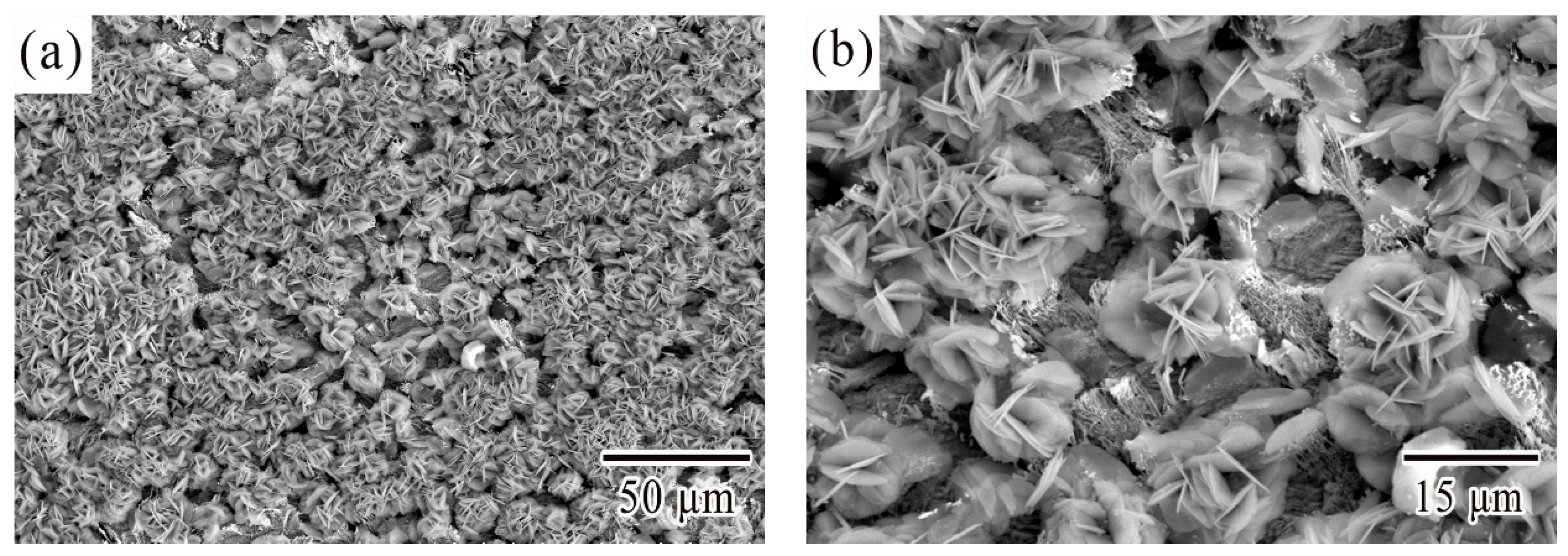
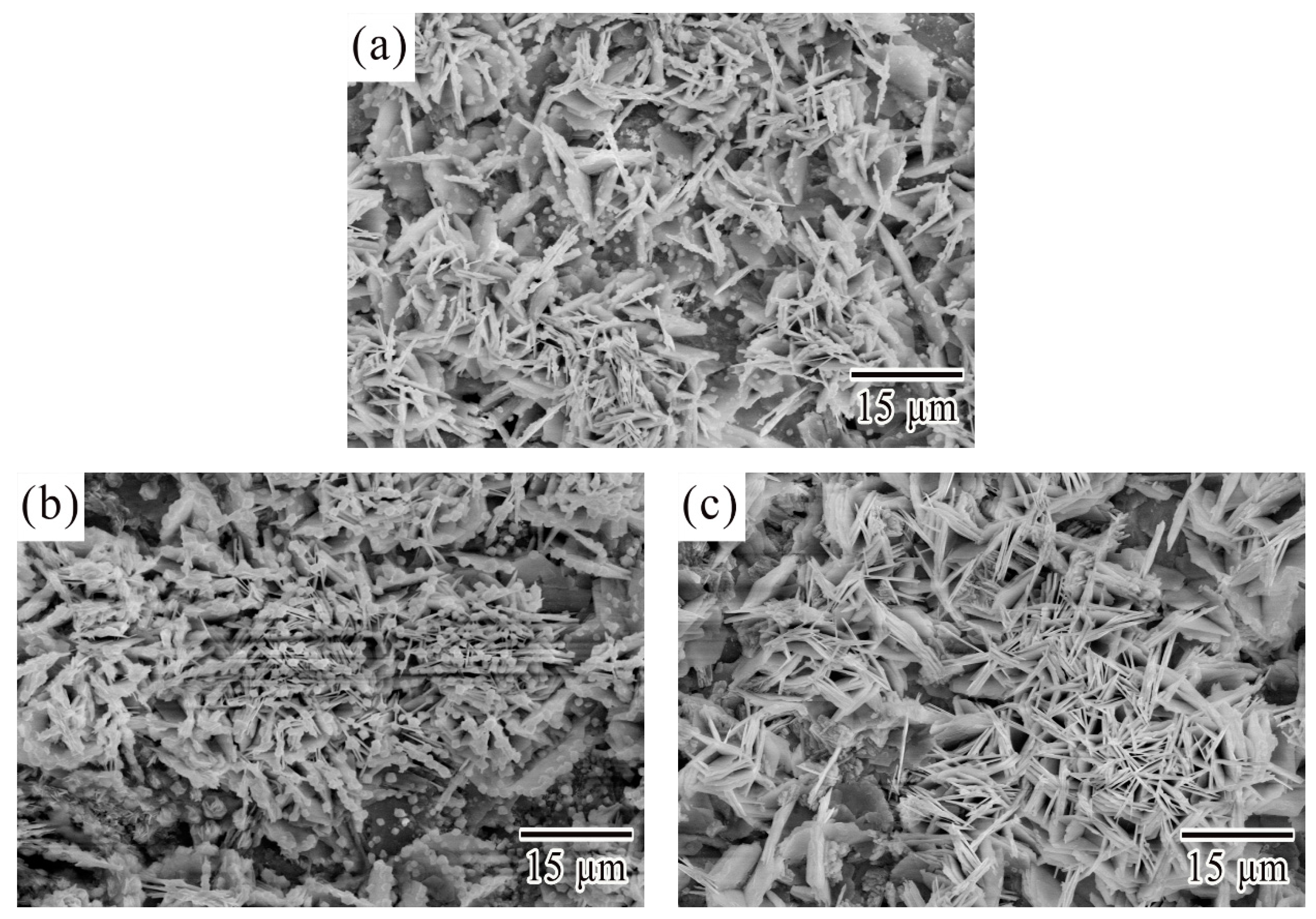
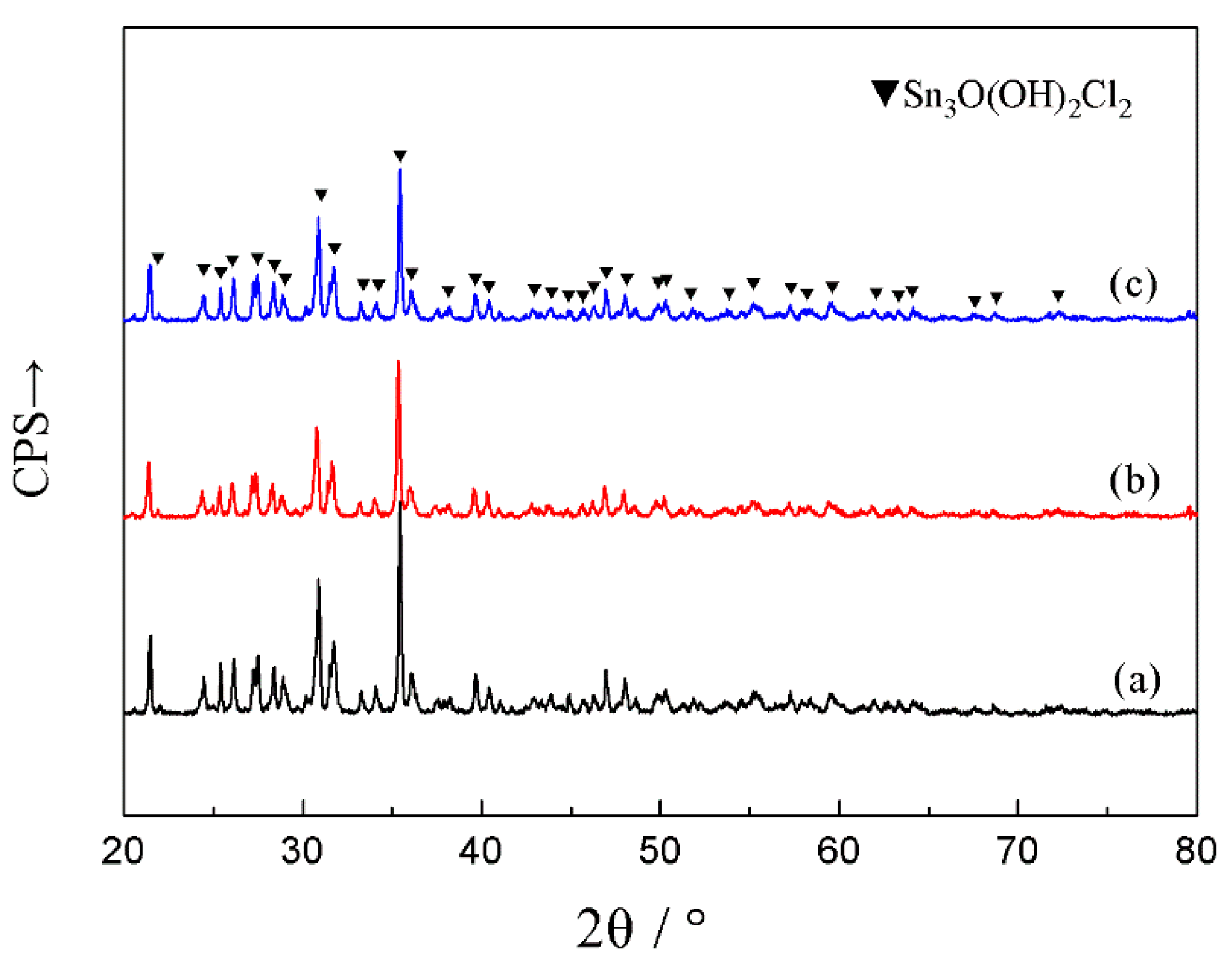
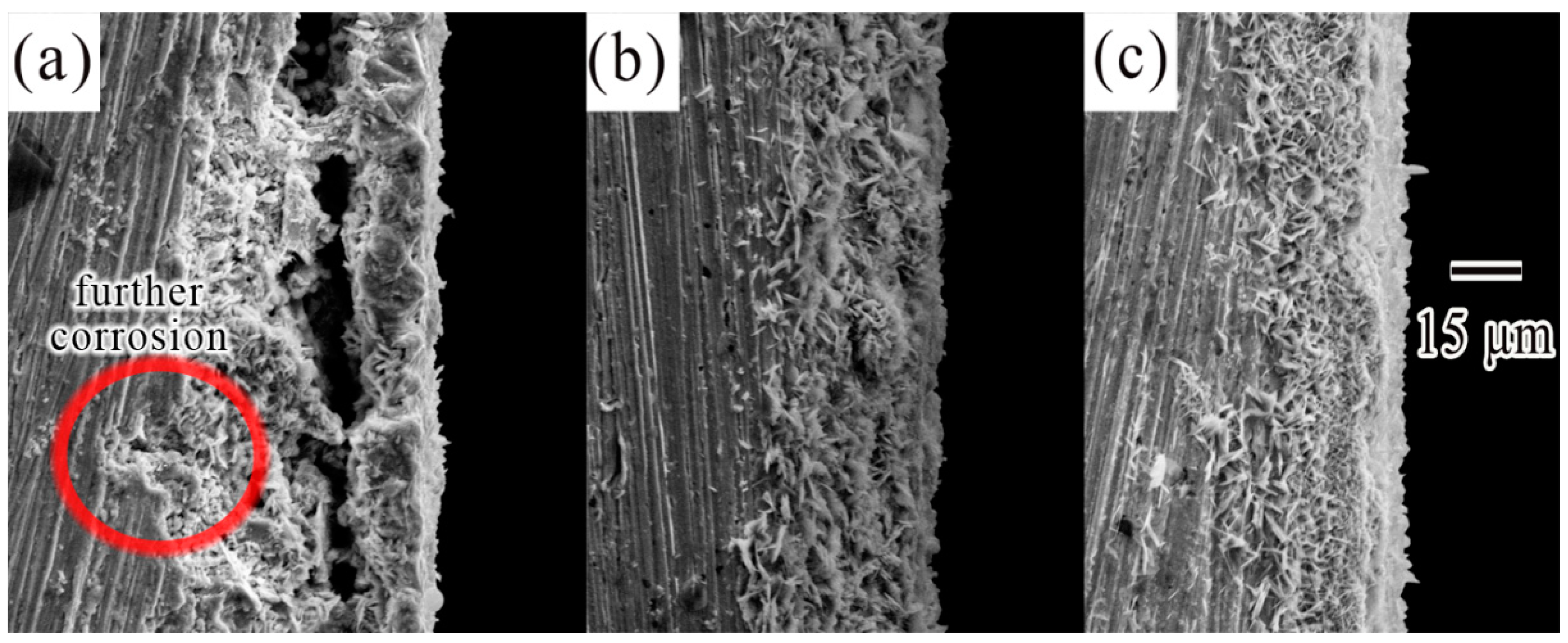
3.1. Microstructural Characterization
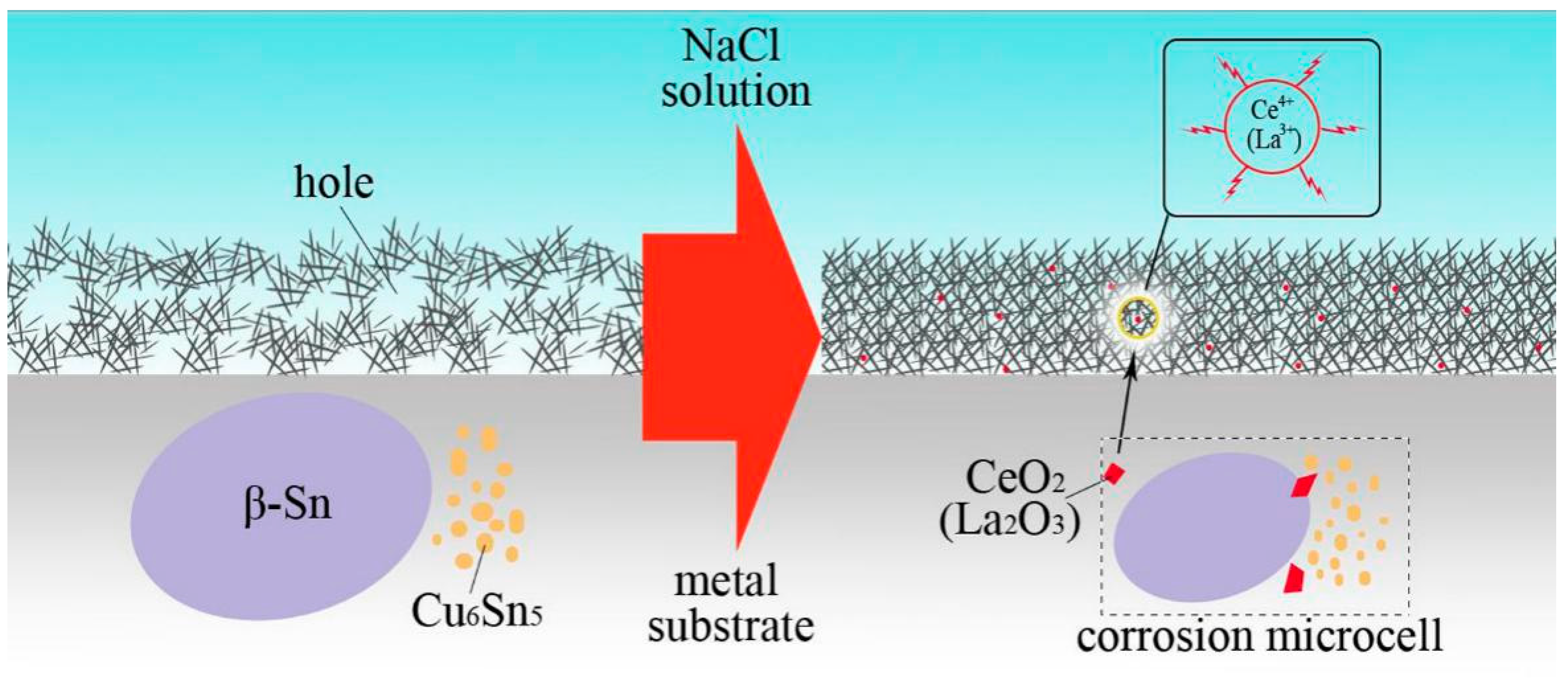
3.2. Electrochemical Corrosion Analysis
3.2.1. Transformation from Cathodic Polarization to Anodic Polarization
3.2.2. Active/Passive Transition Stage
3.2.3. Stable Passivation Stage
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suganuma, K. Advances in lead-free electronics soldering. Curr. Opin. Solid St. Mater. Sci. 2001, 5, 55–64. [Google Scholar] [CrossRef]
- Abtew, M.; Selvaduray, G. Lead-free solders in microelectronics. Mater. Sci. Eng. Rep. 2000, 27, 95–141. [Google Scholar] [CrossRef]
- Suraski, D.; Seelig, K. The current status of lead-free solder alloys. IEEE Trans. Electron. Packag. Manuf. 2001, 24, 244–248. [Google Scholar] [CrossRef]
- Gourlay, C.M.; Nogita, K.; McDonald, S.D.; Nishimura, T.; Sweatman, K.; Dahle, A.K. A rheological assessment of the effect of trace level Ni additions on the solidification of Sn-0.7Cu. Scr. Mater. 2006, 54, 1557–1562. [Google Scholar] [CrossRef]
- Sigelko, J.D.; Subramanian, K.N. Overview of lead-free solders. Adv. Mater. Process. 2000, 157, 47–48. [Google Scholar]
- Harrison, M.R.; Vincent, J.H.; Steen, H.A.H. Lead-free reflow soldering for electronics assembly. Solder. Surf. Mt. Technol. 2001, 13, 21–38. [Google Scholar] [CrossRef]
- Lim, S.R.; Schoenung, J.M. Human health and ecological toxicity potentials due to heavy metal content in waste electronic devices with flat panel displays. J. Hazard. Mater. 2010, 177, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.F.; Cheng, C.Q.; Zhao, J.; Wang, L.H.; Li, X.G. Electrochemical corrosion of Sn–0.75Cu solder joints in NaCl solution. Trans. Nonferrous Metal. Soc. China 2012, 22, 977–982. [Google Scholar] [CrossRef]
- Li, D.; Conway, P.P.; Liu, C. Corrosion characterization of tin–lead and lead free solders in 3.5 wt.% NaCl solution. Corros. Sci. 2008, 50, 995–1004. [Google Scholar] [CrossRef]
- Osorio, W.R.; Peixoto, L.C.; Garcia, L.R.; Garcia, A.; Spinelli, J.E. The effects of microstructure and Ag3Sn and Cu6Sn5 intermetallics on the electrochemical behavior of Sn-Ag and Sn-Cu solder alloys. Int. J. Electrochem. Sci. 2012, 7, 6436–6452. [Google Scholar]
- Osório, W.R.; Freire, C.M.; Caram, R.; Garcia, A. The role of Cu-based intermetallics on the pitting corrosion behavior of Sn–Cu, Ti–Cu and Al–Cu alloys. Electrochim. Acta 2012, 77, 189–197. [Google Scholar] [CrossRef]
- Tsao, L.C.; Chen, C.W. Corrosion characterization of Cu-Sn Intermetallics in 3.5 wt.% NaCl solution. Corros. Sci. 2012, 63, 393–398. [Google Scholar] [CrossRef]
- Moon, K.W.; Johnson, C.E.; Williams, M.E.; Kongstein, O.; Stafford, G.R.; Handwerker, C.A.; Boettinger, W.J. Observed correlation of Sn oxide film to Sn whisker growth in Sn-Cu electrodeposit for Pb-free solders. J. Electron. Mater. 2005, 34, L31–L33. [Google Scholar] [CrossRef]
- Horváth, B.; Illés, B.; Shinohara, T.; Harsányi, G. Copper-oxide whisker growth on tin-copper alloy coatings caused by the corrosion of Cu6Sn5 intermetallics. J. Mater. Sci. 2013, 48, 8052–8059. [Google Scholar] [CrossRef]
- Dudek, M.A.; Sidhu, R.S.; Chawla, N.; Renavikar, M. Microstructure and mechanical behavior of novel rare earth-containing Pb-free solders. J. Electron. Mater. 2006, 35, 2088–2097. [Google Scholar] [CrossRef]
- Wu, C.M.L.; Wong, Y.W. Rare-earth additions to lead-free electronic solders. J. Mater. Sci. Mater. Electron. 2007, 18, 77–91. [Google Scholar] [CrossRef]
- Guo, F.; Zhao, M.K.; Xia, Z.D.; Lei, Y.P.; Li, X.Y.; Shi, Y.W. Lead-free solders with rare earth additions. JOM 2009, 61, 39–44. [Google Scholar] [CrossRef]
- Zhasng, L.; Xue, S.B.; Gao, L.L.; Zeng, G.; Sheng, Z.; Chen, Y.; Yu, S.L. Effects of rare earths on properties and microstructures of lead-free solder alloys. J. Mater. Sci. Mater. Electron. 2009, 20, 685–694. [Google Scholar] [CrossRef]
- Dudek, M.A.; Chawla, N. Effect of rare-earth (La, Ce, and Y) additions on the microstructure and mechanical behavior of Sn-3.9Ag-0.7Cu solder alloy. Metall. Mater. Trans. A 2010, 41, 610–620. [Google Scholar] [CrossRef]
- Noh, B.I.; Choi, J.H.; Yoon, J.W.; Jung, S.B. Effects of cerium content on wettability, microstructure and mechanical properties of Sn-Ag-Ce solder alloys. J. Alloy. Compd. 2010, 499, 154–159. [Google Scholar] [CrossRef]
- Wang, J.X.; Xue, S.B.; Han, Z.J.; Yu, S.L.; Chen, Y.; Shi, Y.P.; Wang, H. Effects of rare earth Ce on microstructures, solderability of Sn-Ag-Cu and Sn-Cu-Ni solders as well as mechanical properties of soldered joints. J. Alloy. Compd. 2009, 467, 219–226. [Google Scholar] [CrossRef]
- Chen, W.X.; Xue, S.B.; Wang, H.; Hu, Y.; Wang, J.X. Effects of rare earth Ce on properties of Sn-9Zn lead-free solder. J. Mater. Sci. Mater. Electron. 2010, 21, 719–725. [Google Scholar] [CrossRef]
- Wu, C.M.L.; Yu, D.Q.; Law, C.M.T.; Wang, L. The properties of Sn-9Zn lead-free solder alloys doped with trace rare earth elements. J. Electron. Mater. 2002, 31, 921–927. [Google Scholar] [CrossRef]
- Liu, M.; Yang, W.; Ma, Y.; Tang, C.; Tang, H.; Zhan, Y. The electrochemical corrosion behavior of Pb-free Sn-8.5Zn-XCr solders in 3.5 wt.% NaCl solution. Mater. Chem. Phys. 2015, 168, 27–34. [Google Scholar] [CrossRef]
- Koleňák, R.; Kostolný, I. Study of direct bonding ceramics with metal using Sn2La solder. Adv. Mater. Sci. Eng. 2015, 2015, 1–13. [Google Scholar]
- Forsyth, M.; Seter, M.; Tan, M.Y.; Hinton, B. Recent developments in corrosion inhibitors based on rare earth metal compounds. Corros. Eng. Sci. Technol. 2014, 49, 130–135. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simoes, A.M.; Carmezim, M.J. Characterization of rare-earth conversion films formed on the AZ31 magnesium alloy and its relation with corrosion protection. Appl. Surf. Sci. 2007, 253, 6922–6931. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.C.; Du, C.C.; Dai, J.; Zhang, N. Effect of aluminum concentration on the microstructure and mechanical properties of Sn-Cu-Al solder alloy. Microelectron. Reliab. 2015, 55, 596–601. [Google Scholar] [CrossRef]
- Osório, W.R.; Spinelli, J.E.; Afonso, C.R.M.; Peixoto, L.C.; Garcia, A. Microstructure, corrosion behaviour and microhardness of a directionally solidified Sn–Cu solder alloy. Electrochim. Acta 2011, 56, 8891–8899. [Google Scholar] [CrossRef]
- Osorio, W.R.; Freitas, E.S.; Spinelli, J.E.; Garcia, A. Electrochemical behavior of a lead-free Sn-Cu solder alloy in NaCl solution. Corros. Sci. 2014, 80, 71–81. [Google Scholar] [CrossRef]
- Freitas, E.S.; Osorio, W.R.; Spinelli, J.E.; Garcia, A. Mechanical and corrosion resistances of a Sn-0.7 wt.%Cu lead-free solder alloy. Microelectron. Reliab. 2014, 54, 1392–1400. [Google Scholar] [CrossRef]
- Hung, F.Y.; Lui, T.S.; Chen, L.H.; He, N.T. Resonant characteristics of the microelectronic Sn-Cu solder. J. Alloy. Compd. 2008, 457, 171–176. [Google Scholar] [CrossRef]
- Wang, F.J.; Xin, M.; Qian, Y.Y. Improvement of microstructure and interface structure of eutectic Sn-0.7Cu solder with small amount of Zn addition. Scr. Mater. 2005, 53, 699–702. [Google Scholar] [CrossRef]
- Li, X.A.F.; Zhang, F.; Zu, F.Q.; Lv, X.; Zhao, Z.X.; Yang, D.D. Effect of liquid-liquid structure transition on solidification and wettability of Sn-0.7Cu solder. J. Alloy. Compd. 2010, 505, 472–475. [Google Scholar] [CrossRef]
- Alam, M.E.; Gupta, M. Development of extremely ductile lead-free Sn-Al solders for futuristic electronic packaging applications. Electron. Mater. Lett. 2014, 10, 515–524. [Google Scholar] [CrossRef]
- Lai, Z.M.; Ye, D. Effect of Al on the microstructure and properties of Sn-0.7Cu solder alloy. J. Mater. Sci. Mater. Electron. 2016, 27, 1177–1183. [Google Scholar] [CrossRef]
- Drienovsky, M.; Trnkova, L.R.; Martinkovic, M.; Ozvold, M.; Cernickova, I.; Palcut, M.; Janovec, J. Influence of cerium addition on microstructure and properties of Sn-Cu-(Ag) solder alloys. Mater. Sci. Eng. A 2015, 623, 83–91. [Google Scholar] [CrossRef]
- Mohran, H.S.; El-Sayed, A.R.; Abd El-Lateef, H.M. Anodic behavior of tin, indium, and tin-indium alloys in oxalic acid solution. J. Solid State Electron. 2009, 13, 1279–1290. [Google Scholar] [CrossRef]
- Sharma, A.; Das, S.; Das, K. Electrochemical corrosion behavior of CeO2 nanoparticle reinforced Sn-Ag based lead free nanocomposite solders in 3.5 wt.% NaCl bath. Surf. Coat. Techol. 2015, 261, 235–243. [Google Scholar] [CrossRef]
- Zhou, X.; Shen, Y. Beneficial effects of CeO2 addition on microstructure and corrosion behavior of electrodeposited Ni nanocrystalline coatings. Surf. Coat. Techol. 2013, 235, 433–446. [Google Scholar] [CrossRef]
- Montemor, M.F.; Simões, A.M.; Ferreira, M.G.S.; Carmezim, M.J. Composition and corrosion resistance of cerium conversion films on the AZ31 magnesium alloy and its relation to the salt anion. Appl. Surf. Sci. 2008, 254, 1806–1814. [Google Scholar] [CrossRef]
- Rosero-Navarro, N.C.; Curioni, M.; Castro, Y.; Aparicio, M.; Thompson, G.E.; Durán, A. Glass-like CexOy sol–gel coatings for corrosion protection of aluminium and magnesium alloys. Surf. Coat. Techol. 2011, 206, 257–264. [Google Scholar] [CrossRef]
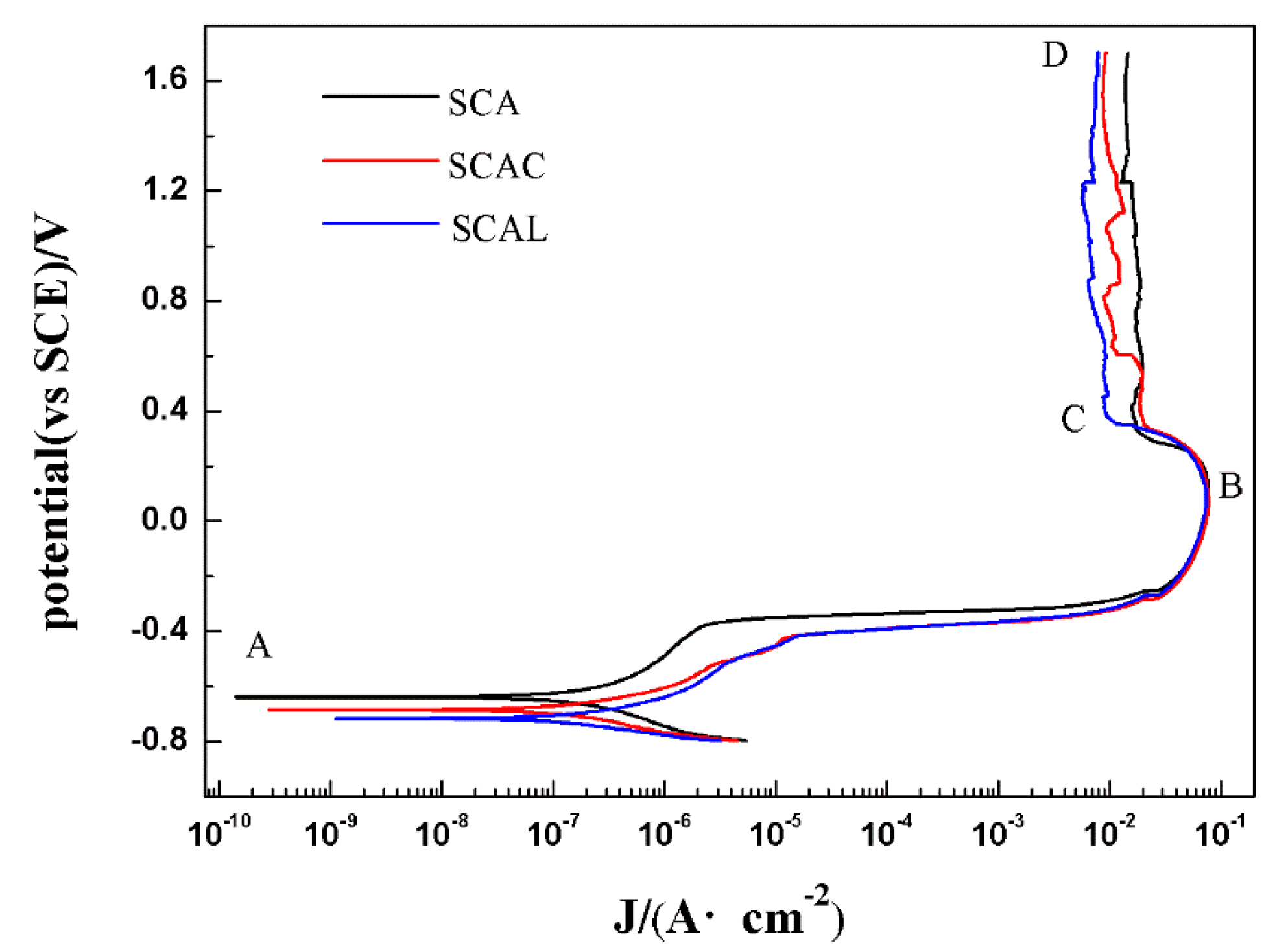

| Material | Ecorr (mV) | Icorr (A·cm−2) | Epp (mV) | Ic (A·cm−2) | Ep (mV) | Ip (A·cm−2) | Corrosion Rate (mm/a) |
|---|---|---|---|---|---|---|---|
| SCA | −639 | 4.16 × 10−7 | 113 | 7.69 × 10−2 | 334 | 1.40 × 10−2 | 0.009 |
| SCAC | −685 | 1.43 × 10−7 | 71 | 7.76 × 10−2 | 344 | 0.87 × 10−2 | 0.004 |
| SCAL | −718 | 1.19 × 10−7 | 84 | 7.41 × 10−2 | 357 | 0.75 × 10−2 | 0.004 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Du, Z.; Yu, S.; Li, Y.; Feng, J.; Wei, X.; Li, Q.; Zhan, Y. The Effect of Rare Earths Additions on the Microstructure and the Corrosion Behavior of Sn-0.7Cu-0.075Al Solder Alloy. Materials 2019, 12, 3731. https://doi.org/10.3390/ma12223731
Yang W, Du Z, Yu S, Li Y, Feng J, Wei X, Li Q, Zhan Y. The Effect of Rare Earths Additions on the Microstructure and the Corrosion Behavior of Sn-0.7Cu-0.075Al Solder Alloy. Materials. 2019; 12(22):3731. https://doi.org/10.3390/ma12223731
Chicago/Turabian StyleYang, Wenchao, Zaixiang Du, Shuyuan Yu, Yitai Li, Junli Feng, Xuanchen Wei, Qiang Li, and Yongzhong Zhan. 2019. "The Effect of Rare Earths Additions on the Microstructure and the Corrosion Behavior of Sn-0.7Cu-0.075Al Solder Alloy" Materials 12, no. 22: 3731. https://doi.org/10.3390/ma12223731
APA StyleYang, W., Du, Z., Yu, S., Li, Y., Feng, J., Wei, X., Li, Q., & Zhan, Y. (2019). The Effect of Rare Earths Additions on the Microstructure and the Corrosion Behavior of Sn-0.7Cu-0.075Al Solder Alloy. Materials, 12(22), 3731. https://doi.org/10.3390/ma12223731




