Recent Progress with In Situ Characterization of Interfacial Structures under a Solid–Gas Atmosphere by HP-STM and AP-XPS
Abstract
1. Introduction
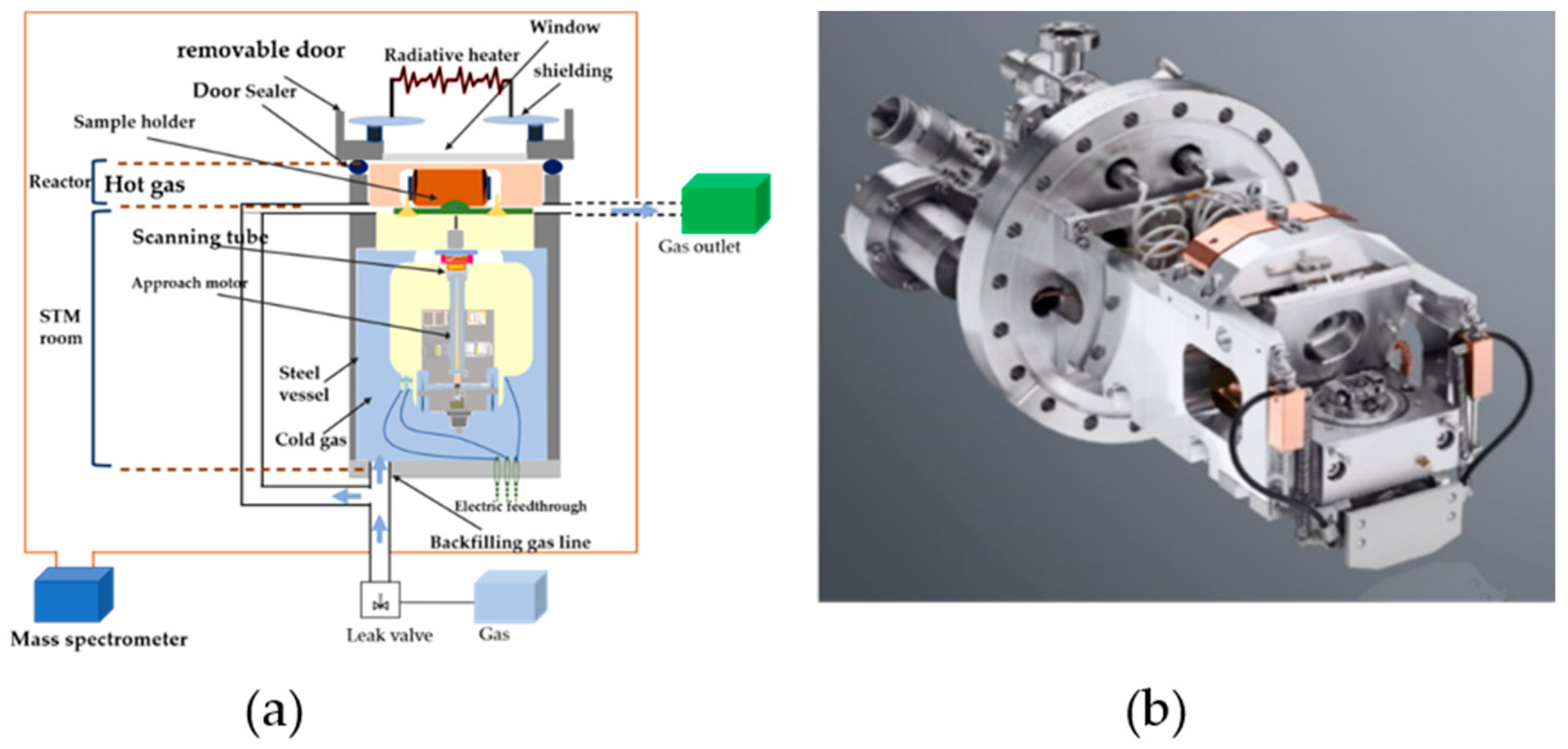
2. Applications of HP-STM in Identifying Surface Catalysis Reactions
2.1. HP-STM Experiments of Single-Crystal Reactions and Reactant Gas
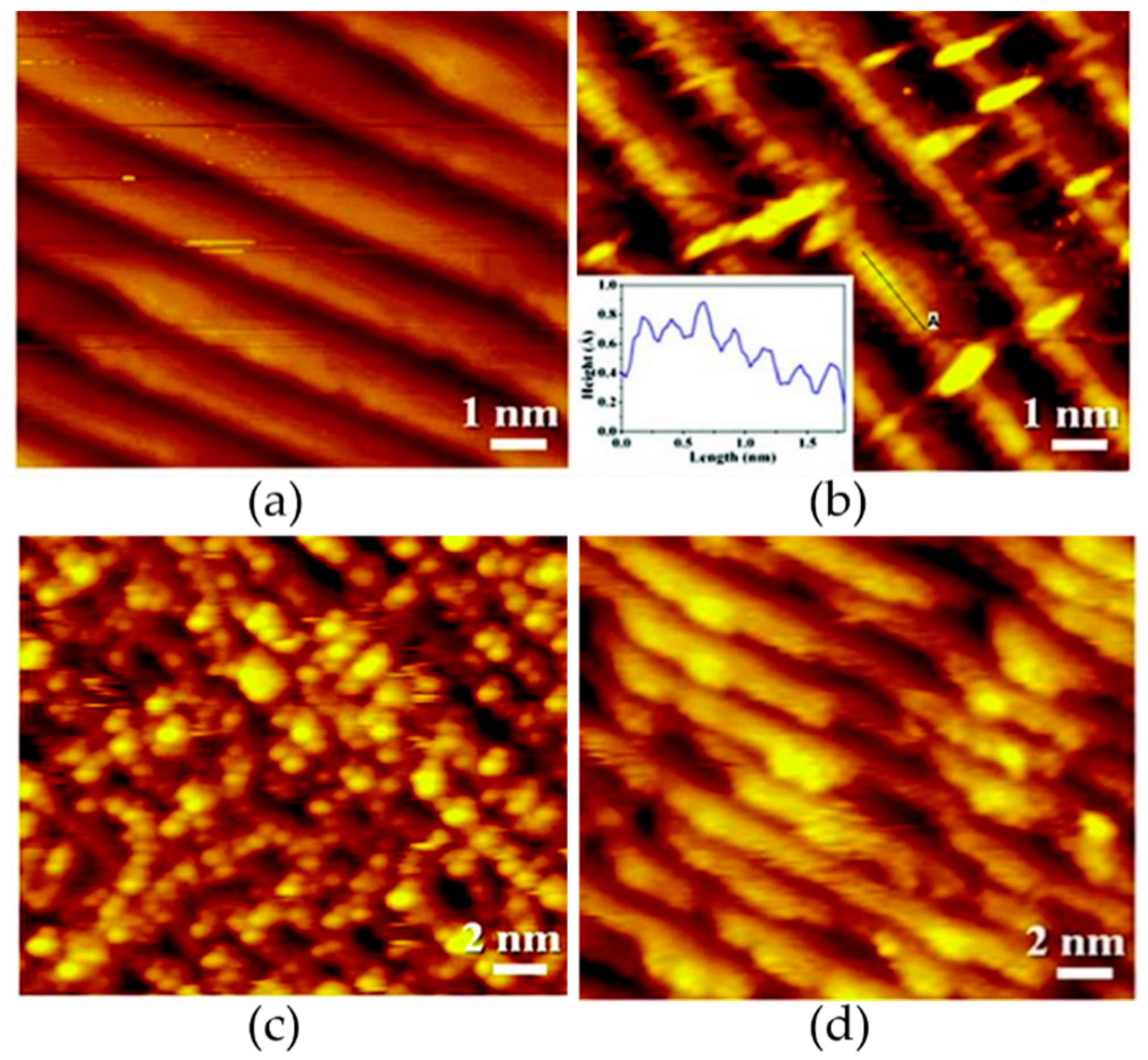
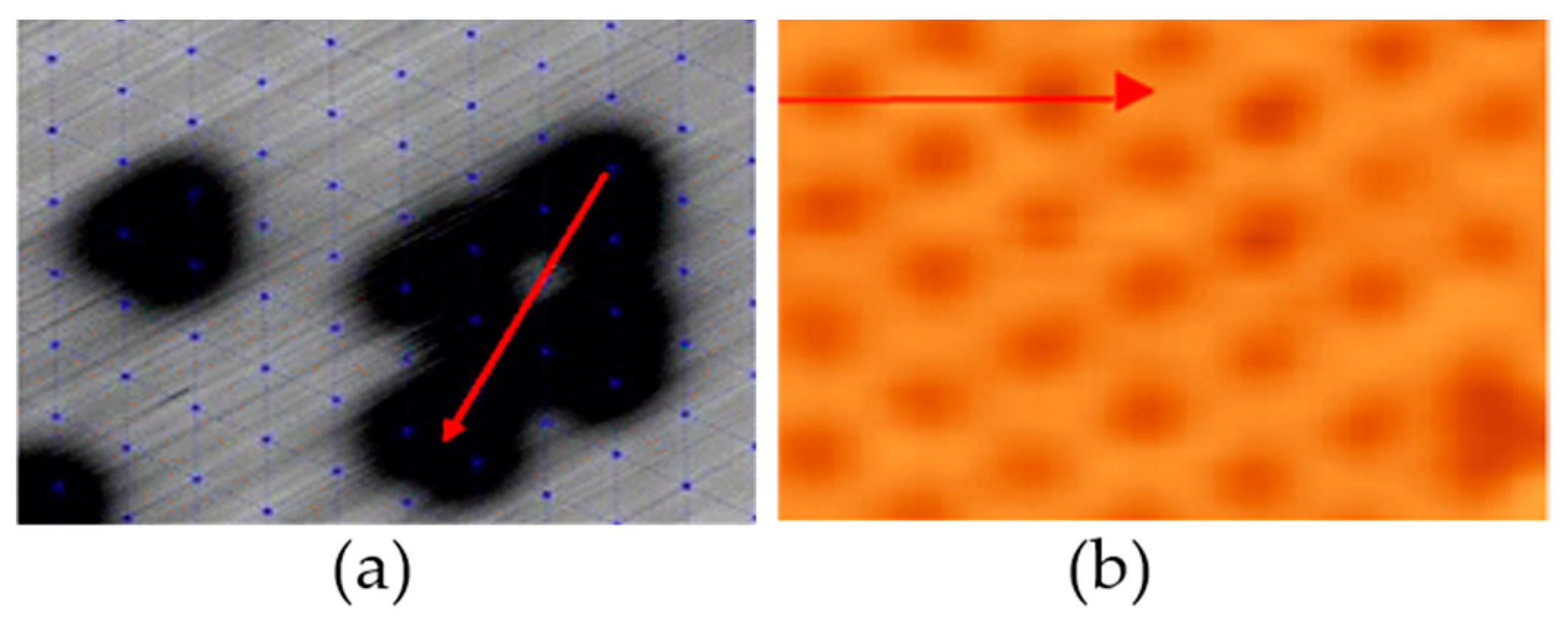
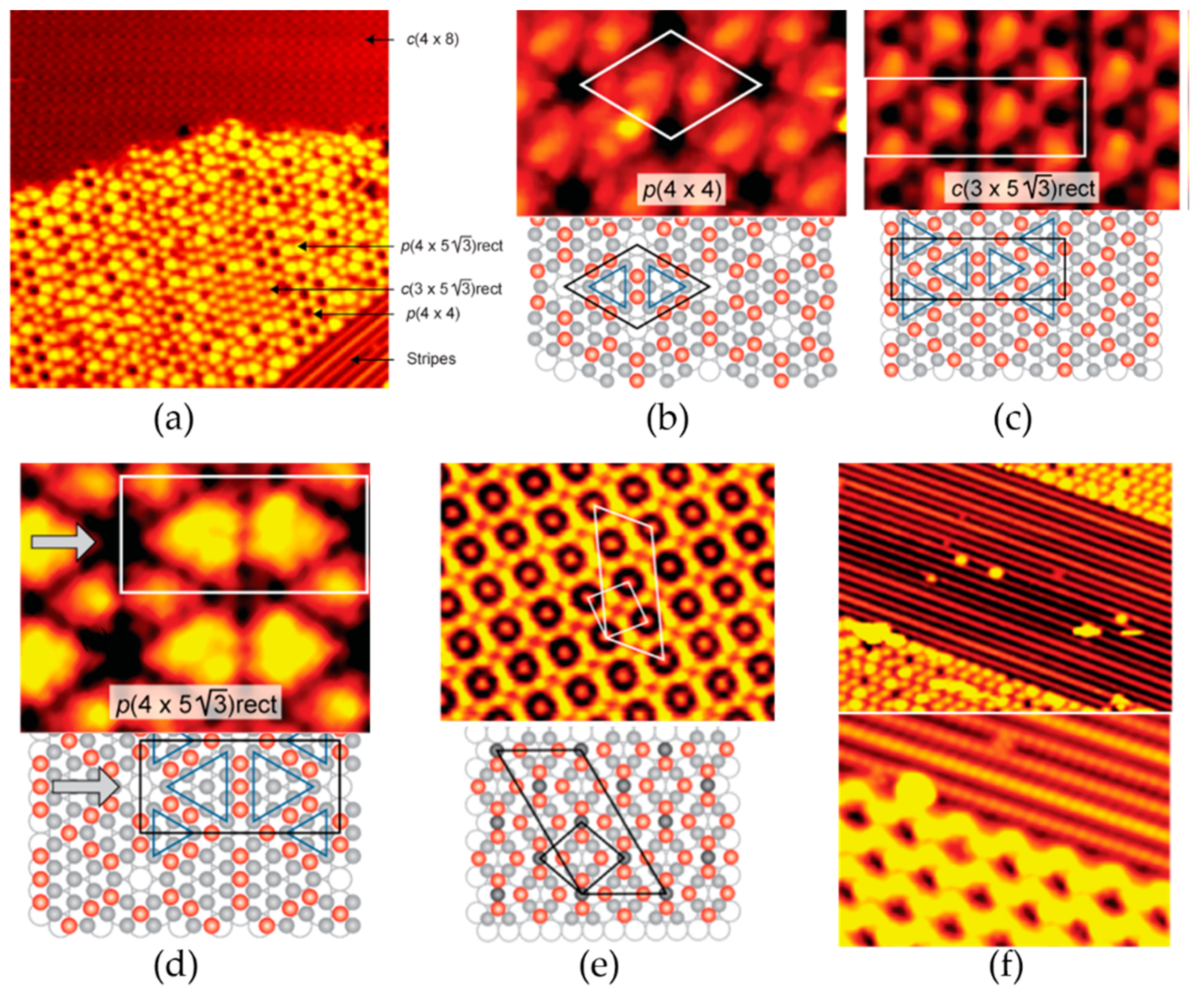
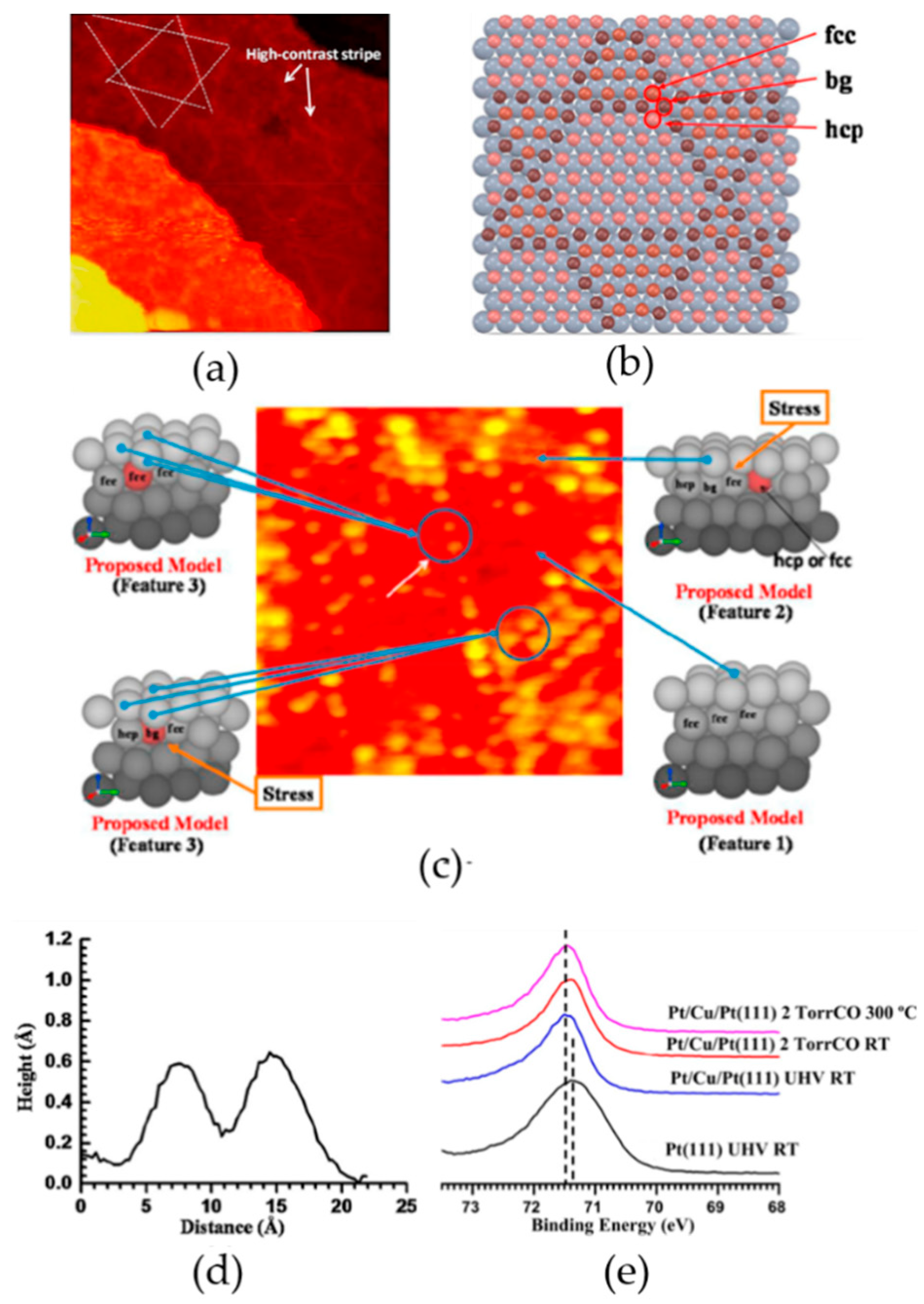
2.1.1. Reconstruction
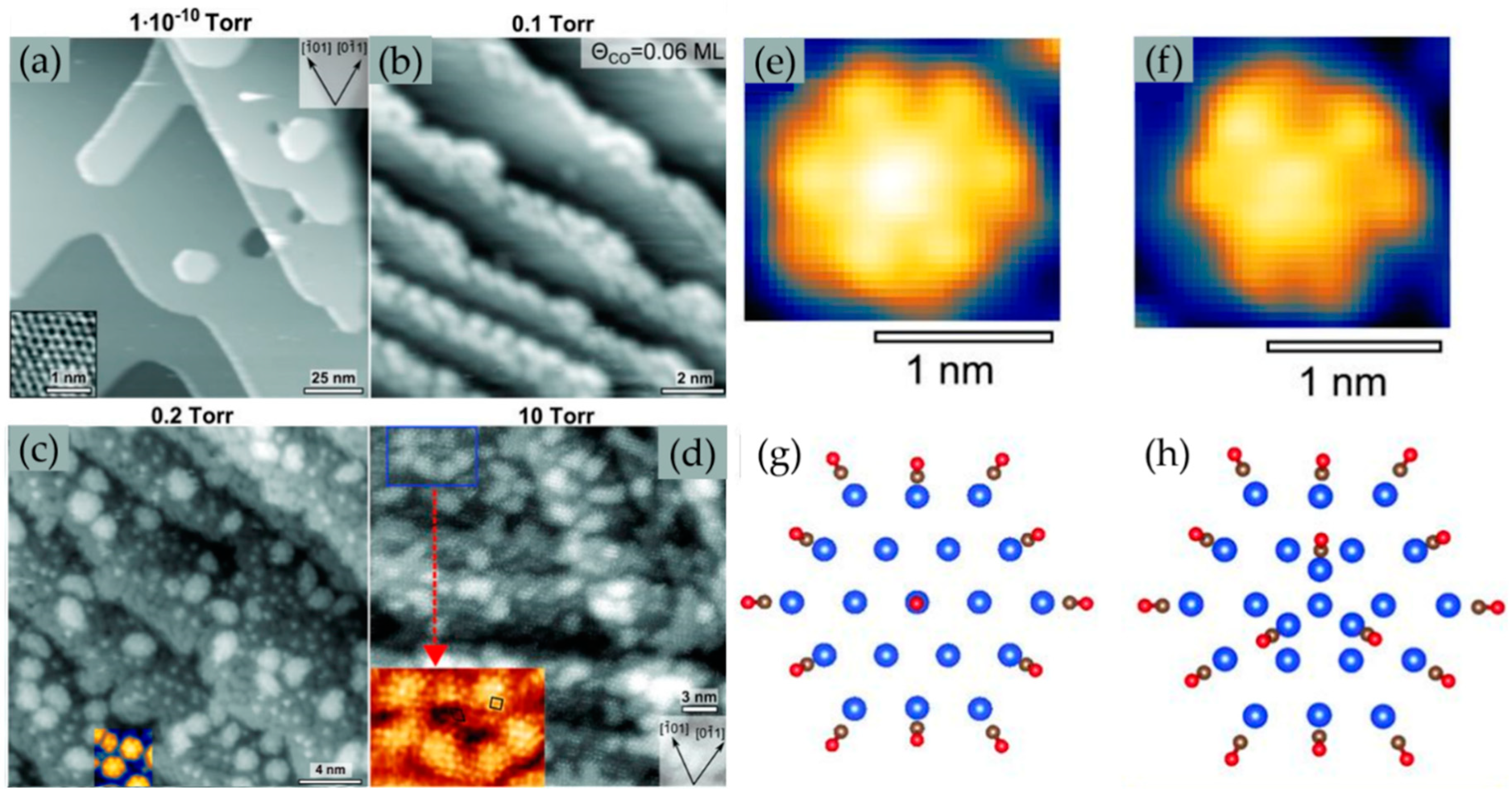
2.1.2. CO Catalytic Oxidation
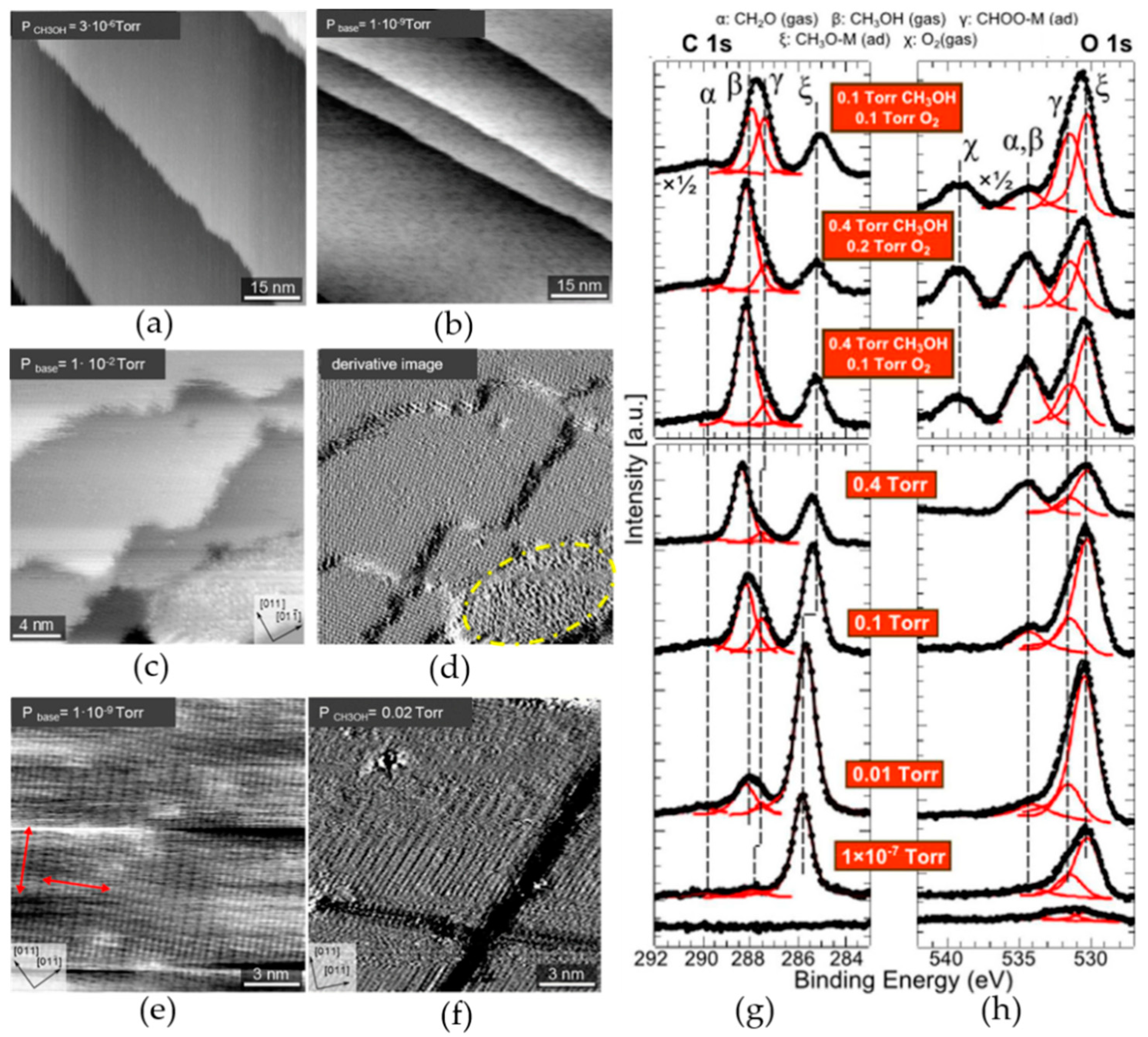
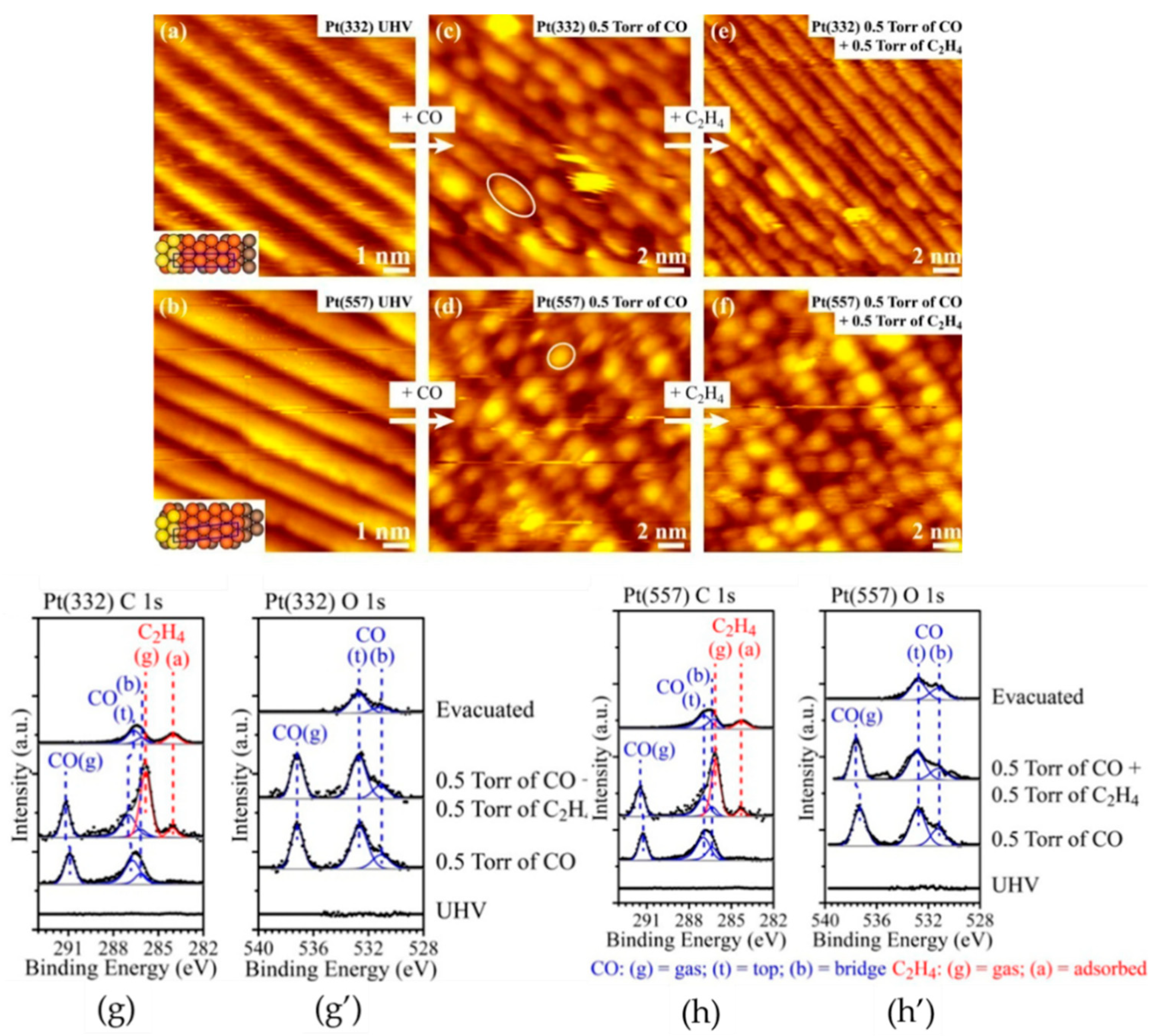
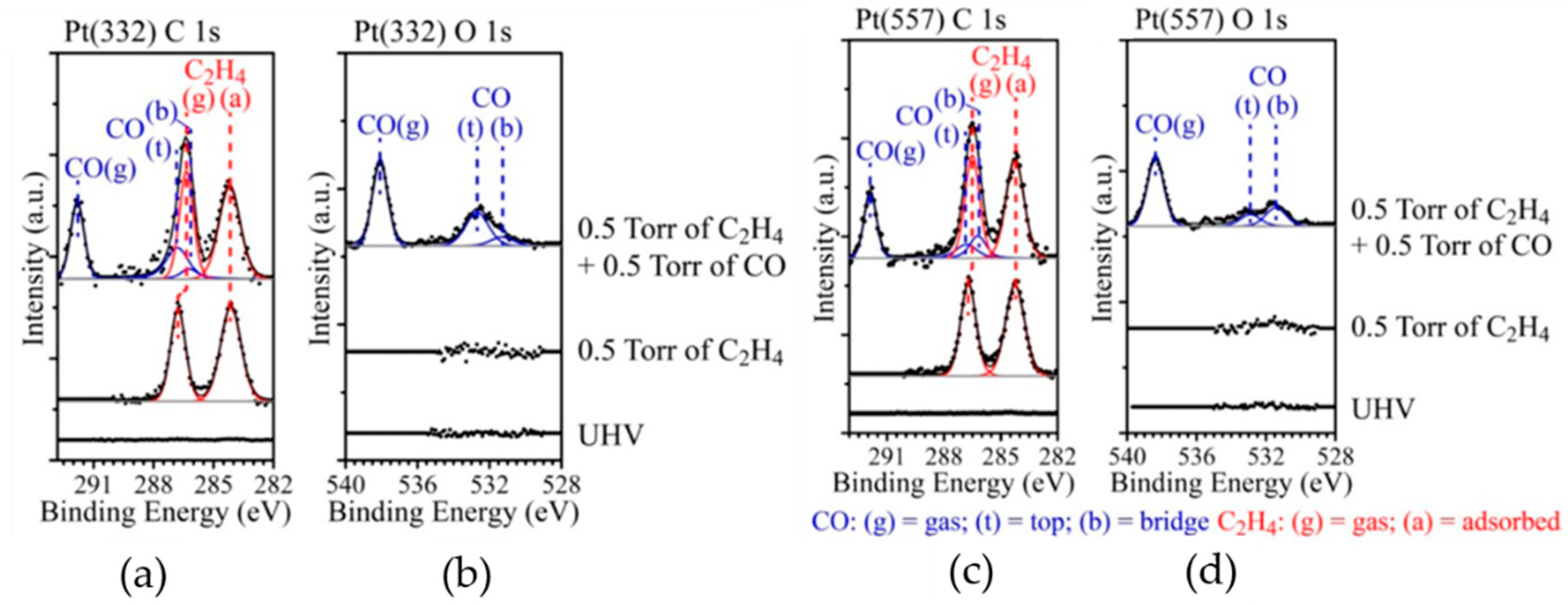
2.2. HP-STM Experiments of Dehydrogenation Reactions
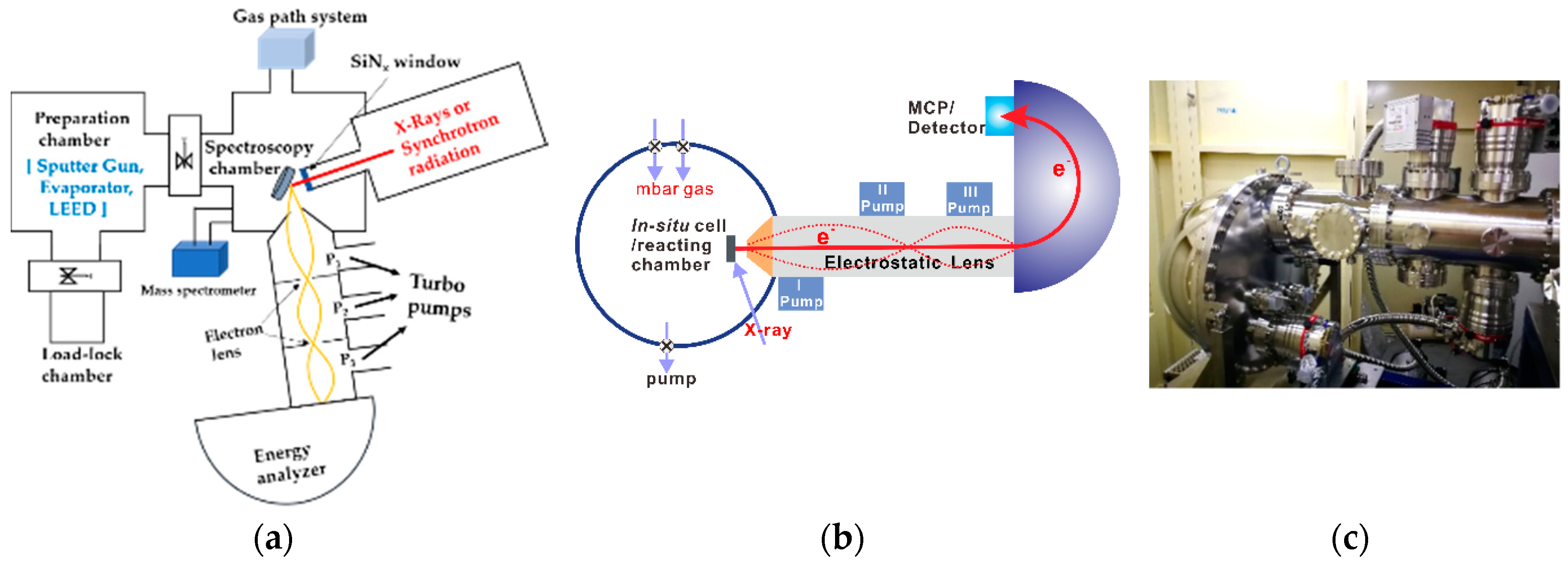
3. The Combination of HP-STM and AP-XPS
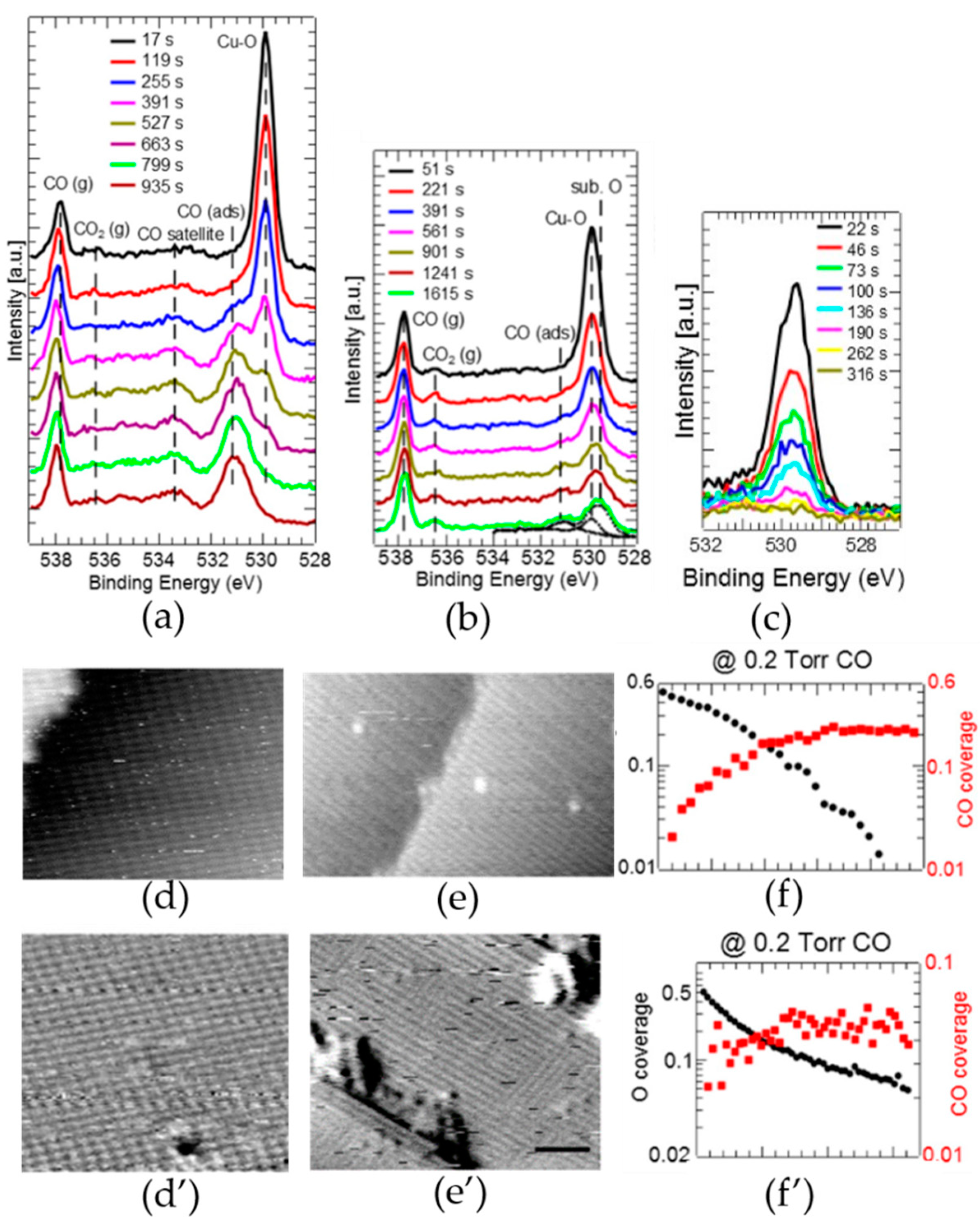
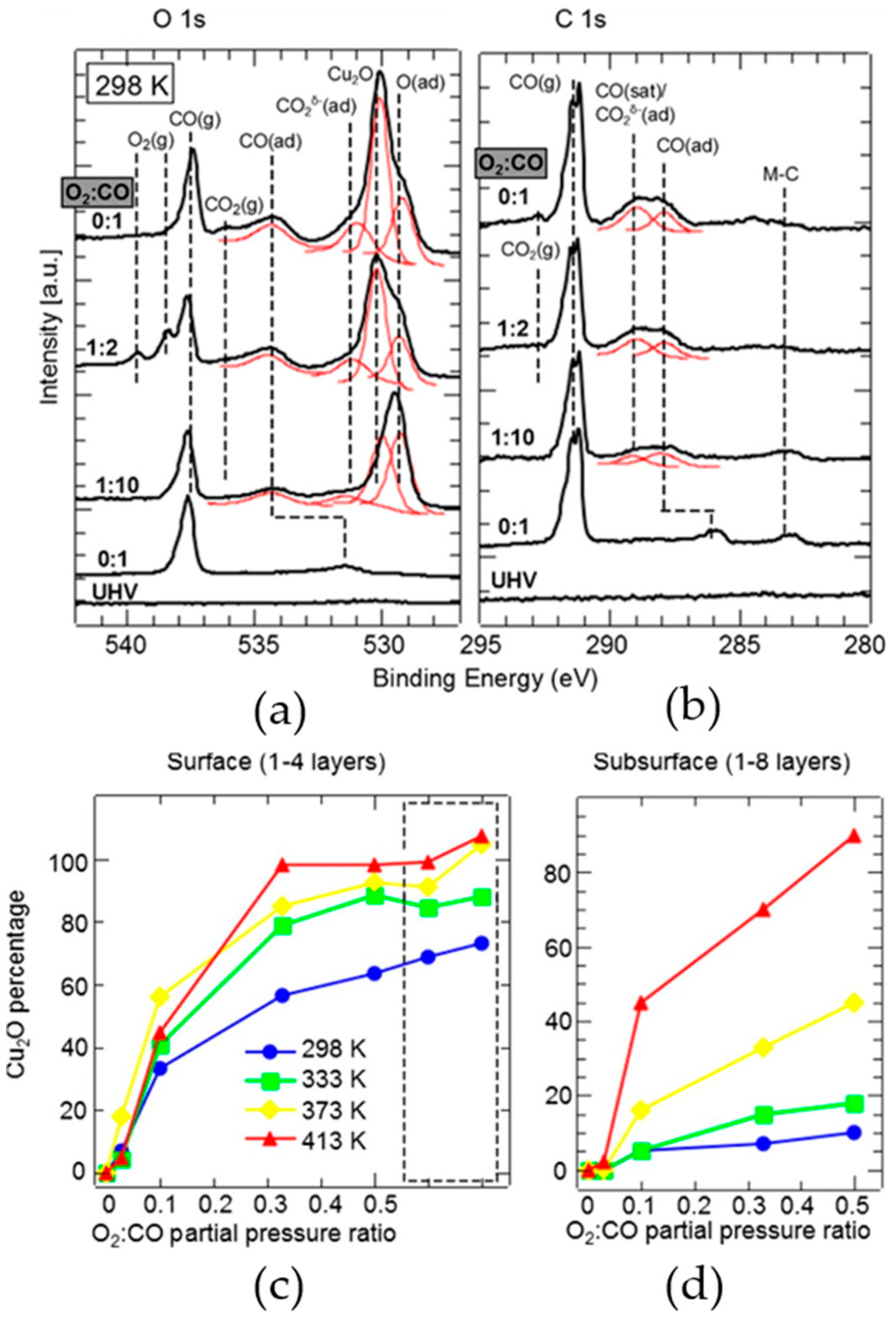
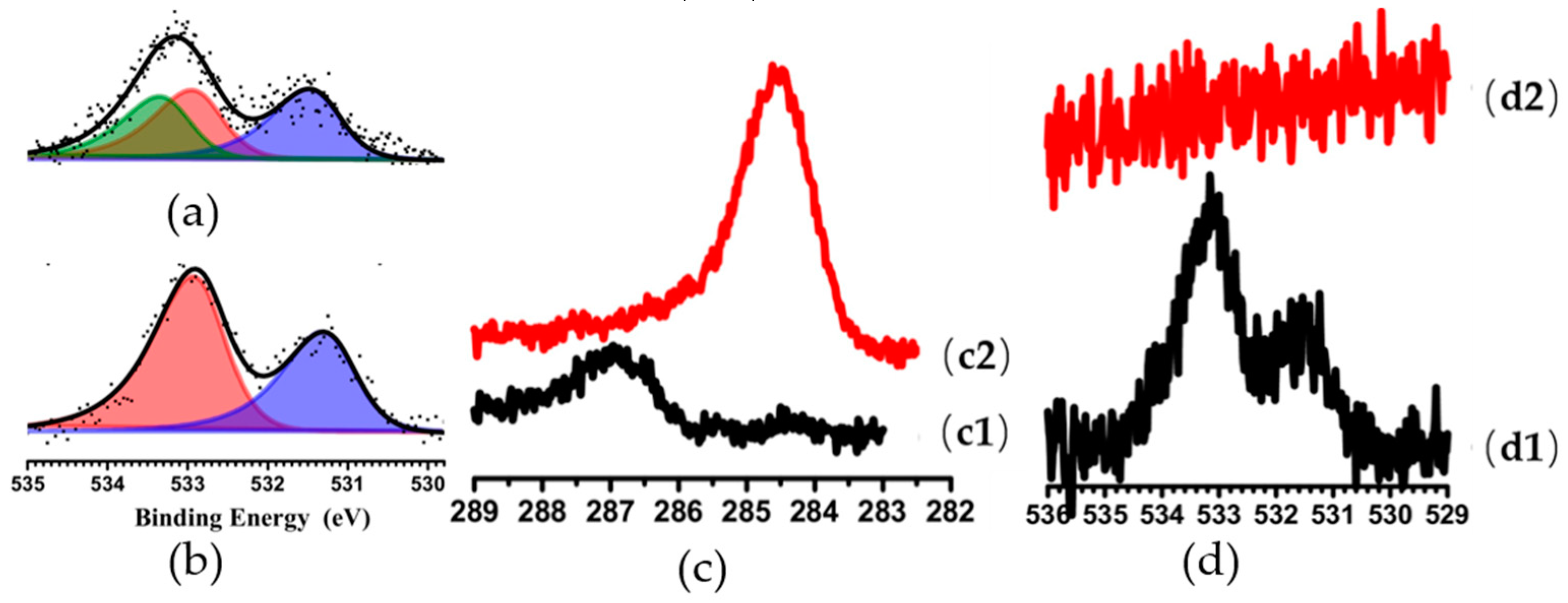
3.1. Investigations of Copper-Based Catalyst Reactions
3.2. Reactions on Platinum-Based Catalysts
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Aberle, A.G. Surface passivation of crystalline silicon solar cells: A review. Prog. Photovolt. Res. Appl. 2000, 8, 473–487. [Google Scholar] [CrossRef]
- Carra, P.; Thole, B.T.; Altarelli, M.; Wang, X. X-ray circular dichroism and local magnetic fields. Phys. Rev. Lett. 1993, 70, 694–697. [Google Scholar] [CrossRef] [PubMed]
- De Groot, F.M.F.; Grioni, M.; Fuggle, J.C.; Ghijsen, J.; Petersen, H.; Sawatzky, G.A. Oxygen 1 s x-ray-absorption edges of transition-metal oxides. Phys. Rev. B 1989, 40, 5715–5723. [Google Scholar] [CrossRef] [PubMed]
- McHale, J.M.; Auroux, A.; Perrotta, A.J.; Navrotsky, A. Surface Energies and Thermodynamic Phase Stability in Nanocrystalline Aluminas. Science 1997, 277, 788–791. [Google Scholar] [CrossRef]
- Shulman, G.R.; Yafet, Y.; Eisenberger, P.; Blumberg, W.E. Observations and interpretation of x-ray absorption edges in iron compounds and proteins. Proc. Natl. Acad. Sci. USA 1976, 73, 1384–1388. [Google Scholar] [CrossRef]
- Blanco, J.M.; Flores, F.; Pérez, R. STM-theory: Image potential, chemistry and surface relaxation. Prog. Surf. Sci. 2006, 81, 403–443. [Google Scholar] [CrossRef]
- Donald, A.M. The use of environmental scanning electron microscopy for imaging wet and insulating materials. Nat. Mater. 2003, 2, 511–516. [Google Scholar] [CrossRef]
- Fischer, Ø.; Berthod, C.; Renner, C.; Maggio-Aprile, I. Scanning tunneling spectroscopy of high-temperature superconductors. Rev. Mod. Phys. 2007, 79, 353–419. [Google Scholar] [CrossRef]
- Altman, E.I.; Tanner, R.E. Using scanning tunneling microscopy to characterize adsorbates and reactive intermediates on transition metal oxide surfaces. Catal. Today 2003, 85, 101–111. [Google Scholar] [CrossRef]
- Diebold, U. The surface science of titanium dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Barth, J.V.; Costantini, G.; Kern, K. Engineering atomic and molecular nanostructures at surfaces. Nature 2005, 437, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Dürr, M.; Biedermann, A.; Hu, Z.; Hofer, U.; Heinz, T.F. Probing high-barrier pathways of surface reactions by scanning tunneling microscopy. Science 2002, 296, 1838–1841. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gobbo, C.; Beurroies, I.; De Ridder, D.; Eelkema, R.; Marrink, S.J.; De Feyter, S.; Van Esch, J.H.; De Vries, A.H. MARTINI Model for Physisorption of Organic Molecules on Graphite. J. Phys. Chem. C 2013, 117, 15623–15631. [Google Scholar] [CrossRef]
- Gross, L.; Moll, N.; Mohn, F.; Curioni, A.; Meyer, G.; Hanke, F.; Persson, M. High-Resolution Molecular Orbital Imaging Using a p-Wave STM Tip. Phys. Rev. Lett. 2011, 107, 086101. [Google Scholar] [CrossRef] [PubMed]
- Hla, S.-W. Scanning tunneling microscopy single atom/molecule manipulation and its application to nanoscience and technology. J. Vac. Sci. Technol. B 2005, 23, 1351–1360. [Google Scholar] [CrossRef]
- Starr, D.E.; Liu, Z.; Hävecker, M.; Knop-Gericke, A.; Bluhm, H. Investigation of solid/vapor interfaces using ambient pressure X-ray photoelectron spectroscopy. Chem. Soc. Rev. 2013, 42, 5833–5857. [Google Scholar] [CrossRef] [PubMed]
- Imparato, C.; Fantauzzi, M.; Passiu, C.; Rea, I.; Ricca, C.; Aschauer, U.; Sannino, F.; D’Errico, G.; De Stefano, L.; Rossi, A.; et al. Unraveling the Charge State of Oxygen Vacancies in ZrO2–x on the Basis of Synergistic Computational and Experimental Evidence. J. Phys. Chem. C 2019, 123, 11581–11590. [Google Scholar] [CrossRef]
- Siegbahn, H.; Siegbahn, K. ESCA applied to liquids. J. Electron Spectrosc. Relat. Phenom. 1973, 2, 319–325. [Google Scholar] [CrossRef]
- Knudsen, J.; Andersen, J.N.; Schnadt, J. A versatile instrument for ambient pressure x-ray photoelectron spectroscopy: The Lund cell approach. Surf. Sci. 2016, 646, 160–169. [Google Scholar] [CrossRef]
- Ogletree, D.F.; Bluhm, H.; Lebedev, G.; Fadley, C.S. A differentially pumped electrostatic lens system for photoemission studies in the millibar range. Rev. Sci. Instrum. 2002, 73, 3872–3877. [Google Scholar] [CrossRef]
- Brown, M.A.; Redondo, A.B.; Jordan, I.; Duyckaerts, N.; Lee, M.-T.; Ammann, M.; Nolting, F.; Kleibert, A.; Huthwelker, T.; Machler, J.-P.; et al. A new endstation at the Swiss Light Source for ultraviolet photoelectron spectroscopy, X-ray photoelectron spectroscopy, and X-ray absorption spectroscopy measurements of liquid solutions. Rev. Sci. Instrum. 2013, 84, 073904. [Google Scholar] [CrossRef] [PubMed]
- Grass, M.E.; Karlsson, P.G.; Aksoy, F.; Lundqvist, M.; Wannberg, B.; Mun, B.S.; Hussain, Z.; Liu, Z. New ambient pressure photoemission endstation at Advanced Light Source beamline 9.3.2. Rev. Sci. Instrum. 2010, 81, 053106. [Google Scholar] [CrossRef] [PubMed]
- Hoshihara, Y.; Kimura, Y.; Matsumoto, M.; Nagasawa, M.; Terazima, M. An optical high-pressure cell for transient grating measurements of biological substance with a high reproducibility. Rev. Sci. Instrum. 2008, 79, 034101. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Crumlin, E.J.; Veith, G.M.; Harding, J.R.; Mutoro, E.; Baggetto, L.; Dudney, N.J.; Zhi, L.; Shao-Horn, Y. In Situ Ambient Pressure X-ray Photoelectron Spectroscopy Studies of Lithium-Oxygen Redox Reactions. Sci. Rep. 2012, 2, 715. [Google Scholar] [CrossRef]
- Pérez-Dieste, V.; Aballe, L.; Ferrer, S.; Nicolàs, J.; Escudero, C.; Milán, A.; Pellegrin, E. In Near Ambient Pressure XPS at ALBA. J. Phys. Conf. Ser. 2013, 425, 072023. [Google Scholar] [CrossRef]
- Velasco-Vélez, J.J.; Pfeifer, V.; Havecker, M.; Wang, R.; Centeno, A.; Zurutuza, A.; Algara-Siller, G.; Stotz, E.; Skorupska, K.; Teschner, D.; et al. Atmospheric pressure X-ray photoelectron spectroscopy apparatus: Bridging the pressure gap. Rev. Sci. Instrum. 2016, 87, 053121. [Google Scholar] [CrossRef]
- Kaya, S.; Ogasawara, H.; Näslund, L.Å.; Forsell, J.-O.; Casalongue, H.S.; Miller, D.J.; Nilsson, A. Ambient-pressure photoelectron spectroscopy for heterogeneous catalysis and electrochemistry. Catal. Today 2013, 205, 101–105. [Google Scholar] [CrossRef]
- Bluhm, H.; Hävecker, M.; Knop-Gericke, A.; Kiskinova, M.; Schlogl, R.; Salmeron, M. In Situ X-Ray Photoelectron Spectroscopy Studies of Gas-Solid Interfaces at Near-Ambient Conditions. MRS Bull. 2007, 32, 1022–1030. [Google Scholar] [CrossRef]
- Zhu, Z.; Tao, F.F.; Zheng, F.; Chang, R.; Li, Y.; Heinke, L.; Liu, Z.; Salmeron, M.; Somorjai, G.A. Formation of Nanometer-Sized Surface Platinum Oxide Clusters on a Stepped Pt(557) Single Crystal Surface Induced by Oxygen: A High-Pressure STM and Ambient-Pressure XPS Study. Nano Lett. 2012, 12, 1491–1497. [Google Scholar] [CrossRef]
- Liu, Q.; Han, Y.; Cao, Y.J.; Li, X.B.; Huang, W.G.; Yu, Y.; Yang, F.; Bao, X.H.; Li, Y.M.; Liu, Z. In situ AP-XPS and STM Study of the Activation of H-2 on ZnO(100) Surface. Acta Phys. Chim. Sin. 2018, 34, 1366–1372. [Google Scholar]
- Dulub, O.; Meyer, B.; Diebold, U. Observation of the Dynamical Change in a Water Monolayer Adsorbed on a ZnO Surface. Phys. Rev. Lett. 2005, 95, 136101. [Google Scholar] [CrossRef] [PubMed]
- Blanco, J.M.; González, C.; Jelinek, P.; Ortega, J.; Flores, F.; Pérez, R.; Rose, M.; Salmeron, M.; Méndez, J.; Wintterlin, J.; et al. Origin of contrast in STM images of oxygen on Pd(111) and its dependence on tip structure and tunneling parameters. Phys. Rev. B 2005, 71, 113402. [Google Scholar] [CrossRef]
- Rovida, G.; Pratesi, F.; Maglietta, M.; Ferroni, E. Chemisorption of oxygen on the silver (111) surface. Surf. Sci. 1974, 43, 230–256. [Google Scholar] [CrossRef]
- Carlisle, C.I.; Fujimoto, T.; Sim, W.S.; King, D.A. Atomic imaging of the transition between oxygen chemisorption and oxide film growth on Ag{1 1 1}. Surf. Sci. 2000, 470, 15–31. [Google Scholar] [CrossRef]
- Schnadt, J.; Knudsen, J.; Hu, X.L.; Michaelides, A.; Vang, R.T.; Reuter, K.; Li, Z.; Lægsgaard, E.; Scheffler, M.; Besenbacher, F. Experimental and theoretical study of oxygen adsorption structures on Ag(111). Phys. Rev. B 2009, 80, 075424. [Google Scholar] [CrossRef]
- Schnadt, J.; Michaelides, A.; Knudsen, J.; Vang, R.T.; Reuter, K.; Laegsgaard, E.; Scheffler, M.; Besenbacher, F. Revisiting the structure of the p(4 x 4) surface oxide on Ag(111). Phys. Rev. Lett. 2006, 96, 146101. [Google Scholar] [CrossRef]
- Helveg, S.; Li, W.X.; Bartelt, N.C.; Horch, S.; Laegsgaard, E.; Hammer, B.; Besenbacher, F. Role of surface elastic relaxations in an O-induced nanopattern on Pt(110)-(1 x 2). Phys. Rev. Lett. 2007, 98, 115501. [Google Scholar] [CrossRef]
- Czupryn, K.; Kocemba, I.; Rynkowski, J. Photocatalytic CO oxidation with water over Pt/TiO2 catalysts. React. Kinet. Mech. Catal. 2017, 124, 187–201. [Google Scholar] [CrossRef]
- Fujiwara, K.; Okuyama, K.; Pratsinis, S.E. Metal–support interactions in catalysts for environmental remediation. Environ. Sci. Nano 2017, 4, 2076–2092. [Google Scholar] [CrossRef]
- Iida, H.; Igarashi, A. Characterization of a Pt/TiO (rutile) catalyst for water gas shift reaction at low-temperature. Appl. Catal. A Gen. 2006, 298, 152–160. [Google Scholar] [CrossRef]
- Diemant, T.; Hartmann, H.; Bansmann, J.; Behm, R. CO adsorption energy on planar Au/TiO2 model catalysts under catalytically relevant conditions. J. Catal. 2007, 252, 171–177. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, Y.; Shangguan, W.; Jiang, Z. Influence of Support and Metal Precursor on the State and CO Catalytic Oxidation Activity of Platinum Supported on TiO2. J. Phys. Chem. C 2012, 116, 19396–19404. [Google Scholar] [CrossRef]
- Lu, J.-L.; Gao, H.-J.; Shaikhutdinov, S.; Freund, H.-J. Gold supported on well-ordered ceria films: Nucleation, growth and morphology in CO oxidation reaction. Catal. Lett. 2007, 114, 8–16. [Google Scholar] [CrossRef]
- Li, N.; Chen, Q.-Y.; Luo, L.-F.; Huang, W.-X.; Luo, M.-F.; Hu, G.-S.; Lu, J.-Q. Kinetic study and the effect of particle size on low temperature CO oxidation over Pt/TiO2 catalysts. Appl. Catal. B Environ. 2013, 142, 523–532. [Google Scholar] [CrossRef]
- Sannino, F.; Pernice, P.; Imparato, C.; Aronne, A.; D’Errico, G.; Minieri, L.; Perfetti, M.; Pirozzi, D. Hybrid TiO2–acetylacetonate amorphous gel-derived material with stably adsorbed superoxide radical active in oxidative degradation of organic pollutants. RSC Adv. 2015, 5, 93831–93839. [Google Scholar] [CrossRef]
- Branda, F.; Silvestri, B.; Costantini, A.; Luciani, G. Effect of exposure to growth media on size and surface charge of silica based Stöber nanoparticles: A DLS and ζ-potential study. J. Sol-Gel Sci. Technol. 2015, 73, 54–61. [Google Scholar] [CrossRef]
- Inukai, J.; Tryk, D.A.; Abe, T.; Wakisaka, M.; Uchida, H.; Watanabe, M. Direct STM Elucidation of the Effects of Atomic-Level Structure on Pt(111) Electrodes for Dissolved CO Oxidation. J. Am. Chem. Soc. 2013, 135, 1476–1490. [Google Scholar] [CrossRef]
- Rudnev, A.V.; Kuzume, A.; Fu, Y.; Wandlowski, T. CO Oxidation on Pt(100): New Insights based on Combined Voltammetric, Microscopic and Spectroscopic Experiments. Electrochim. Acta 2014, 133, 132–145. [Google Scholar] [CrossRef]
- Hendriksen, B.; Bobaru, S.; Frenken, J. Oscillatory CO oxidation on Pd(100) studied with in situ scanning tunneling microscopy. Surf. Sci. 2004, 552, 229–242. [Google Scholar] [CrossRef]
- Hendriksen, B.L.M.; Frenken, J.W.M. CO Oxidation on Pt(110): Scanning Tunneling Microscopy Inside a High-Pressure Flow Reactor. Phys. Rev. Lett. 2002, 89, 046101. [Google Scholar] [CrossRef]
- Li, H.; Weng, X.; Tang, Z.; Zhang, H.; Ding, D.; Chen, M.; Wan, H. Evidence of the Encapsulation Model for Strong Metal–Support Interaction under Oxidized Conditions: A Case Study on TiOx/Pt(111) for CO Oxidation by in Situ Wide Spectral Range Infrared Reflection Adsorption Spectroscopy. ACS Catal. 2018, 8, 10156–10163. [Google Scholar] [CrossRef]
- Peters, K.; Steadman, P.; Isern, H.; Alvarez, J.; Ferrer, S. Elevated-pressure chemical reactivity of carbon monoxide over Au(111). Surf. Sci. 2000, 467, 10–22. [Google Scholar] [CrossRef]
- Jugnet, Y.; Aires, F.C.S.; Deranlot, C.; Piccolo, L.; Bertolini, J. CO chemisorption on Au(110) investigated under elevated pressures by polarized reflection absorption infrared spectroscopy and scanning tunneling microscopy. Surf. Sci. 2002, 521, L639–L644. [Google Scholar] [CrossRef]
- Piccolo, L.; Loffreda, D.; Aires, F.C.S.; Deranlot, C.; Jugnet, Y.; Sautet, P.; Bertolini, J. The adsorption of CO on Au(111) at elevated pressures studied by STM, RAIRS and DFT calculations. Surf. Sci. 2004, 566, 995–1000. [Google Scholar] [CrossRef]
- Starr, D.E.; Shaikhutdinov, S.K.; Freund, H.-J. Gold Supported on Oxide Surfaces: Environmental Effects as Studied by STM. Top. Catal. 2005, 36, 33–41. [Google Scholar] [CrossRef]
- Han, P.; Akagi, K.; Canova, F.F.; Mutoh, H.; Shiraki, S.; Iwaya, K.; Weiss, P.S.; Asao, N.; Hitosugi, T. Bottom-Up Graphene-Nanoribbon Fabrication Reveals Chiral Edges and Enantioselectivity. ACS Nano 2014, 8, 9181–9187. [Google Scholar] [CrossRef]
- Sanchez, C.S.; Martinez, J.I.; Lanzilotto, V.; Biddau, G.; Gómez-Lor, B.; Pérez, R.; Floreano, L.; López, M.F.; Martín-Gago, J.Á. Chemistry and temperature-assisted dehydrogenation of C60H30 molecules on TiO2(110) surfaces. Nanoscale 2013, 5, 11058–11065. [Google Scholar] [CrossRef][Green Version]
- Gaudioso, J.; Lee, H.J.; Ho, W. Vibrational Analysis of Single Molecule Chemistry: Ethylene Dehydrogenation on Ni(110). J. Am. Chem. Soc. 1999, 121, 8479–8485. [Google Scholar] [CrossRef]
- Montano, M.; Salmeron, M.; Somorjai, G.A. STM studies of cyclohexene hydrogenation/dehydrogenation and its poisoning by carbon monoxide on Pt(111). Surf. Sci. 2006, 600, 1809–1816. [Google Scholar] [CrossRef]
- Vestergaard, E.K.; Vang, R.T.; Knudsen, J.; Pedersen, T.M.; An, T.; Lægsgaard, E.; Stensgaard, I.; Hammer, B.; Besenbacher, F. Adsorbate-Induced Alloy Phase Separation: A Direct View by High-Pressure Scanning Tunneling Microscopy. Phys. Rev. Lett. 2005, 95, 126101. [Google Scholar] [CrossRef]
- Longwitz, S.R.; Schnadt, J.; Vestergaard, E.K.; Vang, R.T.; Stensgaard, I.; Brune, H.; Besenbacher, F. High-Coverage Structures of Carbon Monoxide Adsorbed on Pt(111) Studied by High-Pressure Scanning Tunneling Microscopy. J. Phys. Chem. B 2004, 108, 14497–14502. [Google Scholar] [CrossRef]
- Croci, M.; Félix, C.; Vandoni, G.; Harbich, W.; Monot, R. Measurement of macroscopic diffusion of CO on Pt(111) by thermal helium scattering. Surf. Sci. 1993, 290, L667–L672. [Google Scholar] [CrossRef]
- Ma, J.; Xiao, X.; Dinardo, N.J.; Loy, M.M.T. Diffusion of CO on Pt(111) studied by an optical diffraction method. Phys. Rev. B 1998, 58, 4977–4983. [Google Scholar] [CrossRef]
- Eren, B.; Zherebetskyy, D.; Patera, L.L.; Wu, C.H.; Bluhm, H.; Africh, C.; Wang, L.-W.; Somorjai, G.A.; Salmeron, M. Activation of Cu(111) surface by decomposition into nanoclusters driven by CO adsorption. Science 2016, 351, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Eren, B.; Lichtenstein, L.; Wu, C.H.; Bluhm, H.; Somorjai, G.A.; Salmeron, M. Reaction of CO with Preadsorbed Oxygen on Low-Index Copper Surfaces: An Ambient Pressure X-ray Photoelectron Spectroscopy and Scanning Tunneling Microscopy Study. J. Phys. Chem. C 2015, 119, 14669–14674. [Google Scholar] [CrossRef]
- Eren, B.; Heine, C.; Bluhm, H.; Somorjai, G.A.; Salmeron, M. Catalyst Chemical State during CO Oxidation Reaction on Cu(111) Studied with Ambient-Pressure X-ray Photoelectron Spectroscopy and Near Edge X-ray Adsorption Fine Structure Spectroscopy. J. Am. Chem. Soc. 2015, 137, 11186–11190. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Prendergast, D.; Borondics, F.; Porsgaard, S.; Giovanetti, L.; Pach, E.; Newberg, J.; Bluhm, H.; Besenbacher, F.; Salmeron, M. Experimental and theoretical investigation of the electronic structure of Cu2O and CuO thin films on Cu(110) using x-ray photoelectron and absorption spectroscopy. J. Chem. Phys. 2013, 138, 024704. [Google Scholar] [CrossRef]
- Baber, A.E.; Xu, F.; Dvořák, F.; Mudiyanselage, K.; Soldemo, M.; Weissenrieder, J.; Senanayake, S.D.; Sadowski, J.T.; Rodríguez, J.A.; Matolín, V.; et al. In Situ Imaging of Cu2O under Reducing Conditions: Formation of Metallic Fronts by Mass Transfer. J. Am. Chem. Soc. 2013, 135, 16781–16784. [Google Scholar] [CrossRef]
- Eren, B.; Kersell, H.; Weatherup, R.S.; Heine, C.; Crumlin, E.J.; Friend, C.M.; Salmeron, M.B. Structure of the Clean and Oxygen-Covered Cu(100) Surface at Room Temperature in the Presence of Methanol Vapor in the 10-200 mTorr Pressure Range. J. Phys. Chem. B 2018, 122, 548–554. [Google Scholar] [CrossRef]
- Chen, P.; Kung, K.Y.; Shen, Y.R.; Somorjai, G.A. Sum frequency generation spectroscopic study of CO/ethylene coadsorption on the Pt( 111 ja:math ) surface and CO poisoning of catalytic ethylene hydrogenation. Surf. Sci. 2001, 494, 289–297. [Google Scholar] [CrossRef]
- Zhu, Z.; Barroo, C.; Lichtenstein, L.; Eren, B.; Wu, C.H.; Mao, B.; De Bocarmé, T.V.; Liu, Z.; Kruse, N.; Salmeron, M.; et al. Influence of Step Geometry on the Reconstruction of Stepped Platinum Surfaces under Coadsorption of Ethylene and CO. J. Phys. Chem. Lett. 2014, 5, 2626–2631. [Google Scholar] [CrossRef] [PubMed]
- Axnanda, S.; Scheele, M.; Crumlin, E.; Mao, B.; Chang, R.; Rani, S.; Faiz, M.; Wang, S.-D.; Alivisatos, A.P.; Liu, Z. Direct Work Function Measurement by Gas Phase Photoelectron Spectroscopy and Its Application on PbS Nanoparticles. Nano Lett. 2013, 13, 6176–6182. [Google Scholar] [CrossRef] [PubMed]
- Illas, F.; Zurita, S.; Rubio, J.; Márquez, A.M. Origin of the vibrational shift of CO chemisorbed on Pt(111). Phys. Rev. B 1995, 52, 12372–12379. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Tao, F.; Park, J.Y. The Nanoscience Revolution: Merging of Colloid Science, Catalysis and Nanoelectronics. Top. Catal. 2008, 47, 1–14. [Google Scholar] [CrossRef]
- Greeley, J.; Jaramillo, T.F.; Bonde, J.L.; Chorkendorff, I.; Nørskov, J.K. Computational high-throughput screening of electrocatalytic materials for hydrogen evolution. Nat. Mater. 2006, 5, 909–913. [Google Scholar] [CrossRef]
- Besenbacher, F.; Chorkendorff, I.; Clausen, B.S.; Hammer, B.; Molenbroek, A.M.; Nørskov, J.K.; Stensgaard, I. Design of a Surface Alloy Catalyst for Steam Reforming. Science 1998, 279, 1913–1915. [Google Scholar] [CrossRef]
- Tian, G.; Shen, Y.; He, B.; Yu, Z.Q.; Song, F.; Lu, Y.H.; Wang, P.S.; Gao, Y.L.; Huang, H. Effects of Monolayer Bi on the Self-Assembly of DBBA on Au(111). Surf. Sci. 2017, 665, 89–95. [Google Scholar] [CrossRef]
- He, B.C.; Tian, G.; Gou, J.; Liu, B.X.; Shen, K.C.; Tian, Q.W.; Yu, Z.Q.; Song, F.; Xie, H.P.; Gao, Y.L.; et al. Structural and electronic properties of atomically thin Bismuth on Au(111). Surf. Sci. 2019, 679, 147–153. [Google Scholar] [CrossRef]
- Sun, H.L.; Liang, Z.F.; Shen, K.C.; Luo, M.; Hu, J.B.; Huang, H.; Zhu, Y.Y.; Li, Z.J.; Jiang, Z.; Song, F. Fabrication of NiSe2 by direct selenylation of a nickel surface. Appl. Surf. Sci. 2018, 428, 623–629. [Google Scholar] [CrossRef]
- Shen, K.; Narsu, B.; Ji, G.; Sun, H.; Hu, J.; Liang, Z.; Gao, X.; Li, H.; Li, Z.; Song, F. On-surface manipulation of atom substitution between cobalt phthalocyanine and the Cu(111) substrate. RSC Adv. 2017, 7, 13827–13835. [Google Scholar] [CrossRef]
- Zeng, S.; Nguyen, L.; Cheng, F.; Liu, L.; Yu, Y.; Tao, F. Surface structure and chemistry of Pt/Cu/Pt(111) near surface alloy model catalyst in CO. Appl. Surf. Sci. 2014, 320, 225–230. [Google Scholar] [CrossRef]
- Jeff, G.; Mavrikakis, M. Alloy catalysts designed from first principles. Cheminform 2004, 36, 810–815. [Google Scholar]
- Zhang, S.; Shan, J.; Zhu, Y.; Nguyen, L.; Huang, W.; Yoshida, H.; Takeda, S.; Tao, F. Restructuring Transition Metal Oxide Nanorods for 100% Selectivity in Reduction of Nitric Oxide with Carbon Monoxide. Nano Lett. 2013, 13, 3310–3314. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.; Nilekar, A.U.; Vang, R.T.; Schnadt, J.; Kunkes, E.L.; Dumesic, J.A.; Mavrikakis, M.; Besenbacher, F. A Cu/Pt Near-Surface Alloy for Water−Gas Shift Catalysis. J. Am. Chem. Soc. 2007, 129, 6485–6490. [Google Scholar] [CrossRef]
- Andersson, K.J.; Calle-Vallejo, F.; Rossmeisl, J.; Chorkendorff, I. Adsorption-Driven Surface Segregation of the Less Reactive Alloy Component. J. Am. Chem. Soc. 2009, 131, 2404–2407. [Google Scholar] [CrossRef]
- Tao, F.; Dag, S.; Wang, L.-W.; Liu, Z.; Butcher, D.R.; Bluhm, H.; Salmeron, M.; Somorjai, G.A. Break-Up of Stepped Platinum Catalyst Surfaces by High CO Coverage. Science 2010, 327, 850–853. [Google Scholar] [CrossRef]
- Kinne, M.; Führmann, T.; Whelan, C.M.; Zhu, J.F.; Pantforder, J.; Probst, M.; Held, G.; Denecke, R.; Steinrück, H.-P. Kinetic parameters of CO adsorbed on Pt(111) studied by in situ high resolution x-ray photoelectron spectroscopy. J. Chem. Phys. 2002, 117, 10852–10859. [Google Scholar] [CrossRef]
- Montano, M.; Bratlie, K.; Salmeron, M.; Somorjai, G.A. Hydrogen and Deuterium Exchange on Pt(111) and Its Poisoning by Carbon Monoxide Studied by Surface Sensitive High-Pressure Techniques. J. Am. Chem. Soc. 2006, 128, 13229–13234. [Google Scholar] [CrossRef]
- Björneholm, O.; Nilsson, A.; Tillborg, H.; Bennich, P.; Sandell, A.; Hernnäs, B.; Puglia, C.; Mårtensson, N. Overlayer structure from adsorbate and substrate core level binding energy shifts: CO, CCH3 and O on Pt(111). Surf. Sci. 1994, 315, L983–L989. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Sun, H.; Shen, K.; Hu, J.; Hu, J.; Jiang, Z.; Song, F. Recent Progress with In Situ Characterization of Interfacial Structures under a Solid–Gas Atmosphere by HP-STM and AP-XPS. Materials 2019, 12, 3674. https://doi.org/10.3390/ma12223674
Zhang H, Sun H, Shen K, Hu J, Hu J, Jiang Z, Song F. Recent Progress with In Situ Characterization of Interfacial Structures under a Solid–Gas Atmosphere by HP-STM and AP-XPS. Materials. 2019; 12(22):3674. https://doi.org/10.3390/ma12223674
Chicago/Turabian StyleZhang, Huan, Haoliang Sun, Kongchao Shen, Jinping Hu, Jinbang Hu, Zheng Jiang, and Fei Song. 2019. "Recent Progress with In Situ Characterization of Interfacial Structures under a Solid–Gas Atmosphere by HP-STM and AP-XPS" Materials 12, no. 22: 3674. https://doi.org/10.3390/ma12223674
APA StyleZhang, H., Sun, H., Shen, K., Hu, J., Hu, J., Jiang, Z., & Song, F. (2019). Recent Progress with In Situ Characterization of Interfacial Structures under a Solid–Gas Atmosphere by HP-STM and AP-XPS. Materials, 12(22), 3674. https://doi.org/10.3390/ma12223674




