Humidity Driven Transition from Insulator to Ionic Conductor in Portland Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
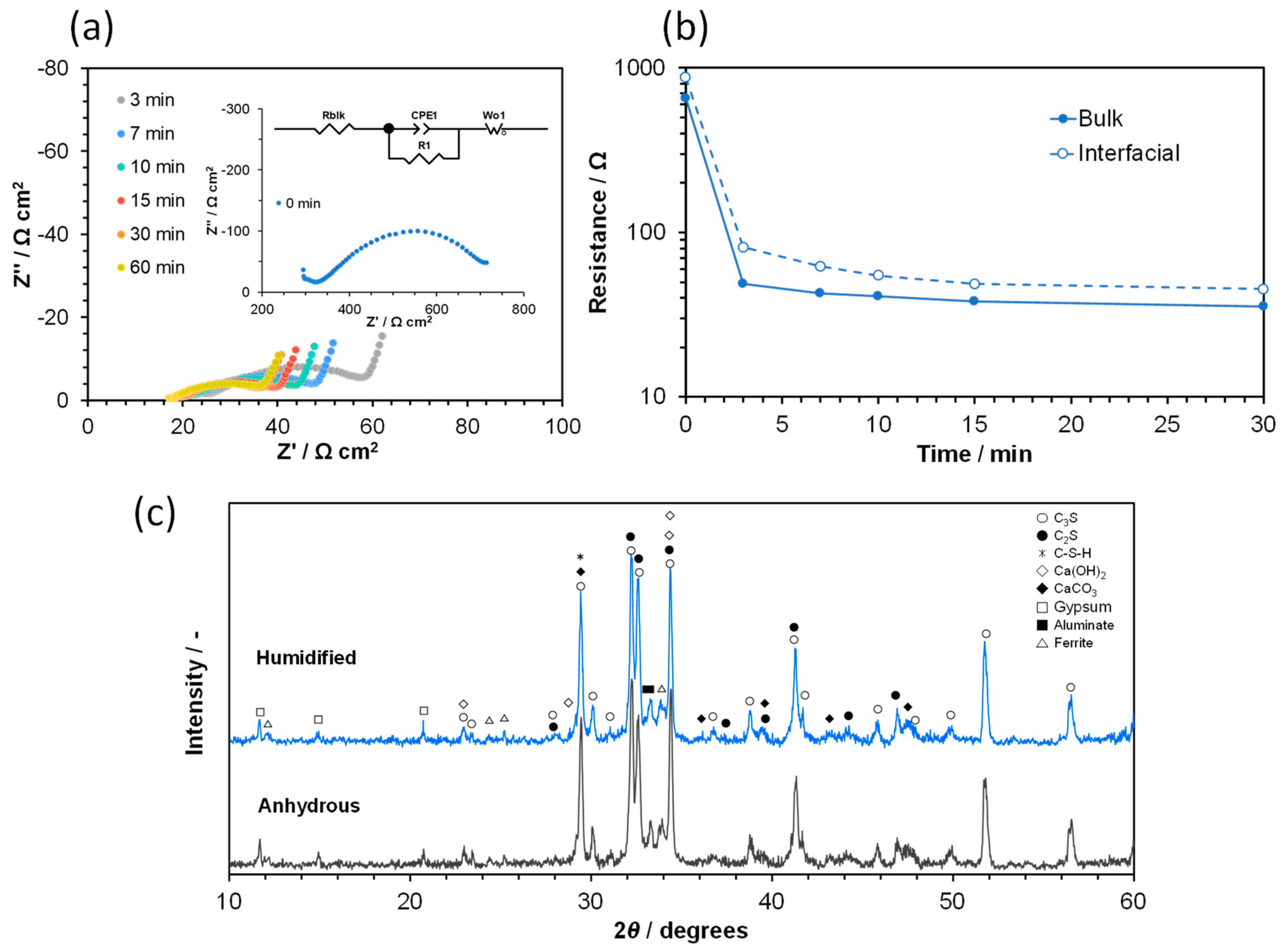
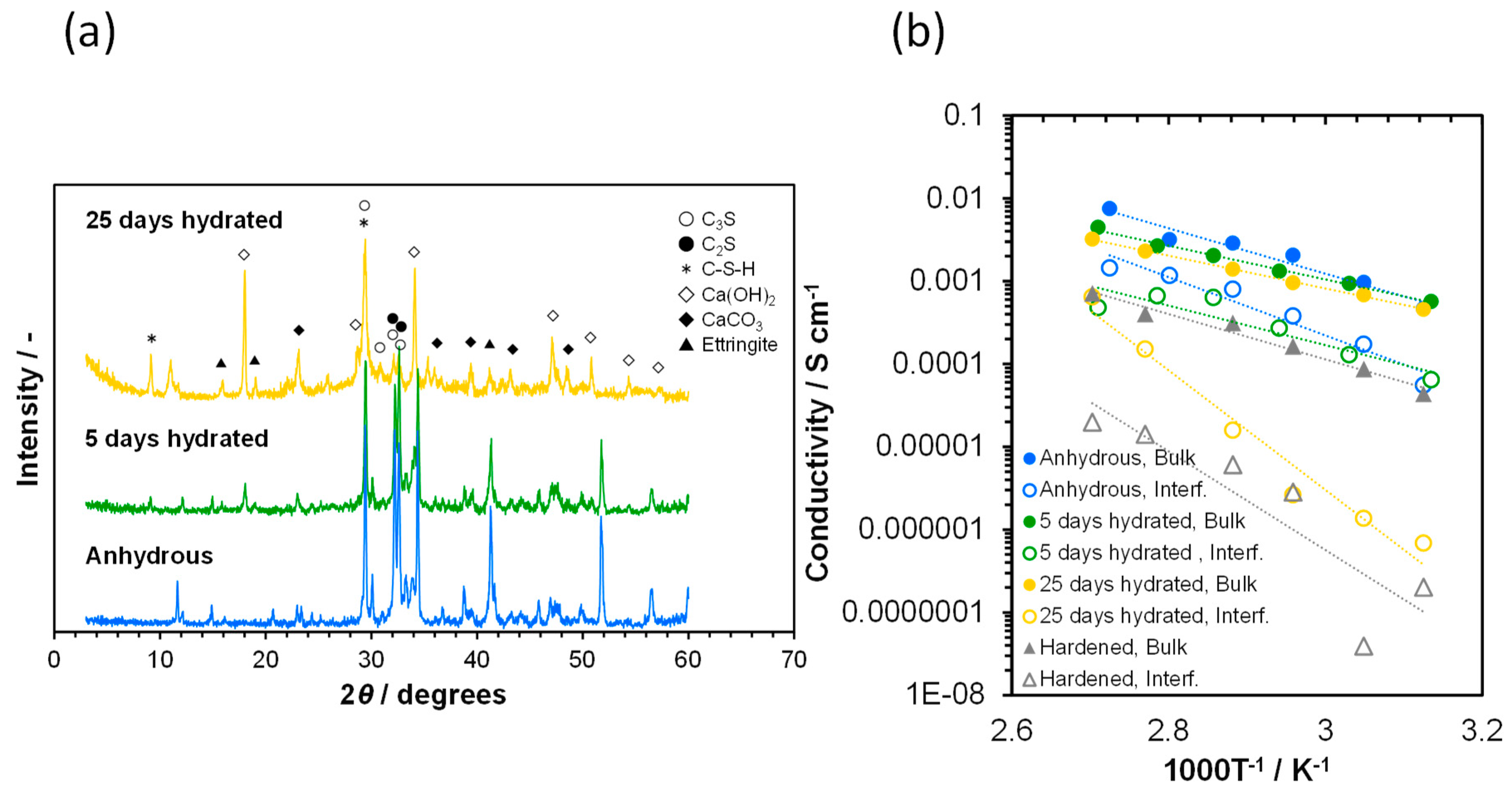
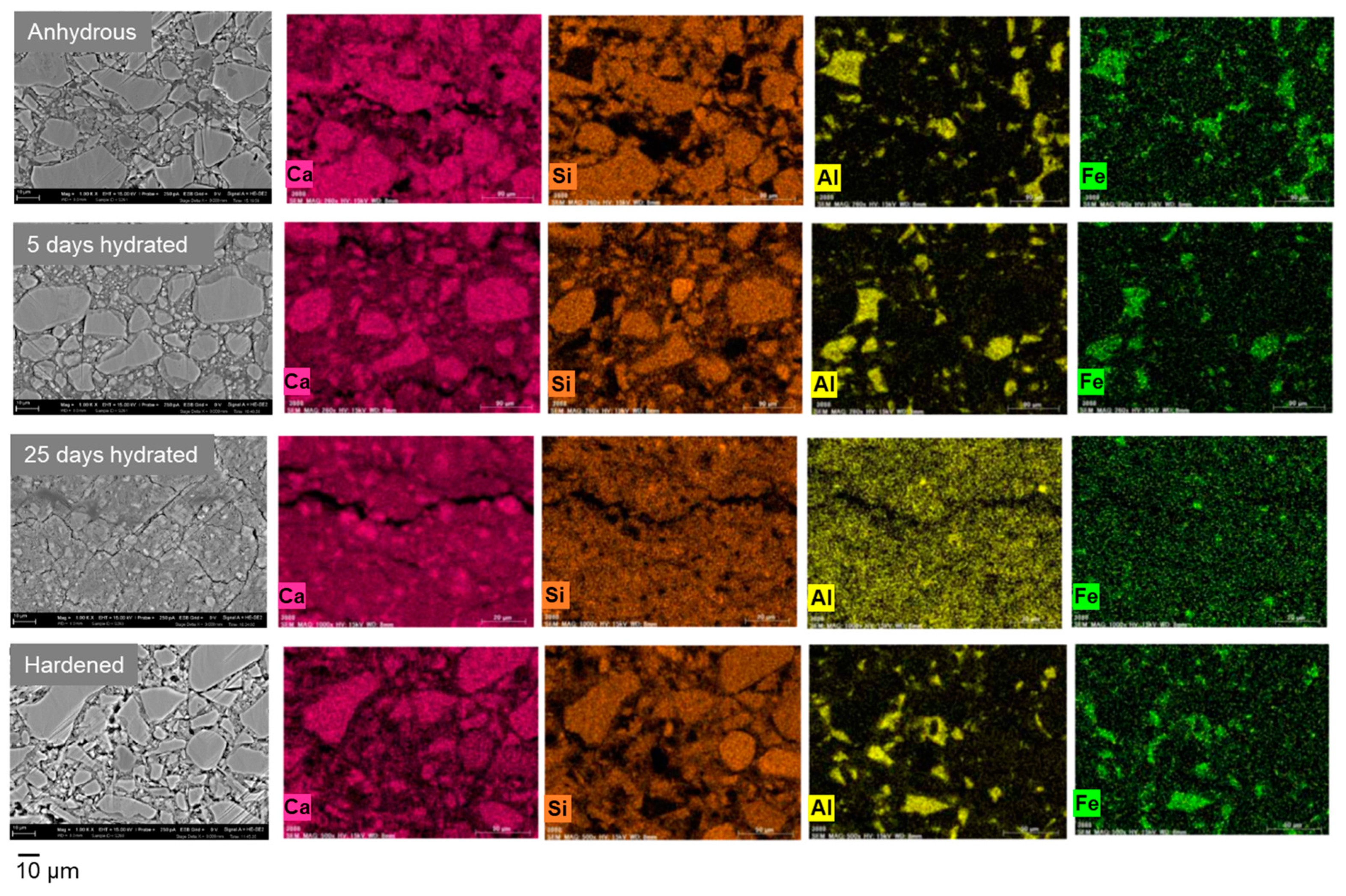
3.1. Ionic Conductivity of Portland Cements with Different Degrees of Hydration
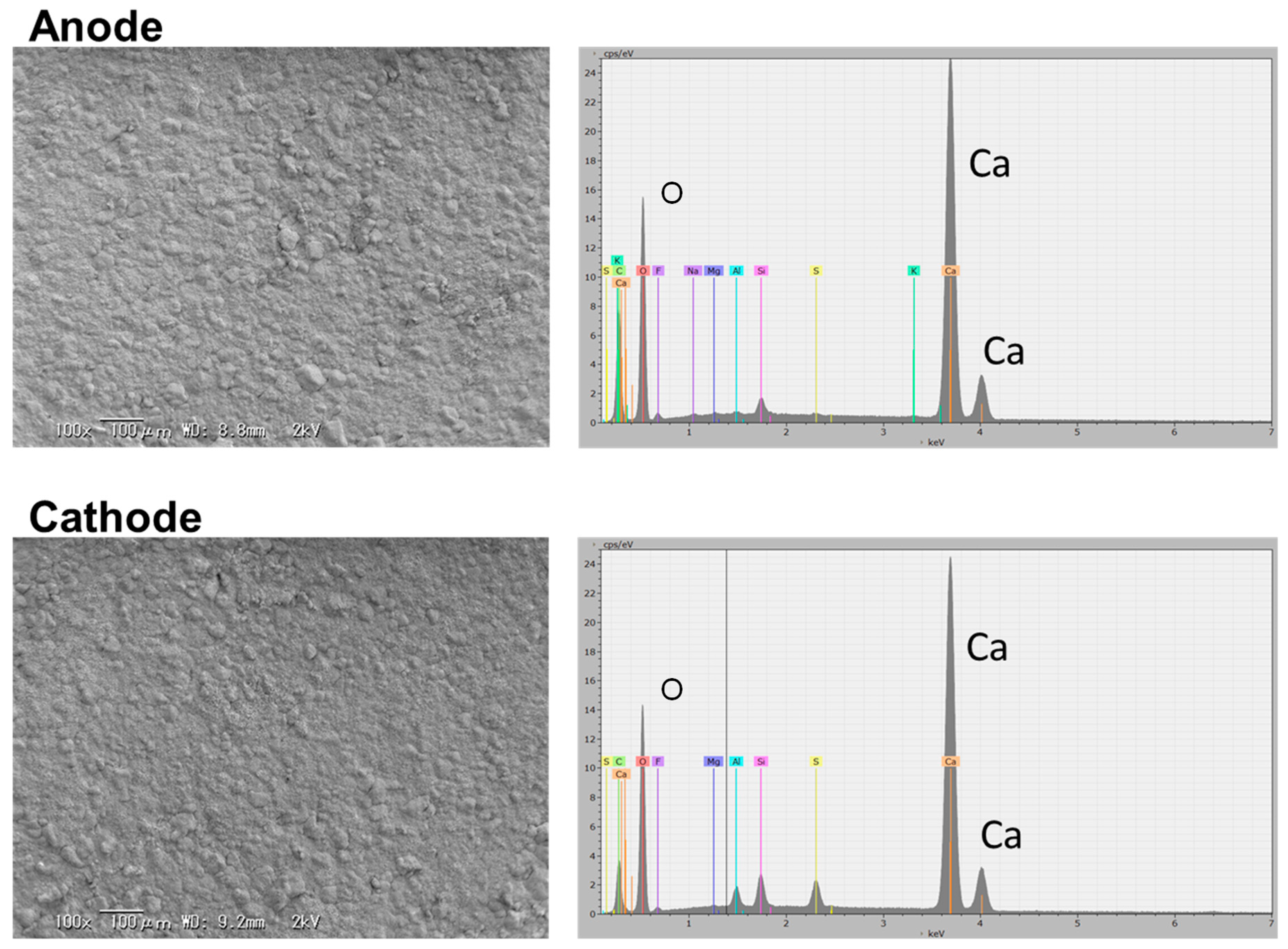
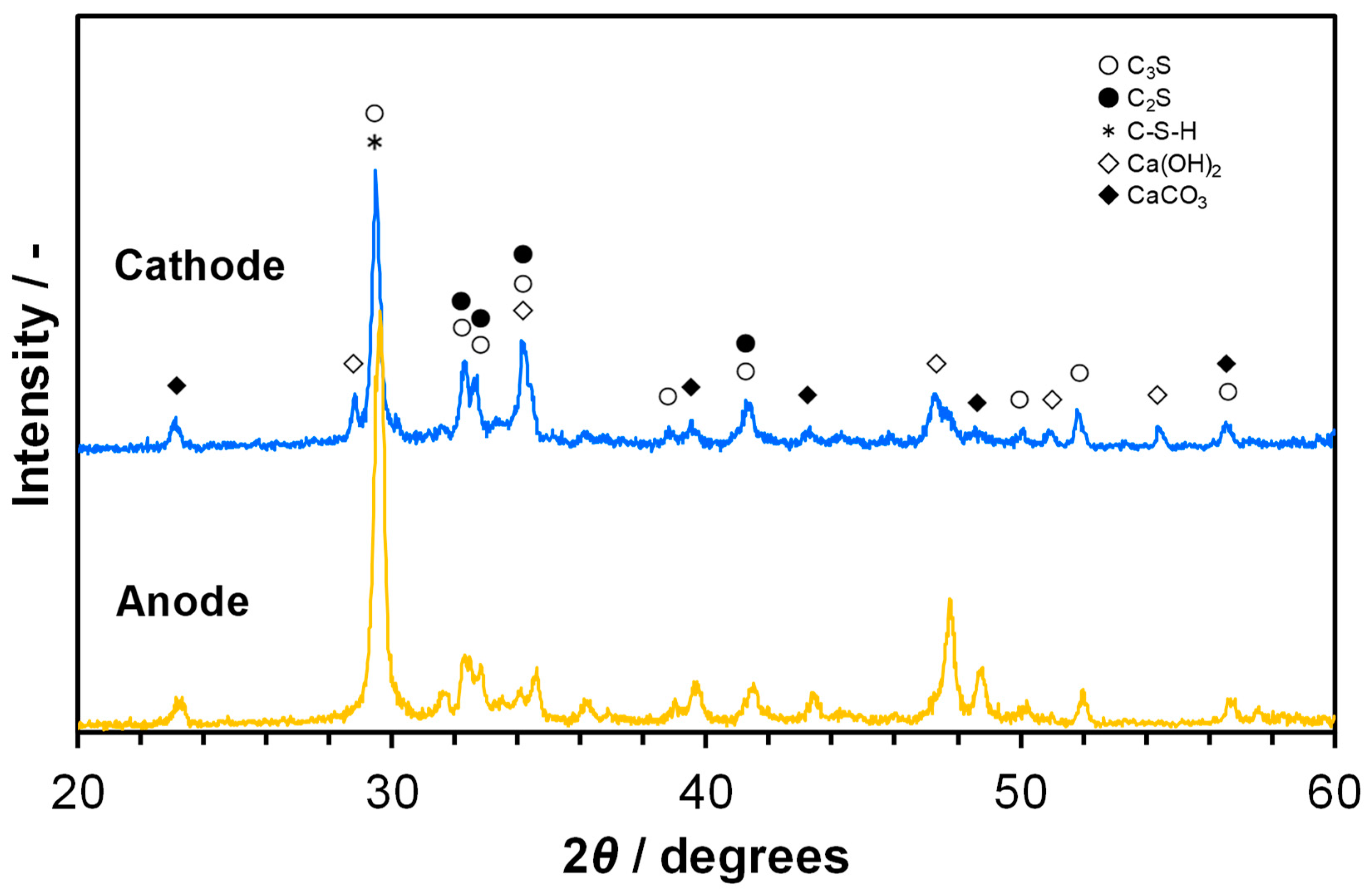
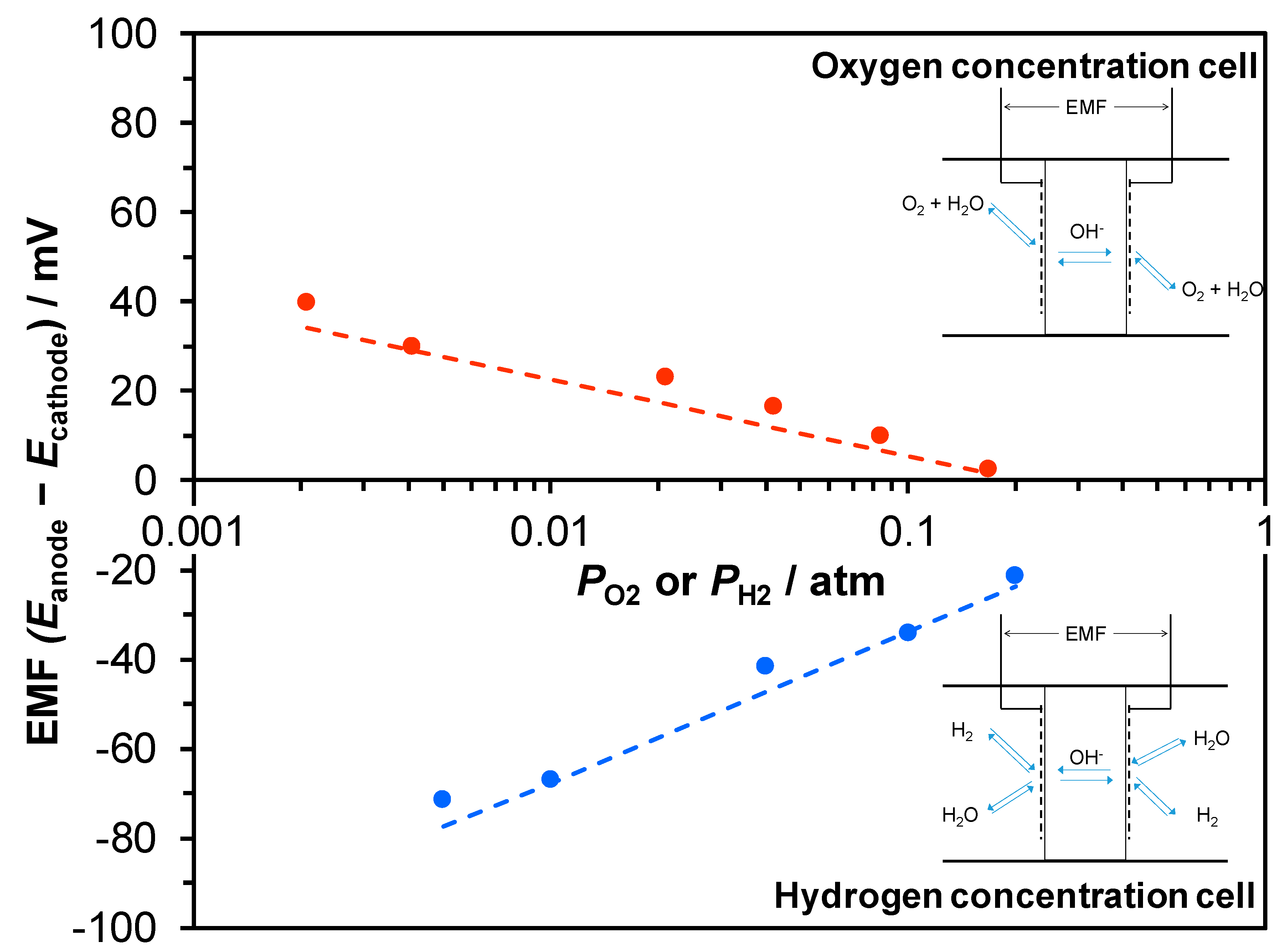
3.2. Ionic Conducting Characteristics of Portland Cement
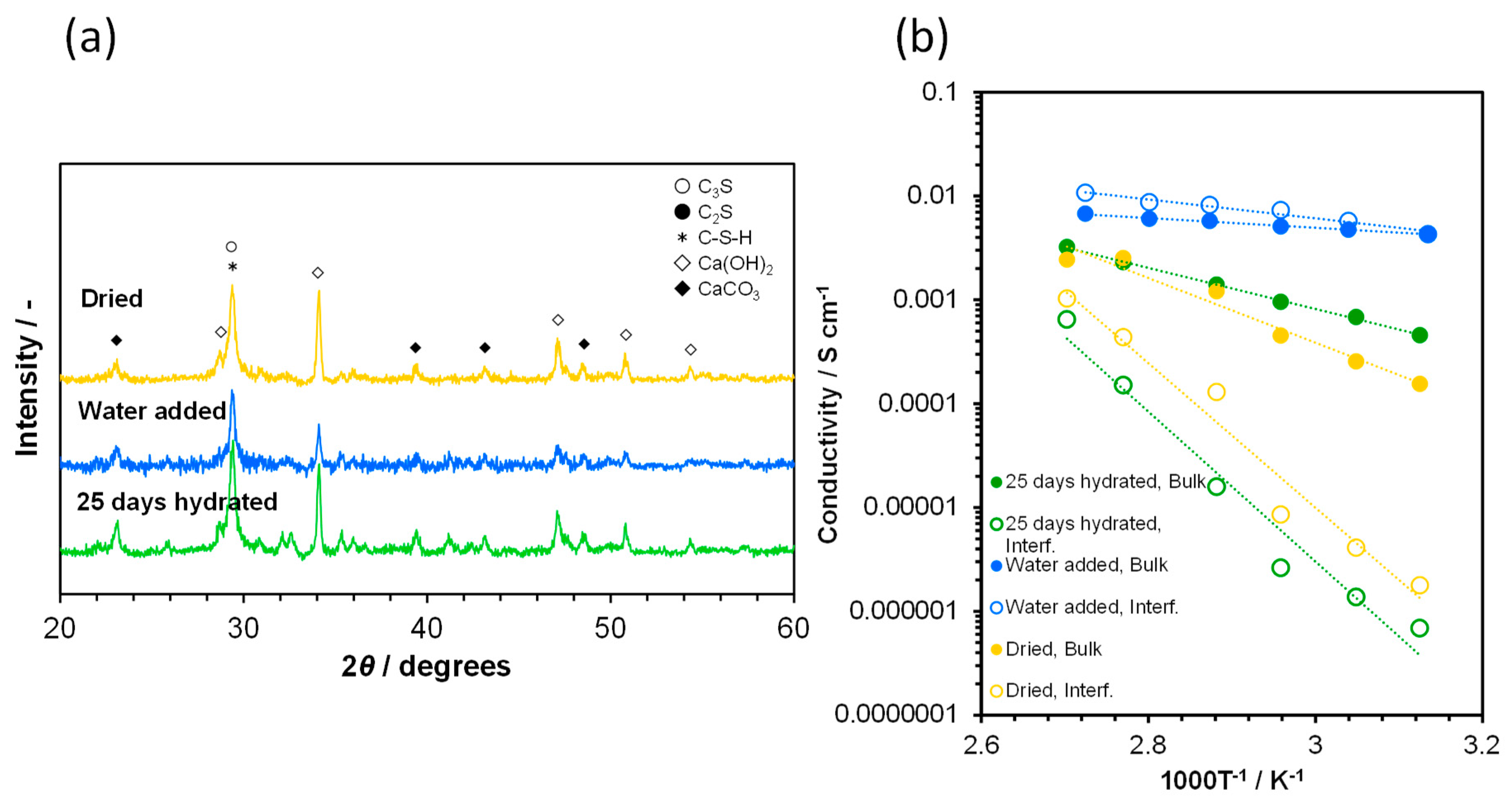
3.3. Degradation of Ionic Conduction in Cement by CO2 and Subsequent Amelioration
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beaudoin, J.J.; Ramachandran, V.S. A new perspective on the hydration characteristics of cement phases. Cem. Concr. Res. 1992, 22, 689–694. [Google Scholar] [CrossRef]
- Chen, Y.; Odler, I. On the origin of portland cement setting. Cem. Concr. Res. 1992, 22, 1130–1140. [Google Scholar] [CrossRef]
- Zanni, H.; Rassem-Bertolo, R.; Masse, S.; Fernandez, L.; Nieto, P.; Bresson, B. A spectroscopic NMR investigation of the calcium silicate hydrates present in cement and concrete. Magn. Reson. Imaging 1996, 14, 827–831. [Google Scholar] [CrossRef]
- Grutzeck, M.W. A new model for the formation of calcium silicate hydrate (C-S-H). Mater. Res. Innov. 1999, 3, 160–170. [Google Scholar] [CrossRef]
- Ménétrier, D.; Jawed, I.; Sun, T.S.; Skalny, J. Surface studies of hydrated β-C2S. Cem. Concr. Res. 1980, 10, 425–432. [Google Scholar] [CrossRef]
- Ectors, D.; Neubauer, J.; Goetz-Neunhoeffer, F. The hydration of synthetic brownmillerite in presence of low Ca-sulfate content and calcite monitored by quantitative in-situ-XRD and heat flow calorimetry. Cem. Concr. Res. 2013, 54, 61–68. [Google Scholar] [CrossRef]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. Thermodynamic properties of Portland cement hydrates in the system CaO-Al2O3-SiO2-CaSO4-CaCO3-H2O. Cem. Concr. Res. 2007, 37, 1379–1410. [Google Scholar] [CrossRef]
- Šavija, B.; Luković, M. Carbonation of cement paste: Understanding, challenges, and opportunities. Constr. Build. Mater. 2016, 117, 285–301. [Google Scholar] [CrossRef]
- Ekolu, S.O. A review on effects of curing, sheltering, and CO2 concentration upon natural carbonation of concrete. Constr. Build. Mater. 2016, 127, 306–320. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Puligilla, S.; Mondal, P. Co-existence of aluminosilicate and calcium silicate gel characterized through selective dissolution and FTIR spectral subtraction. Cem. Concr. Res. 2015, 70, 39–49. [Google Scholar] [CrossRef]
- Fernández, A.; García Calvo, J.L.; Alonso, M.C. Ordinary Portland cement composition for the optimization of the synergies of supplementary cementitious materials of ternary binders in hydration processes. Cem. Concr. Compos. 2018, 89, 238–250. [Google Scholar] [CrossRef]
- Hu, C.; Ruan, Y.; Yao, S.; Wang, F.; He, Y.; Gao, Y. Insight into the evolution of the elastic properties of calcium-silicate-hydrate (C-S-H) gel. Cem. Concr. Compos. 2019, 104. [Google Scholar] [CrossRef]
- Saafi, M.; Gullane, A.; Huang, B.; Sadeghi, H.; Ye, J.; Sadeghi, F. Inherently multifunctional geopolymeric cementitious composite as electrical energy storage and self-sensing structural material. Compos. Struct. 2018, 201, 766–778. [Google Scholar] [CrossRef]
- Cabeza, M.; Merino, P.; Miranda, A.; Nóvoa, X.R.; Sanchez, I. Impedance spectroscopy study of hardened Portland cement paste. Cem. Concr. Res. 2002, 32, 881–891. [Google Scholar] [CrossRef]
- Coppio, G.J.L.; de Lima, M.G.; Lencioni, J.W.; Cividanes, L.S.; Dyer, P.P.O.L.; Silva, S.A. Surface electrical resistivity and compressive strength of concrete with the use of waste foundry sand as aggregate. Constr. Build. Mater. 2019, 212, 514–521. [Google Scholar] [CrossRef]
- Dong, B.Q.; Qiu, Q.W.; Xiang, J.Q.; Huang, C.J.; Xing, F.; Han, N.X.; Lu, Y.Y. Electrochemical impedance measurement and modeling analysis of the carbonation behavior for cementititous materials. Constr. Build. Mater. 2014, 54, 558–565. [Google Scholar] [CrossRef]
- Neithalath, N.; Jain, J. Relating rapid chloride transport parameters of concretes to microstructural features extracted from electrical impedance. Cem. Concr. Res. 2010, 40, 1041–1051. [Google Scholar] [CrossRef]
- Xing, L.; Das, P.K.; Song, X.; Mamlouk, M.; Scott, K. Numerical analysis of the optimum membrane/ionomer water content of PEMFCs: The interaction of Nafion® ionomer content and cathode relative humidity. Appl. Energy 2015, 138, 242–257. [Google Scholar] [CrossRef]
- Liu, L.; Chen, W.; Li, Y. An overview of the proton conductivity of nafion membranes through a statistical analysis. J. Membr. Sci. 2016, 504, 1–9. [Google Scholar] [CrossRef]
- Heo, P.; Shibata, H.; Nagao, M.; Hibino, T.; Sano, M. Performance of an intermediate-temperature fuel cell using a proton-conducting Sn0.9In0.1P2O7 electrolyte. J. Electrochem. Soc. 2006, 153, A897–A901. [Google Scholar] [CrossRef]
- Li, Q.; He, R.; Jensen, J.O.; Bjerrum, N.J. Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem. Mater. 2003, 15, 4896–4915. [Google Scholar] [CrossRef]
- Haile, S.M.; Chisholm, C.R.I.; Sasaki, K.; Boysen, D.A.; Uda, T. Solid acid proton conductors: From laboratory curiosities to fuel cell electrolytes. Faraday Discuss. 2007, 134, 17–39. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.; Zeng, Q.H.; Liu, Q.L. Preparation and characterization of cross-linked quaternized poly (vinyl alcohol) membranes for anion exchange membrane fuel cells. J. Membr. Sci. 2008, 311, 319–325. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Leng, Y.; Chen, G.; Mendoza, A.J.; Tighe, T.B.; Hickner, M.A.; Wang, C.-Y. Solid-state water electrolysis with an alkaline membrane. J. Am. Chem. Soc. 2012, 134, 9054–9057. [Google Scholar] [CrossRef]
- Arges, C.G.; Parrondo, J.; Johnson, G.; Nadhan, A.; Ramani, V. Assessing the influence of different cation chemistries on ionic conductivity and alkaline stability of anion exchange membranes. J. Mater. Chem. 2012, 22, 3733–3744. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Xie, X.; Lv, Y.; Ramani, V.K. Hydroxide-ion induced degradation pathway for dimethylimidazolium groups in anion exchange membranes. J. Membr. Sci. 2014, 462, 112–118. [Google Scholar] [CrossRef]
- Chempath, S.; Einsla, B.R.; Pratt, L.R.; Macomber, C.S.; Boncella, J.M.; Rau, J.A.; Pivovar, B.S. Mechanism of tetraalkylammonium headgroup degradation in alkaline fuel cell membranes. J. Phys. Chem. C 2008, 112, 3179–3182. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, P.K. Recent development of polymer electrolyte membranes for fuel cells. Chem. Rev. 2012, 112, 2780–2832. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Hatanaka, S.; Chareerat, T.; Mishima, N.; Yuasa, Y. Cement paste characteristics and porous concrete properties. Constr. Build. Mater. 2008, 22, 894–901. [Google Scholar] [CrossRef]
- Tikkanen, J.; Cwirzen, A.; Penttala, V. Effects of mineral powders on hydration process and hydration products in normal strength concrete. Constr. Build. Mater. 2014, 72, 7–14. [Google Scholar] [CrossRef]
- Nagao, M.; Kamiya, T.; Heo, P.; Hibino, T.; Sano, M.; Tomita, A. Proton conduction in In 3+-doped SnP2O7 at intermediate temperatures. J. Electrochem. Soc. 2006, 153, A1604–A1609. [Google Scholar] [CrossRef]
- Shi, R.; Liu, J.; Wang, H.; Wu, F.; Miao, H. Intermediate temperature fuel cell durability of Eu-doped SrCeO3-SrZrO3 solid solution/ NaCl-KCl composite electrolyte. Ceram. Int. 2017, 43, 16931–16935. [Google Scholar] [CrossRef]
- Capaccioli, S.; Lucchesi, M.; Casalini, R.; Rolla, P.A.; Bona, N. Effect of water inclusions on charge transport and polarization in porous media. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 454–460. [Google Scholar] [CrossRef]
- Das, A.; Thakur, A.K.; Kumar, K. Evidence of low temperature relaxation and hopping in ion conducting polymer blend. Solid State Ion. 2014, 262, 815–820. [Google Scholar] [CrossRef]
- Bi, L.; Fabbri, E.; Sun, Z.; Traversa, E. Sinteractive anodic powders improve densification and electrochemical properties of BaZr0.8Y0.2O3-δ electrolyte films for anode-supported solid oxide fuel cells. Energy Environ. Sci. 2011, 4, 1352–1357. [Google Scholar] [CrossRef]
- Bui, P.T.; Ogawa, Y.; Nakarai, K.; Kawai, K.; Sato, R. Internal curing of Class-F fly-ash concrete using high-volume roof-tile waste aggregate. Mater. Struct. Constr. 2017, 50, 1–12. [Google Scholar] [CrossRef]
- MacLaren, D.C.; White, M.A. Cement: Its chemistry and properties. J. Chem. Educ. 2003, 80, 623–635. [Google Scholar] [CrossRef]
- Korpa, A.; Kowald, T.; Trettin, R. Hydration behaviour, structure and morphology of hydration phases in advanced cement-based systems containing micro and nanoscale pozzolanic additives. Cem. Concr. Res. 2008, 38, 955–962. [Google Scholar] [CrossRef]
- Grew, K.N.; Chiu, W.K.S. A dusty fluid model for predicting hydroxyl anion conductivity in alkaline anion exchange membranes. J. Electrochem. Soc. 2010, 157, B327–B337. [Google Scholar] [CrossRef]
- Zhang, W.; Van Duin, A.C.T. ReaxFF reactive molecular dynamics simulation of functionalized poly (phenylene oxide) anion exchange membrane. J. Phys. Chem. C 2015, 119, 27727–27736. [Google Scholar] [CrossRef]
- Huo, J.; Qi, W.; Zhu, H.; Yang, B.; He, G.; Bao, J.; Zhang, X.; Yan, X.; Gao, L.; Zhang, N. Molecular dynamics simulation on the effect of water uptake on hydrogen bond network for OH− conduction in imidazolium-g-PPO membrane. Int. J. Hydrogen Energy 2019, 44, 3760–3770. [Google Scholar] [CrossRef]
- Pomiès, M.-P.; Lequeux, N.; Boch, P. Speciation of cadmium in cement. Cem. Concr. Res. 2001, 31, 563–569. [Google Scholar] [CrossRef]
- Cong, X.; Kirkpatrick, R.J. 29Si MAS NMR study of the structure of calcium silicate hydrate. Adv. Cem. Based Mater. 2002, 3, 144–156. [Google Scholar] [CrossRef]
- Ong, B.C.; Kamarudin, S.K.; Basri, S. Direct liquid fuel cells: A review. Int. J. Hydrogen Energy 2017, 42, 10142–10157. [Google Scholar] [CrossRef]
- Cao, Y.C.; Wang, X.; Mamlouk, M.; Scott, K. Preparation of alkaline anion exchange polymer membrane from methylated melamine grafted poly (vinylbenzyl chloride) and its fuel cell performance. J. Mater. Chem. 2011, 21, 12910–12916. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, T.S.; An, L. A high-performance supportless silver nanowire catalyst for anion exchange membrane fuel cells. J. Mater. Chem. A 2015, 3, 1410–1416. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Kutchko, B.G.; Strazisar, B.R.; Dzombak, D.A.; Lowry, G.V.; Thauiow, N. Degradation of well cement by CO2 under geologic sequestration conditions. Environ. Sci. Technol. 2007, 41, 4787–4792. [Google Scholar] [CrossRef]
- Carroll, S.; Carey, J.W.; Dzombak, D.; Huerta, N.J.; Li, L.; Richard, T.; Um, W.; Walsh, S.D.C.; Zhang, L. Review: Role of chemistry, mechanics, and transport on well integrity in CO2 storage environments. Int. J. Greenh. Gas Control 2016, 49, 149–160. [Google Scholar] [CrossRef]
- Naughton, M.S.; Brushett, F.R.; Kenis, P.J.A. Carbonate resilience of flowing electrolyte-based alkaline fuel cells. J. Power Sources 2011, 196, 1762–1768. [Google Scholar] [CrossRef]
- Tewari, A.; Sambhy, V.; Urquidi MacDonald, M.; Sen, A. Quantification of carbon dioxide poisoning in air breathing alkaline fuel cells. J. Power Sources 2006, 153, 1–10. [Google Scholar] [CrossRef]
- Mendes, A.; Gates, W.P.; Sanjayan, J.G.; Collins, F. NMR, XRD, IR and synchrotron NEXAFS spectroscopic studies of OPC and OPC/slag cement paste hydrates. Mater. Struct. Constr. 2011, 44, 1773–1791. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagao, M.; Kobayashi, K.; Hori, T.; Li, Y.; Hibino, T. Humidity Driven Transition from Insulator to Ionic Conductor in Portland Cement. Materials 2019, 12, 3701. https://doi.org/10.3390/ma12223701
Nagao M, Kobayashi K, Hori T, Li Y, Hibino T. Humidity Driven Transition from Insulator to Ionic Conductor in Portland Cement. Materials. 2019; 12(22):3701. https://doi.org/10.3390/ma12223701
Chicago/Turabian StyleNagao, Masahiro, Kazuyo Kobayashi, Tetsuya Hori, Yaorong Li, and Takashi Hibino. 2019. "Humidity Driven Transition from Insulator to Ionic Conductor in Portland Cement" Materials 12, no. 22: 3701. https://doi.org/10.3390/ma12223701
APA StyleNagao, M., Kobayashi, K., Hori, T., Li, Y., & Hibino, T. (2019). Humidity Driven Transition from Insulator to Ionic Conductor in Portland Cement. Materials, 12(22), 3701. https://doi.org/10.3390/ma12223701



